Abstract
BACKGROUND/OBJECTIVE
Dietary assessment in children is difficult, suggesting a need to develop more objective biomarkers of intake. Resonance Raman spectroscopy (RRS) is a non-invasive, validated method of measuring carotenoid status in skin as a biomarker of fruit/vegetable intake. The purpose of this study was to examine the feasibility of using RRS in preschool children, to describe inter-individual variability in skin carotenoid status and to identify factors associated with the biomarker in this population.
SUBJECTS/METHODS
We conducted a cross-sectional study of 381 economically disadvantaged preschoolers in urban centers in Connecticut (USA). In all, 85.5% were black non-Hispanic or Hispanic/Latino, and 14.1% were obese and 16.9% were overweight by age- and sex-specific body mass index (BMI) percentiles. Children had their skin carotenoid status assessed by RRS in the palm of the hand. Fruit/vegetable consumption was assessed by a brief parent/guardian-completed food frequency screener and a liking survey.
RESULTS
We observed inter-individual variation in RRS values that was nearly normally distributed. In multiple regression analysis, higher carotenoid status, measured by RRS, was positively associated with fruit/vegetable consumption (P =0.02) and fruit/vegetable preference (P<0.01). Lower carotenoid status was observed among younger children, those participating in the US Supplemental Nutrition Assistance Program, and those with greater adiposity (P<0.05 for all).
CONCLUSIONS
We observed wide variability in skin carotenoid status in a population of young children, as assessed by RRS. Parent-reported fruit/vegetable intake and several demographic factors were significantly associated with RRS-measured skin carotenoid status. We recommend further development of this biomarker in children, including evaluating response to controlled interventions.
Keywords: carotenoids, biomarker, children, resonance Raman spectroscopy
INTRODUCTION
Dietary assessment is inherently difficult and involves additional complications in children. Self-report methods are subject to bias and measurement error due to problems with reporting, portion size estimation and inaccurate recall.1,2 In preschoolers, dietary assessment requires the assistance of a proxy reporter, usually parents or teachers.3 Direct observation (for example, measured plate waste or digital photography) techniques offer more valid estimation than questionnaires,4,5 but these methods are laborious, limited to small populations and do not reflect usual dietary intake. Literature also exists supporting the use of food liking/preference scales to assess intake, assuming that individuals habitually consume what they like and avoid what they do not.6,7 The invasiveness of more objective measures of nutrition status, typically involving plasma samples for biochemical analysis, limits their feasibility for young children. Thus, a need exists for a valid, rapid, and non-invasive method to assess nutritional status in children using biomarkers, such as carotenoid status for estimating fruit and vegetable intake.
Carotenoids are plant pigments responsible for red, orange and yellow coloring. Classified as antioxidants, carotenoids have a suggested role in the prevention of many diseases, including cancer,8–11 heart disease12–14 and age-related eye disease,15,16 as well as all-cause mortality.17 Because carotenoids are not found in significant concentrations in other foods, but are widely distributed in fruits and vegetables, they are considered to be the best current biomarker for fruit and vegetable intake.18 Traditional approaches for carotenoid assessment often depend on self-reported intake of fruits and vegetables linked to food composition databases for estimation of carotenoid content in foods. However, the results of dietary intervention trials have suggested that self-reported carotenoid intake can have bias and measurement error.19 Alternatively, biochemical analyses such as high-performance liquid chromatography can be used to measure carotenoids in plasma or tissue samples.20,21
The development of non-invasive, less expensive, but sensitive optical monitoring technologies provides an alternative to high-performance liquid chromatography for measurement of carotenoids in living tissues. In particular, resonance Raman spectroscopy (RRS) has emerged as an objective indicator of carotenoid status.22,23 A novel, non-invasive technique for measuring carotenoid status in the skin using visible light, RRS utilizes a small probe with a laser at a blue wavelength (λ =488 nm) to measure total carotenoid concentration in skin.24 The Raman scattered light produces a spectral fingerprint of the carotenoid molecules based on their unique molecular structure (alternating carbon double and single bonds) and vibrational energy levels. One of the preferred body sites for Raman scanning is the palm of the hand because the stratum corneum, the outer skin tissue layer where carotenoids concentrate, is relatively thick, and the melanin content is less variable among individuals of different races and ethnicities.23
Studies around the world are now using the RRS technology to non-invasively measure skin carotenoid status as a promising biomarker of fruit and vegetable intake, but the evidence supporting its use as a biomarker comes primarily from studies of adult populations. Our group has previously shown that RRS is a valid biomarker of skin carotenoid status in healthy adults, compared with biopsies of excised human skin, with correlation coefficients ranging from r =0.7 to 0.9.25,26 RRS was also a reliable indicator of carotenoid status over time and across different body sites (three measured: palm, inner arm and outer arm).25 In adults, RRS has also been found to correlate significantly with dietary intake of fruit and vegetable intake in cross-sectional studies.23,25,27,28 Also, adult studies involving carotenoid or fruit and vegetable interventions have demonstrated that RRS values change accordingly.29,30 Because of growing evidence supporting the validity of RRS as a biomarker of carotenoid status in adults, the purpose of this study was to examine the feasibility of using this biomarker in a population of children, where dietary assessment is notoriously difficult. Specifically, we aimed to use RRS in a field (non-clinical) setting to estimate the inter-individual variability, and to identify predictors of skin carotenoid status in a sample of preschool children.
