Abstract
Sine-wave electrical stimulation at frequencies 2000, 250, and 5 Hz to respectively evaluate Aβ, Aδ, and C sensory neurons has recently been added to the armamentarium used to evaluate sensory neurons. We developed an automated nociception assay using sine-wave stimulation methodology to determine current vocalization threshold in response to 2000, 250, and 5 Hz and examine the effects of sex, analgesics, and anesthetics in mice. At baseline, males had significantly higher mean current vocalization thresholds compared with female mice at 2000, 250, and 5 Hz (p ≤ 0.019). By 1 h after intrathecal injections of morphine there were significant increases in current vocalization threshold percent changes from baseline that varied with doses (p = 0.0001) and frequency used (p < 0.0001). Specifically, with increasing doses of morphine, there were significantly greater increases in current vocalization threshold percent changes from baseline in response to 5 Hz compared with 250 and 2000 Hz stimulation in a significantly ordered pattern: 5 Hz > 250 Hz (p < 0.0001) and 250 Hz > 2000 Hz (p = 0.0002). Forty-five minutes after exposure, there were no effects of isoflurane on current vocalization thresholds at any frequency. Therefore, our findings suggest that this automated nociception assay using sine-wave stimulation in mice, can be valuable for measurements of the effects of sex, opioids, and anesthetics on the response to electrical stimuli that preferentially stimulate Aβ, Aδ, and C-sensory fibers in vivo. This investigation suggests the validation of this assay and supports its use to examine mechanisms of nociception in mice.
Keywords: Nociception, Sine-wave, Sex, Opioid, Anesthesia, Morphine
1. Introduction
In vivo, the evaluation of specific sensory nerve fibers function [Aβ (pressure), Aδ (localized sharp pain), and C (burning pain) fibers] can be performed with the use of variable rates of noxious radiant heat stimulation as Aδ fibers are activated by high rate and C fibers by low rate of skin heating (Yeomans et al., 1996a,b; Yeomans and Proudfit, 1996). Alternatively, sine-wave electrical stimulation at frequencies of 2000, 250, and 5 Hz to respectively stimulate Aβ, Aδ, and C sensory nerve fibers function can also be used to study specific sensory neurons. The specificity of 5, 250, and 2000 Hz to stimulate C, Aδ, Aβ sensory neurons results from the distinct electrophysiological characteristics (diameter, conduction velocity, and refractory period) of each type of afferent neurons (Katims, 1998; Koga et al., 2005). As such, this methodology has been used in clinical and experimental settings in humans and animals for the diagnosis of neuropathies and for the investigations of pharmaco-dynamics of analgesics and mechanisms of nociception (Angst et al., 2001; Finkel et al., 2002; Katims, 1998; Katims et al., 1991; Liem et al., 2005; Masson and Boulton, 1991; Matsutomo et al., 2005; Oishi et al., 2002). In rodents and dogs, sine-wave stimulation has also been used to evaluate the effect of analgesics and inflammatory pain models on specific sensory nerve fibers (Kiso et al., 2001; Matsumoto et al., 2006a,b, 2008; Nagakura et al., 2008a,b; Oda et al., 2005; Watabiki et al., 2010). Therefore sine wave stimulation has been shown to be applicable to several species and has added to our ability to evaluate the function of sensory neurons and nocifensive behavior.
We have developed an automated nociception assay using sine-wave stimulation methodology to determine current vocalization threshold in response to 2000, 250, and 5 Hz and thereby examine the function of Aδ, Aβ, and C sensory fibers respectively in mice. In the present investigation, we aimed at validating the assay for the measurements of the effects of sex, opioid analgesia, and anesthetics in mice.
2. Materials and methods
2.1. Mice
After approval from the NIH Clinical Center Animal Care and Use Committee, National Institutes of Health, 87 female and 47 male B6129Sf/J and C57Bl/6 (Jackson Laboratory, Bar Harbor, ME) mice weighing 18–32 g and aged 6–22 weeks were enrolled in this study (Table 1).
Table 1.
Number of animals enrolled per group.
| Baseline sex difference studies | |||||
| Strain | C57Bl/6a | B6129Sf/J | |||
| Male | 19 | 12 | |||
| Female | 53 | 18 | |||
| Morphine effect studies | |||||
| Morphine dose | 0 μg/mouse | 10 μg/mouse | 20 μg/mouse | 30 μg/mouse | 40 μg/mouse |
| Male | 3 | 4 | 2 | 6 | 4 |
| Female | 16 | 6 | 8 | 10 | 13 |
| Isoflurane anesthesia study | |||||
| Anesthesia | Sham Anesthesia | Isoflurane anesthesia | |||
| Male | 8 | 8 | |||
| Female | 8 | 8 |
For baseline sex difference studies in C57Bl/6 mice, we included all baseline measurements obtained in animals from the morphine study before any intervention was made. Animals in the morphine study were given only one of the 5 morphine doses. Only C57Bl/6 mice were included in the morphine effect study.
2.2. Nociception assay
We designed an assay aiming at eliminating operator variability in the interpretation of nocifensive behavior in mice. As such, in this nociception assay, the delivery of electrical stimuli and the recognition of the nocifensive behavior, here defined as vocalization (audible), are automated. Fig. 1 illustrates the components of the neurospecific nociception assay. Custom hardware and software were designed to control and automate the frequency of electrical stimulation, stimulus delivery/termination, intensity, duration, and duty cycle. The system monitors the progression of experiments, detects mouse vocalizations (here defined as the nocifensive behavior), and records measurements. The electrical stimulus is generated by a neurostimulator (Neurometer, Neurotron, Inc., Baltimore, MD) and is controlled by custom software through a standard RS-232 serial port. A custom built handheld control device is connected through a custom cable to a DAQCard 6533 Digital IO PCMCIA card (National Instruments, Austin, TX) located in the laptop and allows for discontinuation of stimulus. A microphone (AT943-SP, Sound Professionals, Mt Laurel, NJ) is placed on a rubber mount in front of the mouse. The microphone connects to a custom built preamplifier which connects to a DAQ-Card PCMCIA card (National Instruments, Austin, TX). A custom software program controls the devices through drivers provided by the respective companies. The multi-threaded program is written in C++ using object-oriented concepts and uses Microsoft Foundation Classes (MFC) for the graphical interface.
