Abstract
Background
Several factors contribute to the documented racial disparity in head and neck cancer (HNSCC), among which are socioeconomic status, access to care and biologic factors.
Methods
Clinical characteristics of 87 African-American head and neck cancer patients and a random sample of 261 White patients matched on age and smoking dose were associated to outcome.
Results
Black oral cavity and larynx patients were more likely diagnosed with advanced stages than Whites, after adjusting for socioeconomic and insurance status and other confounding factors. There was a significant difference in relapse-free survival between Blacks and Whites with larynx tumors (Hazard Ratio = 3.36, 95% CI: 1.62-7.00), but not with oral cavity or pharynx tumors.
Conclusion
Differences in disease outcome may be attributed to a combination of tumor stage, socioeconomic status and access to health care. The inclusion of biological markers such as Human Papillomavirus status is needed in future studies to further evaluate racial disparities in head and neck cancer outcomes.
Keywords: head and neck cancer, racial disparities, survival, African-American, outcome
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) represented around 3% of all newly diagnosed cancers in the United States in 20091. According to the data from the American Cancer Society, it is estimated that 48,010 cases will be diagnosed with and 11,260 patients will die of cancer of the oral cavity, pharynx and larynx in 20091.
Studies have shown differences in HNSCC in survival between Whites and African-Americans. While there are minor differences in incidence between the two races, 2 there is a large disparity in mortality rates. Oral cavity and oropharyngeal cancer mortality in the White population was 3.7 per 100,000 men as compared to 6.5 per 100,000 African-American men from 2002-2006 3. Mean survival time also varied significantly by ethnicity. In the US, 1-year overall survival for HNSCC is 84%, and 5-year survival is 59% (58% for males and 62% for females), but African-Americans’ 5-year overall survival is 39.5% (34% for males and 52% for females) 4.
Several possible reasons for such outcome differences amongst racial groups have been proposed 5-9. A recent meta-analysis of oral cavity and oropharynx cancer incidence by Conway et al. reported that head and neck cancer risk was associated with low educational attainment, low occupational social class and lower income 10. A lower socioeconomic status amongst minorities has been thought of as one of the possible causes for health disparity and has been associated with less access to health care, less likelihood of being insured and more advanced stages at presentation as compared to patients of higher socioeconomic class. A previous study regarding HNSCC showed that 13% of African-Americans did not have health insurance compared to 1.5% of Hispanics and 0.6% of whites 9. Other published data have also shown that African-American patients with oral cancer have later presentations than their White counterparts 11. Although a limited number of studies have evaluated the independent effect of socioeconomic status 4,7,9,12-16 or access to care7,9,15 on overall or disease-free survival among African-Americans, none of these studies have investigated the role of both socioeconomic and insurance status on outcome. A recent study reported that overall survival in patients diagnosed with head and neck cancer was worse for Blacks compared to Whites; such disparity was observed only for oropharynx tumors and was dependent on a low prevalence of Human papillomavirus (HPV)-positive tumors among Black cases17. The study included cases diagnosed with late stage disease only (106 White, 95 Black), and a small number of Black cases was included in the race stratified analysis according to HPV status (n = 31). Furthermore, the reported hazards ratios were not adjusted for confounders such as age, stage, socioeconomic and insurance status. In this study, we will add to previous literature on survival disparities by reporting findings from a retrospective analysis of consecutive incident head and neck cancer cases non-selected on disease stage, from a tertiary care cancer center. Unique to the study presented is analysis regarding socioeconomic classification, insurance status, and TNM staging. This study will test whether differences in disease outcome remain when tumor stage, socioeconomic status and access to health care are considered simultaneously.
METHODS
Study Population
The study population consisted of patients who were diagnosed with primary squamous cell carcinoma of the head and neck (HNSCC). Patients with recurrent and/or second primary tumors were excluded. A cohort study design was used, consisting of all consecutive African-American patients (n = 87) diagnosed or receiving first course of treatment at the University of Pittsburgh Medical Center (Pittsburgh, PA) from January,1987 to October, 2007; and a random sample of White patients (n = 261) that were frequency matched to African-Americans 3:1 by age category (< 40, 40-44, 45-49, 50-54, 55-59, 60-64, 65-69, 70-74, 75-79, 80-84 years) and smoking dose (never smoker; < 10 pack-year or < 1 pack-per-day or < 10 yrs smoker, > 10 pack-year or > 1 pack-perday or > 10 yrs smoker, and smoker, dose unknown and unknown smoking status), selected from a larger cohort of 2,016 Whites diagnosed or receiving first course of treatment at the same facility during the same time frame. . To achieve the 3:1 matching, all Black (n = 87) and White (n = 2,016) cases were stratified by age and smoking dose, and within each strata, 3 White patients were randomly selected (using a random number generator) for each Black patient. The majority of the cases (n = 317) was also included in a previous study on cancer survival according to insurance status 18. All of the tumors were biopsy-proven, arising in the oral cavity, pharynx, or larynx. The study was exempt approved by the University of Pittsburgh Institutional Review Board.