SUBJECTS AND METHODS
Study population
A community-based sample of economically disadvantaged children was recruited from six federal and state-supported preschool programs in two urban centers in Connecticut, USA. A total of 381 preschool children, aged 3–5 years, from primarily black non-Hispanic and Hispanic/Latino ethnic backgrounds, were enrolled in the sample for RRS testing during August and September 2008. Written informed consent of their child’s voluntary participation was obtained from a parent or caregiver, and oral assent was obtained from the children before RRS scanning. All procedures were approved by the Institutional Review Boards at the Yale University School of Medicine and the University of Connecticut.
Data collection
Participant characteristics
A baseline questionnaire (available in English and Spanish) was completed by parents to collect demographic information about their child, including date of birth, sex, race/ethnicity, parent/caregiver smoking status and current family participation in the US Supplemental Nutrition Assessment Program (SNAP). Children’s date of birth and sex were confirmed with school enrollment records. Height and weight were measured by a registered dietitian using a platform scale and stadiometer (SECA, Hanover, MD, USA). Children were weighed with their shoes on to comply with school’s fire safety regulations. These measurements were used to calculate body mass index (BMI) and age- and sex-specific percentiles (BMI %) from the 2000 CDC standards (with ‘overweight’ ≥85th percentile and ‘obese’ ≥95th percentile of BMI).31,32
Resonance Raman spectroscopy
Details of RRS instrumentation have been reported elsewhere,24 with minor modifications in this study. Briefly, the instrument uses a compact, portable, continuous wave solid-state laser operating at a wavelength of 488 nm to shine blue visible light onto a tissue of interest (that is, skin). This laser is based on a frequency-double near-infrared semiconductor diode laser. The spectrograph is equipped with a linear charge coupled device array operating at room temperature. The array was interfaced to a laptop computer for data acquisition, processing and display. At a power of 0.2 W/cm2 and an elliptical laser spot size of 2.5 × 1.5 mm, it is safe to expose skin to the laser light for 30 h. The light exposure is ~1000 times less than the exposure limit set by the ANSI z136.1-2007 standard, which assures that the instrument was safe for use in a population of young children. The palm of each child was scanned three times for reliability at an exposure time of 30 s per scan, and scans were performed immediately before or after the height/weight screenings. Protective goggles (Kentek Corporation, Pittsfield, NH, USA) were worn by the children and the research assistant to block any laser excitation light. Mean RRS values for total carotenoid status, measured as skin Raman intensity, were obtained to provide an assessment of total skin carotenoid status for each child.
Fruit and vegetable intake and preference
Parents completed a modified Block Kids Questionnaire (available in English and Spanish)–a brief, 2-page, 41-item food frequency screener (FFS) that inquired about their child’s recent dietary intake in the past seven days (NutritionQuest, Berkeley, CA, USA). Intake of total fruits and vegetables was estimated (frequency × portion size, summed across foods). Additionally, intake of total and individual carotenoids was estimated based on the USDA Food and Nutrient Database for Dietary Studies33 (Supplementary Appendix). Because adjusting for total energy intake (kcals) may yield more reliable estimates of self-reported diet,34 we energy-adjusted intake of total fruits and vegetables and total carotenoids estimated from the FFS. Parents also completed the Preschool-Adapted Liking Survey, developed by our group. This two-page, 5 min survey asked parents to rate their child’s likes or dislikes of 18 food and beverage items, as well as four non-food items (for example, a loud siren, taking a bath) on a continuous scale. Liking scores were generated by measuring the ±100 point line from the center rating (‘he/she thinks it’s ok’ at 0) to the liking rating (‘he/she really likes it’ from 30 to 50 and ‘he/she loves it’ from 80 to 100) or to the disliking rating (‘he/she really does not like it’ from–30 to–50 and ‘he/she hates it’ from–80 to–100). An average liking score was computed for total fruits and vegetables, and for high carotenoid foods (Supplementary Appendix).
