Abstract
Oxidative stress plays a key role in the light damage (LD) model of retinal degeneration as well as in age-related macular degeneration (AMD). Since iron can promote oxidative stress, the iron chelator Deferiprone (DFP) was tested for protection against light-induced retinal degeneration. To accomplish this, A/J mice were treated with or without DFP in drinking water, and then were placed in constant bright white fluorescent light (10,000 lux) for 20 hours. Retinas were evaluated at several time points after light exposure. Photoreceptor apoptosis was assessed using the TUNEL assay. Retinal degeneration was assessed by histology 10 days after exposure to damaging white light. Two genes upregulated by oxidative stress, heme oxygenase 1 (Hmox1) and ceruloplasmin (Cp), as well as complement component 3 (C3) were quantified by RT-qPCR. Cryosections were immunolabeled for oxidative stress marker (nitrotyrosine), a microglial marker (Iba1) as well as both heavy (H) and light (L) ferritin. Light exposure resulted in substantial photoreceptor-specific cell death. Dosing with DFP protected photoreceptors, decreasing the numbers of TUNEL-positive photoreceptors and increasing the number of surviving photoreceptors. The retinal mRNA levels of oxidative stress related genes and C3 were upregulated following light exposure and diminished by DFP treatment. Immunostaining for nitrotyrosine indicated that DFP reduced the nitrative stress caused by light exposure. Robust H/L-ferritin-containing microglial activation and migration to the outer retina occurred after light exposure and DFP treatment reduced microglial invasion. DFP is protective against light-induced retinal degeneration and has the potential to diminish oxidative stress in the retina.
Keywords: Deferiprone, iron, light damage, retinal degeneration, chelator
Introduction
Photoreceptor cell death is irreversible and can cause night blindness, constriction of the visual field, and loss of central vision. In age-related macular degeneration (AMD), the most common cause of irreversible vision loss in the elderly worldwide, photoreceptor cell death may be promoted by photo-oxidative stress. This is supported by the finding that dietary antioxidants reduce the risk of developing advanced AMD [1]. Epidemiological studies suggest an association between light exposure and AMD risk [2, 3].
Also, long-term bright light exposure increases oxidative stress and has been shown to change macular pigmentation and decrease visual acuity in humans [4, 5]. Acute light-induced photoreceptor degeneration has been studied in experimental animals for over 40 years as a model for human retinal degenerative diseases [6, 7]. In the light damage (LD) model, the combination of high oxygen tension, focused light, and a high concentration of easily oxidized lipids causes photoreceptors to be particularly susceptible to photic injury. Exposure of rodents to photo-oxidative stress results in an outer retinal degeneration exhibiting some features of late atrophic AMD, including loss of photoreceptors and retinal pigment epithelium (RPE) [8]. This damage can be ameliorated by pretreatments with antioxidants [9, 10].
Although the pathogenesis of AMD is not fully understood, growing evidence suggests that, in addition to inflammation, complement activation, and other hereditary and environmental influences[11–17], oxidative stress [18–22] and iron [23–25] may play important roles. Previously, we have demonstrated higher iron levels in AMD retinas than in age-matched controls, suggesting that iron-mediated oxidative stress may contribute to retinal degeneration in AMD [26]. We also found that iron chelation diminished oxidative stress and protected against hereditary iron overload-induced retinal degeneration in mice [27]. Therefore, iron chelators may serve as protective agents against human retinal disorders in which iron accumulation is involved.
The goal of the present study was to determine whether retinal iron chelation might also protect against retinal oxidative stress induced by photo-oxidative (light) damage in wild-type mice that have normal retinal iron levels. A prior study convincingly showed that the iron chelator deferoxamine protects the rat retina from light damage [28]. Yet, the mechanism of deferoxamine’s retinal protection against light damage has not yet been defined, as it may not have crossed the blood-retinal barrier. Further, deferoxamine's clinical potential for retina protection is limited by the required subcutaneous or intravenous administration, and by toxicity to the retinal pigment epithelium [29]. Deferiprone (DFP) is an attractive alternative as it is orally absorbed, has been shown to cross the blood–brain barrier [30], and has shown efficacy and low toxicity in human clinical trials for diabetic nephropathy and primary glomerulonephritis [31]. It has also been shown to diminish retinal labile iron without retinal toxicity in the mouse [27].
