Abstract
Chronic cocaine use has been proposed to induce long-lasting alterations in cognitive functions dependent on the prefrontal cortex, and these alterations may contribute to the development of addiction. However, the underlying cellular mechanisms remain largely unknown, in part because of the lack of suitable animal models of cocaine-induced cognitive dysfunction that are amenable to molecular manipulations. Here, we characterized the effects of repeated cocaine administration on multiple aspects of cognitive function in C57BL/6 mice. Mice received 14 daily injections of either cocaine or saline, followed by a drug-free period of 2 weeks. They were then assessed for (i) cognitive flexibility in an instrumental reversal learning task; (ii) attentional function and response inhibition in a three-choice serial reaction time task; and (iii) working memory in a delayed matching-to-position task. Prior chronic exposure to cocaine resulted in impairments in reversal learning and working memory. Although there were no effects on attentional function or response inhibition, a shift in the pattern of errors committed was observed. These results indicate that prior chronic cocaine exposure in mice induces long-lasting alterations in cognitive functions associated with the prefrontal cortex.
Keywords: addiction, attention, cocaine, impulsivity, mouse, orbitofrontal, perseveration
Introduction
Chronic use of drugs such as cocaine has been proposed to induce long-lasting alterations in prefrontal cortical (PFC) function, leading to deficits in executive processes such as inhibitory control and decision-making that may contribute to the development of addictive behavior (Jentsch and Taylor, 1999; Volkow and Fowler, 2000; Everitt and Robbins, 2005; Schoenbaum et al., 2006; Olausson et al., 2007). In support of this hypothesis, drug addicts show reductions in PFC volume (Fein et al., 2002; Matochik et al., 2003), as well as decreased PFC blood flow and glucose metabolism both at baseline and during cognitive tasks (Volkow et al., 1988, 1991; Bolla et al., 2003, 2004). In addition, neuropsychological tests have identified deficits in executive function in chronic psychostimulant users (Bolla et al., 1998, 1999; Rogers et al., 1999; Bechara et al., 2001; Rosselli et al., 2001; Jovanovski et al., 2005; Fillmore and Rush, 2006; Verdejo-Garcia et al., 2006; Ersche et al., 2008). In nonhuman primate and rat models of addiction, chronic exposure to cocaine impairs reversal learning (Jentsch et al., 2002; Olausson et al., 2004; Schoenbaum et al., 2004; Calu et al., 2007), a task that is sensitive to lesions of the orbitofrontal cortex (OFC) (Dias et al., 1996; Chudasama and Robbins, 2003; McAlonan and Brown, 2003; Schoenbaum et al., 2003; Boulougouris et al., 2007; Howell et al., 2007).
The molecular mechanisms underlying these persistent drug-induced alterations in cognitive function are receiving increasing attention, and a number of proteins have been identified that are differentially regulated after cocaine exposure (Nestler, 2004; Ron and Jurd, 2005; Hyman et al., 2006). However, the evidence linking these candidate molecules to behavior is frequently correlational, and there is a clear need for experiments that establish a causal role for such candidates in mediating the behavioral effects of cocaine. Such experiments are dependent on the availability of animal models suitable both for the assessment of behavioral functions related to cocaine exposure and for the manipulation of target molecules. Genetic manipulation of mice is one effective strategy commonly used for the latter purposes. However, much of the prior work regarding the effects of chronic cocaine administration on PFC-related cognitive behaviors has been performed using rats or nonhuman primates, and little is currently known about the effects of chronic cocaine exposure in mice.
The aim of this study was to characterize the effects of prior cocaine exposure on cognitive flexibility, attentional function, inhibitory control, and working memory in C57BL/6 mice, a strain that is widely used for genetic manipulation. Three behavioral tasks were used for this purpose, including (i) reversal of a food-motivated instrumental response, (ii) a three-choice serial reaction time task, and (iii) a delayed matching-to-position task. In each experiment, mice received 14 daily injections of cocaine (30 mg/kg) or saline (vehicle), after training but 14 days before testing, on each of these tasks. The results presented here provide evidence for persistent and selective cocaine-induced cognitive deficits in C57BL/6 mice, showing that this mouse model can be used to explore the role of candidate proteins in these cocaine-induced impairments.
Methods
Subjects
Male C57BL/6J mice were obtained from the Jackson Laboratory (USA) and were housed under standard laboratory conditions. The C57BL/6J strain was chosen based on its sensitivity to the rewarding effects of cocaine (Crawley et al., 1997) and its wide-spread use as a background strain for knockout and transgenic mouse models. Mice were approximately 3 months of age at the beginning of the experiment. To avoid any effects of testing order, a separate set of mice was used for each experiment [except for the progressive ratio (PR) test and the assessment of body weight and baseline locomotor activity, which were performed in the same group of animals as the instrumental conditioning experiment and the delayed matching-to-position task, respectively]. All procedures were approved by the Yale University Animal Care and Use Committee and followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Behavioral apparatus and testing conditions
Locomotor activity was assessed using the EthovisionPro Video Tracking System (Noldus Information Technology, Leesburg, Virginia, USA). All subsequent experiments were performed in behavior test chambers (Med Associates, St Albans, Vermont, USA) equipped with a food magazine and pellet dispenser, house light, stimulus light, and, on the opposite wall, three nosepoke apertures. The pellet dispenser delivered food rewards (20 mg purified dustless precision pellets, Bio-Serv, Frenchtown, New Jersey, USA) upon correct completion of a reinforced trial. Before starting each experiment, mice were food restricted to 85% free-feeding body weight. They were then given 2 days of magazine training, in which food pellets were delivered on a fixed-time 15-s schedule after each entry into the food magazine. Each training session lasted for 15 min or until 50 reinforcers were delivered.
