Summary
We describe our preliminary experience on the feasibility of using the Willis covered stent in patients with carotid-cavernous fistulas (CCFs).
Eleven consecutive patients with post-traumatic CCFs referred for treatment with Willis covered stents were enrolled into this prospective study, and were subsequently followed-up at our hospital. Data on technical success, initial and final angiographic results, mortality, morbidity and final clinical outcome, was collected, with follow-up performed at one, three, six and 12 months, and yearly thereafter.
Deployment of the covered stents was technically successful in all patients. Angiographic results following stent placement showed a complete occlusion in eight patients with ten CCFs, and an incomplete occlusion in three. No adverse events occurred either during or after the procedure. Angiographic follow-up (mean 14.73 ± 6.77 months) revealed complete occlusion and no obvious in-stent stenosis in all patients. Clinical follow-up (mean 17.73 ± 6.48 months) demonstrated full recovery in ten patients, and improvement in one.
These preliminary results indicate that the use of the Willis covered stent is a feasible procedure, and that it may therefore serve as an alternative treatment for CCFs. Longer follow-up assessments and an expanded clinical trial are needed.
Key words: carotid-cavernous fistulas, endovascular treatment, trauma, covered stents
Introduction
Carotid-cavernous fistulae (CCFs) are rare complications, representing 0.2% to 0.3% of blunt or penetrating traumatic injuries of the carotid arteries.
Surgical repair and detachable balloon (DBs) are the most commonly used treatment methods.
Direct surgical repair with distal internal carotid artery (ICA) preservation still presents a challenge due to relative inaccessibility 1.
Although embolization with DBs has been widely accepted as a first-line and classical therapeutic option for CCFs, with a reported success rates of 75%-88% 1-3, residual or recurrence of CCFs, as well as pseudoaneurysm formation, frequently occur due to incomplete occlusion of the orifice, especially in patients with difficult or complex CCFs.
Recently, the successful use of covered stents for the management of post-traumatic CCFs has been reported 4-7.
This technique not only preserves and reconstructs the pathologic parent vessel to simplify the endovascular procedure, but also shortens the procedure time and reduces the cost by placing the stent across the ostium of the fistula.
Thus, the purpose of this study was to present our preliminary experience of the evaluation and feasibility of using a Willis covered stent for the treatment of CCFs.
Patients and Methods
Patients
The study was approved by our institutional review board and written informed consent was obtained from all patients or immediate relatives. Between June 2009 and September 2011, 11 consecutive patients with post-traumatic CCFs referred for treatment with a Willis covered stent were enrolled into the study and were subsequently followed up at the hospital. The cohort included eight males and three females, with a mean age of 32.18 ± 11.19 years (range, 15-52 years). The demographics, clinical characteristics, endovascular treatment and follow-up outcomes of the patients are summarized in Table 1.
Table 1.
Demographic, clinical presentation, CT findings, endovascular treatment and follow-up outcomes of 11 patients with CCFs.
| Pa./Age /Sex |
Clinical symptoms |
Etiology | Duration (mos) |
Stent size (mm) |
Immediate angiographic result |
Final angiography follow-up |
Final clinical follow-up |
||
|---|---|---|---|---|---|---|---|---|---|
| months | Result | months | Outcomes | ||||||
| 1/33/M | PE, RB, B | TA | 3 | 4.0×10 | Complete occlusion | 24 | Complete occlusion |
27 | Full recovery |
| 2/32/F | PE, RB, DVA | MVA | 2 | 4.0×10 | Incomplete occlusion | 24 | Complete occlusion |
25 | Full recovery |
| 3/52/M | PE, RB, C | MVA | 1 | 3.5×13 | Complete occlusion | 18 | Complete occlusion |
24 | Full recovery |
| 3.5×10 | |||||||||
| 4/15/M | PE, RB, C, DVA | MVA | 3 | 3.5×10 | Complete occlusion | 18 | Complete occlusion |
22 | Unchanged |
| 5/34/F | PE, RB, C, O | TA | 2 | 4.0×13 | Complete occlusion | 18 | Complete occlusion |
20 | Full recovery |
| 6/38/M | PE, RB, C, | MVA | 1 | 3.0×7 | Complete occlusion | 18 | Complete occlusion |
18 | Full recovery |
| 7/25/M | PE, RB, C, DVA | MVA | 2 | 4.0×10 | Complete occlusion | 12 | Complete occlusion |
15 | Full recovery |
| 4.0×13 | |||||||||
| 8/42/F | PE, RB, DVA | MVA | 3 | 3.5×7 | Incomplete occlusion | 12 | Complete occlusion |
15 | Full recovery |
| 4.0×10 | |||||||||
| 9/16/M | PE, RB, C, | TA | 1 | 4.0×10 | Complete occlusion | 6 | Complete occlusion |
