Abstract
Nitrate and nitrite are precursors in the formation of N-nitroso compounds. We recently found a 40% increased risk of NHL with higher dietary nitrite intake and significant increases in risk for follicular and T-cell lymphoma. It is possible that these compounds also affect NHL prognosis by enhancing cancer progression in addition to development by further impairing immune system function. To test the hypothesis that nitrate and nitrite intake affects NHL survival, we evaluated the association in study participants that have been followed post-disease diagnosis in a population-based case-control study among women in Connecticut. We did not observe a significant increasing trend of mortality for NHL overall or by subtype for nitrate or nitrite intake for deaths from NHL or death from any cause, although a borderline significant protective trend was observed for follicular lymphoma with increasing nitrate intake. We did not identify a difference in overall survival for nitrate (P = 0.39) or for nitrite (P = 0.66) or for NHL specific survival for nitrate (P = 0.96) or nitrite (P = 0.17). Thus, our null findings do not confer support for the possibility that dietary nitrate and nitrite intake impacts NHL survival by promoting immune unresponsiveness.
INTRODUCTION
Although the median survival for non-Hodgkin lymphoma (NHL) is approximately 10 yr, the course of the disease is highly variable, progressing slowly for indolent and very rapidly for aggressive tumors (1). Studies have shown that NHL survival patterns vary by subtype (2,3), suggesting different prognostic risk factors for NHL histological subtypes. Nitrate and nitrite are precursors in the formation of N-nitroso compounds (4), a class of genotoxic compounds, most of which are animal carcinogens that can act systemically and are commonly found in the diet and in contaminated drinking water (5–7). Nitrate is a natural component of plants and is found at high concentrations in leafy vegetables, such as lettuce and spinach, and some root vegetables, such as beets (4). Nitrite and nitrate salts are added to cured meats such as bacon, hot dogs, and ham to prevent the growth of spore-forming bacterium as well as to add color and flavor (6).
We recently found a 40% increased risk of NHL with higher dietary nitrite intake and significant increases in risk for follicular and T-cell lymphoma (8). It is possible that these compounds also affect NHL prognosis by enhancing cancer progression in addition to development by further impairing immune system function (9), although previous investigation in this study population showed improved survival for those who report high prediagnostic vegetable intake (10). However, to date, the impact of nitrate and nitrite intake on NHL survival has not been evaluated. To test the hypothesis that nitrate and nitrite intake affects NHL survival, we evaluated this association in study participants that have been followed post-disease diagnosis in a population-based case-control study among women in Connecticut.
METHODS
The study population has been described elsewhere (10). In brief, a total of 1,122 potential female NHL cases aged between 21 and 84 yr were identified between 1996 and 2000 through the Yale Comprehensive Cancer Center’s Rapid Case Ascertainment Shared Resource, a component of the Connecticut Tumor Registry (CTR). Among those cases, 167 died before they could be interviewed and 123 were excluded because of doctor refusal, previous diagnosis of cancer (excluding nonmelanoma skin cancer), or inability to speak English. Of 832 remaining eligible cases, 601 completed an in-person interview. Pathology slides or tissue blocks were obtained from the hospitals where the cases had been diagnosed. The specimens were reviewed by 2 independent study pathologists. All NHL cases were classified according to the World Health Organization (WHO) classification system (11,12). Vital status for these NHL cases was abstracted at the CTR in May–October 2008. Other follow-up information was also abstracted, including date of death, cause of death, most recent follow-up date, type and date of treatments, B-symptoms, and tumor stage. Of the 601 cases, 13 were not able to be identified in the CTR system, 13 were found to have a cancer history prior to diagnosis of NHL, and 7 had diet information missing, yielding 568 patients with NHL in the final analyses. Of these, 180 had diffuse large B-cell lymphoma; 131 had follicular lymphoma; 63 had chronic lymphocytic leukemia/small lymphocytic lymphoma; 39 had marginal zone B-cell lymphoma; 42 had T/NK-cell lymphoma; and 113 were classified as other. There were 250 deaths from any cause and 140 deaths from NHL in the study population.
