Abstract
Aims
The origin of the pubovisceral muscle (PVM) from the pubic bone is known to be at elevated risk for injury during difficult vaginal births. We examined the anatomy and histology of its enthesial origin to classify its type and see if it differs from appendicular entheses.
Methods
Parasagittal sections of the pubic bone, PVM enthesis, myotendinous junction and muscle proper were harvested from five female cadavers (51 - 98 years). Histological sections were prepared with hematoxylin and eosin, Masson’s trichrome, and Verhoeff-Van Gieson stains. The type of enthesis was identified according to a published enthesial classification scheme. Quantitative imaging analysis was performed in sampling bands 2 mm apart along the enthesis to determine its cross-sectional area and composition.
Results
The PVM enthesis can be classified as a fibrous enthesis. The PVM muscle fibers terminated in collagenous fibers that insert tangentially onto the periosteum of the pubic bone for the most part. Sharpey’s fibers were not observed. In a longitudinal cross-section, the area of the connective tissue and muscle becomes equal approximately 8 mm from the pubic bone.
Conclusion
The PVM originates bilaterally from the pubic bone via fibrous entheses whose collagen fibers arise tangentially from the periosteum of the pubic bone.
Keywords: pubovisceral muscle, avulsion injury, enthesis, myotendinous junction, elastic fiber, levator ani, pelvic floor dysfunction
INTRODUCTION
An enthesis is the specialized arrangement of connective tissue that comprises the attachment of a muscle to bone while permitting the muscle’s fibers to be organized in appropriate pennation. An enthesis may be categorized into a fibrous enthesis or a fibrocartilaginous enthesis according to Benjamin et al.,1 based on the characteristics of the tissue at the bone-tendon interface. A fibrous enthesis is composed with mainly dense fibrous connective tissues and can be further divided into two categories – periosteal and bony, depending on the place to which the tendon attaches. On the other hand, a fibrocartilaginous enthesis appears in the area which is subject to compression and shows two more additional zones between connective tissue and bone – uncalcified fibrocartilage and calcified fibrocartilage. Entheses are often the sites of musculoskeletal overuse injuries that include tennis elbow and jumper’s knee.1 Several different types of entheses have been described within a classification system.1-5
The focus of the present paper is the region of the pubovisceral muscle (PVM, also known as the pubococcygeal muscle as listed in Terminologia Anatomica) nearest its origin from the pubic bone,6 because it is subject to increased risk for injury during difficult vaginal births.7,8 This stretch-related injury, which occurs during labor, is thought to be due to the PVM having to stretch to over three times its original length,9 or more than twice the value that striated muscle can withstand without injury in non-pregnant individuals.10 Such injuries have been implicated in causing pelvic organ prolapse, a common female pelvic floor impairment that is a common cause of surgical treatment later in life.11,12
Several attributes of an enthesis can help protect against injury at the junction of the three structures having differing material properties, striated muscle, collagenous connective tissue, and bone. First, dense embedded fibrous fasciae and/or the periosteum can help dissipate stress concentrations and mitigate against the risk of tensile failure or tearing.4 Second, the enthesis can be anchored to the bone via Sharpey’s fibers, which perforate the superficial lamellae of the bone.2 Thirdly, elastic fibers could play a role in helping to minimize the effect of abrupt increases in load by deforming under load.13 Finally, certain morphological features, such as a flaring near the bone, could systematically reduce high tensile stresses and/or strains by adding greater cross-sectional area in regions prone to injury.3 However, to our knowledge the literature contains no descriptions of the morphology or histology of the PVM enthesis.
The first goal of this paper, therefore, was to classify the PVM enthesis according to a formal enthesial classification system. A second goal was to test the hypothesis that one or more of the above stress-reduction mechanisms would be observed in the PVM enthesis.