Drug administration
Cocaine hydrochloride was generously provided by the National Institute on Drug Abuse. Mice received 14 daily intraperitoneal injections of saline or cocaine (30 mg/kg/day). This dose of cocaine is widely used as the highest dose that can be administered chronically in mice without adversely affecting the animals’ general health, as higher doses carry a significant risk of cocaine-kindled seizures (Marley et al., 1991; Miller et al., 2000). For the initial instrumental conditioning experiment, which was designed to test whether cocaine exposure would facilitate the acquisition of a food-reinforced instrumental response, food restriction and training began immediately after drug administration. For all subsequent experiments, drug administration was followed by a 14-day drug-free period before testing, to ensure assessment of persistent, rather than acute, effects of cocaine or of cocaine withdrawal.
Locomotor activity
To determine whether the chronic cocaine exposure schedule used in this study affected locomotor activity at the time of behavioral testing, that is, after 14 days of cocaine administration and 14 days of withdrawal, mice (n=10 per group) were placed in a clear plastic cage (15 × 26 cm) for 1 h, and total distance traveled was recorded using a video tracking system.
Instrumental conditioning
To assess whether chronic cocaine administration influenced the acquisition of an instrumental response, mice (n=8 for saline, n=6 for cocaine) were exposed to cocaine or saline for 14 days as described above and were then trained to perform a food-reinforced nosepoke response. For each of 14 daily 15-min training sessions, mice were placed in the test chamber containing three illuminated apertures. For each mouse, one of these apertures was designated as active (left, center or right) in a pseudo-randomized and balanced manner for each treatment. A response in the active aperture (‘correct response’) resulted in delivery of a food pellet, whereas a response in the other two apertures (‘incorrect response’) had no effect. The first 10 rewards in each session were delivered on a fixed ratio-1 schedule; all subsequent rewards were delivered on a variable ratio-2 schedule (i.e. 1, 2, or 3 correct responses were required to obtain reinforcement). The number of correct and incorrect responses was recorded as a measure of acquisition of the instrumental response.
Progressive ratio test
To assess whether the effects of chronic cocaine administration were because of a difference in the motivation to respond for a food reinforcer, mice were subjected to a PR test after acquisition of the food-reinforced nosepoke response as described above. In this test, the reinforcement schedule was switched from a variable ratio-2 schedule to a PR schedule, in which the ratio, defined as the number of correct responses required to obtain a food reinforcer, was increased after delivery of each reinforcer. The ratio at any given time was calculated as a function of the number of reinforcers obtained at that time using the formula r = n(n + 1)/2 + 1 (where r is the ratio and n the number of reinforcers obtained), which results in the numerical series 1, 2, 4, 7, 11, 16, 22, 29 etc. The session was terminated when a mouse reached break point, that is, when it made no nosepoke response at all for 5 min. Data were recorded as the total number of correct and incorrect responses made during the test, the total number of reinforcers obtained, and the current ratio at break point.
Reversal learning
Cognitive flexibility was assessed using an instrumental reversal-learning task.
Training
Acquisition of the instrumental response occurred exactly as detailed above for instrumental conditioning.
Testing
On the basis of the data obtained from the acquisition phase, mice were divided into two groups of equal performance (n=12 for saline, n=13 for cocaine). After cocaine administration and a 14-day withdrawal period, they were retrained for 5 days on the instrumental task. Subsequently, a reversal test session was performed in which the position of the active aperture was switched. This session consisted of two phases: in the first 5 min, the active aperture was the same as during acquisition. At the end of these 5 min, the position of the active aperture was switched without any indication to the mice, and the test was continued for an additional 15 min. During these 15 min, the number of responses at the new active aperture (‘correct response’), at the previously active aperture (‘perseverative response’), and at the previously and currently inactive aperture (‘incorrect response’) was recorded. After the reversal test session, mice were retrained on the new active aperture for 4 daily 15-min sessions, and they were then subjected to a second reversal test and retrained for another 4 days.
Three-choice serial reaction time task
This task was based on the five-choice serial reaction time task, developed originally to measure attention in rats (Carli et al., 1983; Robbins, 2002) and then adapted for mice (Greco et al., 2005).
Training
Initially, mice (n=9 per group) were trained to nosepoke in an illuminated aperture to obtain a food reward. At the beginning of each trial, one of the three apertures was randomly designated as the active aperture and illuminated for that trial. This aperture remained illuminated until the mouse made a response in this aperture, upon which a food reward was delivered. Once a mouse had obtained more than 40 rewards and more than 50% correct responses in a 15-min session, training on the attentional component of the task began. For this training, mice received daily 20-min sessions until they had acquired the task. At the beginning of each trial, the mouse was required to perform a magazine entry to initiate the trial, signaled by a light above the magazine. After a prestimulus period, a stimulus was delivered by randomly illuminating one of the three nosepoke apertures, and the mouse was required to make a response in the illuminated aperture to receive a food pellet. Responses in the nonilluminated apertures, failure to respond during or within 5 s after termination of the stimulus, and responses in one of the apertures before the onset of the stimulus resulted in a 2 s timeout, during which the house light and all other lights were turned off. After this timeout, a new trial was initiated as signaled by illumination of the light above the magazine. At the beginning of training, the stimulus lasted for 60 s and the prestimulus period lasted for 2 s. As mice acquired the task, the stimulus duration was decreased and the prestimulus duration was increased until the final parameters of 1 s for the stimulus duration and 5 s for the prestimulus duration were achieved.