Fig. 1.
Components of the nociception assay used to study the effects of sex, opioids and anesthetics in current vocalization threshold to electrical stimuli. The delivery of stimulation and detection of nocifensive behavior recognition is entirely automated.
For current vocalization threshold measurements, animals are placed in a mouse holder (Kent Scientific Corporation, Torrington, CT) such that the tail is accessible to the investigator. The mouse holder was modified to minimize mouse chewing and scratching on hard surfaces, which can be a sources of problematic audio noise. Electrodes are applied to the mouse tail using adhesive tape. A grounding (SDE44; Neurotron Inc., Baltimore, MD) electrode is placed at the most proximal end of the tail and a stimulating (ATE1925; Neurotron Inc.) electrode is placed 1 cm distally to the grounding electrode. Cables are snapped on to grounding and stimulating electrodes and connected to the neurostimulator to enable stimuli delivery.
2.3. Current vocalization threshold
Audible vocalization is the nocifensive behavior end-point used to cease delivery of electrical stimulation. The current vocalization threshold corresponds to the amperage of the electrical stimulus at which vocalization occurs. For ease of data presentation, we defined the unit of measure of current vocalization threshold as units which corresponds to the stimulus intensity (amperage) that yielded nocifensive behavior (vocalization) or the maximum amperage delivered for each frequency multiplied by 100 (Table 2). We conducted pilot studies to examine the characteristics of mouse vocalization in response to the electrical stimulus and found that mouse movements during the experiment could be picked up by the microphone and needed to be distinguished from vocalizations. Pilot studies showed that vocalizations could vary in frequency from 2 kHz to 20 kHz. Amplitudes can vary greatly, even for a given mouse and frequency. Some vocalizations are essentially sine waves of a single frequency, others more closely resemble a chirp (the frequency slowly increases/decreases for the length of the vocalization), and others are more complex and do not fit simple descriptions, although almost all contain a strong sinusoidal component. Also, mouse movements during the stimulus are picked up by the microphone and must be distinguished from real vocalizations. Typically, these signals are of much lower frequency and do not share the same features as a vocalization. For instance, the clicking of a mouse's claws or the chewing of the plastic holder generates very sharp spikes which have some oscillatory features, but are not sinusoidal.
Table 2.
Characteristics of electrical stimulation algorithms at each frequency delivered to determine current vocalization threshold.a
| Frequency |
|||
|---|---|---|---|
| 5 Hz | 250 Hz | 2000 Hz | |
| Stimulus characteristics | |||
| Fiber preferentially stimulated | C fiber | Aδ fiber | Aβ fiber |
| Duration of stimulus at each level | 1 s | 1 s | 1 s |
| Intensity (minimum–maximum, mA) | 0.05–0.5 | 0.14–0.5 | 0.4–1.6 |
| Increment intensity (mA) | 0.05 | 0.04 | 0.1 |
| Respective current vocalization threshold (units) | 5–50 | 14–50 | 40–160 |
One current vocalization threshold unit is equal to 100 mili Amperes (mA).
The audio data processing starts with a high-pass filter (cutoff frequency of 500 Hz) to eliminate vibration noise picked up by the microphone and a median filter which eliminates sharp spikes (caused by chewing for example). A running root-mean-square (RMS) algorithm is applied which effectively calculates the energy of the incoming signal. The program next determines if the signal has exceeded a threshold and the length of time it has exceeded the threshold. If the signal is very short (<5 ms) it is determined to be noise and ignored. If the signal is long (>50 ms) it is determined to be a vocalization since the filters applied at the beginning have eliminated the most serious noise. Signals <50 ms (but >5 ms) need further processing to determine if they are noise or a vocalization. First, the original data is baseline corrected and then passed through a zero crossing detection algorithm which calculates the number of crossings and the mean and standard deviation of the spacing of those crossings. Noisy signals have more random zero crossings while vocalizations will generally have consistent spacing. The algorithm uses this information to determine if the signal is a vocalization and subsequently cease neurostimulation.
2.4. Delivery of electrical stimulus
We used electrical stimuli at three different frequencies, 2000, 250, and 5 Hz, to preferentially (Koga et al., 2005) stimulate Aβ, Aδ, and C sensory nerve fibers. At each frequency, stimuli are delivered at stepwise increments and each stimulus lasts 1 s and is followed by a 1-s stimulus-free interval (50% duty cycle). The electrical stimulus pulse that results in vocalization could be shorter than 1 s if vocalization occurs prior to the end of the normal pulse. A 1-min interval is built in between deliveries of electrical stimuli of different frequencies. The duration of each stimulus as well as stimulation-free intervals of 1 s were based on intervals used in human studies and on mouse pilot studies suggesting adequacy and reproducibility of threshold measurements with 1-s stimulation. The 1-min interval between frequencies was set arbitrarily in order to allow animals to rest between frequencies. In this investigation, current vocalization thresholds were sequentially determined in response to 2000, 250, and 5 Hz and the threshold for each frequency was determined as the average of five consecutive measurements. The reason for this order of stimuli is based on prior studies showing that vocalization thresholds for 2000 Hz and 250 Hz frequencies are lower when they follow the 5 Hz electrical stimulus (Finkel et al., 2006). In addition, five measurements are obtained in order to increase accuracy of threshold measurements as shown in previous studies (Finkel et al., 2006).