Data Collection
Clinical and demographic data for patients diagnosed or treated for head and neck cancer was recorded in the University of Pittsburgh Head and Neck Oncology Registry. Clinical information related to the patients’ tumors, vital status, and treatment was gathered prospectively by the Head and Neck Oncology Registry following initial diagnosis and was regularly updated in order to reflect the patient's status at the most recent follow-up visit. The sources of these data are patient medical records, the UPMC Network Cancer Registry, and the Social Security Death Index (SSDI).
Data on patient health insurance at the time of diagnosis were obtained via the University of Pittsburgh Medical Center billing system as previously described 18. Insurance status was categorized as private, Medicaid, uninsured, Medicare, and Veterans Affairs. Some patients who were uninsured at the time of diagnosis subsequently enrolled in Medicaid in order to cover their treatment expenses and could not be distinguished in this dataset, therefore uninsured and Medicaid enrollees were combined. The Medicare classification was further subdivided into patients younger than 65 years of age (Medicare disability) and those 65 years or older. All of the data included in this study (clinical, demographic and health insurance) were obtained as a de-identified dataset.
Statistical Analysis
Modified Nakao and Treas occupational prestige scores (SEI) 19 were used to produce an indicator of socioeconomic status based on self-reported occupation during working years; patients were categorized according to occupational prestige score as unemployed/disabled (0), 0< SEI ≤ 50, 50< SEI ≤ 100. An additional category was added to include homemakers, who were not accounted for by the original Nakao and Treas scoring system. Alcohol use was classified as never, ≤ 6 drinks/week, 7-14 drinks/week, > 14 drinks/week, and drinker, intensity unknown. Cigarette smoking was classified as never, < 10 pack-year or < 1 pack-per-day or < 10 yrs smoker, > 10 pack-year or > 1 pack-per-day or > 10 yrs smoker, and smoker, dose unknown. Use of other types of tobacco included all non-cigarette forms of tobacco (pipe, cigar, chew) and was coded dichotomously as ever versus never use.
Tumor site was the primary site of the initial tumor, categorized by ICD-10 site codes according to American Joint Committee on Cancer (AJCC) staging manual (6th edition) guidelines 20 as oral cavity (C00.0-C00.6, C00.8, C00.9, C02.0-C02.3, C02.8-C03.1, C03.9-C04.1, C04.8-C05.0, C05.8-C06.2, C06.8, C06.9), pharynx (C01.9, C02.4, C05.1, C05.2, C09.0, C09.1, C09.8-C10.0, C10.2-C10.4, C10.8-C11.3, C11.8, C11.9, C12.9-C13.2, C13.8-C14.2, C14.8), or larynx (C10.1, C32.0-C32.3, C32.8, C32.9). Stage at diagnosis was categorized as stage I, II, III, or IV; according to the AJCC staging for head and neck cancer 20. The AJCC stage group was classified based on pathological TNM staging and when not available, clinical TNM staging was used. Treatment was categorized as surgery only, radiation and/or chemotherapy only, or surgery and radiation and/or chemotherapy. Three patients who refused treatment were grouped with those for which treatment was unknown. The primary outcomes of interest in this study were overall and relapse-free survival by race. The follow-up time for overall survival was defined as the time from initial diagnosis of the primary tumor to death or last contact if the patient was still alive. The follow-up time for relapse-free survival was defined as the time from initial diagnosis of the primary tumor to recurrence, development of a second primary, or metastasis or last contact if the patient remained relapse-free. For relapse-free survival, patients who died without recurrence were considered censored. Presence of lymph node involvement and advanced stage disease at diagnosis were evaluated as secondary outcomes of interest.
Descriptive statistics were generated for demographic and clinical variables by race, using Fisher's exact test to identify differences between Black and White subjects. Kaplan-Meier survival functions were produced for overall and relapse-free survival. Differences in survival by race were tested using the Wilcoxon Log-Rank test. Multivariable Cox proportional hazards models were developed for overall and relapse-free survival, with race as the primary independent variable of interest. The models were adjusted for age, gender, family history of cancer, socioeconomic status, insurance status at diagnosis, alcohol use, any tobacco use (cigarette and non-cigarette forms of tobacco), tumor site, stage at diagnosis, and treatment modality. Proportional hazards assumptions were formally tested using an approach based on scaled Schoenfeld's residuals 21.
Separate multivariable logistic regression models were employed to assess the association of race with lymph node involvement and stage at diagnosis, respectively, for all sites and by specific site (oral cavity, pharynx, and larynx). Stage was categorized as advanced-stage (AJCC stage group III or IV) versus early-stage disease (AJCC stage group I or II). Lymph node involvement was categorized as the presence (N1-N3) versus absence (N0) of positive lymph nodes at diagnosis. The primary predictor in the model was race; other covariates in the model included age (< 61 year vs. ≥ 61 years), any tobacco use (ever vs. never), gender, family history of cancer, socioeconomic status (uninsured/disabled, 0< SEI ≤ 50, 50< SEI ≤ 100, homemaker), insurance status (uninsured/disabled, insured), alcohol use (ever vs. never), and primary tumor site. Model fit was assessed using the Hosmer and Lemeshow goodness-of-fit test 22, and was considered a poor fit if P ≤ 0.05. All statistical analyses were performed using Stata 10 software (StataCorp LP, College Station, TX).