Testing
Once all mice had acquired the task, a pretreatment test set was performed, consisting of two separate tests to measure attention and response inhibition, respectively. (a) The attention test consisted of 80 trials in which the duration of the stimulus was varied in a pseudorandom manner, lasting for 0.25, 0.5, 1, or 2 s. The duration of the prestimulus period was invariant at 5 s. (b) The response inhibition test, conducted on a separate day, consisted of 60 trials in which the duration of the prestimulus period was varied in a pseudorandom manner, lasting for 5, 7.5, 10, or 20 s. The stimulus duration was invariant at 2 s. On the basis of these two pretreatment tests, mice were divided into two groups of equal performance. After cocaine administration and withdrawal, mice were retrained for 5 days on the basic task, and they were then subjected to the same attention and response inhibition tests as previously. For the attention test, data were recorded as the percentage of trials per stimulus duration in which a response was made in the illuminated aperture (‘correct response’), in one of the two nonilluminated apertures (‘incorrect response’), or in which no response was made within 5 s after termination of the stimulus (‘omission’). For the response inhibition test, data were recorded as the percentage of trials per prestimulus duration in which a response was made before stimulus onset (‘premature response’), after stimulus onset in the illuminated aperture (‘correct response’) or nonilluminated apertures (‘incorrect response’), or in which no response was made within 5 s after termination of the stimulus (‘omission’).
Delayed matching-to-position task
Working memory was tested using a delayed matching-to-position task previously described for mice (Estape and Steckler, 2002).
Training
Initially, mice (n=10 for saline, n=8 for cocaine) were trained to nosepoke as described for the three-choice serial reaction time task, except that only the outer two nosepoke apertures were used. Once a mouse had obtained more than 40 rewards and more than 50% correct responses in a 15-min session, training on the working memory task began. For this training, mice received daily 30-min sessions until they had acquired the task. Each trial consisted of the following sequence of events: mice were required to initiate the trial by making a response in the food magazine. This response initiated the ‘sample phase’, in which the left or the right aperture was randomly designated as active for that trial and illuminated. A nosepoke response in this aperture initiated the ‘delay phase’. During this phase, none of the apertures was illuminated, and responding in any of the apertures or the magazine had no programmed consequences. At the end of the delay phase, signaled by the illumination of the stimulus light adjacent to the magazine, mice were required to make a response in the reinforcer magazine to initiate the ‘choice phase’. In this phase, depending on the training stage as described below, one or both of the outer two nosepoke apertures were illuminated. A response in the active aperture resulted in delivery of a food pellet, whereas a response in the inactive aperture or an omission resulted in a 2-s timeout with all lights turned off. Delivery of the pellet or termination of the timeout was followed by a 20-s inter-trial interval, after which the stimulus light adjacent to the magazine was illuminated to signal initiation of the next trial.
On account of the complexity of this task, training was accomplished in three stages. In stage 1, only the active aperture was illuminated during the choice phase and the delay phase was set to 0 s. In stage 2, both outer apertures were illuminated during the choice phase, but all other parameters remained as in stage 1. In stage 3, both outer apertures were illuminated during the choice phase, and the delay phase was set to 2 s. Mice were advanced to the next training stage when they had achieved a criterion of more than 75% correct and more than 30 trials per 30-min session.
Testing
After completion of all three training stages, a pretreatment working memory test was administered, in which a variable delay length of 2, 5, 10, or 20 s was introduced. Mice were required to complete 15 trials at each delay length, that is, 60 trials total, the order of which was randomized across the session. All other parameters were identical to the last training stage. On the basis of this test, mice were divided into two groups of equal performance. After cocaine administration and withdrawal, mice were retrained for 5 days on the basic task, and they were then subjected to the same working memory test as previously detailed. Data were recorded as the percentage of trials per delay length in which a response was made in the illuminated aperture (‘correct response’), in the nonilluminated aperture (‘incorrect response’), or in which no response was made within 5 s after termination of the stimulus (‘omission’).
Statistical analysis
Group comparisons for instrumental conditioning and reversal learning were performed using analysis of variance (ANOVA) with repeated measures for day and treatment. Group comparisons for body weight, locomotor activity, and the PR test were performed using a one-way ANOVA for treatment. Group comparisons for the attention and response inhibition tests were performed using a two-way ANOVA for treatment and stimulus duration (attention) or prestimulus duration (response inhibition). Group comparisons for the working memory test were performed using a one-way ANOVA for treatment at the delay relevant for working memory. Post-hoc analysis was performed using Scheffe’s test. All data are expressed as mean ± SEM.