The computer program reads in the designed experimental protocol which is stored in a spreadsheet format. Experimental protocols contain the permutation of electrical stimulus frequencies, minimum and maximum amperages for each frequency, duration of and interval between stimuli. The investigator has access to a screen that indicates the electrical stimulus frequency, intensity, and repetition throughout a given experiment. The handheld control enables the investigator to manually stop a stimulus (because of missed automatic vocalization detection), inform the software of false negative detection of vocalization, and abort the entire experiment if necessary.
2.5. Study design and experimental protocol
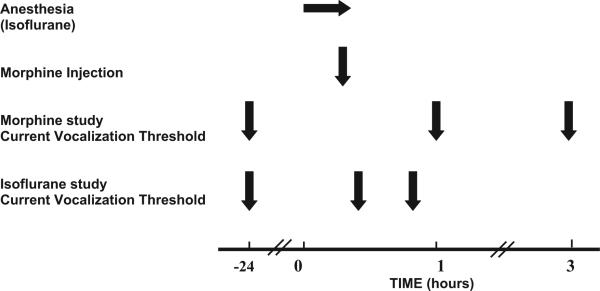
Fig. 2 shows the time course of this study. Current vocalization thresholds were measured in basal conditions in male and female B6129Sf/J and C57Bl/6 mice. In animals enrolled in the morphine study, measurements were obtained before (−24 h) and 1 and 3 h after an intrathecal injection of morphine sulfate (0–40 μg/mouse, Baxter Healthcare Corporation). Different cohorts of animals were used for each dose of morphine (Table 1). Intrathecal injections were performed under brief isoflurane anesthesia as previously described (Hylden and Wilcox, 1980). Briefly, using a 30 G needle and a microliter syringe, morphine or vehicle were injected intrathecally between the L5 and L6 intervertebral space. A tail flick or formation of an S shape by the tail associated with needle placement indicated puncture of the dura as previously reported (Fairbanks, 2003). All drugs were prepared such that a volume of 10 μl was injected intrathecally.
Fig. 2.
Experimental design and time course of the study. Animals participating in the morphine study received an intrathecal injection of morphine at the time indicated and those in the isoflurane study did not.
In a different cohort of animals, we investigated the effect of isoflurane and sham anesthesia on current vocalization threshold in C57Bl/6 mice in order to eliminate any confounding effect of the anesthesia used for intrathecal injections and the effects of morphine. After baseline measurements (−24 h), mice were placed into an anesthesia chamber and exposed to oxygen (FiO2 of 1) only (sham anesthesia) or to 3% isoflurane (Baxter Healthcare Corporation, IL) in oxygen for 10 min. Current vocalization thresholds were then measured at 15 and 45 min after isoflurane or sham anesthesia. These times were chosen to evaluate the effect of isoflurane soon after exposure (15 min) and just before (45 min) the time after intrathecal morphine administration (1 h) when current vocalization threshold measurements were obtained.
2.6. Statistical methods
Statistical analyses were done using Statistical Analysis System Version 9.2 software (SAS Institute, Inc., Cary, NC). Our data involved repeating measures of current vocalization thresholds at 3 different frequencies on the same mice at different times before and after intrathecal injections. For comparing males and females in two different strains of mice at baseline conditions we used 2-sample t-tests. To compare changes in vocalization thresholds between males and females at 1 h and 3 h post treatment we calculated percent changes from baseline threshold for each frequency and performed 2-way ANOVA with treatment (dose of morphine) as a covariate.
We used specialized methods for multivariate repeated measures ANOVA using PROC MIXED (for mixed models) for comparing the three frequencies. For comparing the dependent variable of current vocalization threshold percent changes from baseline among the 3 frequencies studied, we modeled frequency and treatment dose as classification variables with unstructured covariance pattern and mouse was a random effect (i.e., a “random intercept” model). Because of the small sample sizes we used the Kenward and Roger method for computing the degrees of freedom for tests of fixed effects, and all models included interaction terms. The value of the estimator (“least square means” in SAS terminology) and associated standard error of the estimator and p values are reported. After comparing pairs of least square means among the 3 frequencies at each dose level we adjusted p-values using a Bonferroni's correction for multiple comparisons. Two sided p < 0.05 was considered statistically significant for all analyses.
3. Results
Throughout the study, animals showed no signs of injury or lasting discomfort resulting from measurements of current vocalization threshold. All vocalizations recorded contained a sinusoidal component and were observed only in response to neurostimulation. We also observed that while in the restraint device and after placement of electrodes on the tails, most mice displayed random tail movements which were not necessarily associated with the delivery of any electrical stimuli. In addition, in response to electrical stimuli of intensities lower than that yielding vocalization (current vocalization threshold), some animals displayed tail movements perhaps suggesting that perception of some stimulus preceded vocalization. Once the vocalization threshold was reached, the stimulation session at the given frequency was terminated as per the experimental protocol.