RESULTS
Demographic and clinical characteristics
The description of the patient population (N = 348) is provided in Table 1. The mean age was 61 years old (range: 33-84), the majority of cases were > 10 pack-year or > 1 pack-per-day or > 10 yrs smokers (54%); few cases have used other types of tobacco. Black patients were more likely to be identified as unemployed or disabled and less likely to be in the higher socioeconomic classes (SEI > 50). Blacks were also less likely to have private insurance and more likely to have Medicaid/uninsured or Medicare disability, compared to Whites. Gender distribution, family history of cancer, alcohol consumption and other tobacco use was not different between the two races. There were differences in cancer characteristics (Table 2); Black patients were significantly more likely than Whites to develop laryngeal cancers (44.8% vs. 30.7%; p = 0.049), and to be diagnosed with Stage IV disease (59.0% vs. 44.0%; p = 0.045). Although lymph node involvement at diagnosis and tumor grade were not statistically significantly different according to race, White cases were more likely to be treated with surgery plus radiation and/or chemotherapy, while Black cases were more likely to be treated with radiation and/or chemotherapy only. Among the cases that refused treatment, all of them were Black, 3.4%, (3/87) of the Black cases or 0.9%, (3/386) of the entire cohort.
Table 1.
Demographic description of the study population by racea
| Demographic variables | Black N (%) | White N (%) | p-value |
|---|---|---|---|
| Smoking dose | 1.000 | ||
| Never smoker | 7 (8.1) | 21 (8.1) | |
| < 10 pack-year or < 1 pack-per-day or < 10 yrs smoker | 9 (10.3) | 27 (10.3) | |
| > 10 pack-year or > 1 pack-per-day or > 10 yrs smoker | 47 (54.0) | 141 (54.0) | |
| Smoker, dose unknown | 20 (23.0) | 60 (23.0) | |
| Unknown smoking status | 4 (4.6) | 12 (4.6) | |
| Age (years) | 1.000 | ||
| < 45 | 5 (5.7) | 15 (5.7) | |
| 45-54 | 20 (23.0) | 60 (23.0) | |
| 55-64 | 36 (41.4) | 108 (41.4) | |
| 65-74 | 15 (17.2) | 45 (17.2) | |
| 75-84 | 11 (12.6) | 33 (12.6) | |
| Gender | 0.666 | ||
| Male | 64 (73.6) | 199 (76.3) | |
| Female | 23 (26.4) | 62 (23.8) | |
| Family History of Cancer | 0.313 | ||
| Yes | 40 (46.0) | 139 (53.3) | |
| No | 28 (32.2) | 63 (24.1) | |
| Unknown | 19 (21.8) | 59 (22.6) | |
| Socioeconomic Status | 0.055 | ||
| Unemployed/disabled | 21 (24.1) | 39 (14.9) | |
| 0< SEI ≤ 50 | 38 (43.7) | 96 (36.7) | |
| 50 < SEI ≤ 100 | 11 (12.6) | 58 (22.2) | |
| Homemaker | 1 (1.2) | 12 (4.6) | |
| Unknown | 16 (18.4) | 56 (21.5) | |
| Insurance At Diagnosis | 0.152 | ||
| Private insurance | 28 (32.2) | 113 (43.3) | |
| Medicaid/uninsured | 14 (16.1) | 22 (8.4) | |
| Medicare disability (< 65) | 11 (12.6) | 22 (8.4) | |
| Medicare, ≥ 65 | 22 (25.3) | 72 (27.6) | |
| Veterans Affairs | 3 (3.5) | 5 (1.9) | |
| Unknown | 9 (10.3) | 27 (10.3) | |
| Alcohol Consumption | 0.335 | ||
| Never drinker | 23 (26.4) | 65 (24.9) | |
| ≤ 6 drinks/week | 13 (14.9) | 55 (21.1) | |
| 7-14 drinks/week | 4 (4.6) | 25 (9.6) | |
| > 14 drinks/week | 20 (23.0) | 56 (21.4) | |
| Drinker, intensity unknown | 20 (23.0) | 39 (14.9) | |
| Unknown alcohol status | 7 (8.1) | 21 (8.1) | |
| Other Tobacco Useb | 0.861 | ||
| Never | 82 (94.3) | 242 (92.7) | |
| Ever | 1 (1.2) | 7 (2.7) | |
| Unknown | 4 (4.6) | 12 (4.6) | |
| Total | 87 (100) | 261 (100) | |
Study population matched on age and smoking dose,
Other tobacco use includes all non-cigarette forms of tobacco (pipe, cigar, chew).
Table 2.