Dietary intake was assessed using a mailed self-administered semiquantitative food frequency questionnaire (FFQ) developed by the Fred Hutchinson Cancer Research Center (Seattle, Washington), in which subjects were asked to characterize their usual diet in the year before being interviewed (13). The FFQ collects data on consumption frequency and portion size for approximately 120 foods, including 19 vegetables, 11 fruits, and fresh and processed meats. Participants were queried about their frequency of intake in 9 categories ranging from never to 2+ times per day for foods and never to 6+ times per day for beverages. Each line item was accompanied by 3 possible portion size categories (small, medium, or large).
We determined the nitrate and nitrite contents of the foods on the questionnaire by conducting a review of values in published literature (14,15). We included 27 studies that ranged in publication date from 1967 to 2008. The criteria for including a study with published values for nitrate or nitrite were that they focused on U.S. rather than foreign food products, as levels can vary regionally, especially for preserved meats and cheeses, as well as for some vegetables, and that the date of publication was most consistent with the timeline ascertained by the FFQ (particularly for processed meats as additives have changed in past decades). Because of the lack of current values in the literature, it was necessary to expand our search criteria to include values from other countries and published during other time periods for some foods. The identified values were prioritized within each food item according to country of origin and the year of sample collection. As available, information was abstracted regarding cooking or preservation methods, methods of laboratory analysis, number of observations in each sample, ranges of values, means, and standard deviations.
We calculated the mean of the published values for individual foods. Food-specific nitrate and nitrite values were combined using the same methodology that was used for other nutrients. Daily intakes of nitrate and nitrite were calculated by multiplying the frequency of consumption of each food and portion size by the nitrate or nitrite content of the food and summing across all food items. Intake was computed separately for animal and plant sources. We also evaluated intake of nitrate plus nitrite from processed meat sources separately, which included both red and white meat sources of sausage, luncheon meats, cold cuts, ham, and hot dogs. The median daily intake of nitrate (95.9 mg/day) and nitrite (1.1 mg/day) was used as the cutoff point for high and low intake. The major contributors to nitrate intake were lettuce (22.5%), melon (watermelon, cantaloupe, honeydew) (19.8%), and squash (11.1%), and the major contributors to nitrite intake were lunch meats (10.4%), rice and noodles (7.8%), and fresh meat (beef, pork, or lamb) (6.9%). The correlation of nitrate with vegetable intake was 0.61, with fruit intake was 0.34, with vitamin C intake was 0.43, and with vitamin D intake was 0.15.
Survival analyses were conducted for both death from any cause and death from NHL as events. We assumed the Cox proportional hazards model and estimated hazard ratios (HR) and 95% confidence intervals (95% CI) for the association of nitrate and nitrite intake quartiles with overall survival. Age (continuous), caloric intake (continuous), family history of cancer, and vitamin C were included as confounder variables in the final model. Kaplan-Meier survival curves were plotted by high and low nitrate and nitrite intake (above or below the median) for NHL overall and subtypes. We stratified our data by high and low vitamin C intake to evaluate the possibility of nitrosation inhibition. Log-rank statistics were computed to evaluate the difference in survival (16). Statistical analyses were performed using SAS, v. 9.1 (SAS Institute, Cary, NC).
RESULTS
The study population characteristics have been described elsewhere (10). During the follow-up period, 250 patients died (148 from lymphoma and 102 from other causes). Median follow-up time was 3.58 yr for the deceased and 9.07 yr for the survivors. Mean follow-up time was 4.06 yr (SD: 2.73, range: 0.33–11.01) for the deceased and 8.98 yr (SD = 1.56, range: 1.32–11.79) for the survivors. The average age was 61.6 yr; the average body mass index was 26.1 kg/m2; most participants were White (94.7%); most reported some college, college graduation, or additional higher education (57%); and less than 2% reported a family history of NHL.