MATERIALS AND METHODS
Five female cadavers were dissected to examine the detailed morphology and histology of the origin of the PVM at the pubic bone (Fig. 1). The cadaveric specimens ranged in age from 51 to 98 years old (mean 77 years). All the specimens appeared to be parous from inspection of the perineal body and cervix when present. None showed signs of pelvic floor muscle damage associated with maternal birth, as evaluated by a clinician anatomist with experience in evaluating the levator ani muscle (JOLD). Characteristics such as the detailed morphological and structural relationships around the PVM and its attachment area, and the specific fiber orientation and connection of the PVM enthesis were examined before the pubic bones and the attached muscles were removed. The fiber direction in the region where PVM injury is known to occurs7,14,15 was determined by visual inspection, using magnification when necessary.
Fig. 1.
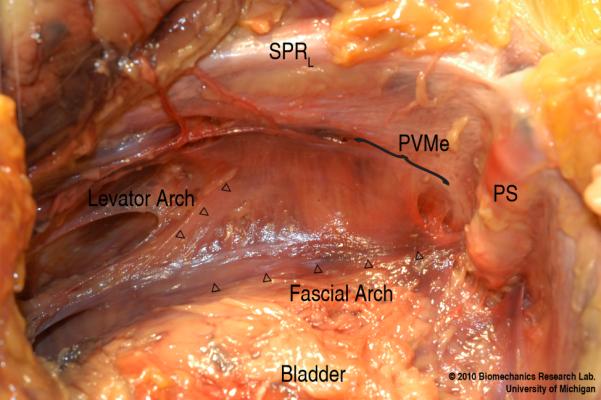
View looking down towards the left pelvic sidewall showing the location of the PVM enthesis (PVMe, below bracket). SPRL denotes superior pelvic ramus (left); and PS, pubic symphysis.
Smaller samples were removed for histochemical processing by excising them parallel to the PVM fiber direction. The samples were fixed in 10% neutral buffered formalin, decalcified in 10% formic acid and dehydrated in 70% ethanol. Paraffin embedding was then applied followed by serial sectioning of samples less than 7 μm in thickness. Sections were stained with hematoxylin and eosin, Masson’s trichrome, and Verhoeff-Van Gieson according to standard procedures for selected slides for analyzing muscle/connective tissue composition and elastic fiber pattern. A ScanScope XT digital slide scanner (Aperio Technologies, Vista, CA) and a Super COOLSCAN 5000 ED film scanner (Nikon, Shinjuku, Tokyo, Japan) were used to convert the slides into digital images.
For the quantitative observation, custom designed software written in Matlab (The MathWorks, Natick, MA) was developed to allow the interactive selection of the quadrangular sampling bands to be assessed. It permitted analysis of changes within the muscles and connective tissue along the PVM enthesis (Fig. 2) at 2 mm intervals, resulting in 6-7 sampled locations depending upon the length of the enthesis in each sample. The location where no muscle was present was defined as the origin of the measurement. At each location, bands with 1 mm width were placed perpendicular to the PVM line-of-action and used to collect pixel image information in the sampling bands. Color-based segmentation using the k-means clustering technique was applied to the trichrome digital images of the specimens to distinguish the muscle (stained in red) from the connective tissue (stained in blue). Descriptive statistics were generated as mean and SD.
Fig. 2.
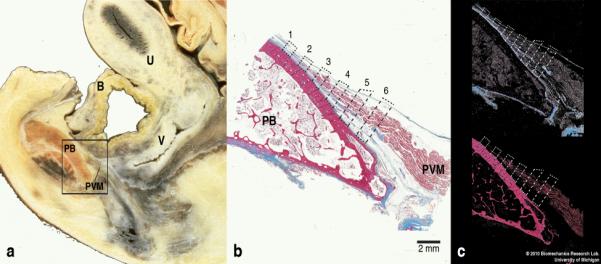
(a) Lateral view of a parasagittal section through a female cadaveric pelvis for orientation. The rectangular box shows an area that includes the pubic bone and the origin of the pubovisceral muscle on which detailed histological and quantitative analyses were performed. (b) The pubovisceral muscle origin showing the sampling bands (dashed quadrangles) at 6 different locations along the pubovisceral muscle, where color-based segmentation was conducted. (Masson’s trichrome stain, scale bar = 2 mm). [Note: this specimen is from a different cadaver from that shown in (a)]. (c) After the image is filtered by color, the pre-defined sampling bands were quantitatively analyzed to compute unit area and relative composition of the collagenous tissue (blue; top image) and the muscle (red, bottom image). In this and the following images, PVM denotes the pubovisceral muscle; OIM, obturator internus muscle; PB, pubic bone; B, bladder; U, uterus; and V, vagina.
