Introduction
Monoclonal antibody-based treatment of cancer has been established as one of the most successful therapeutic strategies for both hematologic malignancies and solid tumors in the last 20 years. The initial combining of serological techniques for cancer cell surface antigen discovery with hybridoma technology led to a series of landmark clinical trials that paved the way for new generation antibodies and subsequent clinical success. Optimization of anti-tumor immune responses through Fc modifications has also made a major contribution to clinical efficacy. The modulation of immune system interplay with tumor cells through targeting of T cell receptors has emerged as a powerful new therapeutic strategy for tumor therapy and to enhance cancer vaccine efficacy. This commentary will provide an overview of the history of antibody identification of tumor surface antigens, antigenic targets suitable for antibody-based therapy, antibody mechanisms of action, and recent successes of antibodies in the clinic.
Cancer serology - the prelude to antibody therapeutics
The concept that antibodies could serve as ‘magic bullets’ in the diagnosis and therapy of cancer dates back to their discovery in the late 19th century. A considerable effort over the ensuing decades involved immunization of a variety of animal species with human cancer in the hope of generating antisera with some degree of cancer specificity (1). Unfortunately, this approach had limited early success, with the notable exception of the discovery of carcinoembryonic antigen (CEA), a marker for colon and other cancers, and α-fetoprotein, a marker for hepatocellular cancer (1, 2).
The development of inbred mice initiated a new era of serological investigation of cancer with the emergence of the cytotoxic test as a powerful tool to analyze the cell surface reactivity of alloantibodies. This subsequently led to the recognition that the cell surface is a highly differentiated structure. During the 1960s and 1970s, Lloyd Old made a series of discoveries that revolutionized our understanding of the immune system. In collaboration with Ted Boyse, he introduced the concept of cell surface differentiation antigens that could distinguish lineage and functional subsets of leukocytes in mice (3). This led to major contributions at the time which include the discovery of the thymus-leukemia (TL) antigen, the linking of the major histocompatibility complex (MHC) and leukemia, and subsequently the Ly series of antigens (4). These discoveries led to the precise and systematic identification of cell surface antigens that distinguished normal cells from malignant cells, and directly to the cluster of differentiation (CD) classification.
Following the development of hybridoma technology by Köhler and Milstein (5), combined with serological techniques and analytical tools such as fluorescence-activated cell sorting (FACS), monoclonal antibodies (mAbs) were used to dissect the surface structure of human cancer cells, thus paving the way for the identification of cancer cell surface antigens suitable for targeting by antibodies. The characterization of the cancer cell “surfaceome” has been enhanced in recent times with proteomic, genomic, and bioinformatic approaches to identifying antigen targets on cancer cells, as well as in cancer stroma and vasculature.
Tumor antigens as targets for antibody therapy
The selection of tumor antigens suitable for antibody targeting and therapy requires a comprehensive analysis of tumor expression (including homogeneity of expression) and normal tissue expression, as well as an understanding of the biologic role of the antigen in tumor growth. If the desired mechanism of action is engagement with cell surface receptors (to either activate or inhibit signaling), or to activate antibody-dependent cell-mediated cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC), then it is desirable that the antigen-mAb complex should not be rapidly internalized. This allows the maximization of the availability of the Fab region to appropriately engage with surface receptors, and of the Fc region to immune effector cells and complement proteins. In contrast, internalization is desirable for antibodies or proteins delivering toxins into the cancer cell and for antibodies whose action is primarily based on downregulation of cell surface receptors (2).
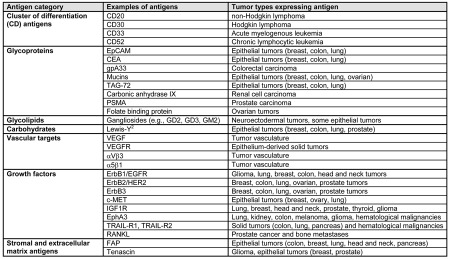
Tumor-associated antigens recognized by therapeutic mAbs are outlined in Table 1. Hematopoietic differentiation antigens are glycoproteins usually associated with CD groupings and include CD20, CD30, CD33, and CD52 (2, 6–8). Cell surface differentiation antigens represent a diverse group of glycoproteins and carbohydrates that are found on the surface of both normal and tumor cells. Growth factors that are targets for antibodies in oncology patients include CEA (2), epidermal growth factor receptor (EGFR; also known as ErbB1) (9), ErbB2 (also known as HER2) (10), ErbB3 (11), MET (12), insulin-like growth factor 1 receptor (IGF1R) (13), ephrin receptor A3 (EphA3) (14), TNF receptor apoptosis-inducing ligand receptor 1 (TRAIL-R1), TRAIL-R2, and receptor activator of nuclear factor κβ ligand (RANKL) (15). Antigens involved in angiogenesis are usually proteins or growth factors that support the formation of new microvasculature, including vascular endothelial growth factor (VEGF), VEGF receptor (VEGFR), and integrins αVβ3 and α5β1 (16). Stromal and extracellular matrix antigens that are therapeutic targets include fibroblast activation protein (FAP) and tenascin (17–19).