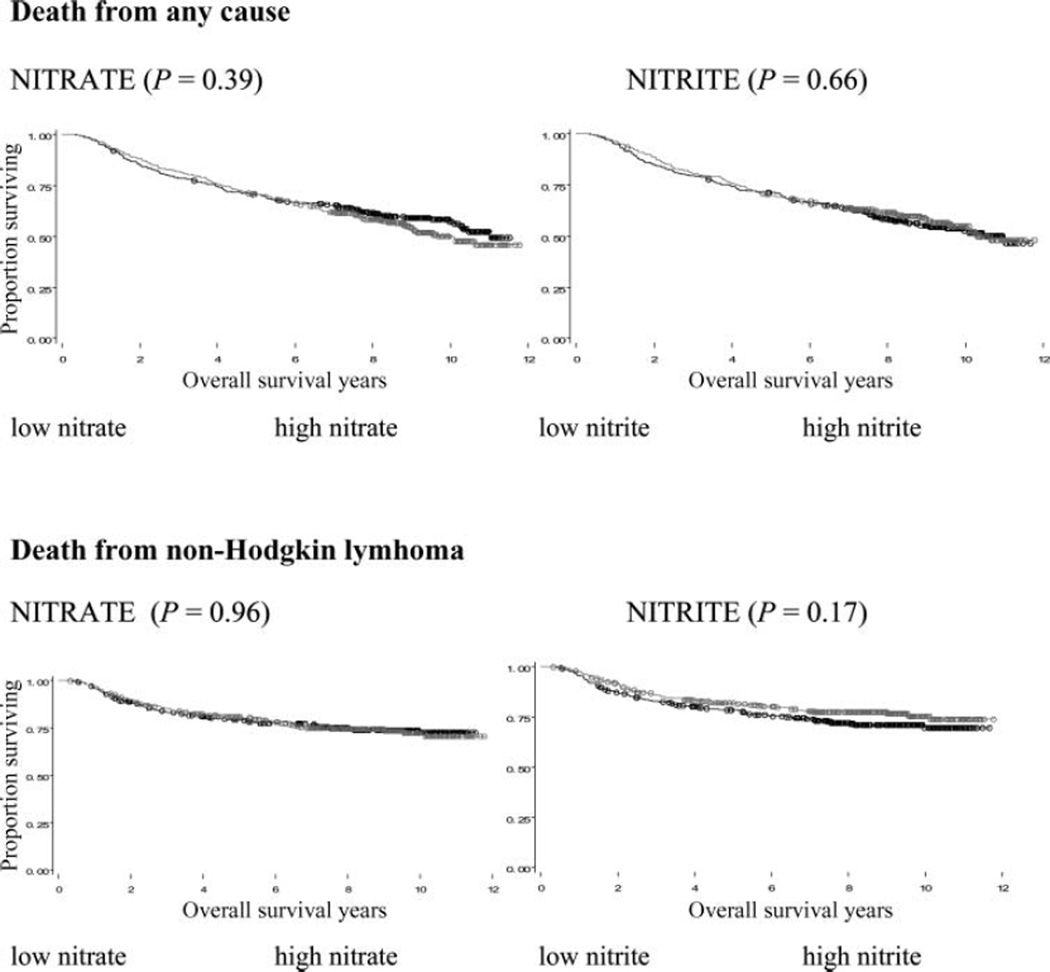
We did not observe a significant increasing trend of mortality for NHL overall or by subtype in relation to nitrate or nitrite intake for deaths from NHL or death from any cause, though a borderline significant protective trend was observed for follicular lymphoma with increasing nitrate intake (P trend = 0.06) (Table 1). We also observed a significant trend for nitrate intake and T-cell lymphoma (P trend = 0.02), but there were no cases in the highest intake quintile. We plotted the Kaplan-Meier survival curves by high and low nitrate and nitrite intake for NHL overall, but we did not identify a difference in overall survival for nitrate (P = 0.39) or for nitrite (P = 0.66) or for NHL specific survival for nitrate (P = 0.96) or nitrite (P = 0.17). The results were unchanged when we stratified by vitamin C intake (data not shown).
