Abstract
Objective
To investigate the role of neuronal nitric oxide synthase (NOS1) in murine polymicrobial peritonitis and sepsis.
Design
Randomized experimental trial.
Setting
Animal research facility.
Subjects
B6129S NOS1+/+ and B6;129S4 NOS–/– mice.
Interventions
NOS1+/+ and NOS1–/– animals underwent cecal ligation and puncture (CLP) or sham surgery and received the NOS1 inhibitor 7-nitroindazole (7-NI) or vehicle.
Measurements and main results
After CLP, genetic deficiency and pharmacologic inhibition of NOS1 significantly increased risk of mortality [8.69 (3.27, 23.1), p < 0.0001 and 1.71 (1.00, 2.92) p = 0.05, hazard ratio of death (95% confidence interval) for NOS1–/– and 7-NI-treated NOS1+/+ respectively] compared with NOS1+/+ animals. In 7-NI-treated NOS1+/+ animals, there were increases (6 h) and then decreases (24 h), whereas in NOS–/– animals persistent increases in blood bacteria counts (p = 0.04 for differing effects of 7-NI and NOS1–/–) were seen compared with NOS1+/+ animals. After CLP, NOS1–/– had upregulation of inducible NOS and proinflammatory cytokines and greater increases in serum tumor necrosis factor-α and interleukin-6 levels compared with NOS1+/+ mice (all p < 0.05). Following CLP, there were similar significant decreases in circulating leukocytes and lung lavage cells (p ≤ 0.0008) and significant increases in peritoneal lavage cells (p = 0.0045) in all groups. Over 6 h and 24 h following CLP, compared with NOS1+/+, NOS–/– mice had significantly higher peritoneal cell concentrations {respectively 0.40 ± 0.09 vs 0.79 ± 0.15 [log(× 104cells/ml)] averaged over both times p = 0.038}.
Conclusions
Deficiency and inhibition of NOS1 increases mortality, possibly by increasing proinflamma-tory cytokine response and impairing bacterial clearance after CLP. These data suggest that NOS1 is important for survival, bacterial clearance, and regulation of cytokine response during infection and sepsis.
Keywords: Sepsis, Peritonitis, NOS1, nNOS, NO, Rodents
Introduction
Most studies of the role of nitric oxide synthases (NOS) in sepsis have focused on inducible NOS (NOS2) and have shown that its expression and, in turn, overproduction of NO are pivotal in sepsis-induced hemodynamic changes and end-organ damage [1–6]. With regards to the constitutive isoforms of NOS, there is experimental evidence that endothelial NOS (NOS3) has an important role in sepsis as well. Researchers have shown that genetic deficiency of NOS3 ameliorates the hypotensive response to endotoxin [7] and over-expression of NOS3 renders mice resistant to endotoxin-induced lung injury, myocardial dys-function, and death [8, 9]. However, little is known about the role of the other constitutively expressed NOS, neuronal NOS (NOS1), during infection and sepsis [10].
NOS1 is expressed in a variety of tissues [11–15] and has been implicated in modulation of cardiac and renal functions [16–18], vascular homeostasis [19, 20], and airway responsiveness and inflammation [15]. With regards to leukocyte function, studies suggest that NOS1 has a role in the regulation of leukocyte–endothelial interactions. Mice congenitally lacking NOS1 have increased leukocyte rolling and adhesion in mesenteric microcirculation during basal conditions and after thrombin stimulation [21]. During chemical peritonitis, NOS1-deficient mice have increased transmigration of leukocytes into the peritoneum which appears to be P-selectin mediated [21]. Others have shown that NOS1 deficiency increased pulmonary neutrophil recruitment after intra-tracheal administration of endotoxin or Klebsiella pneumoniae [22]. In sheep, NOS1 inhibition ameliorated lung injury after smoke inhalation and airway instillation of bacteria [23]. Taken together, these studies suggest that NOS1 might have an important role in regulating leukocyte trafficking at basal conditions and during inflammation.
However, the effect of NOS1 on overall outcome during bacterial infection is unknown. We hypothesized that genetic deficiency and pharmacologic inhibition of NOS1 would lead to increases in leukocyte trafficking to infection site, increases in microbial clearance, and improvement in overall outcome during peritonitis and sepsis.
Methods
Experimental subjects
After approval by the NIH Clinical Center Animal Care and Use Committee, we studied female B6129S NOS1+/+ (F2 hybrids of the parental strains of NOS1–/– mice, C67BL/6J and 129S) and B6;129S4 NOS1–/– mice aged 6–8 weeks and weighing 15–30 g (Jackson Laboratories, Bar Harbor, ME). Animals were housed at 72°F (22.2°C) with free access to food and water. After surgery, animals were observed every 2 h for the first 36 h, every 4 h from 36 h to 48 h, and once daily from 48 h to 168 h. Animals found to be moribund or to display unexpected distress were killed humanely.
Experimental protocol
Survival
We studied eight groups including all combinations of surgery [cecal ligation and puncture (CLP) or sham surgery (laparotomy without cecal ligation and puncture)], genotype (NOS1+/+ or NOS1–/–), and treatment [7-nitroindazole (7-NI) or vehicle]. After induction of anesthesia with isoflurane, animals underwent CLP or sham surgery as previously described [24]. Briefly, after a small midline abdominal incision, the base of the cecum was ligated at 1 cm from its tip with 4–0 silk suture and perforated once at the anti-mesenteric surface with a 16-G needle. A small amount of feces was then expressed through the puncture wound. Fluids were administered at completion of surgery (normal saline, 50 ml/kg) and with each dose of antibiotic [Primaxin® (imipenem/cilastatin sodium), 25 mg/kg of imipenem, Merck, Whitehouse Station, NJ] starting 2 h after surgery and every 12 h for 72 h (each time 50 ml/kg of saline subcutaneously, amounting to 150 ml/kg in the first 24 h and 100 ml/kg from 24 h to 72 h).
7-NI treatment
During surgery, according to experimental group assignments, NOS1+/+ and NOS1–/– animals were given 7-NI (80 mg/kg) in peanut oil or peanut oil (control) i.p. This dose of 80 mg/kg was based on mouse studies showing that it significantly decreases cerebellum cyclic guanosine monophosphate levels [25] and produces detectable serum levels of 7-NI for over 10 h [26].
Cell counts and organ histology
Separate animal cohorts were killed humanely at baseline or at 6 h or 24 h after surgery for collection of blood, lung and peritoneal lavage fluids, and organs. Complete and differential blood cell counts and quantitative blood cultures were obtained. For peritoneal lavage, phosphate-buffered saline (PBS) was injected into the peritoneum and peritoneal fluid retrieved after 3 min. Lung lavage fluid was obtained by cannulating the trachea and sequentially injecting four 1-ml aliquots of PBS. Lavage cell counts were performed with an electronic counter (Z1 Coulter Particle Counter, Coulter Electronics, Miami, FL). Slides for differential counts were prepared (Cytospin 4, Thermo Shandon Inc., Pittsburgh, PA) and stained. Lavage supernatants were passed through a 45-μm filter and protein determinations were made using a Folin–Lowry technique.