RESULTS
Fig. 3 (a) and (b) illustrate geometric relation of the PVM origin with its encompassing structures. The striated PVM muscle fibers mainly insert tangentially onto the periosteum of the superior pubic rami and the posteroinferior margin of the body of the pubic bone (Fig. 3d). The width of the attachment extends over a length of about 30 mm. The length of the tendinous fibers linking the PVM to the pubic bone ranges from tens of micrometers to a few millimeters (Fig. 3c). The PVM is bounded by the superior fascia of the pelvic diaphragm on both its ventral and dorsal surfaces until each merges with the periosteum 5 to 10 mm apart. No flaring of the PVM enthesis was observed in the sagittal plane as it attached onto the pubic bone. The fiber direction in the superficial region of the PVM takes runs perpendicular to the arcus tendineus fascia pelvis, passing lateral to the tendinous arch on its way to attach to the perineal body. Immediately lateral to the PVM enthesis lies the medial origin of the obturator internus muscle, the fiber direction of which is perpendicular to that of the PVM.
Fig. 3.
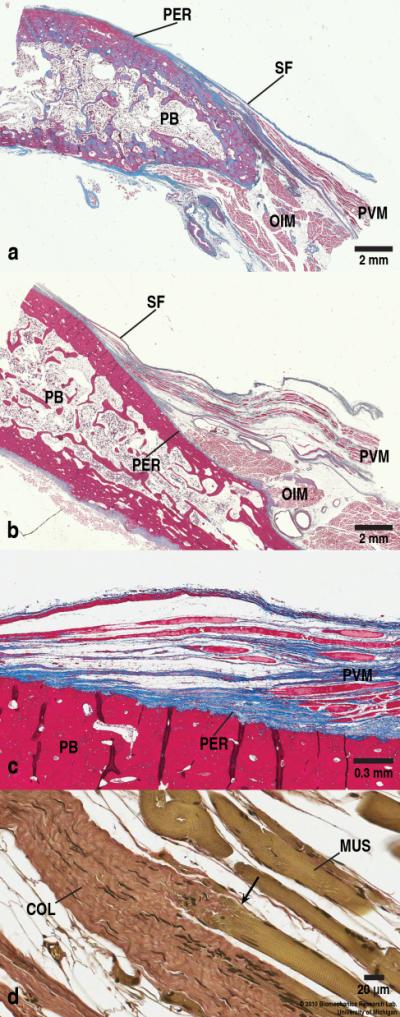
Two examples of entheses: (a) Right parasagittal section through the PVM origin on the pubic bone 2-3 cm from the midline taken from the superior pubic ramus area. The PVM emanates tangentially from the pubic bone and the OIM lies caudally to the PVM and also originates from the PB with its fiber direction being perpendicular to that of the PVM. (Masson’s trichrome stain, scale bars = 2 mm). (b) Another parasagittal section through the PVM origin on the pubic bone 1-2 cm from the midline. Note the varied morphology of the pubic bone due to different harvesting locations as well as different cadaveric samples. (Masson’s trichrome stain, scale bars = 2 mm). (c) Higher magnification view of the PVM origin (in b) showing the enthesis. The collagenous fibers from the PVM blend in with the periosteum of the PB, and can sometimes be seen to insert on irregularities on the surface of the PB. Notice the absence of Sharpey’s fibers penetrating into the bone, and the presence of the periosteum. The vertical dark lines in the PB are artifacts due to tissue folding during the microtome sectioning. Also note that the image is slightly rotated counterclockwise. (Masson’s trichrome stain, Scale bar = 0.3 mm). (d) Myo-tendinous junction (arrow) of the PVM (green) and the collagenous tissue (red). (Verhoeff-Van Gieson stain, scale bar = 20 μm). SF denotes superior fascia of pelvic diaphragm, and PER, periosteum. In this and later figures, COL denotes collagenous fibers, and MUS, muscle fibers.