TABLE 1.
Multivariate adjusted* hazard ratios (HR) and 95% confidence intervals (CI) for risk of death associated with nitrate and nitrite intake quartiles among patients with non-Hodgkin lymphoma by subtype major B-cell subtypes
| Total NHL (n = 568) HR (95% CI) |
DLBCL (n = 180) HR (95% CI) |
FL (n = 131) HR (95% CI) |
CLL/SLL (n = 63) HR (95% CI) |
MZ (n = 39) HR (95% CI) |
T-cell (n = 42) HR (95% CI) |
|
|---|---|---|---|---|---|---|
| Deaths from any cause | ||||||
| Nitrate (mg/day) | ||||||
| <62.8 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 62.8 to <95.9 | 1.0 (0.7–1.4) | 1.0 (0.5–1.9) | 0.6 (0.2–1.4) | 1.0 (0.4–2.9) | 1.1 (0.2–5.0) | 0.7 (0.2–2.2) |
| 95.9 to <141.0 | 1.1 (0.7–1.6) | 1.1 (0.6–2.3) | 1.0 (0.4–2.4) | 1.2 (0.4–3.7) | 1.2 (0.1–10.8) | 0.2 (0.1–1.0) |
| ≥141.0 | 1.0 (0.7–1.5) | 1.2 (0.6–2.5) | 0.4 (0.1–1.0) | 1.3 (0.4–4.0) | 0.6 (0.1–6.2) | — |
| P for trend | 0.88 | 0.51 | 0.06 | 0.27 | 0.69 | 0.02 |
| Nitrite (mg/day) | ||||||
| <0.8 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 0.8 to <1.1 | 1.2 (0.8–1.6) | 0.9 (0.4–1.7) | 2.2 (0.8–6.0) | 0.5 (0.2–1.4) | 0.5 (0.2–1.4) | 1.6 (0.3–8.0) |
| 1.1 to <1.4 | 0.8 (0.6–1.3) | 0.9 (0.4–1.8) | 1.1 (0.4–3.2) | 0.5 (0.1–1.8) | 0.4 (0.1–2.8) | 0.2 (0.0–1.8) |
| ≥1.4 | 1.0 (0.6–1.6) | 1.5 (0.7–3.6) | 0.8 (0.2–2.9) | 0.4 (0.1–1.7) | 0.7 (0.1–4.8) | 0.4 (0.0–5.5) |
| P for trend | 0.69 | 0.73 | 0.84 | 0.81 | 0.47 | 0.16 |
| Deaths from NHL | ||||||
| Nitrate (mg/day) | ||||||
| <62.8 | 1.0 | 1.0 | 1.0 | 1.0 | — | — |
| 62.8 to <95.9 | 1.0 (0.7–1.4) | 1.0 (0.5–1.9) | 0.6 (0.2–1.4) | 1.0 (0.4–2.9) | — | — |
| 95.9 to <141.0 | 1.1 (0.7–1.6) | 1.1 (0.6–2.3) | 1.0 (0.4–2.4) | 1.2 (0.4–3.7) | — | — |
| ≥141.0 | 1.0 (0.7–1.5) | 1.2 (0.6–2.5) | 0.4 (0.1–1.0) | 1.3 (0.4–4.0) | — | — |
| P for trend | 0.88 | 0.51 | 0.06 | 0.27 | — | — |
| Nitrite (mg/day) | ||||||
| <0.8 | 1.0 | 1.0 | 1.0 | 1.0 | — | — |
| 0.8 to <1.1 | 1.2 (0.8–1.6) | 0.9 (0.4–1.7) | 2.2 (0.8–6.0) | 0.5 (0.2–1.4) | — | — |
| 1.1 to <1.4 | 0.8 (0.6–1.3) | 0.9 (0.4–1.8) | 1.1 (0.4–3.2) | 0.5 (0.1–1.8) | — | — |
| ≥1.4 | 1.0 (0.6–1.6) | 1.5 (0.7–3.6) | 0.8 (0.2–2.9) | 0.4 (0.1–1.7) | — | — |
| P for trend | 0.69 | 0.73 | 0.84 | 0.81 | — | — |
Adjusted for calories, age, family history, and vitamin C.