A pathologist blinded to animal groups measured neutrophil infiltration in heart, lung, spleen, liver, and kidney as follows: score 0 indicated no neutrophils, 1+ a low number, 2+ a moderate number, and 3+ a high number of neutrophils.
Quantitative real-time polymerase chain reaction
RNA was isolated from spleens 6 h after CLP using RNeasy Mini kit (Qiagen, Valencia, CA). RNA quality was evaluated by Agilent 2100 Bioanalyzer using RNA 6000 Nano kits (Agilent Technologies, Santa Clara, CA) and quantity was measured using NanoDrop (NanoDrop Technologies, Wilmington, DE). Tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-10, IL-1β, interferon (IFN)-γ, macrophage inflammatory protein (MIP)-1α, and NOS2 gene expressions were quantified by real-time polymerase chain reaction (qPCR) using One-Step qRTPCR Kit in the Abi Prism® 7500 (both from Applied Biosystems, Foster City, CA). Fold changes in gene expression were calculated after normalization to endogenous GAPDH and were expressed relative to that detected in spleens of NOS1+/+ control animals (normalized to 1) using the comparative Ct method [27].
Serum cytokine and chemokine levels
Serum TNF-α, IL-6, IL-10, IL-1β, IFN-γ, and MIP-1α levels were measured using a multiplex sandwich enzyme-linked immunosorbent assay (SearchLight Mouse Cytokine array, ThermoFisher Scientific, Woburn, MA, USA).
In vitro bacterial killing assay
Leukocytes were elicited from a cohort of six NOS1+/+ and six NOS–/– mice, peritoneal lavage cells (> 90% granulocytes and < 10% monocytes and lymphocytes) were obtained and suspended in Dulbecco's PBS without Ca2+ and Mg2+. E. coli 0111:H12 (5 × 106 CFU of a log phase culture) was added to polypropylene tubes containing 2 × 106 leukocytes of the respective mouse strain. Following coincubation for 90 min at 37°C with end-over-end rotation, bacterial viability was determined by plating serial dilutions of each tube's content in triplicates onto MacConkey agar and counting bacterial colonies after overnight growth.
Statistical methods
The survival effect comparison between groups (7-NI treatment versus vehicle in either NOS1+/+ or NOS1–/– mice or NOS1–/– versus NOS1+/++ vehicle or 7-NI treatment) were analyzed using a Cox proportional hazards model and are presented as the hazard ratio of death and 95% confidence interval. Since 7-NI had no significant effect on mortality of NOS1–/– animals, 7-NI-treated NOS1–/– and vehicle-treated NOS–/– mice were combined and compared with NOS1+/+ and 7-NI-treated NOS1+/+ in survival analysis as well as laboratory data. All other laboratory measures were analyzed with ANOVA. Data were averaged over variables when justified based on statistical analysis to increase power and reduce animal usage. Effects were calculated by subtracting the mean of the control group from the mean of the respective experimental group. Data were log transformed where appropriate. All results were expressed as mean ± SEM, and p values ≤ 0.05 were considered significant.
Results
Baseline studies
At baseline, compared with NOS1+/+ mice, NOS1–/– animals had higher circulating neutrophils (p = 0.001, Table 1) and lower circulating platelets (p = 0.03, Table 1). There were no other differences in blood count variables between NOS1+/+ and NOS1–/– mice. In peritoneal lavage fluid, NOS1–/– mice, compared with NOS1+/+, had higher leukocyte counts [0.28 ± 0.11 vs 0.08 ± 0.02 log(× 104/ml), NOSI–/– vs NOS1+/+ respectively, p = 0.002] and higher concentration of total protein (94 ± 17 vs 35 ± 2 mg/10 ml respectively, p = 0.03). In lung lavage fluid, mean number of cells and protein concentration were similar in NOS1–/– and NOS1+/+ mice. Baseline serum cytokine levels were similar in NOS1+/+ and NOS1–/– mice.
Table 1.
Mean ± SEM of circulating and lung lavage variables before or after cecal ligation and puncture