Statistical analysis
Descriptive statistics were obtained for the continuous response variable, mean RRS values for skin carotenoid status, as well as the main effect of demographic covariates and fruit and vegetable intake in relation to the response variable. To estimate variability in skin carotenoid status, a histogram of RRS values (measured in skin Raman intensity) was plotted for the total sample, using the mean of the three replicate values. RRS mean values and medians were estimated for five racial and ethnic groups (Hispanic, black non-Hispanic, white non-Hispanic, biracial and other) and by BMI percentile for sex and age, as a continuous and categorical variable for weight status (underweight (<5th percentile), healthy weight (5th to <85th percentile), overweight (85th to <95th percentile) and obese (≥95th percentile)). An analysis of variance was performed to test whether any of the two-group comparisons in RRS mean values was significantly different at the 0.05 level.
Several variables were selected for examination as the main predictors of total skin carotenoid status, measured by RRS in the preschool population: fruit and vegetable intake estimated from the parent-reported FFS and liking survey, BMI percentile for sex and age, current SNAP participation (yes/no) and age (months). Covariates of interest were specified a priori and included race/ethnicity, sex (male/female) and geographic school site (six-level categorical variable). The main effect of each predictor variable and covariate was examined in univariate linear regression analysis to determine the independent effects on total skin carotenoid status measured by RRS. Next, a multivariate regression analysis was conducted to identify determinants of carotenoid status in our preschool sample, with adjustment for covariates. Dietary intake and liking measures were modeled separately (for example, not included simultaneously in the same models) as possible predictors of RRS. All statistical computations were conducted using SAS v. 9.1 (SAS Institute, Inc., Cary, NC, USA) with a P-value of 0.05 considered statistically significant.
RESULTS
A total of 381 children had their skin carotenoid status assessed by RRS and dietary questionnaires completed by a parent or caregiver. Children were cooperative during measurement, with no unexpected difficulties encountered. Given the ease of use and rapid nature of RRS, we were able to scan up to 60 children in <2 h. Mean RRS palm measures of total carotenoid status by demographic characteristics of the sample are reported in Table 1. Mean age of the children was 3.80 years (range: 2.75–4.83 years), and 85.5% identified as Hispanic/Latino or black non-Hispanic. Almost one third of the sample (31.0%) was overweight or obese, and 54.7% of parents reported their family was currently participating in SNAP. Mean RRS values for total carotenoid status were similar for boys and girls (20.59 vs 20.38; P =0.77). A marginally significant difference was observed in RRS mean values among five racial/ethnic groups (P =0.08), with white non-Hispanics having the highest RRS values. This group also had the highest carotenoid intake, as estimated from the FFS, but the difference was not significant. RRS mean values were significantly lower for children whose parent reported that their family was currently participating in the SNAP, compared with those who were not (19.34 vs 21.83; P<0.01).
Table 1.
Resonance Raman spectroscopy measures for total carotenoids by baseline characteristics of the preschool sample (N =381)
| n (%) | RRS scorea | P-valueb | |
|---|---|---|---|
| Age | 0.23 | ||
| <3.8 years | 189 (49.6) | 20.08±6.82 | |
| ≥3.8 years | 192 (50.4) | 20.90±6.53 | |
| Sex | 0.77 | ||
| Male | 193 (50.7) | 20.59±6.45 | |
| Female | 188 (49.3) | 20.38±6.93 | |
| Race/ethnicity | 0.08 | ||
| White non-Hispanic | 22 (5.8) | 23.95±7.55 | |
| Black non-Hispanic | 98 (25.7) | 19.84±5.85 | |
| Hispanic/Latino | 228 (59.8) | 20.57±6.73 | |
| Biracial (White/Black) | 22 (5.8) | 20.27±7.92 | |
| Other | 11 (2.9) | 18.12±6.99 | |
| SNAP participationc | <0.01 | ||
| Yes | 208 (54.7) | 19.34±6.04 | |
| No | 172 (45.3) | 21.83±7.17 | |
| Current smoking in home | 0.10 | ||
| Yes | 62 (16.5) | 19.18±6.96 | |
| No | 314 (83.5) | 20.72±6.62 | |
| BMI percentile for sex and age | 0.37 | ||
| Underweight (<5th percentile) | 15 (4.1) | 22.13±7.17 | |
| Healthy weight (5th to <85th percentile) | 235 (64.9) | 20.95±6.94 | |
| Overweight (85th to <95th percentile) | 61 (16.9) | 19.48±4.97 | |
| Obese (≥95th percentile) | 51 (14.1) | 20.50±7.09 |
Abbreviations: ANOVA, analysis of variance; BMI, body mass index; RRS, resonance Raman spectroscopy; SNAP, Supplemental Nutrition Assistance Program.
Mean±s.d. RRS palm measures of carotenoid status obtained with portable scanner (unstandardized measures).
P for difference in means derived by Student’s t tests (binary variables) or ANOVA (multilevel variables).
SNAP participation defined as parent-reported family participation in the federal Supplemental Nutrition Assistance Program.