Table 1.
Tumor-associated antigens targeted by monoclonal antibodies
Antibody engineering and mechanisms of action
The development of hybridoma technology led to the first generation of murine antibodies against tumor cell surface antigens. Following initial clinical trials in the 1980s with murine antibodies against CEA and CD3, a range of antibodies against solid tumor and hematologic malignancies were developed and entered clinical trials (20). The development of immune responses against these murine antibodies (human anti-mouse antibodies, HAMA) significantly limited their clinical utility, and as a consequence, apart from the FDA-approved 131I-anti-CD20 antibody tositumomab, and 90Y-anti-CD20 antibody ibritumomab tiuxetan, murine antibodies were not further pursued (20).
The development of humanization approaches by Winter and colleagues (21), whereby murine Fc and Fv framework regions of murine antibodies were replaced by human germline amino acids, revolutionized the field of antibody therapeutics. Through this technology, minimal immune responses to antibodies were observed, allowing the multiple infusions of engineered antibodies, leading to the successful entry of multiple antibodies into the clinic (6, 22). Additional strategies to generate fully human antibodies through phage display techniques, as well as the use of transgenic mice that produce fully human antibodies, have also been successfully implemented (17). More recently, innovative antibody engineering approaches to produce smaller antibody variants, fusion proteins, and bispecific antibodies have been utilized (6, 7, 22). Combined with improved cell line generation and larger scale production techniques, the transition from laboratory scale preclinical testing to large clinical trial batch production has been considerably enhanced.
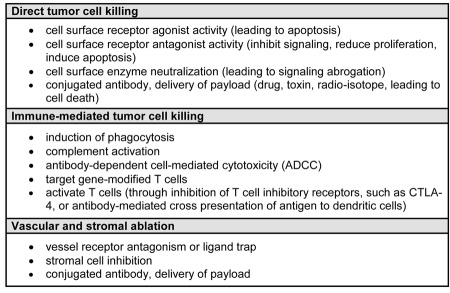
The mechanisms of tumor cell killing by antibodies are outlined in Table 2. These can be due to direct cell killing, such as through receptor blockade or agonist activity, induction of apoptosis, or delivery of a drug, radiation, or cytotoxic agent; immune-mediated cell killing mechanisms; regulation of T cell function; and specific effects on tumor vasculature and stroma. The Fc function of antibodies is particularly important in mediating tumor cell killing through CDC, and immune cell activation (e.g., NK cells) and tumor cell killing through ADCC. The abrogation of tumor cell signaling (e.g., cetuximab and trastuzumab) (9, 10), effector function primarily through ADCC (e.g., rituximab) (23), and immune modulation of T cell function (e.g., ipilimumab) (24) are the approaches that have been most successful and have led to approval of antibodies using these effector mechanisms.
Table 2.
Mechanisms of tumor cell killing by antibodies
“In vivo veritas” - translation of antibodies into the clinic
While the identification of novel target antigens expressed in tumors and the generation of antibodies against these antigens with optimal functional characteristics is the important initial step in developing a potential therapeutic antibody, there are many issues to address before embarking on clinical trials in cancer patients. These include the physical and chemical properties of the antibody, a detailed specificity analysis of antigen expression using panels of normal and malignant tissues, and immune effector function and signaling pathway effects of antibodies. In addition, antibody humanization and in vivo therapeutic activity of the antibody, either alone or conjugated with radioactive isotopes or other toxic agents, are essential in the preclinical evaluation of antibodies (6, 8, 17, 25–29).