| Variable | Time before (baseline) or after (h) cecal ligation and puncture | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| NOS1+/+ | 7-NI-treatedNOS1+/+ | NOS1–/– | |||||||
| Baseline (n = 6) | 6 (n = 9) | 24 (n = 10) | Baseline (n = 6) | 6 (n = 10) | 24 (n = 10) | Baseline (n = 4) | 6 (n = 19) | 24 (n = 15) | |
| Circulating blood cells | |||||||||
| Neutrophils (×103/ul) | 0.46 ± 0.08 | 0.44 ± 0.22 | 0.05 ± 0.04 | 0.46 ± 0.08 | 0.42 ± 0.15 | 0.05 ± 0.03 | 1.21 ± 0.26 | 0.56 ± 0.22 | 0.23 ± 0.13 |
| Lymphocytes (×103/ul) | 5.13 ± 0.84 | 0.77 ± 0.18 | 1.05 ± 0.46 | 5.13 ± 0.84 | 1.03 ± 0.25 | 0.61 ± 0.15 | 3.87 ± 0.94 | 0.79 ± 0.22 | 0.62 ± 0.11 |
| Platelets (×103/ul) | 955 ± 40 | 559 ± 114 | 338 ± 48 | 955 ± 40 | 696 ± 128 | 359 ± 93 | 642 ± 126 | 654 ± 74 | 524 ± 269 |
| Lung lavage cells | |||||||||
| Lymphocytes log(×104 cell/ml) | NA | 1.34 ± 0.24 | 1.45 ± 0.09 | NA | 1.09 ± 0.29 | 0.98 ± 0.07 | NA | 1.03 ± 0.12 | 1.21 ± 0.08 |
SEM, standard error of mean; NOS1+/+, wild-type; NOS1–/–, NOS1-deficient; n, number of samples tested, which is the same at each time point and in each group for all parameters; NA, not available
Survival
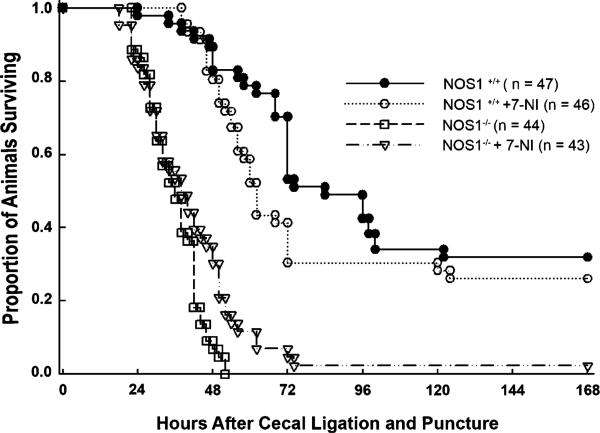
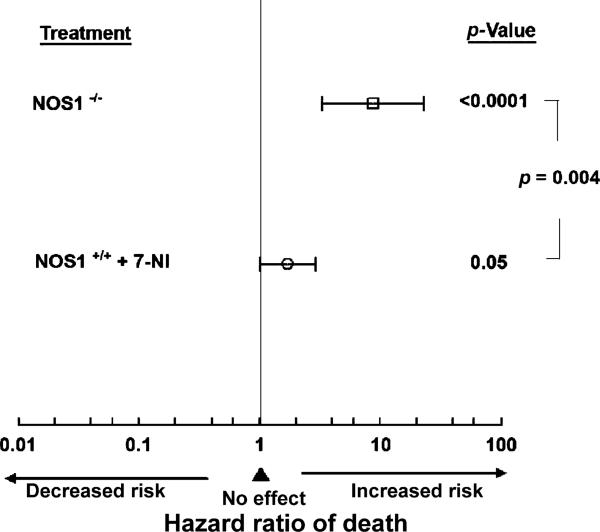
After sham surgery, all NOS1+/+ and NOS1–/– mice survived whereas by 6 h after CLP, all animals showed weakness, lethargy, and decreased mobility. Over 168 h, CLP produced mortality in all groups (Fig. 1). Following CLP, genetic NOS1 deficiency in NOS1–/– and pharmacologic inhibition of NOS1 in 7-NI-treated NOS1–/–, was associated with greater mortality in patterns that were similar (NOS1–/– vs 7-NI-treated NOS1–/–, p = NS) and with increased risk of death (decreased time to death) compared with NOS1+/+ mice [8.69 (3.27, 23.1), hazard ratio for death (95% confidence interval), p < 0.0001, Fig. 2]. In addition, pharmacologic inhibition of NOS1 in 7-NI-treated NOS1+/+ animals, without changing survival rates, significantly decreased time to death compared with NOS1+/+ [1.71 (1.00, 2.92) hazard ratio for death (95% confidence interval), p = 0.05, Fig. 2]. Finally, the overall increase in the hazard ratio of death with genetic deficiency of NOS1 was significantly greater than that of pharmacologic inhibition of NOS1 in 7-NI-treated NOS1+/+ animals (p = 0.004, comparing 7-NI-treated NOS1+/+ animals vs NOS1–/–, Fig. 2).
Fig. 1.
Effect of NOS1 on survival during sepsis. Proportion of animals surviving after cecal ligation and puncture in NOS1+/+, 7-NI-treated NOS1+/+, and NOS1–/– groups
Fig. 2.
Effect of NOS1 on risk of death. Hazard ratio for mortality with NOS1 deficiency (NOS1–/–) and pharmacologic inhibition of NOS1 (NOS1+/+ +7-NI) was compared with normal NOS1 in NOS1+/+ animals. The square and circle represent the hazard ratio of death for the corresponding group and the brackets the 95% confidence interval. Compared with intact NOS1 in NOS1+/+, genetic NOS1 deficiency was associated with significantly greater risk of mortality (p < 0.0001), as was pharmacologic inhibition of NOS1 with 7-NI (p = 0.05). The harmful effect of genetic NOS1 deficiency on risk of death was greater than that of pharmacologic inhibition of NOS1 with 7-NI (p = 0.004)
Bacterial counts
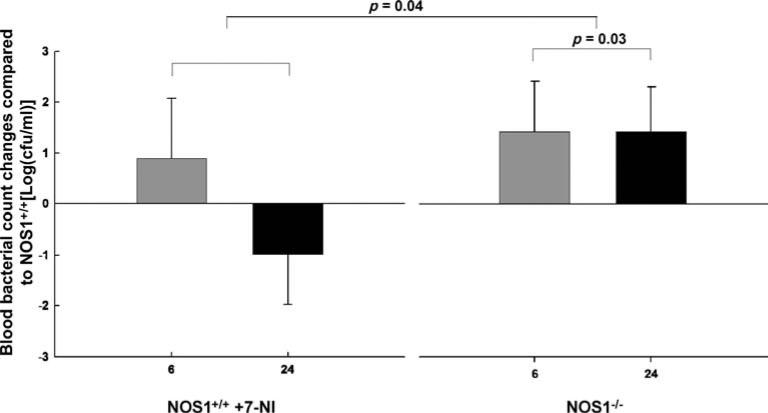
After CLP, compared with NOS1+/+, blood bacteria counts were increased in both 7-NI-treated NOS1+/+ and NOS1–/– animals at 6 h, but decreased in 7-NI-treated NOS1+/+ at 24 h, while still increased in NOS1–/– at 24 h, in patterns that over time were significantly different (p = 0.04, Fig. 3). Furthermore, averaged over 6 h and 24 h, compared with NOS1+/+, NOS–/– mice had significantly greater overall increases in blood bacteria counts [+1.40 ± 0.66 (mean ± SEM log cfu/ml), both time points combined, p = 0.03, Fig. 3]. After sham surgery, in all groups, blood cultures yielded no growth.
Fig. 3.