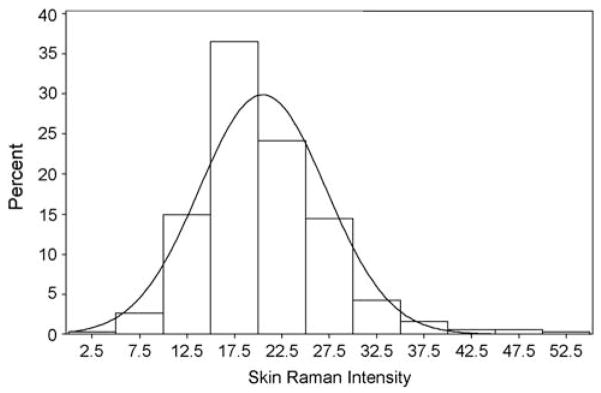
Figure 1 shows the distribution of RRS values, measured as skin Raman intensity values (average of three readings), for the preschool sample. The mean RRS value was 20.48, with a standard deviation of 6.68. The data were approximately normally distributed, with a slight right-skew (Anderson–Darling test for normality, P<0.01; skewness =1.06), and RRS averages for four individuals measured greater than three standard deviations from the mean. RRS values for the total sample ranged from 2.93 to 53.55.
Figure 1.
Histogram showing distribution of total carotenoids in the palm, as assessed using RRS (N =381). Note bell-shaped appearance of histogram indicating a normal distribution, with a slight right-skew (Anderson–Darling test for normality, P<0.01; skewness =1.06).
Following a univariate linear regression analysis to determine independent effects on total skin carotenoid status measured by RRS (data not shown), multiple linear regression was used to identify the main predictors of skin carotenoid status in the preschool population, with adjustment for covariates (Table 2). In adjusted analyses, a significant positive association was observed between total skin carotenoid status and parent-reported fruit and vegetable intake, as measured by the liking survey (P<0.01), as well as energy-adjusted intake of total fruits and vegetables (P =0.02), estimated from the FFS. After accounting for diet, parent-reported SNAP participation was consistently inversely associated with total skin carotenoid status (P<0.01), while age in months and white non-Hispanic race/ethnicity were consistently positively associated (P<0.01), independent of the method used to estimate diet. Finally, an inverse association was observed between total skin carotenoid status and BMI percentile when the liking survey was used to estimate intake, but the relationship was only marginally significant when diet was estimated by the FFS. The partial correlation coefficients (rpartial) between RRS and diet ranged from rpartial = 0.17 for intake of total fruits and vegetables estimated by the FFS to rpartial = 0.23 estimated by the liking survey and intake of high carotenoid foods estimated by the liking survey. Because the distribution of RRS values was slightly right-skewed, we examined the effect of log transformation of the data. No differences were observed in the main outcomes of the study; thus, we retained the original untransformed RRS values for the final analysis.
Table 2.
Main predictors of skin carotenoid status, with adjustment for covariatesa
| Model | β | P-value | Model | β | P-value |
|---|---|---|---|---|---|
| Preference for total fruits and vegetables | 0.05 | <0.01 | Intake of total fruits and vegetablesb | 0.87 | 0.02 |
| Age (months) | 0.15 | <0.01 | Age (months) | 0.18 | <0.01 |
| SNAP participation (yes vs no) | −2.78 | <0.01 | SNAP participation (yes vs no) | −2.57 | <0.01 |
| BMI percentile for sex and age | −0.03 | 0.03 | BMI percentile for sex and age | −0.02 | 0.04 |
| Sex (male vs female) | −0.72 | 0.29 | Sex (male vs female) | −0.38 | 0.59 |
| Race | Race | ||||
| Hispanic/Latino | Reference | Hispanic/Latino | Reference | ||
| White non-Hispanic | 4.07 | 0.01 | White non-Hispanic | 3.72 | 0.02 |
| Black non-Hispanic | −1.16 | 0.16 | Black non-Hispanic | −0.82 | 0.33 |
| Biracial (White/Black) | 1.15 | 0.45 | Biracial (White/Black) | 1.20 | 0.45 |
| Other | −2.50 | 0.22 | Other | −2.57 | 0.23 |
| Preference for high carotenoid foods | 0.04 | <0.01 | Intake of total carotenoidsb | <0.01 | <0.01 |
| Age (months) | 0.15 | <0.01 | Age (months) | 0.16 | <0.01 |
| SNAP participation (yes vs no) | −2.73 | <0.01 | SNAP participation (yes vs no) | −2.55 | <0.01 |
| BMI percentile for sex and age | −0.03 | 0.03 | BMI percentile for sex and age | −0.02 | 0.07 |
| Sex (male vs female) | −0.69 | 0.31 | Sex (male vs female) | −0.38 | 0.59 |
| Race | Race | ||||
| Hispanic/Latino | Reference | Hispanic/Latino | Reference | ||
| White non-Hispanic | 4.05 | 0.01 | White non-Hispanic | 4.12 | 0.01 |
| Black non-Hispanic | −1.28 | 0.12 | Black non-Hispanic | −0.95 | 0.26 |
| Biracial (White/Black) | 0.95 | 0.54 | Biracial (White/Black) | 1.30 | 0.41 |
| Other | −2.72 | 0.19 | Other | −2.00 | 0.35 |
Abbreviations: BMI, body mass index; SNAP, Supplemental Nutrition Assistance Program.