3.1. Effect of sex on current vocalization threshold during basal conditions
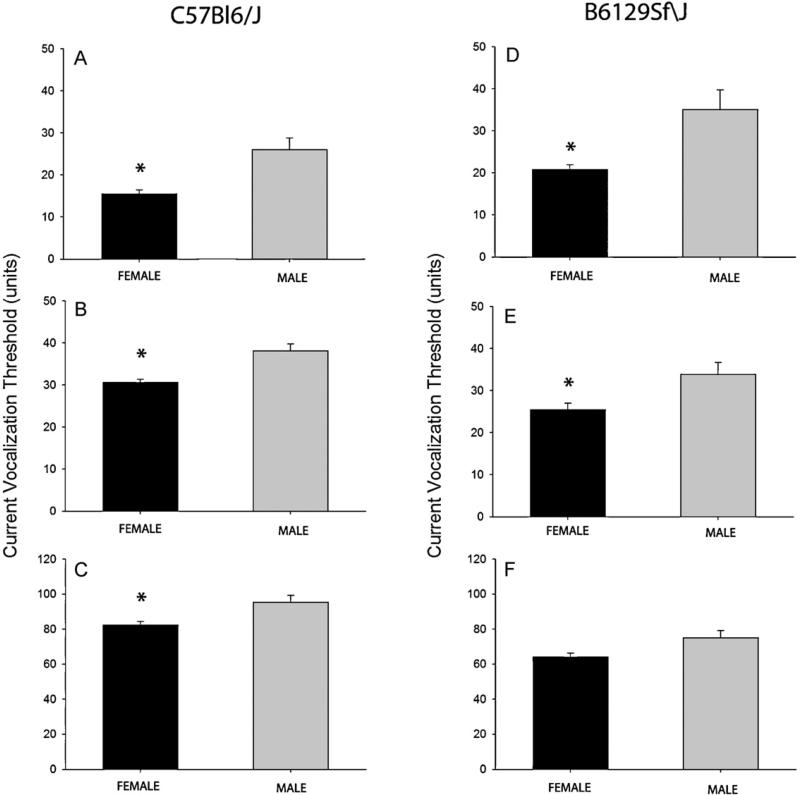
In order to examine whether this nociceptive assay could detect sex-related differences in nociception, we first measured current vocalization threshold in male and female animals during basal condition in two different strains of mice that are representative of the background strains used for a majority of transgenic mice used in nociception research (Fig. 3). C57Bl/6 male mice had significantly higher mean current vocalization thresholds compared with females in response to 2000, 250, and 5 Hz stimulation suggesting that males have higher tolerance to electrical stimulation at all frequencies used (Fig. 3, all male vs. female comparisons p ≤ 0.01). B6129Sf\J male mice had significantly higher mean current vocalization thresholds compared with females in response to 250, and 5 Hz (both p ≤ 0.01) but not to 2000 Hz (Fig. 3).
Fig. 3.
Mean ± SEM baseline current vocalization thresholds in male (gray bars) and female (black bars) C57BL6/J and B6129Sf\J mice in response to 5 (Panels A and D), 250 (Panels B and E), and 2000 (Panels C and F) Hz. * indicates p ≤ 0.01 for male versus female comparisons.
3.2. Effect of morphine on current vocalization thresholds to electrical stimulus
While male and female mice had significantly different current vocalization thresholds at baseline, the percent change from baseline measurements after intrathecal morphine in male (Fig. 4A and C) compared with female (Fig. 4B and D) mice were similar (all p > 0.26) i.e., there was no effect of sex on morphine-induced percent changes from baseline current vocalization threshold. Therefore all comparisons and p values for percent changes from baseline for the morphine effect study reflect male and female mice data combined (Fig. 5).
Fig. 4.
Effect of sex on effect of intrathecal morphine. Panels A and C show least square mean ± SEM current vocalization threshold percent changes from baseline in male and Panels B and D in female C57BL6/J mice at 1 h (Panels A and B) and 3 h (Panels C and D) after administration of escalating doses of intrathecal morphine. Percent changes from baseline after intrathecal morphine were similar comparing male versus female mice (all p > 0.26).
Fig. 5.
Effect of intrathecal morphine expressed as current vocalization threshold percent changes from baseline at 1 (Panel A) and 3 h (Panel B) in response to 5, 250, and 2000 Hz sine-wave stimulation. * represents significant (p < 0.05) percent changes from baseline for 5 Hz stimulation, † (p < 0.05) for 250 Hz, and ‡ (p < 0.05) for 2000 Hz. Open and filled symbols represent least square means current vocalization threshold percent changes from baseline for male and female mice combined (as there was no significant effect of sex) and bars represent the standard error for 5, 250, and 2000 Hz. With increasing doses of morphine, percent changes in current vocalization threshold increased and were significantly greater for 5 Hz compared with 250 and 2000 Hz (Panel A). Current vocalization threshold percent changes from baseline at 1 h (Panel A) were significantly ordered: 5 Hz > 250 Hz (p < 0.0001) and 250 Hz > 2000 Hz (p = 0.0002). The curves represent polynomial smooth regression lines for 5, 250, and 2000 Hz frequencies stimulation as indicated in the legend. Data were modeled using repeated measures to compare the effect of increasing doses of morphine among the 3 frequencies at different doses.
Fig. 5 shows the effect of increasing doses of intrathecal morphine in current vocalization threshold percent changes from baseline (male and female mice combined). At 1 h after intrathecal injections, increasing doses of morphine yielded significantly different increases in mean current vocalization threshold percent changes from baseline among 5, 250, and 2000 Hz stimulations, (all p ≤ 0.0012, Fig. 5A). Specifically, by 1 h after intrathecal injection, in response to 5 Hz stimulation, there were significant increases in current vocalization threshold percent changes from baseline with 10, 20, 30 and 40 μg of morphine (p = 0.02, p < 0.0001, p = 0.0057 and p < 0.0001 respectively, Fig. 5A). In contrast, for 2000 Hz, current vocalization threshold percent changes from baseline were significantly increased at higher (30 and 40 μg) but not lower (10 and 20 μg) doses of morphine (p < 0.0001 for 30 μg and p = 0.03 for 40 μg, Fig. 5A). For the 250 Hz frequency, current vocalization threshold percent changes from baseline significantly increased after 10, 30, and 40 μg of intrathecal morphine (p = 0.02, p = 0.007, and p < 0.0001 respectively, Fig. 5A).
Overall, by 3 h after intrathecal injections, there were no significant effects of morphine on mean current vocalization threshold percent changes from baseline for 2000 and 250 Hz frequencies (both p = 0.4, Fig. 5B). In contrast, by 3 h after intrathecal injections, for the 5 Hz stimulation, there were overall significant effects of morphine in current vocalization threshold changes from baseline (p = 0.019, Fig. 5B). Specifically, at 3 h after morphine injections, current vocalization threshold in response to 5 Hz were still significantly increased from baseline after 10, 20, and 40 μg morphine injections (p = 0.01, p < 0.0001, and p = 0.004 respectively, Fig. 5B).