HR, hazard ratio; CI, confidence interval; NHL, non-Hodgkin lymphoma; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; MZ, marginal zone lymphoma; T-cell, T-cell lymphoma.
DISCUSSION
In this follow-up analysis of a population-based case-control study of NHL in Connecticut women, we did not identify an association between nitrate or nitrite intake and NHL or NHL subtype survival. The null findings do not confer support for the possibility that intake impacts NHL survival by promoting immune unresponsiveness. As these findings are the first to address the role of nitrate and nitrite in NHL survival, they cannot be compared to previous investigation. However, previous evidence from cellular studies of significant effects of nitrate/nitrite on immune functions (i.e., human lymphocyte proliferation and cytokine production) suggested that the role of nitrate and nitrite in NHL survival is of interest. The previous studies showed that although nitrate had no effect on lymphocyte growth, nitrite decreased proliferation (17). However, doses of sodium nitrate and nitrite do not reflect the concomitant consumption in humans of nitrosation inhibitors and additional beneficial components of foods containing nitrate. A previous investigation of the role of vegetable and fruit intake in this study population (10) was the first study to evaluate diet and NHL survival and a protective effect from vegetables and vitamin C was identified. Our null results for nitrate could therefore be the result of the effect on survival from other beneficial nutrients present in vegetables. Our results could also be confounded by additional unmeasured confounders, such as pesticide intake, and could be affected by misclassification of exposure, as total intake was determined from values in the published literature rather than laboratory values for all food items in the year of study enrollment. In addition, the participation rate is also a potential source of bias that could have impacted the results in either direction. Given power limitations, particularly for NHL subtypes, as well as prediagnostic dietary data, we suggest that these findings should be considered in larger study populations with postdiagnostic dietary data.
FIG. 1.
Kaplan-Meier survival curves plotted by high and low nitrate and nitrite intake for non-Hodgkin lymphoma overall.
ACKNOWLEDGMENTS
This research was supported by Hull Argall and Anna Grant 22067A from the Yale Cancer Center, Grant CA62006 from the National Cancer Institute (NCI), the Intramural Research Program of the NCI, National Institutes of Health (NIH), and Fogarty Training Grant 1D43TW008323-01 and 1D43TW007864-01 from the NIH. This publication was made possible by CTSA Grant UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the NIH, and NHL roadmap for medical research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR. This research was approved by the DPH HIC. Certain data used in this study were obtained from the Connecticut Department of Public Health. The authors assume full responsibility for analyses and interpretation of these data.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.tandfonline.com/page/terms-and-conditions
This article may be used for research, teaching, and private study purposes. Any substantial or systematic reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae, and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand, or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Contributor Information
Briseis Aschebrook-Kilfoy, Department of Health Studies, University of Chicago, Chicago, Illinois, USA.
Mary H. Ward, Occupational and Environmental Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Rockville, Maryland, USA
Tongzhang Zheng, Yale School of Public Health, Yale University, New Haven, Connecticut, USA.
Theodore R. Holford, Yale School of Public Health, Yale University, New Haven, Connecticut, USA
Peter Boyle, International Prevention Research Institute, Lyon, France.
Brian Leaderer, Yale School of Public Health, Yale University, New Haven, Connecticut, USA.
Yawei Zhang, Yale School of Public Health, Yale University, New Haven, Connecticut, USA.