Additional covariate: school site (non-significant in all models).
Adjusted for average daily kilocalories. Units are as follows: preference is a continuous scale (−100 to 100); intake of total fruits and vegetables is servings/day; and intake of total carotenoids is μg/day.
DISCUSSION
In this study of underserved preschoolers, we had good acceptance of the technique, and observed an approximately normal distribution of skin carotenoid status, as measured by RRS. In addition to parent-reported fruit and vegetable consumption, age and white non-Hispanic race/ethnicity were also positively associated with skin carotenoid status in our preschool population, while parent-reported SNAP participation and measured BMI predicted lower carotenoid status. Both measures of dietary fruit and vegetable consumption (intake and preference) were significantly correlated with the objective indicator of skin carotenoid status, despite being fairly brief and limited estimates of intake.
Although the evidence that RRS is a reliable and valid biomarker of fruit and vegetable intake comes from many studies in healthy adults as discussed earlier, this was the first study to use RRS to estimate the variability of skin carotenoid status in a large sample of children. This effort involved the use of a portable Raman scanner, developed specifically for use in this study. The Raman scanner used in our prior research was not portable, and the RRS values reported here use a different intensity scale compared with other results we have published, which were obtained using a different instrument (including the type of laser, spectrograph and optical components).23,25,35 We observed that the children were cooperative during scanning, suggesting that RRS is a feasible, non-invasive method for assessing relative fruit and vegetable intake in young populations. The inter-individual variability we detected in this population was similar to that seen in adults and suggests that RRS may be used as a more objective measure of diet to identify children with poor carotenoid status, who may be at risk for nutrition-related diseases.
In addition to the significant correlation observed between the objective indicator of fruit and vegetable intake (RRS) and parent-reported diet in our preschool population, we also identified several demographic and anthropometric predictors of skin carotenoid status, measured by RRS, in this population. Age was positively associated with skin carotenoid status in these children. Data from the Third National Health and Nutrition Examination Survey (NHANES III) indicate serum carotenoid levels in the US population are relatively high in childhood, but decrease in the adolescent years and early adulthood.18 Thus, we may be observing an age trend that is specific to the preschool population. Skin carotenoid status was inversely associated with obesity in our study. Additional studies have found similar results among serum carotenoids in children36 and adipose tissue carotenoids in adults.37 Possible explanations for the inverse association between obesity and carotenoid status include dietary differences compared with healthy weight individuals,38 variability in body compartment size39 and deposition of lipid-soluble carotenoids into adipose tissue for storage.40 Because children identified as overweight or obese comprised almost one third of our sample, the observation that these children also had poorer nutrient status may have important health implications. Finally, we observed an inverse association between skin carotenoid status and reported current SNAP participation. This federal program provides funding assistance and nutrition education to improve the nutritional quality of low-income children and their families in the United States. While the SNAP encourages healthy food selection, growing evidence suggests that issues of access and affordability limit the purchase of fruits and vegetables by low-income consumers.41,42 In our study, we only had information available about current SNAP participation during the time period when these preschool children were evaluated. Thus, we did not have the ability to examine the effect of duration of SNAP use on nutrient status. We also observed a significant positive association between white non-Hispanic race/ethnicity and RRS measures, although this subgroup represented only about 5% of our total population. From our results, it is difficult to disentangle the socioeconomic, racial and income effects that may be driving the inverse association we observed between skin carotenoid status and reported SNAP participation, but may suggest that SNAP participation is insufficient to overcome the adverse effects of poverty on children’s nutrition status. This finding warrants further investigation.