Results
Prior chronic exposure to cocaine enhances acquisition of a food-reinforced instrumental response
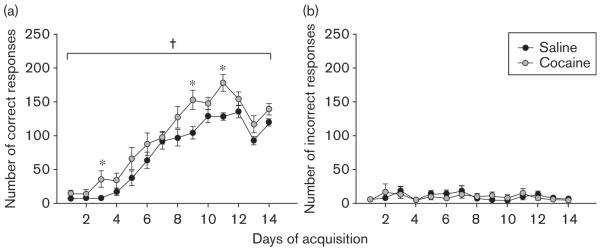
In an initial experiment, chronic cocaine administration occurred before acquisition of the operant conditioning task. This schedule of cocaine administration was found not to affect general health, as assessed by monitoring body weight (Table 1). Across 14 days of training on the operant conditioning task (Fig. 1), mice that had received prior exposure to cocaine made significantly more correct responses than saline-exposed animals [treatment, F(1,12) = 6.10, P < 0.05; treatment × day interaction, F(13,156) = 1.59, P=0.09], indicating that prior chronic cocaine exposure enhanced acquisition of a food-reinforced operant response. No effect on responding at the incorrect apertures was observed [treatment, F(1,12) < 1; treatment × day interaction, F(13,156) = 1.19, NS]. There are several potential explanations for an enhancement in correct responses, including nonspecific increases in locomotor activity, increases in incentive motivation, and increases in reward-related learning. Here, the increase in correct responses was not accompanied by a significant increase in basal locomotor activity (Table 1) or in motivation to respond for the food reward as assessed using a PR test (Table 2). The observed effect is thus likely because of a specific enhancement in a form of reward-related learning, and perhaps action-outcome learning in particular. This finding is consistent with the notion that drugs of abuse augment the function of the mesolimbic dopamine system, an effect thought to contribute to the development of addiction (Jentsch and Taylor, 1999). A similar enhancement has been described in rats (Olausson et al., 2006), but to our knowledge it has not previously been shown in mice. To avoid this potentially confounding factor in the assessment of PFC-dependent cognitive function, drug administration in all subsequent experiments occurred after completion of training on the basic task.
Table 1.
Body weight and locomotor activity
| Saline |
Cocaine |
Statistics |
||
|---|---|---|---|---|
| Measure | Mean ± SEM | Mean ± SEM | P value | F value |
| Body weight (g) | 28.9±0.7 | 28.3±0.4 | 0.45 | <1 |
| Distance traveled (cm) | 8941.7±450.0 | 9041.4±811.7 | 0.92 | <1 |
Data for body weight are presented as the average weight in grams across 14 days of injections and 14 days of withdrawal. Data for baseline locomotor activity at the time of behavioral testing are expressed as centimeters traveled during a 1-h assessment period.
Fig. 1.
Acquisition of instrumental conditioning. After chronic cocaine administration, mice were trained on an instrumental response task for 14 days. On each day, mice were tested for 15 min, and they were required to make a nosepoke response in one of three available illuminated apertures to receive a food reward. Responses in the other apertures had no effect. Data are presented as (a) number of correct responses and (b) number of incorrect responses per 15-min session on each of the 14 days (mean ± SEM). †Significant effect of treatment as determined by repeated-measures analysis of variance, *significant effect of treatment identified by post-hoc analysis.
Table 2.
Motivation to respond for a food reinforcer
| Progressive ratio measure |
Saline |
Cocaine |
Statistics |
|
|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | P value | F value | |
| Active pokes | 1208.8±276.6 | 1778.5±640.1 | 0.41 | <1 |
| Inactive pokes | 340.0±90.2 | 369.3±74.7 | 0.82 | <1 |
| Reinforcers | 1 7.6±1.6 | 19.7±2.5 | 0.51 | <1 |
| Break point ratio | 1 74.5±28.1 | 223.0±56.5 | 0.45 | <1 |
Motivation was assessed using a progressive ratio task, in which the number of correct responses required to obtain a food reinforcer was increased after delivery of each reinforcer. Results are expressed as the total number of correct and incorrect responses made during the test, the total number of reinforcers obtained, and the current ratio at break point.
Prior chronic exposure to cocaine impairs reversal learning without increasing response perseveration
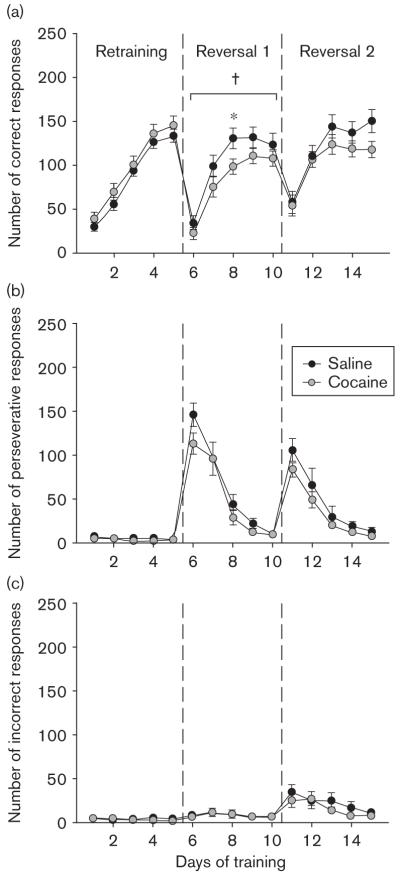
Cognitive flexibility was assessed using an instrumental reversal-learning task. No differences were observed between groups on the 5-day retraining period before reversal, indicating that chronic cocaine exposure post-acquisition does not affect baseline instrumental responding, at least when using the current protocol (Fig. 2). During reversal testing, however, prior cocaine exposure impaired acquisition of the new correct response. This impairment was statistically significant in the first reversal [treatment, F(1,23) = 4.50, P < 0.05; treatment × day interaction, F(4,92) < 1] but not in the second reversal phase [treatment, F(1,23) = 1.26, P=0.27; treatment × day interaction, F(4,92) = 1.80, NS]. No significant effect of prior chronic cocaine exposure on perseverative responding at the previously active aperture was observed during either reversal [first reversal: treatment, F(1,23) = 1.40, NS; treatment × day interaction, F(4,92) < 1; second reversal: treatment, F(1,23) = 2.02, NS; treatment × day interaction, F(4,92) < 1].
Fig. 2.