Effect of NOS1 on bacterial clearance after cecal ligation and puncture (CLP). After CLP, NOS1-deficient mice (NOS1–/–) had significant increases at both 6 h and 24 h (both time points combined p = 0.03) in bacterial blood counts compared with NOS1+/+ mice. After CLP, compared with NOS1+/+, blood bacteria counts were increased in both 7-NI-treated NOS1+/+ and NOS1–/– animals at 6 h, but decreased in 7-NI-treated NOS1+/+ at 24 h while still increased in NOS1–/– at 24 h, in patterns that over time were significantly different (p = 0.03). cfu, Colony-forming units
Gene expression after CLP
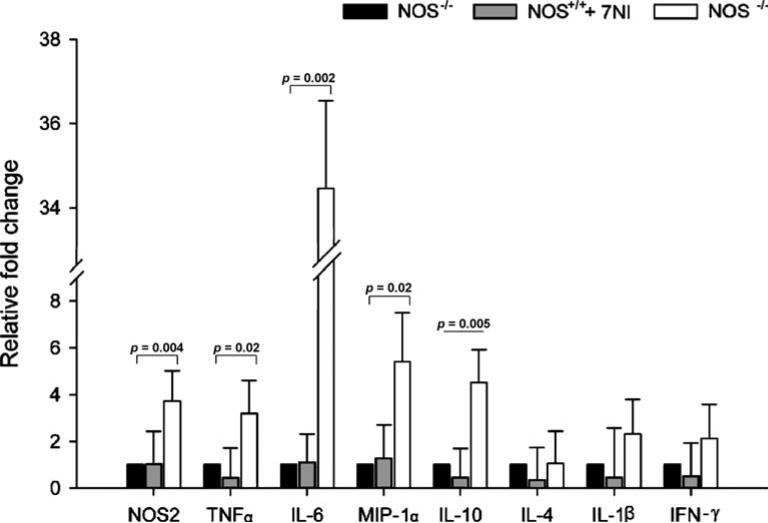
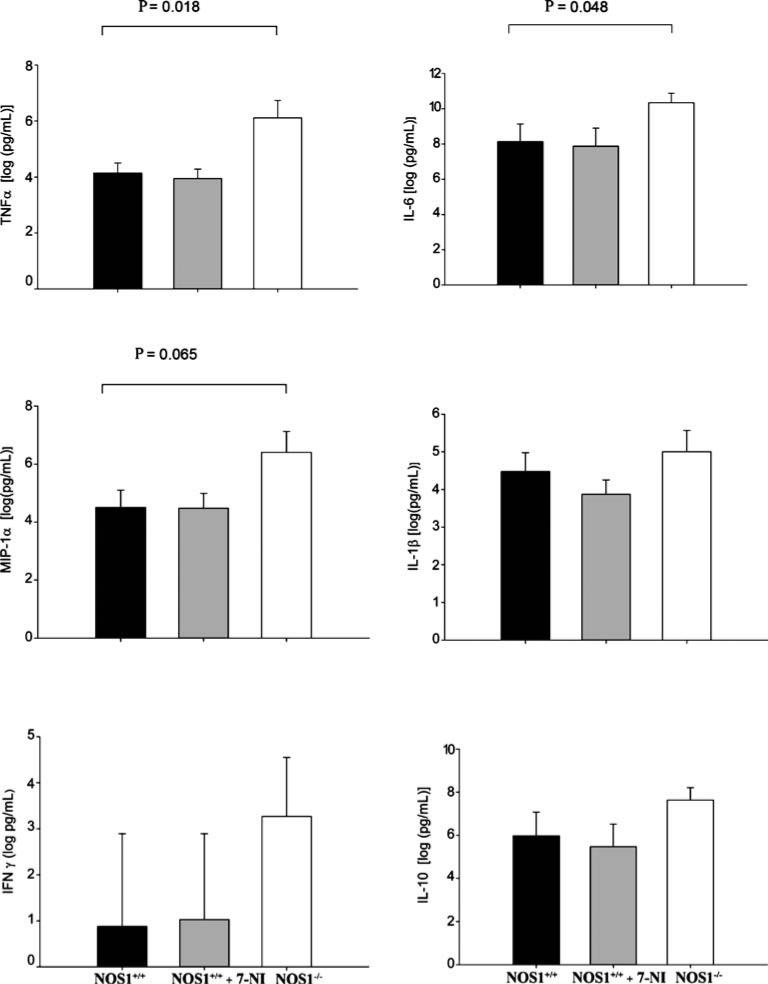
At 6 h after CLP, using real-time quantitative PCR, we examined the expression of a number of genes in spleens of NOS–/–, 7-NI-treated NOS1+/+ and NOS1+/+ animals (Fig. 4). In NOS1–/– animals, compared with NOS1+/+, the expression of NOS2 was upregulated about fourfold (p = 0.004), that of TNF-α about threefold (p = 0.02), IL-6 about 34-fold (p = 0.002), MIP-1α fivefold (p = 0.02), and IL-10 fivefold (p = 0.005). Conversely, at 6 h after CLP, there were no significant differences in gene expressions of IL-4, IL-1β, and IFN-γ between NOS1+/+ and NOS1–/– animals (p = NS). In addition, 7-NI treatment had no significant effect on gene expression in 7-NI-treated-NOS1+/+ animals compared with NOS1+/+ (p = NS, Fig. 4).
Fig. 4.
Effect of NOS1 on gene expression 6 h after cecal ligation and puncture (CLP). Fold changes (y-axis) for each gene were normalized to GAPDH and are relative to the gene expression in vehicle-treated septic NOS1+/+ animals (normalized to 1). Results are means ± SEM of relative fold changes. At 6 h after CLP, in NOS1–/– animals, compared with NOS1+/+ controls, there was upregulation of inducible nitric oxide synthase (NOS2) about fourfold (p = 0.004), of tumor necrosis factor (TNF)-α about threefold (p = 0.02), interleukin (IL)-6 about 34-fold (p = 0.002), macrophage inflammatory protein-1 α (MIP-1α) fivefold (p = 0.02), and IL-10 fivefold (p = 0.005). Conversely, at 6 h after CLP, there were no significant differences in gene expression of IL-4, IL-1β, and interferon (IFN)-γ comparing NOS1+/+ and NOS1–/– animals (p = NS). 7-NI treatment had no significant effect in gene expression in septic animals (p = NS)
Serum cytokines and chemokines
From baseline to 6 h after CLP, there were significant increases in serum TNF-α, IL-6, IL-10, IL-1β, and MIP-1α, in NOS1+/+, 7-NI-treated NOS1+/+, and NOS1–/– animals (all p < 0.0001, Fig. 5), but no significant changes in serum IFN-γ in any of the experimental groups (p = NS, Fig. 5). Further, over 6 h following CLP, compared with NOS1+/+, NOS–/– mice had significantly greater increases in serum levels of TNF-α (p = 0.018) and IL-6 (p = 0.048) and a trend towards greater increases in serum levels of MIP-1α (p = 0.065). Conversely, over 6 h after CLP, there were no significant differences in changes from baseline in serum cytokine levels comparing 7-NI-treated NOS1+/+ with NOS1+/+ animals (p = NS, Fig. 5).
Fig. 5.