Clinical characteristics of the study population by race.
| Clinical variables | Black N (%) | White N (%) | P-Value |
|---|---|---|---|
| Tumor Site | 0.049a | ||
| Oral cavity | 23 (26.4) | 95 (36.4) | |
| Pharynx | 25 (28.7) | 86 (33.0) | |
| Larynx | 39 (44.8) | 80 (30.7) | |
| Stage At Diagnosis | 0.045a | ||
| I | 16 (18.4) | 41 (15.7) | |
| II | 7 (8.1) | 47 (18.0) | |
| III | 11 (12.6) | 48 (18.4) | |
| IV | 49 (56.3) | 107 (41.0) | |
| Unknown | 4 (4.6) | 18 (6.9) | |
| Lymph Node Involvement At Diagnosis | 0.529 | ||
| Yes | 45 (51.7) | 117 (44.8) | |
| No | 38 (43.7) | 126 (48.3) | |
| Unknown | 4 (4.6) | 18 (6.9) | |
| Tumor Grade | 0.129 | ||
| Well differentiated | 4 (4.6) | 28 (10.7) | |
| Moderately differentiated | 59 (67.8) | 154 (59.0) | |
| Poorly differentiated | 11 (12.6) | 30 (11.5) | |
| Not evaluated | 12 (13.8) | 33 (12.7) | |
| Unknown | 1 (1.2) | 16 (6.1) | |
| Treatment | 0.233 | ||
| Surgery only | 25 (28.7) | 84 (32.2) | |
| Radiation and/or chemo only | 29 (33.3) | 60 (23.0) | |
| Surgery + radiation and/or chemo | 26 (29.9) | 99 (37.9) | |
| Unknownb | 7 (8.1) | 18 (6.9) | |
| Total | 87 (100) | 261 (100) | |
p-value <0.05 is considered statistically significant,
Data missing for at least one of the three treatment options and includes three Black cases that refused treatment.
After adjusting for age, gender, family history of cancer, tobacco use, alcohol use, socioeconomic status, insurance status and tumor site, the relative odds of being diagnosed with advanced stage disease was non-statistically significantly increased for Blacks compared to Whites (Adjusted Odds ratio [AdjOR] = 1.47, 95% Confidence Interval [CI]: 0.82-2.65) (Table 3). Blacks with oral cavity tumors were almost four times more likely to be diagnosed with advanced stage disease compared to Whites (AdjOR = 3.60, 95% CI: 0.97-13.41), while lymph node involvement in oral cavity tumors was not associated with race. There were no significant differences in disease stage and lymph node involvement according to race for cases diagnosed with pharynx or larynx cancers. The odds ratio for lymph node involvement for Blacks compared to Whites with larynx cancer was not statistically significantly increased (AdjOR = 2.26, 95% CI: 0.92-5.61).
Table 3.
Association between race and prognostic factors at diagnosis.
| Stage (III, IV)/(I, II) OR (95% CI) | N1, N2, N3/N0 OR (95% CI) | |
|---|---|---|
| All sitesa (n = 326) | ||
| White | 1.00 (reference) | 1.00 (reference) |
| Black | 1.47 (0.82-2.65) | 1.36 (0.78-2.36) |
| Oral Cavityb (n = 113) | ||
| White | 1.00 (reference) | 1.00 (reference) |
| Black | 3.60 (0.97-13.41) | 1.28 (0.41-4.00) |
| Pharynxb* (n = 104) | ||
| White | 1.00 (reference) | 1.00 (reference) |
| Black | 1.38 (0.32-6.06) | 1.12 (0.33-3.83) |
| Larynxb (n = 109) | ||
| White | 1.00 (reference) | 1.00 (reference) |
| Black | 1.10 (0.45-2.72) | 2.26 (0.92-5.61) |
Adjusted for age, any tobacco use, gender, family history of cancer, alcohol use, socioeconomic status, insurance and tumor site
Adjusted for age, any tobacco use, gender, family history of cancer, alcohol use, socioeconomic status and insurance
tobacco eliminated from the model for advanced stage due to collinearity
Overall and relapse-free survival
The median follow-up for the cohort was 24.4 months (10th percentile: 5.4 months; 90th percentile: 82.8 months), during which there were 156 deaths among 348 at-risk patients. For relapse-free survival, the median follow-up was 16.9 months (10th percentile: 4.2 months; 90th percentile: 73.1 months), with 148 recurrences, second primary, or metastasis among 348 at-risk patients.