12 | Full recovery |
| 10/26/M | PE, RB, C, O | MVA | 1 | 3.5×10 | Complete occlusion | 6 | Complete occlusion |
9 | Full recovery |
| 11/41/M | PE, RB, C, O | MVA | 2 | 4.0×10 | Full recovery | 6 | Complete occlusion |
8 | Full recovery |
| 4.0×7 | |||||||||
|
Note: MVA = Motor vehicle accident; TA = Traffic accident; PE = Pulsating exophthalmos; RB = Retroorbital bruit; C = Chemosis; DVA = Decreased visual acuity; O = Ophthalmoplegia; B = Blindness; Duration = from head trauma to Willis covered stent placement. | |||||||||
The diagnosis of CCF was primarily made from the patient's medical history and cerebral angiograms. A delay in diagnosis and endovascular treatment occurred in all patients. Clinical symptoms at presentation included pulsating exophthalmos (n = 11), retroorbital bruit (n = 11), chemosis (n = 8), decreases visual acuity (n = 4), ophthalmoplegia (n = 3), and blindness (n = 1). Angiograms of six arteries performed in all patients revealed bilateral CCFs in two patients and unilateral in nine.
Willis Covered Stent Placement
The Willis balloon-expandable covered stent, made by the MicroPort Medical Company (Shanghai, China), has been described in detail 8-11. Briefly, the stent consists of three parts: a bare stent, an expandable polytetrafluoroethylene (ePTFE) membrane and a balloon catheter. Willis covered stents of 3.5, 4.0, 4.5, and 5.0 mm in diameter and 7, 10, 13, 16, and 19 mm in length are available. The technique of placing the Willis covered stent and periprocedure management have been previously described 8-11. Briefly, all procedures were performed under general anesthesia. After positioning a 6-F Envoy (Cordis, Miami Lakes, FL, USA) guiding catheter in the internal carotid artery (ICA), a microguidewire (Transend floppy, Boston Scientific) was navigated into a distal branch of the middle cerebral artery. With roadmap guidance, the Willis covered stent was navigated over the microguidewire, and then bridged the orifice of the fistula. Angiography was performed immediately after balloon deflation to confirm the correct placement of the stent and satisfactory occlusion of the fistula.
Prior to the procedure, patients received aspirin (100 mg/d) and clopidogrel (75 mg/d) for three consecutive days. Patients received a bolus dose of heparin 5000 IU at the start of the procedure, followed by a continuous infusion of 1000 IU/h with the aim of maintaining the activated clotting time above 300 seconds. Heparin was administered for 48 hrs after the procedure, and patients were instructed to take aspirin (100 mg/d) and clopidogrel (75 mg/d) orally for six months to avoid thrombosis and in-stent stenosis.
Follow-up and Postoperative Outcome Evaluation
The follow-up protocol was performed at one, three, six and 12 months, and thereafter annually, post-stent placement by one of the authors and included a clinical and angiographic assessment. Data on technical success, initial and final angiographic results, mortality, morbidity and the final clinical outcome, were retrospectively collected and analyzed by both authors at the time of discharge and at the end of the follow-up period. The angiographic data were categorized into complete occlusion, with no residual cavity and no endoleak, or incomplete occlusion, with a residual cavity or an endoleak. The clinical follow-up assessment was graded into four types: full recovery from the neurologic symptoms, improved neurologic symptoms, unchanged symptoms, or a deterioration in neurologic symptoms 8-11.
Results
Patients
Primary procedural results
The deployment of the covered stents was technically successful in all patients. Complete occlusion of the fistulas without endoleak was achieved in seven patients with nine CCFs, and transient endoleaks into the sinus cavernosus were observed in four patients with four CCFs immediately after the deployment of the initial Willis covered stent (cases 2, 3, 8 and 11). The endoleak was dramatically reduced to a minimal endoleak (a very small endoleak) in two CCFs (cases 8 and 11) and persisted in two CCFs after balloon reinflation (cases 2 and 3, Figure 1). Additional covered stent placements at the proximal end of the initial stent were performed for two CCFs with persistent endoleak, which resulted in complete occlusion of one fistula (case 3, Figure 1) and one minimal endoleak (case 2).
One covered stent was placed in seven patients and two covered stents in four, including two patients with two CCFs. The angiographies obtained at the end of the initial procedure showed that complete occlusion was achieved in eight patients with ten CCFs (72.7 %), and an incomplete occlusion in three (3/11, 27.3%).