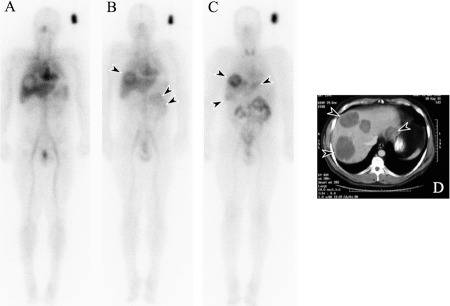
One of the most essential steps in the clinical evaluation of a potential therapeutic antibody is in vivo specificity—determining the biodistribution of antibody (often radiolabeled) in patients to assess the ratio of antibody uptake in the tumor in relation to normal tissues (18, 25, 29) (Figure 1). This information is essential for the design for clinical trials, as knowledge about the targeting of normal tissues is critical for predicting toxicity and determining optimal antibody dose and schedule (8, 29). Under the leadership of Lloyd Old, at the Ludwig Institute for Cancer Research (LICR), we developed a model of a phase I antibody clinical trial that incorporates biodistribution, pharmacokinetics, and pharmacodynamics analyses with toxicity assessment (25). This trial design has been successfully applied to first-in-human clinical trials of more than 15 antibodies in cancer patients (18, 19, 25, 29–34). This approach can identify subtle changes in antibody physicochemical properties (28) that affect biodistribution, which can significantly impact efficacy. In addition, normal tissue and tumor distribution can be quantitated, thus allowing the relationship of the loading dose to tumor concentration to be accurately assessed, rather than relying on plasma concentration and clearance rates to establish an optimal dose. Examples of where this approach was successfully used include the early biodistribution studies of mouse anti-colon cancer antibody A33 (33), the anti-CD33 antibody M195 (30), anti-CAIX antibody G250 (34), anti-FAP antibody F19 (19), anti-GD3 antibody KM871 (31), and anti-Ley antibody hu3S193 (32). This approach has also been applied to recent studies of trastuzumab (which targets ErbB2) biodistribution and in vivo assessment of ErbB2 expression by tumors (35). In non-Hodgkin lymphomas, assessment of the biodistribution of a radioconjugate in both the tumor and through whole body dosimetry was essential in initial trials exploring patient suitability for treatment and treatment dose for the United States Food and Drug Administration (FDA)-approved anti-CD20 radioimmunoconjugates tositumumab and ibritumomab tiuxetan (8, 28, 36).
Figure 1.
Biodistribution of 131I-huA33 in a patient with metastatic colorectal carcinoma. Anterior whole body gamma camera images are shown following infusion of 131I-huA33 at (A) day 0, (B) day 1, and (C) day 5. A standard for quantitation of 131I-huA33 uptake is present, adjacent to the left shoulder. Initial (day 0) images (A) show blood pool appearance only, with large metastatic lesions in the liver demonstrating an initial hypovascular appearance. (B) Excellent targeting of the metastatic lesions in the liver by 131I-huA33 is clearly seen (arrow) as early as day 1, and increasing rapidly with time to day 5. Some central necrosis in the larger tumors is also evident (arrow), also seen on CT scan (D). Gradual bowel uptake (double arrow) of 131I-huA33 is also seen, which gradually decreases with time. No other normal tissue uptake of 131I-huA33 is evident.
The use of patient biopsies can also be utilized to assess the in vivo effect of antibody abrogation of signaling pathways (36). The evaluation of pharmacodynamics in early-phase clinical trials can also involve biological effector function of antibodies, such as ADCC (through optimized FcγR binding) and cytotoxicity (26). The assessment of antibodies as delivery vehicles for toxic agents can also be assessed using this clinical trial design approach (8, 26–29).