Reversal learning. After acquisition of an instrumental task and cocaine administration, mice were retrained for 5 days on the previously rewarded response and subsequently subjected to a reversal-learning phase, in which the position of the active aperture was switched. Mice were retrained on the new response for 5 days, and they were then subjected to a second reversal and retraining. Results are presented as (a) number of correct responses, (b) number of perseverative responses and (c) number of incorrect responses per 15-min session (mean ± SEM). †Significant effect of treatment as determined by repeated-measures analysis of variance, *significant effect of treatment identified by post-hoc analysis.
Prior chronic exposure to cocaine does not impair attentional function
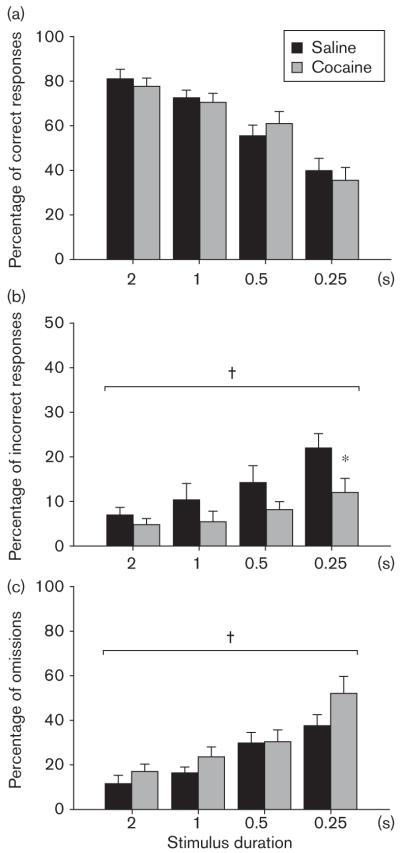
Attentional performance was measured using a modified version of the five-choice serial reaction time task (Greco et al., 2005). Mice were required to attend to a brief light stimulus of variable duration (0.25–2 s duration) presented randomly in one of three apertures, and to make a response in this aperture to obtain a food pellet (Fig. 3). Response accuracy, that is, the number of correct responses versus incorrect responses and omission errors, can be used as a measure of attentional function. The accuracy significantly decreased in all mice in a stimulus duration-dependent manner [stimulus duration, F(3,48) = 33.74, P < 0.01], with performance approximately 80% correct at the longest stimulus duration (2 s) and at almost chance levels (approximately 35%) at the shortest stimulus duration (0.25 s). There was no difference in correct responses between cocaine-exposed and saline-exposed mice at any stimulus duration [treatment, F(1,16) < 1; treatment × stimulus duration interaction, F(3,48) < 1], suggesting that prior chronic exposure to cocaine does not impair attentional function as measured by this paradigm. However, saline animals made significantly more incorrect responses [treatment, F(1,16) = 9.60, P < 0.01; treatment × stimulus duration interaction, F(3,48) < 1], whereas cocaine animals made significantly more omissions [treatment, F(1,16) = 4.47, P < 0.05; treatment × stimulus duration interaction, F(3,48) < 1].
Fig. 3.
Attention. Attentional function was assessed after chronic cocaine administration using a three-choice serial reaction time task. A brief visual stimulus of variable duration (2, 1, 0.5, or 0.25 s) was presented in one of three nosepoke apertures. Mice were required to attend to this stimulus and make a response in the corresponding aperture to receive a food reward. Results are expressed as (a) percentage of correct responses, (b) percentage of incorrect responses and (c) percentage of omissions at each of four stimulus durations (mean ± SEM). †Significant effect of treatment as determined by two-way analysis of variance, *significant effect of treatment identified by post-hoc analysis.
Prior chronic exposure to cocaine does not increase impulsive responding
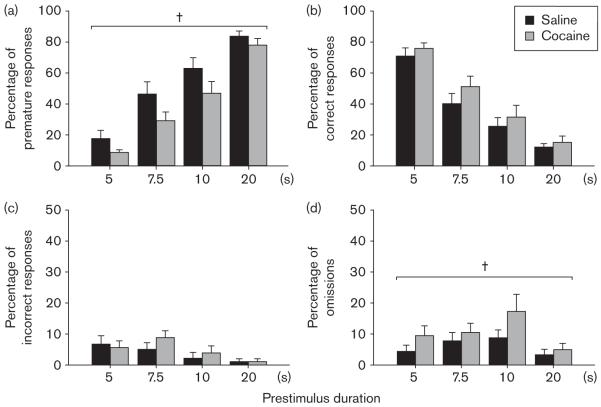
Response inhibition was assessed as the ability to inhibit an instrumental response for food reinforcement until a go-signal was presented (Fig. 4). After initiation of a trial, mice were required to withhold responding for a variable prestimulus period (5–20 s) until the visual stimulus was presented in one of the apertures. The number of premature responses was recorded as a measure of impulsivity. As expected, the number of premature responses increased significantly with increasing prestimulus duration [prestimulus duration, F(3,48) = 56.73, P < 0.001]. Surprisingly, however, cocaine exposure resulted in a significant decrease, rather than the expected increase, in premature responses [treatment, F(1,16) = 9.89, P < 0.01; treatment × prestimulus duration interaction, F(3,48) < 1]. In addition, an increase in omissions was observed after cocaine exposure [treatment, F(1,16) = 4.72, P < 0.05]. There was no significant difference between saline-exposed and cocaine-exposed mice in the number of correct [treatment, F(1,16) = 3.21, P=0.08; treatment × prestimulus duration interaction, F(3,48) < 1] or incorrect [treatment, F(1,16) < 1; treatment × prestimulus duration interaction, F(3,48) < 1] responses.
Fig. 4.