3.3. Comparison of the magnitude of morphine effects on current vocalization thresholds among the three electrical frequencies studied
In order to examine whether morphine differentially affected the response to the three frequencies studied, we compared the magnitude of percent changes from baseline in current vocalization threshold among the three frequencies used. Overall, at 1 h after morphine injection, current vocalization threshold percent changes from baseline significantly varied according to morphine doses (p = 0.0001, Fig. 5A) and frequency used (p < 0.0001), as well as their interaction (dose and frequency, p = 0.0306). Specifically, with increasing doses of intrathecal morphine, the current vocalization threshold percent changes from baseline increased and were significantly greater in response to 5 Hz stimulation compared with 250 and 2000 Hz (Fig. 5A). In fact, current vocalization threshold percent changes from baseline at 1 h were significantly ordered: 5 Hz > 250 Hz (p < 0.0001) and 250 Hz > 2000 Hz (p = 0.0002). Pairwise comparisons were made for each frequency pair at each dose level and after Bonferroni's corrections 5 comparisons remained significant: 5 Hz versus both 250 Hz and 2000 Hz at dose 20 μg, and 5 Hz versus both 250 Hz and 2000 Hz and 250 Hz versus 2000 Hz at dose 40 μg (all adjusted p < 0.012). These results indicate that intrathecal morphine had significantly greater impact on the response to 5 Hz (C sensory nerve fiber) compared to 250 (Aδ fiber) and 2000 Hz (Aβ fiber) simulations (Fig. 5).
By 3 h after morphine injection, there was no overall effect of morphine dose on current vocalization threshold percent changes from baseline (p = 0.1263, Fig. 5B), but frequency and the interaction (dose and frequency) term remained significant (p < 0.0001 and p = 0.0279, respectively). Specifically, when we examined the effect of dose in each frequency, at 3 h after, intrathecal morphine still had significant effects on current vocalization threshold percent changes from baseline in response to 5 Hz stimulation (p = 0.019) but not to 250 Hz or 2000 Hz (both p > 0.39). Further, similar to the observation at 1 h, by 3 h after morphine administration, current vocalization threshold percent changes from baseline were significantly greater in response to 5 Hz compared with 250 and 2000 Hz, i.e., 5 Hz > 250 Hz, 5 Hz > 2000 Hz, and 250 Hz > 2000 Hz (all p < 0.0001). Pairwise comparisons were performed for each frequency pair at each dose level and after adjustment only 2 comparisons remained significant. At dose 20 μg, 5 Hz > both 2000 Hz and 250 Hz (both adjusted p = 0.0015).
3.4. Effect of sex and anesthetics on current vocalization thresholds to electrical stimulus
Given that mice were anesthetized for the intrathecal injections, in a separate cohort of male and female C57B16J mice (Table 1 and Table 3), we investigated the effect of isoflurane on current vocalization threshold in response to 2000, 250, and 5 Hz stimulations to be certain that there were no residual effects of isoflurane on current vocalization thresholds at the time we measured morphine effects. Similar to the other cohort of mice enrolled in this investigation, males had higher current vocalization thresholds at baseline compared with females in response to all frequencies (all p < 0.002, data not shown). At 15 and 45 min after isoflurane or sham anesthesia, there was no significant difference in current vocalization threshold percent changes from baseline comparing male and females and isoflurane and sham anesthesia in response to all frequencies (p > 0.18, data not shown). Table 3 shows the percent changes from baseline after 15 and 45 min after isoflurane anesthesia. As there were no sex-related effects on these changes, percent changes from baseline male and female data were combined. At 15 min after anesthesia, there was near significant difference in magnitude of percent changes from baseline among the 3 frequencies (p = 0.05) that were not different comparing sham and isoflurane anesthesia (p = 0.8), however there was a significant treatment/frequency interaction (p = 0.03). Specifically, the percent changes from baseline in response to 5 Hz were positive compared to negative responses in both 2000 and 250 Hz (both p < 0.05) after sham anesthesia whereas after isoflurane, the percent changes in response to 250 Hz did not decrease as much as those in response to 2000 Hz (p = 0.006). Importantly, at 45 min, there was no significant effect of isoflurane on current vocalization threshold in any of the 3 frequencies (p > 0.1). These data suggest that at 1 h after intrathecal injection of morphine (the first time of measurements of morphine effects), there were no measurable effects of isoflurane in response to electrical stimulation.
Table 3.
Least square means ± SE current vocalization threshold percent changes from baseline after isoflurane or sham anesthesia.a
| Isoflurane anesthesia |
Sham anesthesia |
|||
|---|---|---|---|---|
| Percent change from baseline | Percent change from baseline | |||
| Frequency | 15 min | 45 min | 15 min | 45 min |
| 5 Hz | –4.5 ± 8.0 | 8.2 ± 7.1 | 1.9 ± 8.0 | 9.8 ± 7 |
| 250 Hz | –4.4 ± 4.4 | 5.6 ± 5.7 | –14.8 ± 4.4 | –5.7 ± 5.7 |
| 2000 Hz | –15.0 ± 3.9 | –4.0 ± 3.8 | –13.7 ± 3.9 | –4.4 ± 3.8 |
Sham anesthesia constitutes exposure to similar gas mixture and procedures except for the addition of isoflurane.
4. Discussion
The present study shows that in strains of mice often used in nociception investigations of transgenic animals, females have lower current vocalization thresholds in response to electrical stimulation compared to males at baseline. Interestingly, while females have lower vocalization thresholds than males at baseline, sex did not affect the response to intrathecal opioid. In addition, we found that intrathecal morphine, an opioid known to preferentially attenuate C-fiber mediated nociceptive input to the somatosensory cortex(Kalliomaki et al., 1998), had greater effects on the response to 5 Hz (a frequency known to preferentially stimulate C sensory nerve fibers) compared to 250 (Aδ fibers) and 2000 Hz (Aβ fibers). Therefore, these data support the use of this assay to measure the effects of sex, opioids, and anesthetics on studies of nociception. Further, given that we were able to quantify the differential effects of intrathecal morphine on the response to 5 Hz compared to 250 and 2000 Hz neurostimulation, our findings support the use of this assay for the study of specific sensory nerve fibers in mice.