Success of antibodies in the clinic
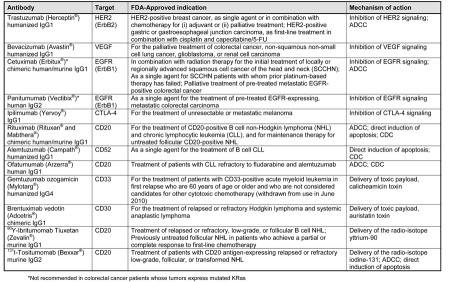
There have been twelve antibodies that have received approval from the FDA for the treatment of a variety of solid tumors and hematological malignancies (Table 3). In addition, there are a large number of additional therapeutic antibodies that are currently being tested in early- and late-stage clinical trials. The use of therapeutic antibodies in patients with solid tumors has been most successful with classes of antibodies targeting the ErbB family (which includes EGFR) and VEGF (9, 16, 20, 37–39). Recent evidence shows that patients with colorectal cancer bearing wild-type KRas tumors who were treated with anti-EGFR antibodies have improved responses (9, 40), disease control (40), and survival (41, 42). These findings have resulted in the FDA-approved use of these agents restricted to patients with colorectal cancer in which KRas is not mutated. The use of trastuzumab has also been restricted to patients with high levels of ErbB2 expression, as studies have shown that this is the group that derives maximum benefit from trastuzumab treatment (6, 10). As a result of the clinical success of these antibodies and preclinical data demonstrating the improved tumor response (and reversal of resistance to single agent) of combined signaling blockade with antibodies to different receptors, or to different epitopes on the same receptor (e.g., trastuzumab and pertuzumab), numerous clinical trials of antibodies as combination therapies are currently under way (20).
Table 3.
Monoclonal antibodies currently FDA-approved in oncology
A number of antibodies have also been approved for the treatment of hematological malignancies, both as unconjugated antibodies and for delivery of isotopes and drugs or toxins to cancer cells (Table 3). Antibody-drug or -toxin conjugates have been shown to have high potency in hematological malignancies, and there have been two approved by the FDA: gemtuzumab ozogamicin in elderly patients with CD33-positive AML (although this drug was voluntarily withdrawn in June 2010 following a post-marketing phase III trial), and more recently brentuximab vedotin in patients with CD30-positive Hodgkin lymphoma (27, 43). A similar approach in patients with advanced ErbB2-positive breast cancer with the antibody-drug conjugate trastuzumab-emtansine (T-DM1) (44) is currently being explored in phase III trials.
There are other antibodies approved for cancer indications outside the U.S. Catumaxomab, a mouse bispecific antibody against CD3 and EpCAM, is approved in the European Union for use in patients with malignant ascites generated by an EpCAM-positive tumor (45). Nimotuzumab, a humanized IgG antibody against EGFR, is approved for use in some countries in Asia, South America, and Africa for the treatment of head and neck cancer, glioma, and nasopharyngeal cancer (46). Vivatuxin (131I-chTNT), a radiolabeled IgG1κ chimeric monoclonal antibody against intracellular DNA-associated antigens, has also been approved by the Chinese drug regulator for the treatment of malignant lung cancer (47).
Immune regulation by antibodies
In addition to targeting antigens involved in cancer cell physiology, antibodies can also function to modulate immunological pathways that are critical to immune surveillance. Antigen-specific immune responses result from a complex dynamic interplay between antigen presenting cells, T lymphocytes, and target cells. Immunologic signal 1, the recognition of specific antigenic peptides bound to MHC by the T cell receptor (TCR) is insufficient for T cell activation. Signal 2, ligation of CD28 by a member of the B7 family of costimulatory molecules (CD80, CD86), initiates T cell activation via signaling pathways resulting in autocrine IL-2 production. Just after T cell activation, CTLA-4, a molecule normally found in intracellular stores, translocates to the immunologic synapse, where it serves to inhibit the activated T cell by binding with high avidity to the same B7 molecules and stopping activation signals mediated by CD28. The role for blockade of CTLA-4 with an antibody as a means to potentiating T cell activation and initiating responses to targets on tumor cells was proposed in 1996 (48) and provided the scientific foundation for the development of two fully human monoclonal antibodies blocking CTLA-4 (ipilimumab and tremelimumab). Ipilimumab was approved by the U.S. FDA, European Medicines Agency (EMA), and a number of other national regulatory agencies for treatment of patients with metastatic melanoma after a pivotal phase III trial demonstrated significant improvement in overall survival resulting from its use, making it the first treatment to be shown to enhance survival and the first newly approved medicine in 13 years for melanoma (24). CTLA-4 blockade does present new paradigms in terms of treatment-related toxicity. The immune-related adverse events are inflammatory and largely confined to the skin and gastrointestinal tract but can more rarely affect the liver and endocrine glands. With prompt diagnosis, these events are generally manageable with immunosuppressive medications such as corticosteroids, which fortunately do not seem to interfere with the anti-tumor effect (24).