Effect of NOS1 on serum cytokine levels after cecal ligation and puncture (CLP). Bars represent mean changes from baseline cytokine levels. After CLP compared with baseline, there were significant increases in serum tumor necrosis factor (TNF-α), interleukin (IL)-6, IL-10, IL-1β, and macrophage inflammatory protein-1 α (MIP-1α) levels in NOS1+/+-type, 7-NI-treated NOS1+/+, and NOS1–/– animals; all p < 0.0001. Over 6 h following CLP, compared with NOS1+/+, NOS–/– mice had significantly greater increases in serum levels of TNF-α (p = 0.018) and IL-6 (p = 0.048) and a trend towards greater increases in serum levels of MIP-1α (p = 0.065). Pharmacologic inhibition with 7-NI in 7-NI-treated- NOS1+/+ had no effect on serum cytokine changes from baseline levels compared with NOS1+/+ animals (p = NS)
Complete blood cell counts
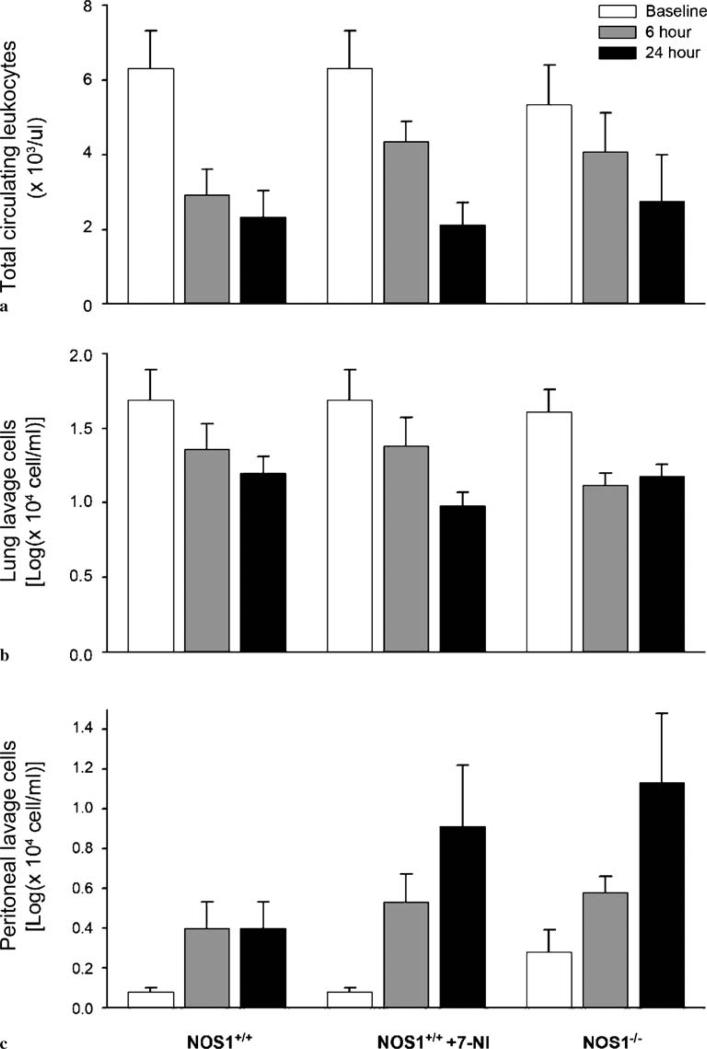
CLP, compared with sham surgery, produced decreases in total circulating leukocytes, lymphocytes, neutrophils, and platelets (p < 0.05, data not shown) in all animals. Following CLP, there were similar significant decreases in total circulating leukocytes (p = 0.0008, Fig. 6a) in NOS1+/+, 7-NI-treated NOS1+/+, and NOS1–/– animals. Over 24 h following CLP, total circulating neutrophils, lymphocytes, and platelet counts (Table 1) and hemoglobin (data not shown) similarly decreased in NOS1+/+, 7-NI-treated NOS1+/+, and NOS1–/– animals (all p < 0.02). From 6 h to 24 h after sham surgery, total circulating leukocytes and lymphocytes similarly increased (p < 0.05) and circulating neutrophils and platelets similarly decreased (p < 0.05) in both NOS1+/+ and NOS1–/– mice. There was no effect of genetic deficiency or pharmacologic inhibition of NOS1 in these measurements (p = NS, data not shown).
Fig. 6.
Effect of NOS1 on cell trafficking after cecal ligation and puncture (CLP). Following CLP, there were similar significant decreases in total circulating leukocytes (a; p = 0.0008), similar significant decreases in total lung lavage cells (log total cells in lung lavage, b; p = 0.0001), and significant increases in total peritoneal lavage cells (c; p = 0.0045, log total cells in peritoneal lavage) in all groups – NOS1+/+, 7-NI-treated NOS1+/+, and NOS1–/– animals. Over 6 h and 24 h following CLP, compared with NOS1+/+, NOS–/– had significantly higher total cell concentrations (p = 0.038) and 7-NI-treated NOS1+/+ mice a trend towards higher cell concentrations (p = 0.09) in peritoneal lavage fluid
Lung lavage
Following CLP, there were similar significant decreases in lung lavage cells (p = 0.0001, Fig. 6b) in NOS1+/+, 7-NI-treated NOS1+/+, and NOS1–/– animals. Over 6 h and 24 h following CLP, compared with NOS1+/+, 7-NI-treated NOS1+/+ (p = 0.02) and NOS1–/– (p = 0.03) mice had significant reductions in lung lavage lymphocyte concentrations [1.42 ± 0.09, 1.02 ± 0.12, 1.11 ± 0.07 log(× 104 cells/ml) NOS1+/+, 7-NI-treated NOS1+/+ and NOS1–/– mice respectively, averaged over 6 h and 24 h)]. There were no significant differences in any other lung lavage measures comparing study groups after CLP. After sham surgery, NOS1+/+, 7-NI-treated NOS1+/+, and NOS1–/– showed no significant changes in cell counts and protein concentration in lung lavage (data not shown).
Peritoneal lavage
Following CLP, there were significant increases in peritoneal lavage cells (p = 0.0045, Fig. 6c) in NOS1+/+, 7-NI-treated NOS1+/+, and NOS1–/– animals. In addition, over 6 h and 24 h following CLP, compared with NOS1+/+ animals, NOS–/– mice had significantly higher total cell concentrations [respectively 0.40 ± 0.09 vs 0.79 ± 0.15 [log(× 104 cells/ml)] averaged over both time points p = 0.038] and 7-NI-treated NOS1+/+ mice a trend towards higher cell concentrations (0.40 ± 0.09 vs 0.68 ± 0.15 [log(× 104 cells/ml)], p = 0.09) in peritoneal lavage fluid. From 6 h to 24 h after CLP, all animals showed similar decreases (p = NS) in protein concentration in peritoneal lavage fluid (data not shown). After sham surgery, NOS1+/+, 7-NI-treated NOS1+/+, and NOS1–/– mice showed no significant changes in cell counts and protein concentration in peritoneal lavage fluid.
Histopathology
In order to evaluate the impact of NOS1 in non-infected organ injury, we evaluated the degree of neutrophil infiltration in spleen, heart, kidney, lung, and liver at 6, 24, and 168 h after sham surgery and CLP. The degree of neutrophil infiltration in these organs was similar among all animals undergoing CLP (Table 2, p = NS).
Table 2.