While neurospecificity of sine-wave stimulation for each of the sensory nerve fibers is a matter of some controversy, in vivo and in vitro studies support the notion that electrical stimuli delivered at 2000, 250, and 5 Hz preferentially activate Aβ, Aδ, and C fibers respectively (Kiso et al., 2001; Nagakura et al., 2008b; Oda et al., 2005; Koga et al., 2005, p. 506). Researchers recording intracellular action potential from isolated dorsal root ganglia neurons showed that 2000 Hz stimulation at low intensity selectively activated Aβ fibers and only at intensities significantly higher (approximately fourfold) did it activate Aδ or C fibers neurons (Finkel et al., 2006; Koga et al., 2005). In the same study, 250 Hz stimulation at lower intensities was shown to selectively stimulate Aδ fibers (Koga et al., 2005). Other in vivo studies in rats showing that morphine, capsaicin, and local analgesics selectively alter response threshold in response to 5 Hz electrical stimulation support that the 5 Hz stimulus preferentially activates C fibers (Kiso et al., 2001; Matsumoto et al., 2006b; Nagakura et al., 2008b; Oda et al., 2005). Therefore, those studies as well as our findings that intrathecal morphine has greater effect on the 5 Hz current vocalization threshold add to the body of evidence suggesting that 2000, 250, and 5 Hz preferentially activate Aβ, Aδ, and C sensory nerve fibers respectively.
Using this assay, we are able to measure differences in current vocalization threshold between male and female mice. Interestingly, sex-related differences in nociceptive responses have been reported in investigations using different nociceptive methodologies (Fillingim and Gear, 2004; Fillingim et al.,1998, 2009; Greenspan et al., 2007; Mogil and Chanda, 2005). However, while a lot is known about sex-related differences in nociception both in humans and animals, the impact of sex on specific sensory (Aβ, Aδ, and C) nerve fibers is less understood. Our findings suggest that in the strains of mice studied, there are sex-related differences in response to neurostimulation with 2000, 250, and 5 Hz (Aβ, Aδ, and C-fibers). These findings are in concert with human studies suggesting the existence of sex-related differences in C-fiber (Fillingim et al., 1998, 2009) and in Aδ fiber mediated thermal pain. (Hashmi and Davis, 2010). Our results are also in concert with animal studies showing sex-related differences in nociception (Hurley and Adams, 2008; Wilson et al., 2003). In a combined sample of approximately 8000 mice, researchers showed that females have significantly lower tail withdrawal latency to radiant heat compared to males (Chesler et al., 2002; Mogil and Chanda, 2005). Others, using von Frey hairs, showed that mechanical thresholds are higher in male compared to female mice (Li et al., 2009). Here we show that in response to electrical stimulation that preferentially stimulate Aβ, Aδ, and C sensory nerve fibers, female mice have lower current vocalization threshold in all sensory nerve fibers compared with males. Those findings suggest that measuring current vocalization threshold could be valuable for the study of sex-related differences in sensory nerve function.
With regards to opioid analgesia, we observed that current vocalization thresholds are higher at 1 h and are back to near baseline levels by 3 h after injection of intrathecal morphine. As expected, the effect of morphine on current vocalization threshold was significantly greater in the response to 5 Hz stimulation (the frequency believed to preferentially stimulate C-fibers) compared to 250 and 2000 Hz believed to stimulate Aδ and Aβ fibers. With 2000 Hz stimulation, only the higher doses of morphine yielded a significant change in vocalization threshold at 1 h. We postulate that this effect could conceivably reflect a sedative effect of higher doses of intrathecal morphine. Therefore, these results suggests that our assay allows for the study of the effects of analgesics and given the pattern of response to 5 Hz stimulation, further supports its use for the study of specific sensory nerve fibers in mice.
The finding that sex did not influence current vocalization threshold changes after intrathecal morphine in mice in this investigation adds to the existing contradicting results on the effect of opioids in rodents (Bodnar and Kest, 2009; Kest et al., 1999, 2000; Mogil et al., 2006; Wilson et al., 2003). While most studies suggest male rodents have increased sensitivity to opioid anal-gesia compared with females (Hurley and Adams, 2008), others show that in different strains males and females mice have equal response (Kest et al., 2000), and still others show that males have lesser antinociceptive response to opioids (Mogil et al., 2000). A number of subsequent studies have shown that genotypes, nociceptive methodology applied, and opioid utilized can affect mouse response to opioid analgesia (Fillingim et al., 2009; Kest et al., 1999; Mogil et al., 2000; Wilson et al., 2003). Nevertheless, the nociception assay described here is able to measure morphine-related increases and decreases in mouse vocalization thresholds and could possibly be valuable for screening of novel analgesics.