As can be seen in Fig. 3 (c), the PVM enthesis seems to be a fibrous enthesis rather than a fibrocartilaginous enthesis, because neither the fibrocartilaginous zone nor the tidemark - a basophilic line separating the fibrocartilage into the calcified zone and the uncalcified zone - was present. The PVM attaches to the pubic bone at an acute angle. While the superficial layer of the collagenous fibers blends in with the periosteum of the pubic bone, penetration of the deeper collagen through the periosteum directly to the pubic bone was not seen. The thickness of the periosteum in the region of the PVM enthesis does not appear to be different than elsewhere on the pubic bone. No Sharpey’s fibers extending into the bone itself were observed anywhere in the enthesial region.
Fig. 4 demonstrates the change in composition of the muscle fibers and the connective tissue in the PVM in the sampling quadrangles. Moving from the muscle towards the bone, the unit area occupied by the muscle fibers gradually decreases as it goes towards the bone. The connective tissue area shows a lesser change than the muscle fiber does. It is notable that the areas of the connective tissue and the muscle become equal in location 5, which is approximately 8 mm apart from the origin. The table also shows that muscular composition increases from about 2 % at the origin to approximately 60 % at the last sampling band.
Fig. 4.
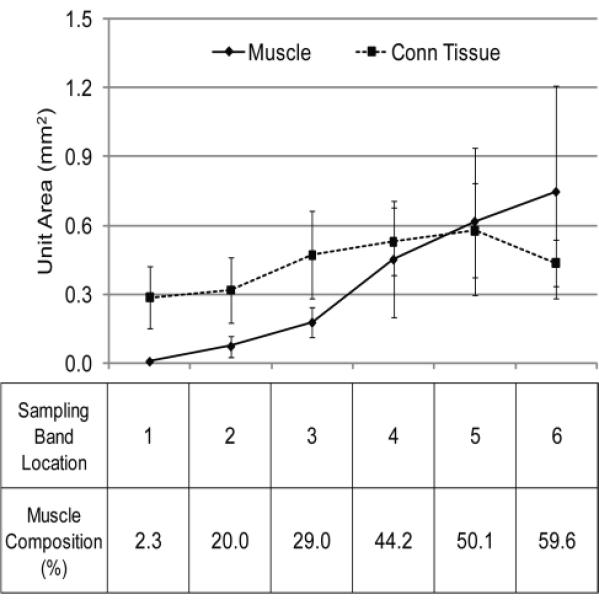
Longitudinal distribution of the muscle and the connective tissue at the sampling bands along the PVM from its origin. Values are mean (bars indicate SD) areas within the 1 mm-wide sampling bands taken normal to the line-of-action of the PVM. The table shows the composition of the muscle in each location. The unit area of the muscle matches that of the connective tissue at a point approximately 8 mm (location 5) from the pubic bone origin.
Fig. 5 shows the myotendinous junction stained with Verhoeff-Van Gieson staining method, which renders elastic fibers black. The elastic fibers appear to be settled at widely spaced intervals. They are found in very small amounts in fibrillar form as they are present in tendons in general.
Fig. 5.