Degree of neutrophil infiltration (mean ± SEM) in lung, liver, kidney, spleen, and heart after cecal ligation and puncture
| Organ | Time (h) after cecal ligation and puncture | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| NOS1+/+ | 7-NI-treated NOS1+/+ | NOS1–/– | |||||||
| 6 (n = 6) | 24 (n = 9) | 168 (n = 10) | 6 (n = 6) | 24 (n = 10) | 168 (n = 10) | 6 (n = 4) | 24 (n = 19) | 168 (n = 15) | |
| Lung | 0 | 0.11 ± 0.11 | 0 | 0 | 0.11 ± 0.11 | 0 | 0.10 ± 0.10 | 0.07 ± 0.07 | NA |
| Liver | 0 | 0.33 ± 0.24 | 0.50 ± 0.29 | 0 | 0.11 ± 0.11 | 0 | 0.40 ± 0.31 | 0.21 ± 0.11 | NA |
| Kidney | 0 | 0.11 ± 0.11 | 0 | 0 | 0 | 0 | 0.30 ± 0.30 | 0.10 ± 0.43 | NA |
| Spleen | 1.17 ± 0.40 | 1.11 ± 0.26 | 0.25 ± 0.25 | 1.40 ± 0.51 | 0.89 ± 0.35 | 1.00 ± 1.00 | 0.90 ± 0.28 | 0.43 ± 0.17 | NA |
| Heart | 0 | 0.11 ± 0.11 | 0 | 0 | 0.11 ± 0.11 | 0 | 0 | 0.07 ± 0.07 | NA |
For explanations see Table 1
In vitro bacterial killing
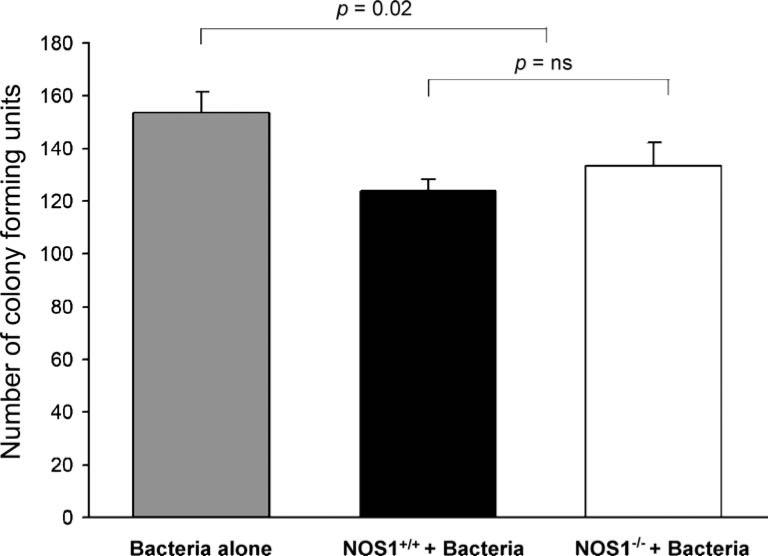
In order to evaluate neutrophil bactericidal abilities in vitro, we evaluated number of bacteria colony-forming units after coincubation of bacteria with elicited leukocytes from NOS1–/– and NOS1+/+ animals. Plates with bacteria and elicited leukocytes from NOS1–/– and NOS1+/+ animals had similar (p = NS) number of bacterial colonies that were both lower (p = 0.02) than with control plates (Fig. 7).
Fig. 7.
In vitro bactericidal activity of elicited neutrophils. Aliquots of elicited neutrophils were incubated with bacteria and were plated overnight. Plates containing elicited neutrophils alone from NOS1+/+ and NOS1–/– animals yielded no growth. Plates with leukocytes from NOS1–/– and NOS1+/+ animals had similar (p = NS) numbers of bacterial colonies that were lower (p = 0.02) than those in control plates containing bacteria alone
Discussion
Genetic deficiency and pharmacologic inhibition of NOS1 increase leukocyte trafficking to the infection site but worsen survival and decrease blood bacterial clearance in CLP-induced sepsis. Further, increased mortality in NOS1–/– animals is associated with changes in inflammatory cytokine response pattern relative to NOS1+/+ animals. Specifically, at 6 h after onset of infection, relative to NOS1+/+ animals, NOS1 deficiency in NOS1–/– animals was associated with significantly greater expression of the NOS2 gene and of proinflammatory cytokines (TNF, IL-6) and chemokines (MIP-1α), as well as upregulation of IL-10. Further, this upregulation in gene expression was coupled with increases in serum levels of proinflammatory (TNF and IL-6) and chemotactic cytokines (MIP-1α) in NOS1-deficient animals. These results indicate that NOS1 deficiency has an overall harmful effect during bacterial peritonitis and sepsis in mice and suggest that NOS1 impacts on survival, possibly by regulating inflammatory cell trafficking, altering microbial clearance, and increasing proinflammatory cytokine response.
While leukocytes from NOS1–/– mice showed normal bactericidal activity in vitro, our findings of in vivo bacterial clearance impairment in these animals are in agreement with that of others [22]. Researchers showed that after K. pneumoniae inhalation, NOS1–/– mice had increased neutrophil recruitment to the lung, yet higher pulmonary K. pneumoniae content, than did NOS1+/+ animals [22]. The discrepancy between our in vivo and in vitro findings suggests that in vitro studies do not entirely reflect bacterial clearance in vivo and that factors other than bacterial killing alone play a role in clearing bacteria from the blood after CLP in NOS–/– animals. Interestingly, NOS1, by a mechanism or mechanisms incompletely understood, has been shown to have a role in clearance of parasite infections in mice [28]. Taken together, our findings and those of others suggest that NOS1 is important for microbial clearance both at the infection site and from the blood during bacterial infection and sepsis.
In this investigation, we found that genetic NOS1 deficiency was associated with upregulation of key pro-inflammatory and chemotactic cytokines as well as upregulation of NOS2. Our findings are in agreement with those showing that chronic pharmacologic inhibition of NOS1 can alter the expression of NOS2 and NF-κB [29], a factor that regulates transcription of proinflammatory cytokines. Both in vitro [30] and in vivo [31], chronic pharmacologic inhibition of NOS1 with 7-NI causes increases in NOS2 mRNA and NOS2 protein associated with upregulation of NF-κB and increases in myeloperoxidase activity (a marker for neutrophil sequestration). Interestingly, in addition to upregulation of pro-inflammatory cytokines, we found that NOS1-deficient animals also had upregulation of the anti-inflammatory cytokine IL-10 compared with controls. One could, then, postulate that by altering expression of cytokine profile after infection, NOS1 deficiency might create an imbalance among proand anti-inflammatory cytokines such that early in sepsis this possible imbalance results in increased risk of death and impaired bacterial clearance.
Given the reported increased leukocyte adhesion, rolling, and transmigration in NOS1-deficient mice [21], we hypothesized that NOS1–/– animals could have increased neutrophil-induced inflammatory organ injury during sepsis. However, contrary to reports suggesting that increased neutrophil adhesion during extravascular infection can aggravate injury [32, 33], we found no histological evidence that NOS1 alters organ neutrophil infiltration during bacterial peritonitis. Conceivably, because we examined organ histology early (up to 24 h) during infection and most deaths in NOS1+/+ and NOS1–/– occurred after 24 h, we were unable to detect differences in organ injury. Contrary to our findings, others found that pharmacologic inhibition of NOS1 decreased physiologic and histologic measures of lung injury in an ovine smoke inhalation and bacteria challenge model [23]. The discrepancy between those results and ours may be explained by differences in species and model studied. Nevertheless, our findings suggest that the detrimental effects of NOS1 deficiency and pharmacologic inhibition of NOS1 are unrelated to noticeable increases in end-organ neutrophil infiltration early in the course of sepsis.