The therapeutic success of ipilimumab has led to enthusiasm for the development of other immune modulating antibodies. The next most advanced products target PD-1, a marker of activated or exhausted T cells that can trigger apoptosis when bound by its ligand, PD-L1 (B7-H1) (49). Interestingly, this ligand is found not only on antigen presenting cells, but also on many tumor cells. PD-1 blockade has been shown in early clinical trials to result in durable responses in patients with melanoma, renal cell carcinoma, non-small cell lung cancer, and colorectal cancer (49). Several antibodies that target the PD-1 axis are in development. Agonistic antibodies are also being explored, including two fully human antibodies to CD137 (4-1BB), an activator of T cells, from Pfizer and Bristol-Myers Squibb (BMS). The BMS antibody has been in phase I trials, demonstrating anti-tumor efficacy across a wide dose range. Trials were temporarily suspended due to severe hepatic toxicity at high doses but are now opening again using low doses. This highlights an important aspect of antibody drug development as higher doses of a blocking antibody may yield better therapeutic results while low doses of agonistic antibodies may allow for a better risk-benefit profile. Other pathways of interest for agonistic antibodies include CD40, where favorable preclinical and clinical results have been noted particularly in pancreatic cancer (50), and the glucocorticoid-induced TNF receptor (GITR).
Antibody therapeutics might also have a role in generation of de novo immune responses to the antigen targeted by the antibody through promoting antigen presentation to Fc receptor-bearing cells (51–53). De novo induction of secondary immune responses may therefore allow for the effects of antigen-specific antibodies to persist after the dosing is completed.
Conclusion
The use of monoclonal antibodies for the therapy of cancer is one of the major contributions of tumor immunology to cancer patients. This success is built on decades of scientific research aimed at serological characterization of cancer cells, techniques for generating optimized antibodies to tumor targets, detailed investigation of signaling pathways relevant to cancer cells, and an understanding of the complex interplay between cancer cells and the immune system (20, 54). The clinical development of antibodies is inextricably linked to disciplined and detailed exploration of the properties of antibodies in vivo and assessment of functional effects on cancer cells. One of our major challenges is now to fully exploit antibody therapies in cancer patients by combining the two major immune-based treatment approaches—antibodies and vaccines. Trials combining ipilimumab with vaccines have shown mixed results thus far (24, 55). The Cancer Vaccine Collaborative, a joint academic clinical trials infrastructure established by LICR and the Cancer Research Institute (CRI), is about to embark on a series of trials exploring NY-ESO-1 vaccines along with ipilimumab to further investigate this important area. In this way, the full promise of tumor immunology in controlling and treating cancer will hopefully be realized.
Acknowledgments
A.M.S. is supported by the Ludwig Institute for Cancer Research (LICR), NHMRC grants 487922 and 1030469, and OIS funding from the Victorian Government. J.P.A. has been supported by the Ludwig Center at MSKCC and the National Institutes of Health. J.D.W. is supported by LICR, the Cancer Research Institute (CRI), National Institutes of Health, Swim Across America, and the Melanoma Research Alliance.
Abbreviations
- ADCC
antibody-dependent cell-mediated cytotoxicity;
- MHC
major histocompatibility complex
References
- 1.Rettig WJ, Old LJ. Immunogenetics of human cell surface differentiation. Annu Rev Immunol. 1989;7:481–511. doi: 10.1146/annurev.iy.07.040189.002405. [DOI] [PubMed] [Google Scholar]