There were no adverse events related to navigation of the covered stent in any of the patients. Neurologic examination after the procedure and before discharge exhibited no new neurologic symptoms and no recurrent pulsating exophthalmos or retroorbital bruit in any of the patients.
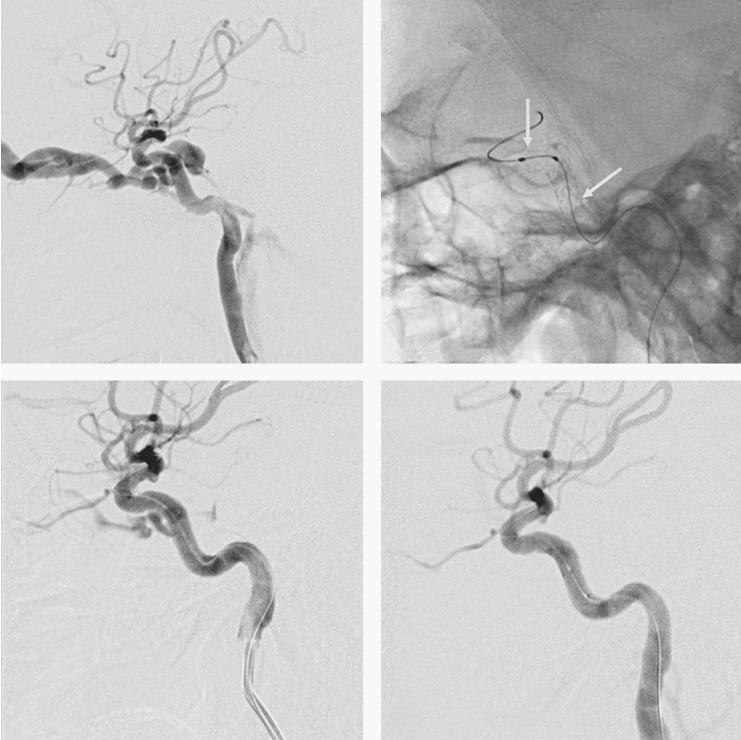
Figure 1.
A 52-year-old man with a pulsatile exophthalmos, an orbital bruit and decreased visual acuity in the left eye after a motor vehicle accident 1 month earlier. A) Cerebral angiography of the left internal carotid artery (lateral projections) confirmed a CCF draining into the inferior petrosal sinus and the superior ophthalmic vein. B) Two Willis covered stent deployments (between the two arrows) for the treatment of the CCF. C) Immediate cerebral angiography of the left ICA after the first Willis covered stent placement confirms an endoleak of the fistula (arrow). D) Lateral cerebral angiography immediately after the second Willis covered stent deployment demonstrates disappearance of the fistula with patency of the parent artery.
One covered stent was placed in seven patients and two covered stents in four, including two patients with two CCFs. The angiographies obtained at the end of the initial procedure showed that complete occlusion was achieved in eight patients with ten CCFs (72.7 %), and an incomplete occlusion in three (3/11, 27.3%).
There were no adverse events related to navigation of the covered stent in any of the patients. Neurologic examination after the procedure and before discharge exhibited no new neurologic symptoms and no recurrent pulsating exophthalmos or retroorbital bruit in any of the patients.
Follow-up angiographic and clinical results
Angiography follow-up (mean, 14.73 ± 6.77 months; range, 6-24 months] was completed in all patients. All 11 subjects underwent the three and six-month follow-up angiography, while nine underwent the 12-month follow-up angiography, and two patients the 24-month post-procedure angiography. No morbidity or mortality occurred in any of the patients in the follow-up study period.
On the three-month follow-up angiography, eight patients with 10 CCFs and initial complete exclusion, complete occlusion of the fistula was still observed with reconstruction of the ICA. In three patients with three CCFs with an initial residual minimal endoleak, all three CCFs observed a spontaneous resolution of the endoleak with reconstruction of the ICA. No obvious in-stent stenoses were noted in any of the 11 patients. In the nine patients with a 12-month follow-up angiography, complete occlusion of the fistula and parent artery patency persisted without in-stent stenosis. Angiographies obtained at the final follow-up demonstrated complete occlusion in all 11 patients with 13 CCFs.
Clinical follow-up data was obtained from all 11 patients between eight and 27 months post-stent placement with a mean of 17.73 ± 6.48 months. Clinical evaluations performed at the final follow-up showed a full recovery in ten patients, and unchanged clinical status in one patient who was blind. During the available follow-up period, no occurrence of pulsating exophthalmos, retroorbital bruit or chemosis was reported by any of patients.