Herein, we examined light damaged retinas to determine whether systemic administration of DFP can protect against light-induced retinal degeneration. We also compared the mRNA levels of genes upregulated by retinal oxidative stress: ceruloplasmin (Cp) and heme oxygenase 1 (Hmox1), as well as levels of the nitrosative stress marker nitrotyrosine after injury-inducing illumination with and without DFP treatment.
MATERIALS AND METHODS
Animals
Adult female albino A/J mice (weight, 15–25g) were purchased from Jackson laboratory (Bar Harbor, ME). All mice were fed a standard laboratory diet with 300 ppm iron and free access to water with or without DFP (1mg/ml) ad libitum and were maintained in a temperature-controlled room at 21–23 °C with a 12h: 12h light-dark photoperiod. Mice were treated with DFP in the drinking water for 2 weeks pre-illumination and also post-illumination until sacrifice. Experimental procedures were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in ophthalmology and vision research. All protocols were approved by the animal care review board of the University of Pennsylvania.
Light-Damage paradigm
In addition to DFP in the drinking water, mice were gavaged with or without 75mg/kg DFP (14mg/ml) in 0.5% carboxymethyl cellulose (CMC) 3 hours before illumination, and were gavaged again 7 hours after the illumination. Mice were exposed to 10k lux of cool white fluorescent light in a well-ventilated room continuously for 20 hours as we have described previously [32] from 2:00PM to 10:00AM next day. After the exposure to light, mice were either sacrificed or placed in the normal light/dark cycle for 72h or 10 days. Eyes were enucleated after sacrifice immediately following light exposure for qPCR, at 72h after light for immunofluorescence and terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) analysis, and at 10 days following light injury for morphologic analysis.
TUNEL analysis
Eyes enucleated 72h following light damage were immersion fixed in 4% paraformaldehyde for 10min. Cryosections were cut in the sagittal plane through the optic nerve head (ONH). The fluorescein-conjugated TUNEL in situ cell death detection kit (Roche, Mannheinm, Germany) was used for these sections, followed by fluorescence microcopy using a Nikon Eclipse TE-300 microscope (Nikon Inc., Melville, NY, USA). Digitized images were acquired with a Spot RT Slider camera (Diagnostic Instrument Inc., Sterling Heights, MI, USA) with ImagePro Plus v.4.1 software (Media Cybernetics, Silver Springs, MD, USA). For each retina, the number of TUNEL-positive photoreceptors was counted in ten sections, spaced every 300 µm centered on the ONH. The mean number of TUNEL-positive photoreceptors per retina was compared within DFP treated, untreated LD and wild-type controls with GraphPad Prism 5.03 (San Diego CA).
Morphologic analysis
Eyes enucleated 10 days following light damage were immersion fixed in 2% paraformaldehyde/2% glutaraldehyde overnight. For standard histology, 3-µm-thick plastic sections were cut in the sagittal plane and toluidine blue-stained as we have described [27]. The number of nuclei per column of outer nuclear layer (ONL) photoreceptors was counted in triplicate at 200 µm intervals from the ONH to 2000 µm from the ONH, using image analysis software (ImagePro Plus 4.1; Media Cybernetics) to calculate distances from manually set lengths. Comparison of ONL thickness (nuclei) measured within 2000 µm from ONH was performed using analysis of variance for repeated measures. The correlations from repeated measures at every 200 µm distances were accounted for by using the generalized estimating equation [33].
Quantitative Real-Time PCR
Gene expression in the neurosensory retina and RPE samples obtained from DFP-treated and untreated mice after LD plus mice without light exposure were analyzed by quantitative RT-PCR as we have described [27]. Probes used were heme oxygenase 1 (Hmox1, Mm00516005_m1), ceruloplasmin (Cp, Mm00432654_m1), complement component 3 (C3, Mm00437858_m1), and retinal pigment epithelium 65 (Rpe65, Mm00504133_m1). Eukaryotic 18S rRNA (Hs99999901_s1) was used as an endogenous control. Real-time qPCR (Taqman; ABI) was performed on a sequence detection system (Prism model 7500; ABI) using the ΔΔCT method, which provides normalized expression values. The amount of target mRNA was compared among the groups of interest. All reactions were performed in biological (four mice) and technical (three qPCR replicates per biological sample) triplicates.