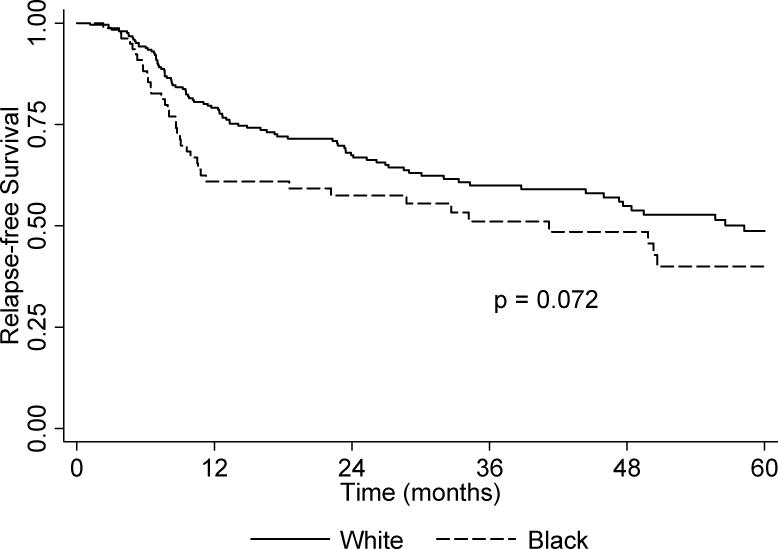
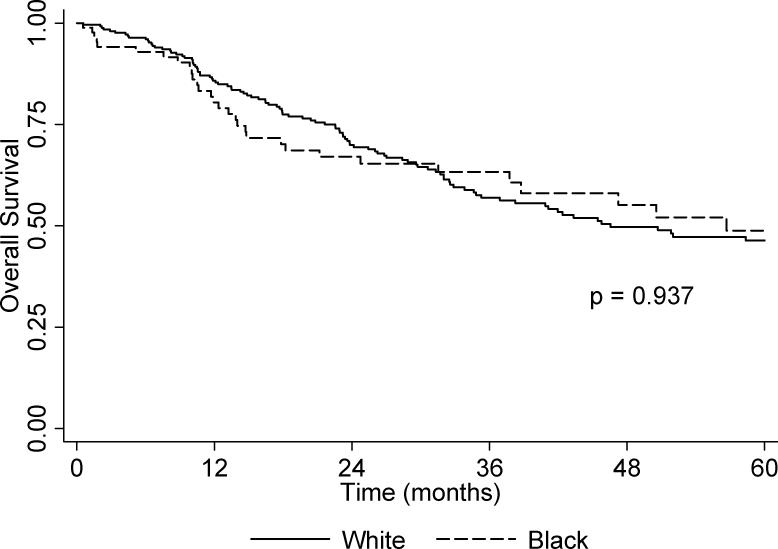
The unadjusted overall and relapse-free Kaplan-Meier 5-year survival curves are presented in Figure 1 and Figure 2, respectively. There was no difference between Blacks and Whites for overall survival (p = 0.937). However, Blacks had borderline poorer 5-year relapse-free survival relative to Whites (p = 0.072). The multivariable Cox proportional hazards models (Table 4), shows a marginal association between self-reported African-American ancestry and relapse-free survival (Adjusted Hazard ratio [AdjHR] = 1.47, 95% CI: 0.99-2.18) but not for overall survival (AdjHR = 0.96, 95% CI: 0.64-1.45). Decreased overall survival was independently associated with being unemployed or disabled, having Medicaid or no insurance; there was also a trend in decreased survival with increasing stage at diagnosis; decreased relapse-free survival was independently associated with treatment via radiation and/or chemotherapy only. When cases were stratified according to tumor site, there was no difference in overall and relapse-free survival between Blacks and Whites diagnosed with oral cavity or pharynx tumors (data not shown). In contrast, the risk of recurrence second primary, or metastasis for Black cases with larynx tumors was statistically significantly increased in comparison to White cases (AdjHR = 3.36, 95% CI: 1.62-7.00).
Figure 1.
Kaplan-Meier unadjusted overall 5-year survival by race.
Figure 2.
Kaplan-Meier unadjusted 5-year relapse-free survival by race.
Table 4.
Predictors of overall and relapse-free survival in HNSCC cases
| Overall survival (all-cause mortality) | Relapse-free survival | |
|---|---|---|
| Deaths/Recurrences (%) | 156 (44.8 %) | 148 (42.5%) |
| At risk | 348 | 348 |
| Hazard Ratio (95% CI)a | Hazard Ratio (95% CI)a | |
| Gender | ||
| Male | 1.00 (reference) | 1.00 (reference) |
| Female | 0.86 (0.56-1.34) | 0.81 (0.52-1.28) |
| Race | ||
| White | 1.00 (reference) | 1.00 (reference) |
| Black | 0.96 (0.64-1.45) | 1.47 (0.99-2.18) |
| Socioeconomic Status | ||
| 50 < SEI ≤ 100 | 1.00 (reference) | 1.00 (reference) |
| 0< SEI ≤ 50 | 1.47 (0.86-2.52) | 1.01 (0.63-1.61) |
| Unemployed/disabled | 2.07 (1.03-4.19) | 0.76 (0.38-1.51) |
| Homemaker | 1.70 (0.63-4.55) | 1.03 (0.35-3.03) |
| Unknown | 1.27 (0.66-2.41) | 0.93 (0.51-1.71) |
| Family History of Cancer | ||
| No | 1.00 (reference) | 1.00 (reference) |
| Yes | 0.72 (0.48-1.08) | 0.93 (0.62-1.38) |
| Unknown | 1.14 (0.66-2.41) | 0.82 (0.44-1.52) |
| Tumor Site | ||
| Oral cavity | 1.00 (reference) | 1.00 (reference) |
| Pharynx | 1.03 (0.66-1.62) | 0.79 (0.49-1.27) |
| Larynx | 0.77(0.51-1.18) | 0.91 (0.59-1.41) |
| AJCC Stage | ||
| I | 1.00 (reference) | 1.00 (reference) |
| II | 1.66 (0.82-3.37) | 0.89 (0.45-1.76) |
| III | 1.80 (0.85-3.80) | 1.37 (0.71-2.65) |
| IV | 2.49 (1.23-5.03) | 1.13 (0.59-2.19) |
| Unknown | 1.88 (0.74-4.77) | 3.04 (1.28-7.20) |
| Treatment | ||
| Surgery only | 1.00 (reference) | 1.00 (reference) |
| Radiation and/or chemo only | 1.68 (0.91-3.08) | 1.93 (1.04-3.58) |
| Surgery + radiation and/or chemo | 1.46 (0.86-2.48) | 1.20 (0.69-2.09) |
| Unknown* | 2.88 (1.21-6.84) | 1.64 (0.65-4.15) |
| Alcohol Use | ||
| Never | 1.00 (reference) | 1.00 (reference) |
| Ever | 1.13 (0.74-1.74) | 1.18 (0.76-1.83) |
| Unknown | 0.24 (0.06-0.96) | 0.31 (0.08-1.19) |
| Insurance At Diagnosis | ||
| Private insurance | 1.00 (reference) | 1.00 (reference) |
| Medicaid/uninsured | 2.11 (1.18-3.77) | 1.22 (0.63-2.34) |
| Medicare disability (< 65) | 1.06 (0.56-2.18) | 1.08 (0.54-2.15) |
| Medicare, ≥ 65 | 1.37 (0.80-2.34) | 0.73 (0.40-1.31) |
| Veterans Affairs | 2.32 (0.86-6.23) | 1.21 (0.36-4.12) |
| Unknown | 0.42 (0.21-0.85) | 0.71 (0.38-1.31) |
Covariates included: age, race, gender, family history of cancer, alcohol use, socioeconomic status, insurance, tobacco use, stage at diagnosis, and treatment modality;