With regards to cell trafficking after CLP, our findings are in agreement with others describing increases in leukocyte transmigration into the peritoneum, during chemical peritonitis [21] and into the lung after tracheal bacterial inhalation [22]. Here we show that during bacterial peritonitis, genetic deficiency and pharmacologic inhibition of NOS1 was associated with greater cell migration into the peritoneum. Therefore, our findings that NOS1 deficiency impacts on cell transmigration into the peritoneum during bacterial peritonitis adds to the existing body of literature suggesting that NOS1 has a regulatory role in leukocyte recruitment to extravascular infection sites. In addition, the finding that NOS1-deficient animals have higher circulating neutrophils and lower platelet counts at baseline may suggest that, by mechanisms incompletely understood, NOS1 might also have a role on regulation of certain cell types at basal conditions that ultimately impacts on circulating neutrophils and platelets. Taken together, these findings support the notion that NOS1 plays a significant role in the regulation of leukocyte–endothelium interaction both in basal conditions and during live bacterial extravascular infection.
In summary, perturbations of NOS1, both by genetic alteration and by pharmacologic inhibition of the enzyme, increase risk of mortality, increase leukocyte recruitment to the infection site, decrease bacterial clearance from the blood, and increase proinflammatory cytokine response in sepsis after CLP. While one has to be circumspect about extrapolating rodent data to other species, our results suggest that NOS1 is important for bacterial clearance, cytokine expression, and survival during sepsis, and therapeutic strategies that inhibit NOS1 could be detrimental.
Acknowledgements
The authors wish to thank Yu Cao, Yvonne Fitz, and LaShon Middleton for their expert technical support. This research was supported by the Intramural Research Program of the NIH Clinical Center, National Institutes of Health. The authors have no conflict of interest to report.
Footnotes
Drs. Cui and Besch contributed equally to this work.
Contributor Information
Xizhong Cui, National Institutes of Health Clinical Center, National Institutes of Health, Critical Care Medicine Department, Bethesda MD, USA.
Virginia Besch, National Institutes of Health Clinical Center, National Institutes of Health, Department of Anesthesia and Surgical Services, 10 Center Drive, Building 10, Room 2C624, MSC-1512, Bethesda 20892-1512, MD, USA.
Alfia Khaibullina, National Institutes of Health Clinical Center, National Institutes of Health, Department of Anesthesia and Surgical Services, 10 Center Drive, Building 10, Room 2C624, MSC-1512, Bethesda 20892-1512, MD, USA.
Adrienne Hergen, National Institutes of Health Clinical Center, National Institutes of Health, Department of Anesthesia and Surgical Services, 10 Center Drive, Building 10, Room 2C624, MSC-1512, Bethesda 20892-1512, MD, USA.
Martha Quezado, National Cancer Institute, National Institutes of Health, Laboratory of Pathology, Bethesda MD, USA.
Peter Eichacker, National Institutes of Health Clinical Center, National Institutes of Health, Critical Care Medicine Department, Bethesda MD, USA.
Zenaide M. N. Quezado, National Institutes of Health Clinical Center, National Institutes of Health, Department of Anesthesia and Surgical Services, 10 Center Drive, Building 10, Room 2C624, MSC-1512, Bethesda 20892-1512, MD, USA
References
- 1.Albuszies G, Vogt J, Wachter U, Thiemermann C, Leverve XM, Weber S, Georgieff M, Radermacher P, Barth E. The effect of iNOS deletion on hepatic gluconeogenesis in hyperdynamic murine septic shock. Intensive Care Med. 2007;33:1094–1101. doi: 10.1007/s00134-007-0638-7. [DOI] [PubMed] [Google Scholar]
- 2.Siegemund M, van Bommel J, Schwarte LA, Studer W, Girard T, Marsch S, Radermacher P, Ince C. Inducible nitric oxide synthase inhibition improves intestinal microcirculatory oxygenation and CO2 balance during endotoxemia in pigs. Intensive Care Med. 2005;31:985–992. doi: 10.1007/s00134-005-2664-7. [DOI] [PubMed] [Google Scholar]
- 3.Hollenberg SM, Broussard M, Osman J, Parrillo JE. Increased microvascular reactivity and improved mortality in septic mice lacking inducible nitric oxide synthase. Circ Res. 2000;86:774–778. doi: 10.1161/01.res.86.7.774. [DOI] [PubMed] [Google Scholar]
- 4.Ullrich R, Scherrer-Crosbie M, Bloch KD, Ichinose F, Nakajima H, Picard MH, Zapol WM, Quezado ZM. Congenital deficiency of nitric oxide synthase 2 protects against endotoxin-induced myocardial dysfunction in mice. Circulation. 2000;102:1440–1446. doi: 10.1161/01.cir.102.12.1440. [DOI] [PubMed] [Google Scholar]
- 5.Cobb JP, Hotchkiss RS, Swanson PE, Chang K, Qiu Y, Laubach VE, Karl IE, Buchman TG. Inducible nitric oxide synthase (iNOS) gene deficiency increases the mortality of sepsis in mice. Surgery. 1999;126:438–442. [PubMed] [Google Scholar]
- 6.Ichinose F, Hataishi R, Wu JC, Kawai N, Rodrigues AC, Mallari C, Post JM, Parkinson JF, Picard MH, Bloch KD, Zapol WM. A selective inducible NOS dimerization inhibitor prevents systemic, cardiac, and pulmonary hemodynamic dysfunction in endotoxemic mice. Am J Physiol Heart Circ Physiol. 2003;285:H2524–2530. doi: 10.1152/ajpheart.00530.2003. [DOI] [PubMed] [Google Scholar]
- 7.Connelly L, Madhani M, Hobbs AJ. Resistance to endotoxic shock in endothelial nitric-oxide synthase (eNOS) knock-out mice: a proinflammatory role for eNOS-derived no in vivo. J Biol Chem. 2005;280:10040–10046. doi: 10.1074/jbc.M411991200. [DOI] [PubMed] [Google Scholar]
- 8.Yamashita T, Kawashima S, Ohashi Y, Ozaki M, Ueyama T, Ishida T, Inoue N, Hirata K, Akita H, Yokoyama M. Resistance to endotoxin shock in transgenic mice overexpressing endothelial nitric oxide synthase. Circulation. 2000;101:931–937. doi: 10.1161/01.cir.101.8.931. [DOI] [PubMed] [Google Scholar]
- 9.Ichinose F, Buys ES, Neilan TG, Furutani EM, Morgan JG, Jassal DS, Graveline AR, Searles RJ, Lim CC, Kaneki M, Picard MH, Scherrer-Crosbie M, Janssens S, Liao R, Bloch KD. Cardiomyocyte-specific overexpression of nitric oxide synthase 3 prevents myocardial dysfunction in murine models of septic shock. Circ Res. 2007;100:130–139. doi: 10.1161/01.RES.0000253888.09574.7a. [DOI] [PubMed] [Google Scholar]