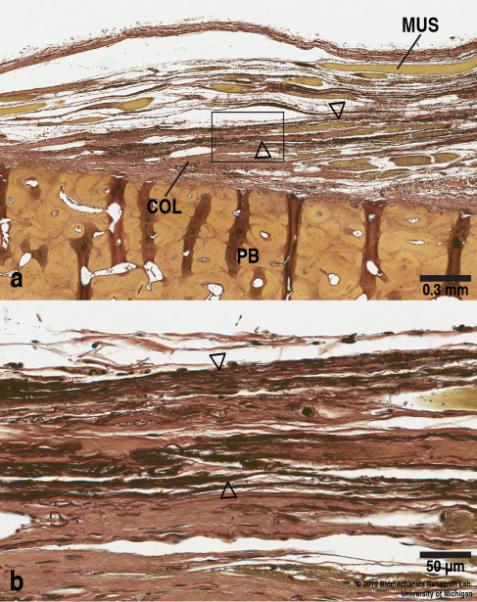
(a) Elastic fibers (stained in black, arrowhead) are sparsely distributed in a fibrillar form along the collagenous fibers (red). (Verhoeff-Van Gieson stain, Scale bar = 0.3 mm). (b) Higher power view of the boxed region in (a) showing the elastic fibers in more detail. (Verhoeff-Van Gieson stain, Scale bar = 50 μm).
DISCUSSION
The current study has shown that the PVM is attached to the pubic bone by short collagenous fibers, the proximal ending of which forms a fibrous enthesis with observation of the absence of the fibrocartilaginous zones and the tidemark. Quantitative analysis demonstrates that the proportion of muscular tissue becomes equal to that of the connective tissue at location 5 about 8 mm away from the osteotendinous junction. Neither flaring of the enthesis at the muscle-bone interface nor Sharpey’s fibers penetrating into the bone was seen in enthesial region. Therefore, the hypothesis that flaring and Sharpey’s fibers form an important part of its structure was rejected. Finally, elastic fibers appear to be widely distributed along the tendinous fibers.
The anatomy of the levator ani muscle have been described by several authors and these findings have been summarized.16 The findings of our research extends earlier studies on the PVM and its surrounding area6,17,18 by describing the detailed histology of the PVM attachment to the pubic bone. The overall morphological features of the levator hiatus including the PVM have been demonstrated with the help of magnetic resonance as well as three-dimensional ultrasound.6,8 These studies show the loss of muscle that occurs after vaginal birth, however, lack the resolution needed to examine the precise details of muscle fiber connection to the bone. The present study is more concerned with the nature of the normal muscle’s origin with a view to better understanding the structure that is likely to be involved in the injury.
The PVM enthesis arises tangentially from the periosteum of the pubic bone to which it is connected through a fibrous enthesis in the manner of the insertion of the pronator teres on the mid-shaft of the radius.3 Taking into account that form is derived from structure and function,3,4 the absence of the tidemark and the fibrocartilaginous zones in the PVM enthesis might mean that tensional loading rather than compressive loading is predominant in this area. This makes sense considering that the PVM is subjected to considerable posteroinferior stretch during vaginal delivery.9
There is growing knowledge concerning injury to the portion of the levator ani muscle that is attached to the pubis. Avulsion injury is associated with the development of pelvic organ prolapse,11,12 and arises as a result of vaginal birth.7,8 In addition the nature of the levator ani damage itself may not be the only consideration. Recent observations have disclosed “architectural distortion”, which may be defined as a characteristic abnormal appearance of the vagina on axial magnetic resonant scans that is associated with an increased occurrence of pelvic organ prolapse compared to women who have muscle injury but no distortion.19 It is possible that the architectural distortion signifies that connective tissues and the muscles around the PVM, which are responsible for pelvic organ support, become defected or even detached at the time of a birth-related PVM avulsion injury. These elements are interconnected to each other by the periosteum of the pelvis, the arcus tendineus fascia pelvis, the arcus tendineus levator ani, and other fasciae. Therefore, if one of these structures is affected by abnormal external loading, the input work can be transmitted to others.
We acknowledge several limitations in this research. First, the sample size is modest and the samples were likely parous individuals who, although not having any distortion or injury to a trained eye, might have some changes secondary to birth. Therefore, the results of the present study might have affected by this possible histological changes consequent upon vaginal delivery. Observing younger nulliparous women would be ideal but young nulliparous cadavers are so rare in anatomical donation programs as to prevent analysis of more than one or two specimens. Second, the sample age ranges from 51 years and up, so one cannot necessarily extrapolate the results to younger individuals. Thirdly, more detailed histological descriptions are needed including the different types of collagens, decorin, biglycan, aggrecan and other constituents that may form this particular enthesis.20 Fourthly, considering the size of elastic fibers being in the order of a few micrometers, observation through a scanning electron microscope could give more comprehensive images of these elastic fibers along the collagenous tissue.