Data missing for at least one of the three treatment options and includes three Black cases that refused treatment.
DISCUSSION
This study evaluates the clinical characteristics of head and neck cancer cases according to race and suggests that after matching for smoking dose and age, and adjusting for socioeconomic status and insurance status, oral cavity and larynx cases with self-defined African ancestry are more likely to be diagnosed with advanced stage disease. Since primary risk factors for head and neck cancer are tobacco and alcohol 23-27, all of the cases included in this study were matched on smoking dose, in order to account for the effect of smoking in the study design. Alcohol use was similarly distributed between Black and White patients. The analysis was also adjusted for socioeconomic status and access to care by using insurance status as a surrogate. Therefore, none of these factors should have significantly contributed to the observed differences in stage with race. Shiboski, et al. reported somewhat similar findings; African-Americans were diagnosed with a significantly higher proportion of tongue cancer compared to Whites, which had spread to a regional node or to a distant site; these data however were not adjusted for socioeconomic and insurance status or smoking11. In our study Black cases with oral cavity tumors were more likely to present with advanced stage disease, but did not have an increased likelihood of nodal involvement. This suggests that the increased likelihood of advanced stage disease observed for Blacks with oral cavity tumors may not necessarily be attributed to differences in behavioral risk factors, poor socioeconomic status or access to care.
In contrast to the oral cavity, Black and White cases diagnosed with pharynx or larynx tumors did not significantly differ in the disease stage or nodal involvement; yet, Black patients were significantly more likely than Whites to develop laryngeal cancers. Studies have reported that Blacks are less likely to stop smoking and are more likely to smoke mentholated cigarettes compared to Whites 28,29. Smoking mentholated cigarettes result in deeper and longer inhalations 30,31 which would allow tobacco smoke to reach the larynx. This may also help to explain the reason why Black cases are more likely to develop larynx cancers compared to Whites. Nevertheless, we cannot rule out the possibility that host factors related to tobacco metabolism may also play a role in the observed tumor site-specific risk and advanced stage disease between Blacks and Whites.
Arbes et al. evaluated disease-specific survival for 1,165 Blacks and 7,338 Whites diagnosed with either oral cavity or pharynx cancer 5. After adjusting for age, geographic area, socioeconomic status, stage and treatment the study reported no significant difference according to race (HR = 1.1, 95% CI = 0.9-1.4). Our study reported similar results after additional adjustments for smoking and insurance status. In addition, we report that the risk of recurrence, second primary or metastasis was almost two-fold for Blacks compared to Whites. Differences in treatment modalities between Blacks and Whites may explain the increased risk of recurrence, second primary or metastasis among Black cases. Murdock, et al. examined differences in treatment modalities for HNSCC in 54 African-American and 52 Whites, and showed higher incidence of surgical intervention amongst Whites compared to Blacks 8, but did not evaluate relapse-free survival. We observed a higher incidence of surgical intervention among Whites; and relapse-free survival was independently associated with treatment by radiation and/or chemotherapy only and was not associated with socioeconomic status or a lack of health insurance.