Impulsivity. Impulsivity was measured after chronic cocaine administration using a three-choice serial reaction time task. A brief stimulus in one of three nosepoke apertures was preceded by a prestimulus period of variable duration (5, 7.5, 10, and 20 s). Mice were required to withhold responding during this period and then make a correct response so as to receive a food reward. Results are expressed as (a) percentage of premature responses, (b) percentage of correct responses, (c) percentage of incorrect responses, and (d) percentage of omissions at each of four prestimulus durations (mean ± SEM). †Significant effect of treatment as determined by two-way analysis of variance.
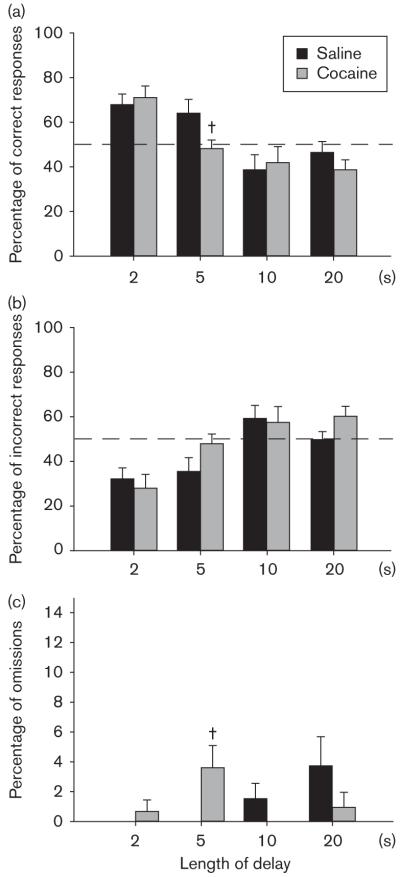
Prior chronic exposure to cocaine impairs working memory performance
Working memory was tested using an operant-delayed matching-to-position task (Fig. 5). Mice were required to retain the location of a previously illuminated nosepoke aperture across a delay of 2–20 s, and to make a response in this aperture to obtain a food pellet. At the shortest delay (2 s), both groups of mice performed at the same levels as during training in the absence of any delay (approximately 70% correct), implying that this short delay length did not impose a significant working memory requirement on the mice. Conversely, at the longest delays (10 and 20 s), both groups performed at chance levels (approximately 50% correct), suggesting that these trials overwhelmed the working memory capacity of the mice. Therefore, data analysis was restricted to the intermediate delay (5 s) as being the only delay length that placed a reasonable working memory demand on the mice. At this delay duration, cocaine exposure resulted in a significant decrease in correct responses [treatment, F(1,15) = 4.79, P < 0.05]. In addition, a significant increase in omissions was observed [treatment, F(1,15) = 7.89, P < 0.05], although this finding should be interpreted with caution because of the small number of omissions made (on average approximately one per animal across 60 trials). No significant effect of cocaine exposure on incorrect responses [treatment, F(1,15) =2.61, NS] was observed.
Fig. 5.
Working memory. Working memory was assessed after chronic cocaine administration using a delayed matching-to-position task. Mice were presented with a visual stimulus in one of two nosepoke apertures, and they were required to retain the correct location across a delay period of 2, 5, 10, or 20 s. They were then required to make a response in the previously indicated aperture to receive a food reward. Results are presented as (a) percentage of correct responses, (b) percentage of incorrect responses, and (c) percentage of omissions at each of four delay lengths (mean ± SEM). †Significant effect of treatment as determined by one-way analysis of variance.
Discussion
This study aimed to characterize the effects of chronic cocaine administration in mice on performance in a number of tasks related to prefrontal-associated cognitive function. Prior chronic exposure to cocaine was found to impair reversal learning, possibly through a mechanism that interferes with the acquisition of an alternate response rather than increasing perseverative responding. Chronic cocaine exposure also impaired working memory function at an intermediate delay length. No significant effects of the present cocaine administration schedule on attentional function or impulsivity were observed as measured by overall correct trials or premature responses, respectively. However, chronic cocaine exposure consistently shifted the pattern of errors in the three-choice serial reaction time task towards an increase in omissions accompanied by a decrease in other types of errors as attentional demands increased.
An important aspect of this study was to assess how the effects of chronic cocaine exposure on cognitive function in mice compare to prefrontal-associated deficits previously reported in human cocaine addicts as well as nonhuman primates and rats. One of the most reproducible findings in both humans and animal models of cocaine exposure has been a deficit in cognitive flexibility, that is, the ability to alter responding in discrimination tasks when reward contingencies are reversed (Jentsch et al., 2002; Olausson et al., 2004, 2007; Fillmore and Rush, 2006; Calu et al., 2007; Ersche et al., 2008). On the basis of the resemblance between these deficits and those observed after lesions of the OFC (Iversen and Mishkin, 1970; Dias et al., 1996; Chudasama and Robbins, 2003; Schoenbaum et al., 2003; Hornak et al., 2004; Izquierdo et al., 2004; Boulougouris et al., 2007; Howell et al., 2007), it has been proposed that cocaine-induced alterations in OFC function may potentially play an important role in addiction (Jentsch and Taylor, 1999; Volkow and Fowler, 2000; Schoenbaum et al., 2006; Everitt et al., 2007; Olausson et al., 2007). Consistent with this hypothesis, we found that prior chronic exposure to cocaine in mice selectively induced deficits in the reversal phase of an instrumental response task, suggesting that cocaine in mice, as in other species, may impair cognitive flexibility by disrupting OFC function. It should be noted, however, that the pattern of deficits observed here was different from that reported for other species (Jentsch et al., 2002; Olausson et al., 2004; Schoenbaum et al., 2004), as the reversal learning impairment was associated with a decrease in correct responses without a corresponding increase in perseverative responses. Mice may use a different strategy to solve the task, resulting in a different pattern of behavior that may reflect a specific deficit in encoding the reversed cue-outcome association. Subtle methodological differences between our task and those used in rat, or dose-related differences between species, may also be relevant to the observed results. In particular, the task used here uses a spatial strategy to indicate a correct response, whereas other studies have used visual (Jentsch et al., 2002; Olausson et al., 2004) or odor (Schoenbaum et al., 2004; Calu et al., 2007) discriminations that may require different and/or more taxing cognitive resources.