In our previous study of adults, total carotenoids assessed by RRS were significantly correlated (r =0.7) with total carotenoids assessed by high-performance liquid chromatography analysis of skin biopsies and with plasma carotenoids.25 As noted earlier, other groups have shown similar findings for adults. The purpose of this study was not to repeat our previous work in adults, but to assess the feasibility of the RRS biomarker in children, examine the distribution of skin carotenoid status in this population, and identify other factors in addition to diet that track with the biomarker. The correlation coefficients we observed between RRS and both measures of diet (intake and preference for fruits/vegetables) in our sample of children were lower than the correlations previously observed in adult populations. A number of factors could provide an explanation for these findings. First, the brief FFS used in our study was a crude measure that attempted to capture diet in young children, which is especially difficult to measure. The FFS, which was completed by a parent/guardian for their child, assessed fruit and vegetable intake over 1 week only, whereas tissue levels reflect weeks to months of intake. Moreover, this study was an ancillary project to an ongoing study in preschool children, and the FFS was not specifically designed to assess carotenoid intake. It is known that common sources of carotenoids in children’s diets include many foods besides fruits and vegetables, including lycopene from tomato-based products (for example, ketchup, spaghetti sauce and pizza sauce). While we attempted to incorporate these other food sources into our dietary measures, it is possible that we are not capturing all of the main sources of carotenoids in children’s diets. To optimize correlations between self-reported diet and the RRS measure of carotenoid status in children, future research should utilize more comprehensive and quantitative dietary measures designed specifically for this population.
There is the possibility that melanin content in the skin could impact the RRS measures of carotenoid status, especially in this diverse population of children. In our previous work, we closely examined the RRS data from participants who self-identified as dark skin as compared with light skin tones.25 When the RRS outer arm (area of most pigmentation) to palm (area of least pigmentation) ratios were compared between participants with dark vs light skin pigmentation, there was no evidence that RRS ratios varied significantly with skin pigmentation, as might be expected if a filtering effect on the laser and Raman scattered light was occurring. However, we had limited power to detect an effect of melanin with relatively few dark-skinned participants. In the present study, we scanned the palm of the hand, with one of the reasons being that melanin in the palm is less variable among children of different races and ethnicities. In future studies of RRS in adults and children, we plan to systematically examine the effect of melanin content on the RRS measures using established procedures for quantifying melanin in the skin.
A major strength of the present study was the use of RRS to objectively assess skin carotenoid status in a community-based sample of preschool children, where dietary assessment is difficult. We have used these data to gain an understanding of the inter-individual variability of skin carotenoid status within the population, since very little knowledge currently exists. We have also identified significant predictors of carotenoid status in our low-income preschool sample, so more targeted interventions can be created to better improve nutritional quality of children with poor carotenoid status. At this time, the RRS values measured are internally valid within a study, but external validity will depend on the use of an accepted external standard.
In summary, results from this study suggest that there is wide variability in skin carotenoid status in a population of young children, as assessed by RRS. In addition to parent-reported fruit and vegetable intake, which was positively associated with skin carotenoid status, several demographic factors also tracked with the biomarker, including some that have been shown to track with the biomarker in studies of adults (for example, obesity). In our experience, RRS was a feasible biomarker of fruit and vegetable intake that may facilitate research on diet and health in children. Future controlled intervention studies are needed in children to continue the development of RRS as a biomarker of nutritional status.
Supplementary Material
Acknowledgments
We thank Angela Corcoran for her contributions to data collection, and the preschool staff, children and parents who participated in this study. This work was supported by funding from R01 CA096838 (STM) and American Diabetes Association (VBD); Sub-Award No. A 05202 and M03A00158 (IVE and WG), R01 EY11600 (PSB) and an unrestricted departmental grant from Research to Prevent Blindness (New York, NY) (PSB).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Livingstone MB, Robson PJ, Wallace JM. Issues in dietary intake assessment of children and adolescents. Br J Nutr. 2004;92 (Suppl 2):S213–S222. doi: 10.1079/bjn20041169. [DOI] [PubMed] [Google Scholar]