Immunofluorescence
The globes fixed in 4% PFA were rinsed in PBS, and the eyecups were generated by removing the anterior segment. The eyecups were infiltrated in 30% sucrose overnight and embedded in Tissue-Tek OCT (Sakura Finetek, Torrance, CA). Immunofluorescence was performed on 10-µm-thick sections as previously published [34]. The primary antibody against a microglia/macrophage marker, ionized calcium binding adapter 1 (Iba1) was used at 1:100 dilution (Wako Chemicals USA, Inc., VA), 1:2500 dilution for rabbit anti-light ferritin (F17) and 1:250 dilution for rabbit anti-heavy ferritin (Y17) (generously provided by Paolo Santambrogio and Paolo Arosio, Instituto di Ricovero e Cura a Carattere Scientifico, Milan, and Universiti of Brescia, Brescia, Italy respectively). Rabbit anti-nitrotyrosine antibody was used at 1:200 dilution (Academy Biomedical Company, Inc. TX). Primary antibody was detected using fluorophore labeled secondary antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Control sections were treated identically but without primary antibody. The sections were analyzed by fluorescence microscopy with identical exposure parameters (model TE300 microscope; Nikon, Tokyo, Japan, with ImagePro software; Media Cybernetics, Silver Springs, MD).
Statistical analysis
The mean ± SE were calculated for each comparison pair. Statistical analyses for TUNEL, relative pixel density, microglial quantification and qPCR in the present study were performed in GraphPad Prism 5.03 (San Diego CA) by one-way ANOVA using the Tukey method. Mean numbers of remaining photoreceptor nuclei were compared using SAS v9.2 (SAS Institute Inc, Cary, NC). *P<0.05 was considered statistically significant.
Results
DFP diminishes the number of TUNEL-positive photoreceptors following LD
As an initial assessment of the photoreceptor-protective activity of DFP, we quantified the number of TUNEL-positive photoreceptors in LD mice with or without DFP treatment. The TUNEL reaction detects DNA fragmentation, a step in the apoptotic program. Seventy-two hours following light exposure, many TUNEL-positive photoreceptors were present in the thinner ONL of LD retinas (Figure 1A), but only a few TUNEL-positive photoreceptors were present in retinas of DFP-treated mice. To quantify the difference in cell death, the number of TUNEL-positive photoreceptors was counted in sagittal sections throughout the eye separated by an interval of 300µm. DFP treated mice had significantly fewer TUNEL-positive photoreceptors than untreated WT, P < 0.0001 (Figure 1B).
Fig. 1.
Fluorescence photomicrographs showing TUNEL label in mouse retinas. There are fewer TUNEL-positive photoreceptor nuclei (green, arrow) 72hr following light-damage (LD) in mice treated with DFP compared to retinas from LD mice not treated with DFP (A). Nuclei are counterstained with DAPI (blue). Photoreceptor nuclei reside in the outer nuclear layer (ONL). Scale bars=100µm. (B) Histogram comparing numbers of TUNEL-positive photoreceptor from no light damage (NLD) control (n=3), LD (n=3) and LD+DFP (n=3) mice. The histogram displays the mean (±S.E.M) of total numbers of TUNEL-positive photoreceptors counted in ten sections per retina with an interval of 300µm in the sagittal plane. *Significant difference (P<0.05).