- 10.Quezado Z, Besch V, Hergen A, Rivas R, Gerstenberger E, Cui X, Fitz Y, Eichacker P. Neuronal nitric oxide synthase (NOS1) is protective during bacterial peritonitis and sepsis in mice. Am J Resp Crit Care Med. 2003;167:A553. [Google Scholar]
- 11.Saini R, Patel S, Saluja R, Sahasrabuddhe AA, Singh MP, Habib S, Bajpai VK, Dikshit M. Nitric oxide synthase localization in the rat neutrophils: immunocytochemical, molecular, and biochemical studies. J Leukoc Biol. 2006;79:519–528. doi: 10.1189/jlb.0605320. [DOI] [PubMed] [Google Scholar]
- 12.Danson EJ, Choate JK, Paterson DJ. Cardiac nitric oxide: emerging role for nNOS in regulating physiological function. Pharmacol Ther. 2005;106:57–74. doi: 10.1016/j.pharmthera.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg SS, Ouyang J, Zhao X, Giles TD. Human and rat neutrophils constitutively express neural nitric oxide synthase mRNA. Nitric Oxide. 1998;2:203–212. doi: 10.1006/niox.1998.0176. [DOI] [PubMed] [Google Scholar]
- 14.Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- 15.De Sanctis GT, MacLean JA, Hamada K, Mehta S, Scott JA, Jiao A, Yandava CN, Kobzik L, Wolyniec WW, Fabian AJ, Venugopal CS, Grasemann H, Huang PL, Drazen JM. Contribution of nitric oxide synthases 1, 2, and 3 to airway hyperresponsiveness and inflammation in a murine model of asthma. J Exp Med. 1999;189:1621–1630. doi: 10.1084/jem.189.10.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ortiz PA, Garvin JL. Cardiovascular and renal control in NOS-deficient mouse models. Am J Physiol Regul Integr Comp Physiol. 2003;284:R628–638. doi: 10.1152/ajpregu.00401.2002. [DOI] [PubMed] [Google Scholar]
- 17.Saraiva RM, Minhas KM, Raju SV, Barouch LA, Pitz E, Schuleri KH, Vandegaer K, Li D, Hare JM. Deficiency of neuronal nitric oxide synthase increases mortality and cardiac remodeling after myocardial infarction: role of nitroso-redox equilibrium. Circulation. 2005;112:3415–3422. doi: 10.1161/CIRCULATIONAHA.105.557892. [DOI] [PubMed] [Google Scholar]
- 18.Mattson DL, Meister CJ. Renal cortical and medullary blood flow responses to L-NAME and ANG II in wild-type, nNOS null mutant, and eNOS null mutant mice. Am J Physiol Regul Integr Comp Physiol. 2005;289:R991–997. doi: 10.1152/ajpregu.00207.2005. [DOI] [PubMed] [Google Scholar]
- 19.Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 20.Ward ME, Toporsian M, Scott JA, Teoh H, Govindaraju V, Quan A, Wener AD, Wang G, Bevan SC, Newton DC, Marsden PA. Hypoxia induces a functionally significant and translationally efficient neuronal NO synthase mRNA variant. J Clin Invest. 2005;115:3128–3139. doi: 10.1172/JCI20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lefer DJ, Jones SP, Girod WG, Baines A, Grisham MB, Cockrell AS, Huang PL, Scalia R. Leukocyte–endothelial cell interactions in nitric oxide synthase-deficient mice. Am J Physiol. 1999;276:H1943–1950. doi: 10.1152/ajpheart.1999.276.6.H1943. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg SS, Ouyang J, Zhao X, Parrish C, Nelson S, Giles TD. Effects of ethanol on neutrophil recruitment and lung host defense in nitric oxide synthase I and nitric oxide synthase II knockout mice. Alcohol Clin Exp Res. 1999;23:1435–1445. [PubMed] [Google Scholar]
- 23.Enkhbaatar P, Murakami K, Shimoda K, Mizutani A, McGuire R, Schmalstieg F, Cox R, Hawkins H, Jodoin J, Lee S, Traber L, Herndon D, Traber D. Inhibition of neuronal nitric oxide synthase by 7-nitroindazole attenuates acute lung injury in an ovine model. Am J Physiol Regul Integr Comp Physiol. 2003;285:R366–372. doi: 10.1152/ajpregu.00148.2003. [DOI] [PubMed] [Google Scholar]
- 24.Alexander HR, Doherty GM, Fraker DL, Block MI, Swedenborg JE, Norton JA. Human recombinant interleukin-1 alpha protection against the lethality of endotoxin and experimental sepsis in mice. J Surg Res. 1991;50:421–424. doi: 10.1016/0022-4804(91)90018-h. [DOI] [PubMed] [Google Scholar]
- 25.Ichinose F, Mi WD, Miyazaki M, Onouchi T, Goto T, Morita S. Lack of correlation between the reduction of sevoflurane MAC and the cerebellar cyclic GMP concentrations in mice treated with 7-nitroindazole. Anesthesiology. 1998;89:143–148. doi: 10.1097/00000542-199807000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Bush MA, Pollack GM. Pharmacokinetics and protein binding of the selective neuronal nitric oxide synthase inhibitor 7-nitroindazole. Biopharm Drug Dispos. 2000;21:221–228. doi: 10.1002/bdd.230. [DOI] [PubMed] [Google Scholar]
- 27.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li E, Zhou P, Singer SM. Neuronal nitric oxide synthase is necessary for elimination of Giardia lamblia infections in mice. J Immunol. 2006;176:516–521. doi: 10.4049/jimmunol.176.1.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.May MJ, Ghosh S. Signal transduction through NF-kappa B. Immunol Today. 1998;19:80–88. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- 30.Togashi H, Sasaki M, Frohman E, Taira E, Ratan RR, Dawson TM, Dawson VL. Neuronal (type I) nitric oxide synthase regulates nuclear factor kappaB activity and immunologic (type II) nitric oxide synthase expression. Proc Natl Acad Sci U S A. 1997;94:2676–2680. doi: 10.1073/pnas.94.6.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qu XW, Wang H, De Plaen IG, Rozenfeld RA, Hsueh W. Neuronal nitric oxide synthase (NOS) regulates the expression of inducible NOS in rat small intestine via modulation of nuclear factor kappa B. FASEB J. 2001;15:439–446. doi: 10.1096/fj.99-0343com. [DOI] [PubMed] [Google Scholar]
- 32.Quezado Z, Parent C, Karzai W, Depietro M, Natanson C, Hammond W, Danner RL, Cui X, Fitz Y, Banks SM, Gerstenberger E, Eichacker PQ. Acute G-CSF therapy is not protective during lethal E. coli sepsis. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1177–1185. doi: 10.1152/ajpregu.2001.281.4.R1177. [DOI] [PubMed] [Google Scholar]
- 33.Freeman BD, Quezado Z, Zeni F, Natanson C, Danner RL, Banks S, Quezado M, Fitz Y, Bacher J, Eichacker PQ. rG-CSF reduces endotoxemia and improves survival during E. coli pneumonia. J Appl Physiol. 1997;83:1467–1475. doi: 10.1152/jappl.1997.83.5.1467. [DOI] [PubMed] [Google Scholar]