Cocaine abusers have also been found to show deficits in performance and brain activation patterns during tasks of attention, response inhibition and working memory (Bolla et al., 1999; Rosselli et al., 2001; Hester and Garavan, 2004; Jovanovski et al., 2005; Verdejo-Garcia et al., 2006; Tomasi et al., 2007; Verdejo-Garcia et al., 2007), functions associated with the dorsolateral PFC in humans and monkeys and with the medial PFC in rodents (Brown and Bowman, 2002; Uylings et al., 2003; Dalley et al., 2004). Consistent with these reports, chronic exposure to cocaine in our study resulted in an impairment in working memory at an intermediate delay length. In contrast, attention and response inhibition were not affected by the current schedule of cocaine administration. Although this observation is somewhat surprising in light of the studies in human addicts, our findings are consistent with the limited existing information regarding the effects of prior chronic cocaine exposure on these cognitive functions in previous animal models, in which effects on attentional function, impulsivity, and set-shifting were found to be either transient or absent altogether (Paine et al., 2003; Olausson et al., 2004; Dalley et al., 2005). One potential explanation for the apparent discrepancy between findings in human drug addicts and in animal models may be that certain aspects of the cognitive deficits induced by cocaine are more robust in humans or may develop only after extended exposure to the drugs. This notion is consistent with recent studies in rats showing that deficits in attention (Briand et al., 2008) and working memory (George et al., 2007) developed only after extended, but not limited, access to self-administered cocaine. Alternatively, it is possible that the deficits observed in human addicts are not exclusively a consequence of drug exposure, but also include a preexisting condition that increases the risk for drug taking and hence the transition to addiction. In support of this hypothesis, impulsivity in rats has been shown to predict increased acquisition of cocaine self-administration (Perry et al., 2005; Dalley et al., 2007).
An unexpected but consistent consequence of prior chronic cocaine exposure was a shift in the pattern of errors committed. In both the attention test and the response inhibition test, cocaine-exposed mice omitted more trials than saline-exposed animals, accompanied by a decrease in incorrect responses (attention test) or a decrease in premature responses (response inhibition test). As there was no significant effect on the percentage of correct responses, one possible interpretation of these data is that cocaine-exposed mice were more likely to omit a trial specifically when the correct response was unclear to them, whereas saline-exposed animals were more willing to attempt a response that may be incorrect. This effect may also potentially have contributed to the reversal-learning deficit described above. Overall, it thus seems that after chronic cocaine exposure, mice were less likely to make a response specifically when they were uncertain of receiving a reward. Nonetheless, this was not because of a general decrease in reward-related learning or motivation, because chronic cocaine exposure enhanced, rather than impaired, the initial acquisition of the instrumental task as we have previously observed in rats (Olausson et al., 2006), and it did not significantly affect performance on a PR test. Although the neural substrates underlying this shift in the pattern of errors are currently unknown, it may provide a useful additional measure in the assessment of cocaine-induced behavioral alterations.
In conclusion, chronic cocaine administration in C57BL/6 mice results in deficits in cognitive functions associated with several distinct PFC areas. Further research aimed at identifying regional sensitivity to the detrimental consequences of cocaine exposure and at characterizing the contribution of preexisting PFC dysfunction to the development of cognitive impairments in addicts is warranted. Moreover, the results presented here provide an important basis for the use of transgenic mice to study the role of candidate molecules in specific cocaine-induced cognitive impairments. Such studies will hopefully aid in elucidating questions that are fundamental for understanding addiction.
Acknowledgements
The authors would like to thank Dr. Jennifer Quinn and Shannon Gourley for valuable advice and discussions. This work was supported by NIH grants DA 010044 (A.C.N.) and DA 011717 (J.R.T.).