The enthesial region of the PVM lies in the area where avulsion injury during the second stage of labor occurs.8,21 The details of the exact injury mechanism have not yet been fully elucidated. This research provides a detailed picture of the structural basis for the attachment of the muscle to the bone and suggests hypotheses helpful in predicting that failure location. It seems likely that it would occur in the muscular portion of the enthesis before the cross-sectional area of the enthesis becomes big enough to endure the external loads. This is because collagenous tissues are known to be approximately two orders stronger than striated muscle.22,23 In addition, they should be more resistant to stretch-related injuries than muscle, because they are highly aligned in the enthesis. Now that the anatomical details of this region are apparent, research can progress to add further detail to our growing understanding. For example, one can exercise the detailed morphology found in this study to create micromechanical models of the PVM enthesis and explore the effect of the enthesis on this injury-prone region. This work may also be extended and compared to the connective tissue attachment of the levator ani muscle along the pelvic sidewall.
CONCLUSION
The pubovisceral portion of the levator ani muscle originates tangentially from the pubic bone through fibrous entheses. Collagenous fibers, which lie between the pubovisceral muscle and the pubic bone and connect each other, mainly arise from the periosteum of the pubic bone. In a longitudinal cross-section, the amount of the connective tissue and muscular tissue becomes equal at approximately 8 mm from the pubic bone.
ACKNOWLEDGMENTS
We would like to thank the histology core facility at the University of Michigan, School of Dentistry for the histological services. Digital conversion of the slides was performed in the Microscopy and Image-analysis Laboratory (MIL), Department of Cell & Developmental Biology and the Virtual Microscopy Facility, Department of Pathology at the University of Michigan with the assistance of Shelley Almburg and Ronald Craig, PhD.
FUNDING National Institutes of Health Office of Research on Women’s Health (ORWH) grant, “Specialized Center of Research (SCOR) on Sex and Gender Factors Affecting Women’s Health,” P50 HD044406.
Footnotes
This study was presented at the 31st American Urogynecologic Society Annual Scientific Meeting, Long Beach, California, USA, September 2010.
REFERENCES
- 1.Benjamin M, Kumai T, Milz S, Boszczyk BM, Boszczyk AA, Ralphs JR. The skeletal attachment of tendons--tendon ‘entheses’. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:931–945. doi: 10.1016/s1095-6433(02)00138-1. [DOI] [PubMed] [Google Scholar]
- 2.Woo SL, Maynard JA, Butler DL, Lyon R, Torzilli P, Akeson WH, et al. Ligament, tendon, and joint capsule insertions to bone. In: Woo SL, Buckwalter JA, editors. Injury and repair of the musculoskeletal soft tissues. American Academy of Orthopaedic Surgeons. Symposium; Savannah, GA: 1987. pp. 133–166. [Google Scholar]
- 3.Benjamin M, Toumi H, Ralphs JR, Bydder G, Best TM, Milz S. Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J Anat. 2006;208:471–490. doi: 10.1111/j.1469-7580.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin M, Kaiser E, Milz S. Structure-function relationships in tendons: a review. J Anat. 2008;212:211–228. doi: 10.1111/j.1469-7580.2008.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.François RJ, Braun J, Khan MA. Entheses and enthesitis: a histopathologic review and relevance to spondyloarthritides. Curr Opin Rheumatol. 2001;13:255–264. doi: 10.1097/00002281-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Margulies RU, Huebner M, DeLancey JO. Origin and insertion points involved in levator ani muscle defects. Am J Obstet Gynecol. 2007;196:251.e1–251.e5. doi: 10.1016/j.ajog.2006.10.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kearney R, Miller JM, Ashton-Miller JA, DeLancey JO. Obstetric factors associated with levator ani muscle injury after vaginal birth. Obstet Gynecol. 2006;107:144–149. doi: 10.1097/01.AOG.0000194063.63206.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietz HP, Lanzarone V. Levator trauma after vaginal delivery. Obstet Gynecol. 2005;106:707–712. doi: 10.1097/01.AOG.0000178779.62181.01. [DOI] [PubMed] [Google Scholar]