REFERENCES
- 1.Clarke C, O’Malley C. Non-Hodgkin lymphoma. In: Ries LAG, Yong JL, Keel GE, Eisner MP, Lin YD, Horner M-JD, editors. Cancer survival among adults: U.S. SEER Program, 1988–2001, patient and tumor characteristics. Bethesda: National Cancer Institutes, SEER Program, NIH; 2007. pp. 235–242. [Google Scholar]
- 2.Han X, Kilfoy B, Zheng T, Holford TR, Zhu C, et al. Lymphoma survival patterns by WHO subtype in the United States: 1973–2003. Cancer Causes Control. 2008;19:841–858. doi: 10.1007/s10552-008-9147-4. [DOI] [PubMed] [Google Scholar]
- 3.Groves FD, Linet MS, Travis LB, Devesa SS. Cancer surveillance series: non-Hodgkin’s lymphoma incidence by histologic subtype in the United States from 1978 through 1995. J Natl Cancer Inst. 2000;92:1240–1251. doi: 10.1093/jnci/92.15.1240. [DOI] [PubMed] [Google Scholar]
- 4.Gangolli SD, van den Brandt PA, Feron VJ, Janzowsky C, Koeman JH, et al. Nitrate, nitrite and N-nitroso compounds. Eur J Pharmacol. 1994;292:1–38. doi: 10.1016/0926-6917(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 5.Bogovski P, Bogovski S. Animal species in which N-nitroso compounds induce cancer. Int J Cancer. 1981;27:471–474. doi: 10.1002/ijc.2910270408. [DOI] [PubMed] [Google Scholar]
- 6.Lück E. [Chemical preservation of food]. Zentralblatt für Bakteriologie, Mikrobiologie und Hygiene. 1. Abt Originale B Hygiene. 1985;180:311–318. [PubMed] [Google Scholar]
- 7.McKnight GM, Duncan CW, Leifart C, Golden MH. Dietary nitrate in man: friend or foe? Br J Nutr. 1999;81:349–358. doi: 10.1017/s000711459900063x. [DOI] [PubMed] [Google Scholar]
- 8.Kilfoy BA, Ward MH, Zheng T, Holford TR, Boyle P, et al. Risk of non-Hodgkin lymphoma and nitrate and nitrite from the diet in Connecticut women. Cancer Causes Control. 2010;21:889–896. doi: 10.1007/s10552-010-9517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser P, Chilvers C, Beral V, Hill MJ. Nitrate and human cancer: a review of the evidence. Int J Epidemiol. 1980;9:3–11. doi: 10.1093/ije/9.1.3. [DOI] [PubMed] [Google Scholar]
- 10.Han X, Zheng T, Foss F, Holford TR, Ma S, et al. Vegetable and fruit intake and non-Hodgkin lymphoma survival in Connecticut women. Leuk Lymphoma. 2010 doi: 10.3109/10428191003690364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma: the Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- 12.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting—Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 13.Willett W, Lenart E, et al. Nutritional Epidemiology. New York, NY: Oxford University Press; 1998. Reproducibility and validity of food-frequency questionnaires. [Google Scholar]
- 14.Ward MH, Zahm SH, Weisenburger DD, Gridley G, Cantor KP, et al. Dietary factors and non-Hodgkin’s lymphoma in Nebraska (United States) Cancer Causes Control. 1994;5:422–432. doi: 10.1007/BF01694756. [DOI] [PubMed] [Google Scholar]
- 15.Kilfoy BA, Zhang Y, Park Y, Holford TR, Schatzkin A, et al. Dietary nitrate and nitrite and the risk of thyroid cancer in the NIH–AARP Diet and Health Study. Int J Cancer. 2011;129:160–172. doi: 10.1002/ijc.25650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrington D. Encyclopedia of Biostatistics. Wiley Interscience; 2005. Linear rank tests in survival analysis. [Google Scholar]
- 17.Ustyugova IV, Zeman C, Dhanwada K, Beltz LA. Nitrates/nitrites alter human lymphocyte proliferation and cytokine production. Arch Environ Contam Toxicol. 2002;43:270–276. doi: 10.1007/s00244-002-0214-7. [DOI] [PubMed] [Google Scholar]