- 2.Baranowski T, Domel SB. A cognitive model of children’s reporting of food intake. Am J Clin Nutr. 1994;59 (1 Suppl):212S–217S. doi: 10.1093/ajcn/59.1.212S. [DOI] [PubMed] [Google Scholar]
- 3.Livingstone MB, Robson PJ. Measurement of dietary intake in children. Proc Nutr Soc. 2000;59:279–293. doi: 10.1017/s0029665100000318. [DOI] [PubMed] [Google Scholar]
- 4.Kirks BA, Wolff HK. A comparison of methods for plate waste determinations. J Am Diet Assoc. 1985;85:328–331. [PubMed] [Google Scholar]
- 5.Martin CK, Newton RL, Jr, Anton SD, Allen HR, Alfonso A, Han H, et al. Measurement of children’s food intake with digital photography and the effects of second servings upon food intake. Eat Behav. 2007;8:148–156. doi: 10.1016/j.eatbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Yatsuya H, Ohwaki A, Tamakoshi K, Wakai K, Koide K, Otsuka R, et al. Reproducibility and validity of a simple checklist-type questionnaire for food intake and dietary behavior. J Epidemiol. 2003;13:235–245. doi: 10.2188/jea.13.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duffy VB, Hayes JE, Sullivan BS, Faghri P. Surveying food and beverage liking: a tool for epidemiological studies to connect chemosensation with health outcomes. Ann NY Acad Sci. 2009;1170:558–568. doi: 10.1111/j.1749-6632.2009.04593.x. [DOI] [PubMed] [Google Scholar]
- 8.Michaud DS, Feskanich D, Rimm EB, Colditz GA, Speizer FE, Willett WC, et al. Intake of specific carotenoids and risk of lung cancer in 2 prospective US cohorts. Am J Clin Nutr. 2000;72:990–997. doi: 10.1093/ajcn/72.4.990. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M, Holman CD, Binns CW. Intake of specific carotenoids and the risk of epithelial ovarian cancer. Br J Nutr. 2007;98:187–193. doi: 10.1017/S0007114507690011. [DOI] [PubMed] [Google Scholar]
- 10.Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst. 1995;87:1767–1776. doi: 10.1093/jnci/87.23.1767. [DOI] [PubMed] [Google Scholar]
- 11.Le Marchand L, Hankin JH, Kolonel LN, Beecher GR, Wilkens LR, Zhao LP. Intake of specific carotenoids and lung cancer risk. Cancer Epidemiol Biomarkers Prev. 1993;2:183–187. [PubMed] [Google Scholar]
- 12.Wang L, Gaziano JM, Norkus EP, Buring JE, Sesso HD. Associations of plasma carotenoids with risk factors and biomarkers related to cardiovascular disease in middle-aged and older women. Am J Clin Nutr. 2008;88:747–754. doi: 10.1093/ajcn/88.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voutilainen S, Nurmi T, Mursu J, Rissanen TH. Carotenoids and cardiovascular health. Am J Clin Nutr. 2006;83:1265–1271. doi: 10.1093/ajcn/83.6.1265. [DOI] [PubMed] [Google Scholar]
- 14.Ito Y, Kurata M, Suzuki K, Hamajima N, Hishida H, Aoki K. Cardiovascular disease mortality and serum carotenoid levels: a Japanese population-based follow-up study. J Epidemiol. 2006;16:154–160. doi: 10.2188/jea.16.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SanGiovanni JP, Chew EY, Clemons TE, Ferris FL, III, Gensler G, Lindblad AS, et al. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Arch Ophthalmol. 2007;125:1225–1232. doi: 10.1001/archopht.125.9.1225. [DOI] [PubMed] [Google Scholar]
- 16.Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA. 1994;272:1413–1420. [PubMed] [Google Scholar]
- 17.Ray AL, Semba RD, Walston J, Ferrucci L, Cappola AR, Ricks MO, et al. Low serum selenium and total carotenoids predict mortality among older women living in the community: the women’s health and aging studies. J Nutr. 2006;136:172–176. doi: 10.1093/jn/136.1.172. [DOI] [PubMed] [Google Scholar]
- 18.Institute of Medicine, National Academy of Sciences; Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. National Academy Press; Washington, DC: 2000. Panel on Dietary Antioxidants and Related Compounds. [Google Scholar]
- 19.Natarajan L, Flatt SW, Sun X, Gamst AC, Major JM, Rock CL, et al. Validity and systematic error in measuring carotenoid consumption with dietary self-report instruments. Am J Epidemiol. 2006;163:770–778. doi: 10.1093/aje/kwj082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker RS. Analysis of carotenoids in human plasma and tissues. Methods Enzymol. 1993;214:86–93. doi: 10.1016/0076-6879(93)14056-o. [DOI] [PubMed] [Google Scholar]
- 21.Karppi J, Nurmi T, Olmedilla-Alonso B, Granado-Lorencio F, Nyyssonen K. Simultaneous measurement of retinol, alpha-tocopherol and six carotenoids in human plasma by using an isocratic reversed-phase HPLC method. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;867:226–232. doi: 10.1016/j.jchromb.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Hata TR, Scholz TA, Ermakov IV, McClane RW, Khachik F, Gellermann W, et al. Non-invasive Raman spectroscopic detection of carotenoids in human skin. J Invest Dermatol. 2000;115:441–448. doi: 10.1046/j.1523-1747.2000.00060.x. [DOI] [PubMed] [Google Scholar]