Previous studies suggest that current vocalization threshold can be used for longitudinal evaluation of nociception as over two-week intervals at different ages, thresholds for all frequencies studied remain unchanged when measured every other day, suggesting that animals do not develop habituation to these electrical stimuli (Finkel et al., 2006). However, some might question whether vocalization reflects nocifensive behavior in mice and argue that vocalization is a less desirable end-point than paw withdrawal for nociception studies (Matsumoto et al., 2006b; Nagakura et al., 2008a,b). In fact, researchers have shown that the current threshold for paw withdrawal is lower than that for vocalization in rats (Nagakura et al., 2008b) and mice (Matsumoto et al., 2006b) in response to 5, 250, and 2000 Hz sine-wave stimulation to the hind paw. We postulate that vocalization in response to electrical stimulation most likely reflects nocifensive behavior, rather than a startle response, as it only occurs in association with higher intensities of neurostimulation, it ceases upon discontinuation of the stimulus, and in the presence of analgesics such as morphine, its occurrence requires higher stimulus intensities. The findings of others that morphine changes nocifensive response to heat (hot-plate) but not the acoustic startle response lends support to our hypothesis (Blaszczyk et al., 2010). Our findings also suggest that there might be some advantages of using vocalization as a nocifensive behavior endpoint. One of the advantages is that by using vocalization, one eliminates the possibility that paw withdrawal represents motor flexion in response to electrical stimulation to the paw instead of true nocifensive response. Contrary to what is reported by others (Matsumoto et al., 2006b; Nagakura et al., 2008b), here we found that the current vocalization threshold in response to tail stimulation was significantly lower than that reported in response to hind paw stimulation (Matsumoto et al., 2006b). We also observed that in response to sine-wave electrical stimulation, mice display random tail movements in response to stimulation at intensities below vocalization threshold. While we cannot eliminate the possibility that these random tail movements represent a startle response, we postulate that these random tail movements at subthreshold stimulus intensity reflect perception of the stimulus which precedes the point at which the stimulus becomes noxious and vocalization ensues. Such pattern appears to mirror the paradigm reported in humans tested with this methodology where current perception threshold is reportedly lower than current tolerance threshold (Katims et al., 1986; Matsutomo et al., 2005).
5. Conclusion
We describe an automated nociception assay using sine-wave stimulation that can be useful to evaluate the effect of analgesics and of sex-related differences in current vocalization threshold to electrical stimuli believed to preferentially stimulate Aβ, Aδ, and C-sensory nerve fibers in mice. Using this assay, we showed that male and female mice have different current vocalization thresholds in basal conditions but C57BL/6J mice have similar analgesic responses to intrathecal opioid. This study supports the use of this automated nociceptive assay in studies of nociception in mice and adds to the body of literature indicating that there are sex-related changes in nociception that affect all sensory nerve fibers.
Acknowledgments
Funding
This research was supported by the Intramural Research Program of the NIH Clinical Center, National Institutes of Health, Bethesda, MD, and The Sheikh Zayed Institute for Pediatric Surgical Innovation, Children's National Medical Center, Washington, DC.
Footnotes
Conflict of interest
The authors have no conflict of interest to report.
References
- Angst MS, Drover DR, Lotsch J, Ramaswamy B, Naidu S, Wada DR, et al. Pharmacodynamics of orally administered sustained-release hydromorphone in humans. Anesthesiology. 2001;94:63–73. doi: 10.1097/00000542-200101000-00014. [DOI] [PubMed] [Google Scholar]
- Blaszczyk JW, Lapo IB, Werka T, Sadowski B. Differential startle magnitude in mice selected for high and low swim analgesia is not related to difference in nociception. Acta Neurobiol Exp (Wars) 2010;70:398–405. doi: 10.55782/ane-2010-1812. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Kest B. Sex differences in opioid analgesia, hyperalgesia, tolerance and withdrawal: central mechanisms of action and roles of gonadal hormones. Horm Behav. 2009;58:72–81. doi: 10.1016/j.yhbeh.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS. Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive. Neurosci Biobehav Rev. 2002;26:907–23. doi: 10.1016/s0149-7634(02)00103-3. [DOI] [PubMed] [Google Scholar]
- Fairbanks CA. Spinal delivery of analgesics in experimental models of pain and analgesia. Adv Drug Deliv Rev. 2003;55:1007–41. doi: 10.1016/s0169-409x(03)00101-7. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Gear RW. Sex differences in opioid analgesia: clinical and experimental findings. Eur J Pain. 2004;8:413–25. doi: 10.1016/j.ejpain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–85. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, Maixner W, Kincaid S, Silva S. Sex differences in temporal summation but not sensory-discriminative processing of thermal pain. Pain. 1998;75:121–7. doi: 10.1016/S0304-3959(97)00214-5. [DOI] [PubMed] [Google Scholar]
- Finkel JC, Besch VG, Hergen A, Kakareka J, Pohida T, Melzer JM, et al. Effects of aging on current vocalization threshold in mice measured by a novel nociception assay. Anesthesiology. 2006;105:360–9. doi: 10.1097/00000542-200608000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel JC, Yang CI, Yarvitz JL, Patel KM. Neuroselective sensory electrodiagnostic evaluation of 4% liposomal topical lidocaine. Anesth Analg. 2002;94:1259–62. doi: 10.1097/00000539-200205000-00039. table of contents. [DOI] [PubMed] [Google Scholar]
- Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, et al. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132(Suppl. 1):S26–45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi JA, Davis KD. Noxious heat evokes stronger sharp and annoying sensations in women than men in hairy skin but not in glabrous skin. Pain. 2010;151:323–9. doi: 10.1016/j.pain.2010.06.026. [DOI] [PubMed] [Google Scholar]