Discussion
In this study, we found that: 1) the use of the Willis covered stent was a feasible procedure for CCFs; 2) complete occlusion of the fistula was achieved in all patients without recanalization on final angiograms; and 3) the parent artery was patent without obvious stenosis. The significant improvement in complete occlusion rates observed during the angiographic follow-up period seemed to be predominantly attributable to occlusion of the orifice of the fistula.
Post-traumatic CCF is an infrequent complication resulting from craniomaxillofacial injuries or basilar skull fracture. The optimal treatment is exclusion of the fistula from the circulation while preserving ICA patency. Transarterial balloon occlusion of CCFs has been widely accepted and used in many centers around the world, as it is relatively easy and inexpensive. In addition, it is associated with high rates of successful fistula occlusion and preservation of the ICA 1-3,12,13. However, residual or recurrent CCF, as well as pseudoaneurysm formation, have often been major problems in balloon occlusion of the fistula, due to incomplete occlusion of the orifice with DBs. Although coil embolization is another relatively easy and widely used approach for the treatment of post-traumatic CCFs 1,4,14-18, especially for recurrent or complex CCFs, it is expensive, and not always safe or effective for large or high-flow fistulas, and associated with sacrifice of the ICA in certain instances 2,18,19.
Today, the covered stent has been successfully used for the treatment of aneurysms, pseudoaneurysms, dissections, and CCFs, with promising results achieved 4-11. Using a covered stent, closure of the CCF orifice is potentially quick and relatively straightforward, and without the occurrence of endoleaks. The drawbacks are residual or recurrence of the CCFs, whereas pseudoaneurysm formation does not occur once the orifice of the CCF is completely occluded. In this study, placement of the Willis covered stent was technically successful in all patients with no procedure-related complications. Complete occlusion of the fistula was achieved in all patients without the occurrence of recanalization on final angiograms. These results indicate that the use of the Willis covered stent was a feasible approach for the treatment of CCFs.
Compared with the established embolization technique with coils, a covered stent has the following advantages: 1) a relatively simple and rapid performance; 2) no coil herniation, delayed migration, and coil loop protrusion; 3) disappearance or reduction of mass effects in large CCFs; and 4) no residual or recurrence of CCFs as well as pseudoaneurysm formation.
Our results have potentially important clinical implications. The use of a covered stent rather than detachable balloons or coils in patients with CCFs will substantially increase the complete occlusion and anatomic cure rates, and eliminate recanalization and mass effect. In addition, considerable savings can be achieved by avoiding the costs of repeated coiling, as well as the costs of treating recurrent CCFs.
The present study had limitations. The number of patients treated remains small. Thus, expanded clinical trials are required to determine the long-term outcomes. Secondly, in-stent stenosis might occur in patients who do not receive regular anticoagulation medication after stent placement. We emphasize that all patients must comply with standard anticoagulant and antiplatelet therapy (aspirin 100 mg/d and clopidogrel 75 mg/d orally) for six months after stent placement. We used the same doses for traumatic CCF patients with and without complete occlusion of the fistulae after stent placement, which did not lead to problems such as bleeding or symptom deterioration. This reflects our use of the minimum dose for the prevention of in-stent thrombosis or stenosis. In addition, the CCF disappeared without recurrence after placement of the covered stent, as the orifice of the fistula was covered by the stent with diversion of blood flow, even in the presence of an endoleak.
In conclusion, our preliminary results indicate that the use of the Willis covered stent is a feasible procedure, and may serve as an alternative treatment for CCFs. Longer term follow-up and expanded clinical trials are needed.