While tobacco and alcohol use are primary risk factors for all head and neck tumors, HPV is also thought to be an independent risk factor for most pharynx tumors 32-34. In a previous study, we reported that patients with HPV-positive tumors have an improved survival and are less likely to develop recurrent tumors; and the improved survival and recurrence, second primary, or metastasis rate was restricted to tumors diagnosed in the pharynx 35. A recent study by Chen et. al. reported that among oropharynx cancer patients, African-Americans and Hispanics had lower overall survival compared to White patients but this study did not include or adjust for socioeconomic or access to care in the analysis, nor evaluate the role of HPV 36. In our study the contribution of HPV involvement could not be evaluated as well, and this was a limitation. Nevertheless, after adjusting for the covariates in the model there was no difference in the risk of death or recurrence, second primary, or metastasis for Black patients diagnosed with oral cavity or pharynx tumors, compared to White patients. Further research of overall survival and relapse-free survival that include both HPV status and socio-demographic variables are needed to further evaluate racial disparities in head and neck cancer outcomes.
Although differences in disease outcome according to race may be attributed to a combination of tumor stage, socioeconomic status and access to health care, our findings suggest that the disparity still exists after adjusting for these factors. The inclusion of biological markers such as Human Papillomavirus status, and the extension of the study to a larger sample of Black patients are needed to further evaluate racial disparities in head and neck cancer outcomes.
Acknowledgments
Funding: Supported in part by Grant Number KL2 RR024154-03 from the National Center for Research Resources (NCRR) http://www.ncrr.nih.gov/, a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. This work was also supported in part by grant number R13CA130596A to CR, P20CA132385-01 to ET and P50CA097190 to JG.
Reference List
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A, Miller BA, Lewis DR, Eisner MP, Stinchcomb DG, Edwards BK. SEER Cancer Statistics Review, 1975-2006. National Cancer Institute; Bethesda, MD: 2009. [Google Scholar]
- 3.American Cancer Society . Cancer Facts & Figures 2009. American Cancer Society; Atlanta: 2009. [Google Scholar]
- 4.Molina MA, Cheung MC, Perez EA, et al. African American and poor patients have a dramatically worse prognosis for head and neck cancer: an examination of 20,915 patients. Cancer. 2008;113:2797–2806. doi: 10.1002/cncr.23889. [DOI] [PubMed] [Google Scholar]
- 5.Arbes SJ, Jr., Olshan AF, Caplan DJ, et al. Factors contributing to the poorer survival of black Americans diagnosed with oral cancer (United States). Cancer Causes Control. 1999;10:513–523. doi: 10.1023/a:1008911300100. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin WJ, Thomas GR, Parker DF, et al. Unequal burden of head and neck cancer in the United States. Head Neck. 2008;30:358–371. doi: 10.1002/hed.20710. [DOI] [PubMed] [Google Scholar]
- 7.Gourin CG, Podolsky RH. Racial disparities in patients with head and neck squamous cell carcinoma. Laryngoscope. 2006;116:1093–1106. doi: 10.1097/01.mlg.0000224939.61503.83. [DOI] [PubMed] [Google Scholar]
- 8.Murdock JM, Gluckman JL. African-American and white head and neck carcinoma patients in a university medical center setting. Are treatments provided and are outcomes similar or disparate? Cancer. 2001;91:279–283. doi: 10.1002/1097-0142(20010101)91:1+<279::aid-cncr19>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 9.Shavers VL, Harlan LC, Winn D, et al. Racial/ethnic patterns of care for cancers of the oral cavity, pharynx, larynx, sinuses, and salivary glands. Cancer Metastasis Rev. 2003;22:25–38. doi: 10.1023/a:1022255800411. [DOI] [PubMed] [Google Scholar]
- 10.Conway DI, Petticrew M, Marlborough H, et al. Socioeconomic inequalities and oral cancer risk: a systematic review and meta-analysis of case-control studies. Int J Cancer. 2008;122:2811–2819. doi: 10.1002/ijc.23430. [DOI] [PubMed] [Google Scholar]
- 11.Shiboski CH, Schmidt BL, Jordan RC. Racial disparity in stage at diagnosis and survival among adults with oral cancer in the US. Community Dent.Oral Epidemiol. 2007;35:233–240. doi: 10.1111/j.0301-5661.2007.00334.x. [DOI] [PubMed] [Google Scholar]
- 12.Funk GF, Karnell LH, Robinson RA, et al. Presentation, treatment, and outcome of oral cavity cancer: a National Cancer Data Base report. Head Neck. 2002;24:165–180. doi: 10.1002/hed.10004. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman HT, Karnell LH, Funk GF, et al. The National Cancer Data Base report on cancer of the head and neck. Arch.Otolaryngol.Head Neck Surg. 1998;124:951–962. doi: 10.1001/archotol.124.9.951. [DOI] [PubMed] [Google Scholar]