- 2.Van den Eynde BJ, Scott AM. Tumor Antigens. Encyclopedia of Immunology. 1998:2424–2431. [Google Scholar]
- 3.Old LJ, Boyse EA, Stockert E. Typing of mouse leukemias by serological methods. Nature. 1964;22:777–779. doi: 10.1038/201777a0. [DOI] [PubMed] [Google Scholar]
- 4.Boyse EA, Old LJ, Luell S. Genetic determination of the TL (Thymus-leukemia) antigen in the mouse. Nature. 1964;22:779. doi: 10.1038/201779a0. [DOI] [PubMed] [Google Scholar]
- 5.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 6.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol. 2010;10:301–316. doi: 10.1038/nri2761. [DOI] [PubMed] [Google Scholar]
- 8.Cheson BD, Leonard JP. Monoclonal antibody therapy for B-cell non-Hodgkin’s lymphoma. N Engl J Med. 2008;359:613–626. doi: 10.1056/NEJMra0708875. [DOI] [PubMed] [Google Scholar]
- 9.Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 10.Hudis CA. Trastuzumab-mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 11.Schoeberl B, Pace EA, Fitzgerald JB, Harms BD, Xu L, Nie L, Linggi B, Kalra A, Paragas V, Bukhalid R, Grantcharova V, Kohli N, West KA, Leszczynicka M, Feldhaus MJ, Kudla AJ, Nielsen UB. Therapeutically targeting ErbB3: a key node in ligand-induced activation of the ErbB receptor-PI3K axis. Sci Signal. 2009;2:ra31. doi: 10.1126/scisignal.2000352. [DOI] [PubMed] [Google Scholar]
- 12.Canadas I, Rojo F, Arumí-Uría M, Rovira A, Albanell J, Arriola E. C-MET as a new therapeutic target for the development of novel anticancer drugs. Clin Transl Oncol. 2010;12:253–260. doi: 10.1007/s12094-010-0501-0. [DOI] [PubMed] [Google Scholar]
- 13.Scartozzi M, Bianconi M, Maccaroni E, Giampieri R, Berardi R, Cascinu S. Dalotuzumab, a recombinant humanized mAb targeted against IGFR1 for the treatment of cancer. Curr Opin Mol Ther. 2010;12:361–371. [PubMed] [Google Scholar]
- 14.Vearing C, Lee FT, Wimmer-Kleikamp S, Spirkoska V, To C, Styli-anou C, Spanevello M, Brechbiel M, Boyd AW, Scott AM, Lackmann M. Concurrent binding of anti-EphA3 antibody and ephrin-A5 amplifies EphA3 signaling and downstream responses: potential as EphA3-specific tumor targeting reagents. Cancer Res. 2005;65:6745–6754. doi: 10.1158/0008-5472.CAN-05-0758. [DOI] [PubMed] [Google Scholar]
- 15.Fox NL, Humphreys R, Luster TA, Klein J, Gallant G. Tumor Necrosis Factor-related apoptosis-inducing ligand (TRAIL) Receptor-1 and Receptor-2 agonists for cancer therapy. Expert Opin Biol Ther. 2010;10:1–18. doi: 10.1517/14712590903319656. [DOI] [PubMed] [Google Scholar]
- 16.Schliemann C, Neri D. Antibody-based vascular tumor targeting. Recent Results Cancer Res. 2010;180:201–216. doi: 10.1007/978-3-540-78281-0_12. [DOI] [PubMed] [Google Scholar]
- 17.Deckert PM. Current constructs and targets in clinical development for antibody-based cancer therapy. Curr Drug Targets. 2009;10:158–175. doi: 10.2174/138945009787354502. [DOI] [PubMed] [Google Scholar]
- 18.Scott AM, Wiseman G, Welt S, Adjei A, Lee FT, Hopkins W, Divgi CR, Hanson LH, Mitchell P, Gansen DN, Larson SM, Ingle JN, Hoffman EW, Tanswell P, Ritter G, Cohen LS, Bette P, Arvay L, Amelsberg A, Vlock D, Rettig WJ, Old LJ. A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin Cancer Res. 2003;9:1639–1647. [PubMed] [Google Scholar]
- 19.Welt S, Divgi CR, Scott AM, Garin-Chesa P, Finn RD, Graham M, Carswell EA, Cohen A, Larson SM, Old LJ, Rettig WJ. Antibody targeting in metastatic colon cancer: A phase I study of monoclonal antibody F19 against a cell surface protein of reactive tumor stromal fibroblasts. J Clin Oncol. 1994;12:1193–1203. doi: 10.1200/JCO.1994.12.6.1193. [DOI] [PubMed] [Google Scholar]
- 20.Pillay V, Gan HK, Scott AM. Antibodies in oncology. N Biotechnol. 2011;28:518–529. doi: 10.1016/j.nbt.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 21.Riechmann L, Clark M, Waldmann H, Winter G. Reshaping human antibodies for therapy. Nature. 1988;332:323–327. doi: 10.1038/332323a0. [DOI] [PubMed] [Google Scholar]