References
- 1.Higashida RT, Halbach VV, Tsai FY, et al. Interventional neurovascular treatment of traumatic carotid and vertebral lesion: results in 234 patients. Am J Roentgenol. 1989;153:577–582. doi: 10.2214/ajr.153.3.577. [DOI] [PubMed] [Google Scholar]
- 2.Luo CB, Teng MM, Yen DH, et al. Endovascular embolization of recurrent traumatic carotid-cavernous fistulas managed previously with detachable balloons. J Trauma. 2004;56:1214–1220. doi: 10.1097/01.ta.0000131213.93205.57. [DOI] [PubMed] [Google Scholar]
- 3.Gupta AK, Purkayastha S, Krishnamoorthy T, et al. Endovascular treatment of direct carotid cavernous fistulae: a pictorial review. Neuroradiology. 2006;48:831–839. doi: 10.1007/s00234-006-0132-x. [DOI] [PubMed] [Google Scholar]
- 4.Felber S, Henkes H, Weber W, et al. Treatment of extra cranial and intracranial aneurysms and arteriovenous fistulae using stent grafts. Neurosurgery. 2004;55:631–638. doi: 10.1227/01.neu.0000134455.02947.1f. [DOI] [PubMed] [Google Scholar]
- 5.Gomez F, Escobar W, Gomez AM, et al. Treatment of carotid cavernous fistulas using covered stents: midterm results in seven patients. Am J Neuradiol. 2007;28:1762–1768. doi: 10.3174/ajnr.A0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C, Xie X, You C, et al. Placement of covered stents for the treatment of direct carotid cavernous fistulas. Am J Neuradiol. 2009;30:1342–1346. doi: 10.3174/ajnr.A1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang W, Li YD, Li MH, et al. Endovascular treatment of posttraumatic direct carotid-cavernous fistulas: a single-center experience. J Clin Neurosci. 2010;18:24–28. doi: 10.1016/j.jocn.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Li MH, Li YD, Gao BL, et al. A new covered stent designed for intracranial vasculature: application in the management of pseudoaneurysms of the cranial internal carotid artery. Am J Neuroradiol. 2007;28:1579–1585. doi: 10.3174/ajnr.A0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li MH, Li YD, Tan HQ, et al. Treatment of distal internal carotid artery aneurysm with the Willis covered stent: a prospective pilot study. Radiology. 2009;253:470–477. doi: 10.1148/radiol.2532090037. [DOI] [PubMed] [Google Scholar]
- 10.Li YD, Li MH, Gao BL, et al. Endovascular treatment of recurrent intracranial aneurysms with re-coiling or covered stents. J Neurol Neurosurg Psychiatry. 2010;81:74–79. doi: 10.1136/jnnp.2009.171967. [DOI] [PubMed] [Google Scholar]
- 11.Li MH, Leng B, Li YD, et al. Comparative study of covered stent with coil embolization in the treatment of cranial internal carotid artery aneurysm: a nonrandomized prospective trial. Eur Radiol. 2010;20:2732–2739. doi: 10.1007/s00330-010-1854-z. [DOI] [PubMed] [Google Scholar]
- 12.Debrun G, Lacour P, Viñuela F, et al. Treatment of 54 traumatic carotid-cavernous fistulas. J Neurosurg. 1981;55:678–692. doi: 10.3171/jns.1981.55.5.0678. [DOI] [PubMed] [Google Scholar]
- 13.Lewis AI, Tomsick TA, Tew JM, et al. Long-term results in direct carotid-cavernous fistulas after treatment with detachable balloons. J Neurosurg. 1996;84:400–404. doi: 10.3171/jns.1996.84.3.0400. [DOI] [PubMed] [Google Scholar]
- 14.Jansen Q, Dorfler A, Forsting M, et al. Endovascular therapy of arteriovenous fistulae with electrolytically detachable coils. Neuroradiology. 1999;41:951–957. doi: 10.1007/s002340050875. [DOI] [PubMed] [Google Scholar]
- 15.Morön FE, Klucznik RP, Mawad ME, et al. Endovascular treatment of high-flow carotid cavernous fistulas by stent-assisted coil placement. Am J Neuroradiol. 2005;26:1399–1404. [PMC free article] [PubMed] [Google Scholar]
- 16.Lin CJ, Luo CB, Chang FC, et al. Combined transarterial, transvenous, and direct puncture of the cavernous sinus to cure a traumatic carotid cavernous fistula. J Clin Neurosci. 2009;16:1663–1665. doi: 10.1016/j.jocn.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 17.Luo CB, Teng MMH, Chang FC, et al. Transarterial balloon-assisted n-Butyl- 2-Cyanoacrylate embolization of direct carotid cavernous fistulas. Am J Neuroradiol. 2006;27:1535–1540. [PMC free article] [PubMed] [Google Scholar]
- 18.Siniluoto T, Seppanen S, Kuurne T, et al. Transarterial embolization of a direct carotid cavernous fistula with Guglielmi detachable coils. Am J Neuroradiol. 1997;18:519–523. [PMC free article] [PubMed] [Google Scholar]
- 19.Klisch J, Huppertz HJ, Spetzger U, et al. Transvenous treatment of carotid cavernous and dural arteriovenous fistulae: results for 31 patients and review of the literature. Neurosurgery. 2003;53:836–856. doi: 10.1227/01.neu.0000083551.26295.ab. [DOI] [PubMed] [Google Scholar]