- 14.Tomar SL, Loree M, Logan H. Racial differences in oral and pharyngeal cancer treatment and survival in Florida. Cancer Causes Control. 2004;15:601–609. doi: 10.1023/B:CACO.0000036166.21056.f9. [DOI] [PubMed] [Google Scholar]
- 15.Moore RJ, Doherty DA, Do KA, et al. Racial disparity in survival of patients with squamous cell carcinoma of the oral cavity and pharynx. Ethn.Health. 2001;6:165–177. doi: 10.1080/13557850120078099. [DOI] [PubMed] [Google Scholar]
- 16.Keller AZ. Survivorship with mouth and pharynx cancer and their association with cirrhosis of the liver, marital status, and residence. Am.J Public Health Nations.Health. 1969;59:1139–1153. doi: 10.2105/ajph.59.7.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Settle K, Posner MR, Schumaker LM, et al. Racial survival disparity in head and neck cancer results from low prevalence of human papillomavirus infection in black oropharyngeal cancer patients. Cancer Prev Res (Phila Pa) 2009;2:776–781. doi: 10.1158/1940-6207.CAPR-09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwok J, Langevin S, Argiris A, et al. The impact of health insurance status on the survival of patients with head and neck cancer. Cancer. 2009 doi: 10.1002/cncr.24774. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakao K, Treas J. The 1989 socioeconomic index of occupations: construction from the 1989 occupational prestige scores. General Social Survey Methodological Reports. 1992;74:1–32. [Google Scholar]
- 20.American Joint Committee on Cancer . AJCC Cancer Staging Manual. 6th ed. Springer-Verlag; New York: 2002. [Google Scholar]
- 21.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 22.Hosmer DW. Applied Logistic Regression. 2nd ed. John Wiley & Sons; New York: 2000. [Google Scholar]
- 23.IARC Tobacco smoking IARC Monogr Eval Carcinog Risk Chem Hum. 1986;38:35–394. [PubMed] [Google Scholar]
- 24.WYNDER EL, BROSS IJ, FELDMAN RM. A study of the etiological factors in cancer of the mouth. Cancer. 1957;10:1300–1323. doi: 10.1002/1097-0142(195711/12)10:6<1300::aid-cncr2820100628>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 25.Choi SY, Kahyo H. Effect of cigarette smoking and alcohol consumption in the aetiology of cancer of the oral cavity, pharynx and larynx. Int.J.Epidemiol. 1991;20:878–885. doi: 10.1093/ije/20.4.878. [DOI] [PubMed] [Google Scholar]
- 26.Franceschi S, Levi F, La Vecchia C, et al. Comparison of the effect of smoking and alcohol drinking between oral and pharyngeal cancer. Int J Cancer. 1999;83:1–4. doi: 10.1002/(sici)1097-0215(19990924)83:1<1::aid-ijc1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Macfarlane GJ, Zheng T, Marshall JR, et al. Alcohol, tobacco, diet and the risk of oral cancer: a pooled analysis of three case-control studies. Eur J Cancer B Oral Oncol. 1995;31B:181–7. doi: 10.1016/0964-1955(95)00005-3. [DOI] [PubMed] [Google Scholar]
- 28.Giovino GA, Sidney S, Gfroerer JC, et al. Epidemiology of menthol cigarette use. Nicotine Tob Res. 2004;6:S67–S81. doi: 10.1080/14622203710001649696. [DOI] [PubMed] [Google Scholar]
- 29.Gandhi KK, Foulds J, Steinberg MB, et al. Lower quit rates among African American and Latino menthol cigarette smokers at a tobacco treatment clinic. Int J Clin Pract. 2009;63:360–367. doi: 10.1111/j.1742-1241.2008.01969.x. [DOI] [PubMed] [Google Scholar]
- 30.Clark PI, Gautam S, Gerson LW. Effect of menthol cigarettes on biochemical markers of smoke exposure among black and white smokers. Chest. 1996;110:1194–1198. doi: 10.1378/chest.110.5.1194. [DOI] [PubMed] [Google Scholar]
- 31.Kabat GC, Morabia A, WYNDER EL. Comparison of smoking habits of blacks and whites in a case-control study. Am J Public Health. 1991;81:1483–1486. doi: 10.2105/ajph.81.11.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ragin CC, Taioli E, Weissfeld JL, et al. 11q13 amplification status and human papillomavirus in relation to p16 expression defines two distinct etiologies of head and neck tumours. Br J Cancer. 2006;95:1432–1438. doi: 10.1038/sj.bjc.6603394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J.Natl.Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 34.Hobbs CG, Sterne JA, Bailey M, et al. Human papillomavirus and head and neck cancer: a systematic review and meta-analysis. Clin.Otolaryngol. 2006;31:259–266. doi: 10.1111/j.1749-4486.2006.01246.x. [DOI] [PubMed] [Google Scholar]
- 35.Ragin C, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to Human Papillomavirus infection: Review and Meta analysis. Int.J.Cancer. 2007;121:1813–1820. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 36.Chen LM, Li G, Reitzel LR, et al. Matched-pair analysis of race or ethnicity in outcomes of head and neck cancer patients receiving similar multidisciplinary care. Cancer Prev Res (Phila Pa) 2009;2:782–791. doi: 10.1158/1940-6207.CAPR-09-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]