- 22.Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nature Rev Drug Discov. 2010;9:767–774. doi: 10.1038/nrd3229. [DOI] [PubMed] [Google Scholar]
- 23.Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010;47:115–123. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott AM, Lee FT, Tebbutt N, Herbertson R, Gill SS, Liu Z, Skrinos E, Murone C, Saunder TH, Chappell B, Papenfuss AT, Poon AM, Hopkins W, Smyth FE, MacGregor D, Cher LM, Jungbluth AA, Ritter G, Brechbiel MW, Murphy R, Burgess AW, Hoffman EW, Johns TG, Old LJ. A Phase I clinical trial with monoclonal antibody ch806 targeting transitional state and mutant epidermal growth factor receptor. Proc Natl Acad Sci U S A. 2007;104:4071–4076. doi: 10.1073/pnas.0611693104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravetch J. In vivo veritas: the surprising roles of Fc receptors in immunity. Nat Immunol. 2010;11:183–185. doi: 10.1038/ni0310-183. [DOI] [PubMed] [Google Scholar]
- 27.Hughes B. Antibody-drug conjugates for cancer: poised to deliver? Nat Rev Drug Discov. 2010;9:665–667. doi: 10.1038/nrd3270. [DOI] [PubMed] [Google Scholar]
- 28.Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol. 2005;23:1137–1146. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- 29.Herbertson RA, Tebbutt NC, Lee FT, MacFarlane DJ, Chappell B, Micallef N, Lee ST, Saunder T, Hopkins W, Smyth FE, Wyld DK, Bellen J, Sonnichsen DS, Brechbiel MW, Murone C, Scott AM. Phase I biodistribution and pharmacokinetic study of Lewis Y targeting immunoconjugate CMD-193 in patients with advanced epithelial cancers. Clin Cancer Res. 2009;15:6709–6715. doi: 10.1158/1078-0432.CCR-09-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caron PC, Jurcic JG, Scott AM, Finn RD, Divgi CR, Graham MC, Jureidini IM, Sgouros G, Tyson D, Old LJ, Larson SM, Scheinberg DA. A phase IB trial of humanized monoclonal antibody M195 (anti-CD33) in myeloid leukemia: specific targeting without immunogenicity. Blood. 1994;83:1760–1768. [PubMed] [Google Scholar]
- 31.Scott AM, Lee FT, Hopkins W, Cebon JS, Wheatley JM, Liu Z, Smyth FE, Murone C, Sturrock S, MacGregor D, Hanai N, Inoue K, Yamasaki M, Brechbiel MW, Davis ID, Murphy R, Hannah A, Lim-Joon M, Chan T, Chong G, Ritter G, Hoffman EW, Burgess AW, Old LJ. Specific targeting, biodistribution and lack of immunogenicity of chimeric anti-GD3 monoclonal antibody KM871 in patients with metastatic melanoma - results of a phase I trial. J Clin Oncol. 2001;19:3976–3987. doi: 10.1200/JCO.2001.19.19.3976. [DOI] [PubMed] [Google Scholar]
- 32.Scott AM, Tebbutt N, Lee FT, Cavicchiolo T, Liu Z, Gill S, Poon AM, Hopkins W, Smyth FE, Murone C, MacGregor D, Papenfuss AT, Chappell B, Saunder TH, Brechbiel MW, Davis ID, Murphy R, Chong G, Hoffman EW, Old LJ. A Phase I biodistribution and pharmacokinetic trial of humanized monoclonal antibody hu3S193 in patients with advanced epithelial cancers which express the Lewis-y antigen. Clin Cancer Res. 2007;13:3286–3292. doi: 10.1158/1078-0432.CCR-07-0284. [DOI] [PubMed] [Google Scholar]
- 33.Welt S, Divgi CR, Real FX, Yeh SD, Garin-Chesa P, Finstad CL, Sakamoto J, Cohen A, Sigurdson ER, Kemeny N, Old LJ. Quantitative analysis of antibody localization in human metastatic colon cancer: a phase I study of moncolonal antibody A33. J Clin Oncol. 1990;8:1894–1906. doi: 10.1200/JCO.1990.8.11.1894. [DOI] [PubMed] [Google Scholar]
- 34.Oosterwijk E, Bander NH, Divgi CR, Welt S, Wakka JC, Finn RD, Carswell EA, Larson SM, Warnaar SO, Fleuren GJ, Old LJ. Antibody localization in human renal cell carcinoma: a phase I study of monoclonal antibody G250. J Clin Oncol. 1993;11:738–750. doi: 10.1200/JCO.1993.11.4.738. [DOI] [PubMed] [Google Scholar]
- 35.Dijkers EC, Oude Munnink TH, Kosterink JG, Brouwers AH, Jager PL, de Jong JR, van Dongen GA, Schröder CP, Lub-de Hooge MN, de Vries EG. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther. 2010;87:586–592. doi: 10.1038/clpt.2010.12. [DOI] [PubMed] [Google Scholar]