Footnotes
Current address: Dilja D. Krueger, Department of Brain and Cognitive Sciences, The Picower Institute for Learning and Memory, Massachusetts Institute of Technology, Cambridge, Massachusetts, USA
References
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Cadet JL, London ED. The neuropsychiatry of chronic cocaine abuse. J Neuropsychiatry Clin Neurosci. 1998;10:280–289. doi: 10.1176/jnp.10.3.280. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Rothman R, Cadet JL. Dose-related neurobehavioral effects of chronic cocaine use. J Neuropsychiatry Clin Neurosci. 1999;11:361–369. doi: 10.1176/jnp.11.3.361. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, et al. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, et al. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav Brain Res. 2007;179:219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Briand LA, Flagel SB, Garcia-Fuster MJ, Watson SJ, Akil H, Sarter M, Robinson TE. Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Neuropsychopharmacology. 2008;33:2969–2980. doi: 10.1038/npp.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VJ, Bowman EM. Rodent models of prefrontal cortical function. Trend Neurosci. 2002;25:340–343. doi: 10.1016/s0166-2236(02)02164-1. [DOI] [PubMed] [Google Scholar]
- Calu DJ, Stalnaker TA, Franz TM, Singh T, Shaham Y, Schoenbaum G. Withdrawal from cocaine self-administration produces long-lasting deficits in orbitofrontal-dependent reversal learning in rats. Learn Mem. 2007;14:325–328. doi: 10.1101/lm.534807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, et al. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Laane K, Pena Y, Theobald D, Everitt BJ, Robbins TW. Attentional and motivational deficits in rats withdrawn from intravenous self-administration of cocaine or heroin. Psychopharmacology. 2005;182:579–587. doi: 10.1007/s00213-005-0107-3. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology (Berl) 2008;197:421–431. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estape N, Steckler T. Cholinergic blockade impairs performance in operant DNMTP in two inbred strains of mice. Pharmacol Biochem Behav. 2002;72:319–334. doi: 10.1016/s0091-3057(01)00747-x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt B, Hutcheson DM, Everitt Y, Dalley J, Robbins TW. The orbital prefrontal cortex and drug addiction in animals and humans. Ann NY Acad Sci. 2007;1121:576–597. doi: 10.1196/annals.1401.022. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68:87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Polydrug abusers display impaired discrimination-reversal learning in a model of behavioural control. J Psychopharmacol. 2006;20:24–32. doi: 10.1177/0269881105057000. [DOI] [PubMed] [Google Scholar]
- George O, Mandyam CD, Wee S, Koob GF. Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology. 2007;33:2474–2482. doi: 10.1038/sj.npp.1301626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco B, Invernizzi RW, Carli M. Phencyclidine-induced impairment in attention and response control depends on the background genotype of mice: reversal by the mGLU2/3 receptor agonist LY379268. Psychopharmacology. 2005;179:68–76. doi: 10.1007/s00213-004-2127-9. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornak J, O’Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, Polkey CE. Reward-related reversal learning after surgical excisions in orbitofrontal or dorsolateral prefrontal cortex in humans. J Cogn Neurosci. 2004;16:463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Howell JL, Gourley SL, Taylor JR. Differential effects of medial orbitofrontal and prefrontal lesions on motivated responding in mice. Society for Neuroscience; Washington DC: 2007. Abstract Viewer/Itinerary Planner. Program # 934.11. [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Ann Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R, 2nd, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- Jovanovski D, Erb S, Zakzanis KK. Neurocognitive deficits in cocaine users: a quantitative review of the evidence. J Clin Exp Neuropsychol. 2005;27:189–204. doi: 10.1080/13803390490515694. [DOI] [PubMed] [Google Scholar]
- Marley RJ, Witkin JM, Goldberg SR. Genetic factors influence changes in sensitivity to the convulsant properties of cocaine following chronic treatment. Brain Res. 1991;542:1–7. doi: 10.1016/0006-8993(91)90989-9. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Miller KA, Witkin JM, Ungard JT, Gasior M. Pharmacological and behavioral characterization of cocaine-kindled seizures in mice. Psychopharmacology (Berl) 2000;148:74–82. doi: 10.1007/s002130050027. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47:24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Olausson P, Tronson NC, Krueger DD, Nairn AC, Taylor JR. Selective cognitive-motivational alterations following prior cocaine exposure in monkeys. Society for Neuroscience Online; Washington, DC: 2004. Program # 671 10, 2004 Abstract Viewer/Itinerary Planner. [Google Scholar]
- Olausson P, Jentsch JD, Tronson N, Neve RL, Nestler EJ, Taylor JR. {Delta}FosB in the nucleus accumbens regulates food-reinforced instrumental behavior and motivation. J Neurosci. 2006;26:9196–9204. doi: 10.1523/JNEUROSCI.1124-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson P, Jentsch D, Krueger DD, Tronson N, Nairn AC, Taylor JR. Orbitofrontal cortex and cognitive-motivational impairments in psychostimulant addiction: evidence from experiments in the non-human primate. Ann NY Acad Sci. 2007;1121:610–638. doi: 10.1196/annals.1401.016. [DOI] [PubMed] [Google Scholar]
- Paine TA, Dringenberg HC, Olmstead MC. Effects of chronic cocaine on impulsivity: relation to cortical serotonin mechanisms. Behav Brain Res. 2003;147:135–147. doi: 10.1016/s0166-4328(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Robbins T. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, et al. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Ron D, Jurd R. The ups and downs of signaling cascades in addiction. Sci STKE. 2005;309:re14. doi: 10.1126/stke.3092005re14. [DOI] [PubMed] [Google Scholar]
- Rosselli M, Ardila A, Lubomski M, Murray S, King K. Personality profile and neuropsychological test performance in chronic cocaine-abusers. Int J Neurosci. 2001;110:55–72. doi: 10.3109/00207450108994221. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learn Mem. 2003;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci. 2004;19:1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 2006;29:116–124. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Widespread disruption in brain activation patterns to a working memory task during cocaine abstinence. Brain Res. 2007;1171:83–92. doi: 10.1016/j.brainres.2007.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings HBM, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A, Recknor EC, Perez-Garcia M. Executive dysfunction in substance dependent individuals during drug use and abstinence: an examination of the behavioral, cognitive and emotional correlates of addiction. J Int Neuropsychol Soc. 2006;12:405–415. doi: 10.1017/s1355617706060486. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia AJ, Perales JC, Perez-Garcia M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addict Behav. 2007;32:950–966. doi: 10.1016/j.addbeh.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Mullani N, Gould KL, Adler S, Krajewski K. Cerebral blood flow in chronic cocaine users: a study with positron emission tomography. Br J Psychiatry. 1988;152:641–648. doi: 10.1192/bjp.152.5.641. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, et al. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry. 1991;148:621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]