- 23.Ermakov IV, Sharifzadeh M, Ermakova M, Gellermann W. Resonance Raman detection of carotenoid antioxidants in living human tissue. J Biomed Opt. 2005;10:064028. doi: 10.1117/1.2139974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ermakov IV, Ermakova MR, McClane RW, Gellermann W. Resonance Raman detection of carotenoid antioxidants in living human tissues. Opt Lett. 2001;26:1179–1181. doi: 10.1364/ol.26.001179. [DOI] [PubMed] [Google Scholar]
- 25.Mayne ST, Cartmel B, Scarmo S, Lin H, Leffell DJ, Welch E, et al. Noninvasive assessment of dermal carotenoids as a biomarker of fruit and vegetable intake. Am J Clin Nutr. 2010;92:794–800. doi: 10.3945/ajcn.2010.29707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ermakov IV, Gellermann W. Validation model for Raman based skin carotenoid detection. Arch Biochem Biophys. 2010;504:40–49. doi: 10.1016/j.abb.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 27.Rerksuppaphol S, Rerksuppaphol L. Effect of fruit and vegetable intake on skin carotenoid detected by non-invasive Raman spectroscopy. J Med Assoc Thai. 2006;89:1206–1212. [PubMed] [Google Scholar]
- 28.Darvin M, Fluhr J, Caspers P, van der Pool A, Richter H, Patzelt A, et al. In vivo distribution of carotenoids in different anatomical locations of human skin: comparative assessment with two different Raman spectroscopy methods. Exp Dermatol. 2009;18:1060–1063. doi: 10.1111/j.1600-0625.2009.00946.x. [DOI] [PubMed] [Google Scholar]
- 29.Meinke M, Darvin M, Vollert H, Lademann J. Bioavailability of natural carotenoids in human skin compared to blood. Eur J Pharm Biopharm. 2010;76:269–274. doi: 10.1016/j.ejpb.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Blume-Peytavi U, Rolland A, Darvin ME, Constable A, Pineau I, Voit C, et al. Cutaneous lycopene and beta-carotene levels measured by resonance Raman spectroscopy: high reliability and sensitivity to oral lactolycopene deprivation and supplementation. Eur J Pharm Biopharm. 2009;73:187–194. doi: 10.1016/j.ejpb.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 31.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. Advance Data from Vital and Health Statistics. US Department of Health and Human Services, National Center for Health Statistics; Hyattsville, MD: 2000. CDC growth charts: United States. [Google Scholar]
- 32.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agricultural Research Service, Food Surveys Research Group. USDA Food and Nutrient Database for Dietary Studies, 1.0. Agricultural Research Service, Food Surveys Research Group; Beltsville, MD: 2004. [Google Scholar]
- 34.Schatzkin A, Kipnis V, Carroll RJ, Midthune D, Subar AF, Bingham S, et al. A comparison of a food frequency questionnaire with a 24-h recall for use in an epidemiological cohort study: results from the biomarker-based Observing Protein and Energy Nutrition (OPEN) study. Int J Epidemiol. 2003;32:1054–1062. doi: 10.1093/ije/dyg264. [DOI] [PubMed] [Google Scholar]
- 35.Ermakov IV, Ermakova MR, Gellermann W, Lademann J. Noninvasive selective detection of lycopene and beta-carotene in human skin using Raman spectroscopy. J Biomed Opt. 2004;9:332–338. doi: 10.1117/1.1646172. [DOI] [PubMed] [Google Scholar]
- 36.Neuhouser ML, Rock CL, Eldridge AL, Kristal AR, Patterson RE, Cooper DA, et al. Serum concentrations of retinol, alpha-tocopherol and the carotenoids are influenced by diet, race and obesity in a sample of healthy adolescents. J Nutr. 2001;131:2184–2191. doi: 10.1093/jn/131.8.2184. [DOI] [PubMed] [Google Scholar]
- 37.Virtanen SM, van’t Veer P, Kok F, Kardinaal AF, Aro A. Predictors of adipose tissue carotenoid and retinol levels in nine countries. The EURAMIC Study. Am J Epidemiol. 1996;144:968–979. doi: 10.1093/oxfordjournals.aje.a008867. [DOI] [PubMed] [Google Scholar]
- 38.Strauss RS. Comparison of serum concentrations of alpha-tocopherol and beta-carotene in a cross-sectional sample of obese and nonobese children (NHANES III). National Health and Nutrition Examination Survey. J Pediatr. 1999;134:160–165. doi: 10.1016/s0022-3476(99)70409-9. [DOI] [PubMed] [Google Scholar]
- 39.Brady WE, Mares-Perlman JA, Bowen P, Stacewicz-Sapuntzakis M. Human serum carotenoid concentrations are related to physiologic and lifestyle factors. J Nutr. 1996;126:129–137. doi: 10.1093/jn/126.1.129. [DOI] [PubMed] [Google Scholar]
- 40.Parker RS. Carotenoids in human blood and tissues. J Nutr. 1989;119:101–104. doi: 10.1093/jn/119.1.101. [DOI] [PubMed] [Google Scholar]
- 41.Dibsdall LA, Lambert N, Bobbin RF, Frewer LJ. Low-income consumers’ attitudes and behaviour towards access, availability and motivation to eat fruit and vegetables. Public Health Nutr. 2003;6:159–168. doi: 10.1079/PHN2002412. [DOI] [PubMed] [Google Scholar]
- 42.Rose D, Richards R. Food store access and household fruit and vegetable use among participants in the US Food Stamp Program. Public Health Nutr. 2004;7:1081–1088. doi: 10.1079/PHN2004648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.