- 36.de Bono JS, Ashworth A. Translating cancer research into targeted therapeutics. Nature. 2010;467:543–549. doi: 10.1038/nature09339. [DOI] [PubMed] [Google Scholar]
- 37.Divgi CR, Welt S, Kris M, Real FX, Yeh SD, Gralla R, Merchant B, Schweighart S, Unger M, Larson SM, Mendelsohn J. Phase I and imaging trial of indium 111-labeled anti-epidermal growth factor receptor monoclonal antibody 225 in patients with squamous cell lung carcinoma. J Natl Cancer Inst. 1991;83:97–104. doi: 10.1093/jnci/83.2.97. [DOI] [PubMed] [Google Scholar]
- 38.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumor activity. Nat Rev Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 39.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 40.Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 41.Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 42.Lievre A, Bachet JB, Boige V, Cayre A, Le Corre D, Buc E, Ychou M, Bouché O, Landi B, Louvet C, André T, Bibeau F, Diebold MD, Rougier P, Ducreux M, Tomasic G, Emile JF, Penault-Llorca F, Laurent-Puig P. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26:374–379. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- 43.Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, Forero-Torres A. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 44.Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, Blättler WA, Lambert JM, Chari RV, Lutz RJ, Wong WL, Jacobson FS, Koeppen H, Schwall RH, Kenkare-Mitra SR, Spencer SD, Sliwkowski MX. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 45.Heiss MM, Murawa P, Koralewski P, Kutarska E, Kolesnik OO, Ivanchenko VV, Dudnichenko AS, Aleknaviciene B, Razbadauskas A, Gore M, Ganea-Motan E, Ciuleanu T, Wimberger P, Schmittel A, Schmalfeldt B, Burges A, Bokemeyer C, Lindhofer H, Lahr A, Parsons SL. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int J Cancer. 2010;127:2209–2221. doi: 10.1002/ijc.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boland WK, Bebb G. Nimotuzumab: a novel anti-EGFR monoclonal antibody that retains anti-EGFR activity while minimizing skin toxicity. Expert Opin Biol Ther. 2009;9:1199–1206. doi: 10.1517/14712590903110709. [DOI] [PubMed] [Google Scholar]
- 47.Chen S, Yu L, Jiang C, Zhao Y, Sun D, Li S, Liao G, Chen Y, Fu Q, Tao Q, Ye D, Hu P, Khawli LA, Taylor CR, Epstein AL, Ju DW. Pivotal study of iodine-131-labeled chimeric tumor necrosis treatment radioimmunotherapy in patients with advanced lung cancer. J Clin Oncol. 2005;23:1538–1547. doi: 10.1200/JCO.2005.06.108. [DOI] [PubMed] [Google Scholar]
- 48.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 49.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O’Dwyer PJ, Vonderheide RH. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor C, Hershman D, Shah N, Suciu-Foca N, Petrylak DP, Taub R, Vahdat L, Cheng B, Pegram M, Knutson KL, Clynes R. Augmented HER-2 specific immunity during treatment with Trastuzumab and chemotherapy. Clin Cancer Res. 2007;15:5133–5143. doi: 10.1158/1078-0432.CCR-07-0507. [DOI] [PubMed] [Google Scholar]
- 52.Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J Clin Oncol. 2010;28:4390–4399. doi: 10.1200/JCO.2009.27.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beck A, Wurch T, Bailly C, Corvaia N. Strategies and challenges for the next generation of therapeutic antibodies. Nat Rev Immunol. 2010;10:345–352. doi: 10.1038/nri2747. [DOI] [PubMed] [Google Scholar]
- 54.Pillay V, Allaf L, Wilding AL, Donoghue JF, Court NW, Greenall SA, Scott AM, Johns TG. The plasticity of oncogene addiction: implications for targeted therapies directed to receptor tyrosine kinases. Oncogene. 2009;11:448–458. doi: 10.1593/neo.09230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, Steinberg SM, Tsang KY, Poole DJ, Parnes HL, Wright JJ, Dahut WL, Schlom J, Gulley JL. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase I dose-escalation trial. Lancet Oncol. 2012. epub ahead of print. [DOI] [PMC free article] [PubMed]