- 9.Lien K, Mooney B, DeLancey JO, Ashton-Miller JA. Levator ani muscle stretch induced by simulated vaginal birth. Obstet Gynecol. 2004;103:31–40. doi: 10.1097/01.AOG.0000109207.22354.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks SV, Zerba E, Faulkner JA. Injury to muscle fibres after single stretches of passive and maximally stimulated muscles in mice. J Physiol. 1995;488:459–469. doi: 10.1113/jphysiol.1995.sp020980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeLancey JO, Morgan DM, Fenner DE, Kearney R, Guire K, Miller JM, et al. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;109:295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 12.Dietz HP, Simpson JM. Levator trauma is associated with pelvic organ prolapse. BJOG. 2008;115:979–984. doi: 10.1111/j.1471-0528.2008.01751.x. [DOI] [PubMed] [Google Scholar]
- 13.Nordin M, Lorenz T, Campello M. Biomechanics of tendons and ligaments. In: Nordin M, Lorenz T, editors. Basic biomechanics of the musculoskeletal system. 3rd ed Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 102–125. [Google Scholar]
- 14.Margulies RU, Hsu Y, Kearney R, Stein TA, Umek WH, DeLancey JO. Appearance of the levator ani muscle subdivisions in magnetic resonance images. Obstet Gynecol. 2006;107:1064–1069. doi: 10.1097/01.AOG.0000214952.28605.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shobeiri SA, Chesson RR, Gasser RF. The internal innervation and morphology of the human female levator ani muscle. Am J Obstet Gynecol. 2008;199:686.e1–686.e6. doi: 10.1016/j.ajog.2008.07.057. [DOI] [PubMed] [Google Scholar]
- 16.Kearney R, Sawhney R, DeLancey JO. Levator ani muscle anatomy evaluated by origin-insertion pairs. Obstet Gynecol. 2004;104:168–173. doi: 10.1097/01.AOG.0000128906.61529.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawson JO. Pelvic anatomy. I. Pelvic floor muscles. Ann R Coll Surg Engl. 1974;54:244–252. [PMC free article] [PubMed] [Google Scholar]
- 18.Albright TS, Gehrich AP, Davis GD, Sabi FL, Buller JL. Arcus tendineus fascia pelvis: A further understanding. Am J Obstet Gynecol. 2005;193:677–681. doi: 10.1016/j.ajog.2005.02.129. [DOI] [PubMed] [Google Scholar]
- 19.Huebner M, Margulies RU, DeLancey JO. Pelvic architectural distortion is associated with pelvic organ prolapse. Int Urogynecol J. 2008;19:863–867. doi: 10.1007/s00192-007-0546-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomopoulos S, Williams GR, Gimbel JA, Favata M, Soslowsky LJ. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res. 2003;21:413–419. doi: 10.1016/S0736-0266(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 21.Dietz HP, Gillespie AV, Phadke P. Avulsion of the pubovisceral muscle associated with large vaginal tear after normal vaginal delivery at term. Aust N Z J Obstet Gynaecol. 2007;47:341–344. doi: 10.1111/j.1479-828X.2007.00748.x. [DOI] [PubMed] [Google Scholar]
- 22.Brooks SV, Faulkner JA. The magnitude of the initial injury induced by stretches of maximally activated muscle fibres of mice and rats increases in old age. J Physiol. 1996;497:573–580. doi: 10.1113/jphysiol.1996.sp021790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woo SL, Levine RE. Ligament, tendon and fascia. In: Black J, Hastings G, editors. Handbook of biomaterial properties. Chapman & Hall; London, UK: 1998. pp. 59–65. [Google Scholar]