- Hurley RW, Adams MC. Sex, gender, and pain: an overview of a complex field. Anesth Analg. 2008;107:309–17. doi: 10.1213/01.ane.0b013e31816ba437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylden J, Wilcox G. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–6. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Kalliomaki J, Luo XL, Yu YB, Schouenborg J. Intrathecally applied morphine inhibits nociceptive C fiber input to the primary somatosensory cortex (SI) of the rat. Pain. 1998;77:323–9. doi: 10.1016/S0304-3959(98)00115-8. [DOI] [PubMed] [Google Scholar]
- Katims J. Electrodiagnostic functional sensory evaluation of the patient with pain: a review of the neuroselective current perception threshold and pain tolerance threshold. Pain Digest. 1998;8:219–30. [Google Scholar]
- Katims JJ, Naviasky EH, Ng LK, Rendell M, Bleecker ML. New screening device for assessment of peripheral neuropathy. J Occup Med. 1986;28:1219–21. [PubMed] [Google Scholar]
- Katims JJ, Taylor DN, Weseley SA. Sensory perception in uremic patients. ASAIO Trans. 1991;37:M370–2. [PubMed] [Google Scholar]
- Kest B, Sarton E, Dahan A. Gender differences in opioid-mediated analgesia: animal and human studies. Anesthesiology. 2000;93:539–47. doi: 10.1097/00000542-200008000-00034. [DOI] [PubMed] [Google Scholar]
- Kest B, Wilson SG, Mogil JS. Sex differences in supraspinal morphine analgesia are dependent on genotype. J Pharmacol Exp Ther. 1999;289:1370–5. [PubMed] [Google Scholar]
- Kiso T, Nagakura Y, Toya T, Matsumoto N, Tamura S, Ito H, et al. Neurometer measurement of current stimulus threshold in rats. J Pharmacol Exp Ther. 2001;297:352–6. [PubMed] [Google Scholar]
- Koga K, Furue H, Rashid MH, Takaki A, Katafuchi T, Yoshimura M. Selective activation of primary afferent fibers evaluated by sine-wave electrical stimulation. Mol Pain. 2005;1:13. doi: 10.1186/1744-8069-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Fan X, Warner M, Xu X-J, Gustafsson J-A, Wiesenfeld-Hallin Z. Ablation of estrogen receptor [alpha] or [beta] eliminates sex differences in mechanical pain threshold in normal and inflamed mice. Pain. 2009;143:37–40. doi: 10.1016/j.pain.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Liem EB, Joiner TV, Tsueda K, Sessler DI. Increased sensitivity to thermal pain and reduced subcutaneous lidocaine efficacy in redheads. Anesthesiology. 2005;102:509–14. doi: 10.1097/00000542-200503000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson EA, Boulton AJ. The Neurometer: validation and comparison with conventional tests for diabetic neuropathy. Diabet Med. 1991;8:S63–6. doi: 10.1111/j.1464-5491.1991.tb02159.x. Spec No. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Inoue M, Hald A, Xie W, Ueda H. Inhibition of paclitaxel-induced A-fiber hypersensitization by gabapentin. J Pharmacol Exp Ther. 2006a;318:735–40. doi: 10.1124/jpet.106.103614. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Inoue M, Hald A, Yamaguchi A, Ueda H. Characterization of three different sensory fibers by use of neonatal capsaicin treatment, spinal antagonism and a novel electrical stimulation-induced paw flexion test. Mol Pain. 2006b;2:16. doi: 10.1186/1744-8069-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Xie W, Ma L, Ueda H. Pharmacological switch in Abeta-fiber stimulation-induced spinal transmission in mice with partial sciatic nerve injury. Mol Pain. 2008;4:25. doi: 10.1186/1744-8069-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsutomo R, Takebayashi K, Aso Y. Assessment of peripheral neuropathy using measurement of the current perception threshold with the neurometer in patients with type 2 diabetes mellitus. J Int Med Res. 2005;33:442–53. doi: 10.1177/147323000503300410. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Chanda ML. The case for the inclusion of female subjects in basic science studies of pain. Pain. 2005;117:1–5. doi: 10.1016/j.pain.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci Biobehav Rev. 2000;24:375–89. doi: 10.1016/s0149-7634(00)00015-4. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Ritchie J, Sotocinal SG, Smith SB, Croteau S, Levitin DJ, et al. Screening for pain phenotypes: analysis of three congenic mouse strains on a battery of nine nociceptive assays. Pain. 2006;126:24–34. doi: 10.1016/j.pain.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Nagakura Y, Jones TL, Malkmus SA, Sorkin L, Yaksh TL. The sensitization of a broad spectrum of sensory nerve fibers in a rat model of acute postoperative pain and its response to intrathecal pharmacotherapy. Pain. 2008a;139:569–77. doi: 10.1016/j.pain.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagakura Y, Malkmus S, Yaksh TL. Determination of current threshold for paw withdrawal with sine-wave electrical stimulation in rats: effect of drugs and alteration in acute inflammation. Pain. 2008b;134:293–301. doi: 10.1016/j.pain.2007.04.030. [DOI] [PubMed] [Google Scholar]
- Oda M, Kitagawa N, Yang BX, Totoki T, Morimoto M. Quantitative and fiber-selective evaluation of dose-dependent nerve blockade by intrathecal lidocaine in rats. J Pharmacol Exp Ther. 2005;312:1132–7. doi: 10.1124/jpet.104.076893. [DOI] [PubMed] [Google Scholar]
- Oishi M, Mochizuki Y, Suzuki Y, Ogawa K, Naganuma T, Nishijo Y, et al. Current perception threshold and sympathetic skin response in diabetic and alcoholic polyneuropathies. Intern Med. 2002;41:819–22. doi: 10.2169/internalmedicine.41.819. [DOI] [PubMed] [Google Scholar]
- Watabiki T, Nagakura Y, Wegner K, Kakimoto S, Tozier NA, Malkmus SA, et al. Assessment of canine sensory function by using sine-wave electrical stimuli paradigm. Physiol Behav. 2010;101:327–30. doi: 10.1016/j.physbeh.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Wilson SG, Smith SB, Chesler EJ, Melton KA, Haas JJ, Mitton B, et al. The heritability of antinociception: common pharmacogenetic mediation of five neurochemically distinct analgesics. J Pharmacol Exp Ther. 2003;304:547–59. doi: 10.1124/jpet.102.041889. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Cooper BY, Vierck CJ., Jr Effects of systemic morphine on responses of primates to first or second pain sensations. Pain. 1996a;66:253–63. doi: 10.1016/0304-3959(96)03082-5. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Pirec V, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: behavioral evidence. Pain. 1996b;68:133–40. doi: 10.1016/S0304-3959(96)03176-4. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: electrophysiological evidence. Pain. 1996;68:141–50. doi: 10.1016/S0304-3959(96)03177-6. [DOI] [PubMed] [Google Scholar]