Preservation of photoreceptor nuclei by DFP
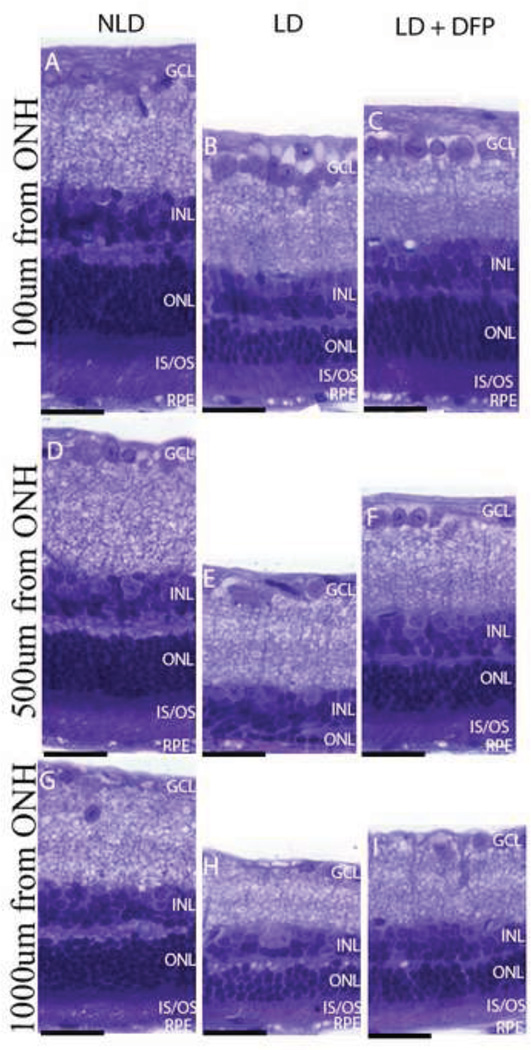
Since the decrease in the number of TUNEL-positive photoreceptors after treatment of DFP implies a pro-survival effect, we sought to confirm this and to determine whether the reduction of TUNEL-positive cells corresponded to preservation of photoreceptors. Morphologic analysis was performed 10 days following light exposure and the numbers of photoreceptor nuclei were counted in three different sagittal sections through ONH. DFP provided significant preservation of photoreceptors throughout the retina. Compared to eyes without light damage (NLD) (Figure 2A), eyes after LD had profound thinning of the photoreceptor nuclear layer and disorganization and shortening of the photoreceptor inner/outer segments (Figure 2B), but the mice treated with DFP had a thicker ONL and better preserved photoreceptor inner and outer segments (Figure 2C). The most severely damaged part of the retina was located centrally near the ONH, especially the superior side of the retina, with relative preservation of the peripheral retina. The most severe degeneration was 500µm from the ONH (Figure 3), compared with 100µm and 1000µm from the ONH. However, the photoreceptors in DFP treated mice at these distances from the ONH were significantly protected (P=0.03) (Figure 2D).
Fig. 2.
Photomicrographs of plastic sections of mouse retinas and plot showing morphologic protection 10 days following LD in LD+DFP retinas. Photomicrographs of plastic sections showing superior retinas of mice in NLD (A), LD (B) and LD+DFP (C) groups. Sagittal plane sections pass through ONH. The photoreceptor nuclei in the ONL and inner/outer segment (IS/OS, labeled with*), are protected from light induced thinning observed in the untreated LD retina. Scale bars=100µm. (D) Plot of the thickness of the ONL, measured in numbers of photoreceptor nuclei per column. Measurements are made in triplicate every 200µm away from the ONH. LD (n=4, red), LD+DFP (n=4, blue) and NLD (n=3, green) retinas 10 days following LD are displayed as mean values (±S.E.M).
Fig. 3.
Photomicrographs of mouse retinas showing protection of DFP against photoreceptor cell loss and inner/outer segment shortening and disruption 10 days following LD. Photomicrographs of 3µm thick plastic section at the indicated distances superior to the ONH from (A, D, G) NLD control, (B, E, H) LD and (C, F, I) LD+DFP retinas 10 days following LD. At 100µm from the ONH (A, B, C), DFP treated retinas have similar numbers of photoreceptor nuclei, compared to NLD, although the IS/OS are shortened. In LD mice without DFP, there is photoreceptor nuclei loss, disorganization and shortening of IS/OS. At 500µm from the ONH (D, E, F), LD retina has marked loss of photoreceptors and IS/OS ablation, but this is diminished by DFP. Light damage is less severe in the periphery. Scale bars=10µm.
DFP inhibited the LD-mediated increase in Heme oxygenase 1, Ceruloplasmin and C3 mRNA levels
To investigate the effect of DFP on gene expression, qPCR with Hmox1, and Cp primers, two genes upregulated by oxidative stress [32, 35] was performed following light injury. Both Hmox1 and Cp mRNA levels increased significantly after light exposure. This increase was diminished by treatment with DFP (Figure 4A, B). Consistent with the change in Hmox1 mRNA levels in the neurosensory retina, light injury significantly increased RPE Hmox1 mRNA and there was a trend toward decrease in DFP treated mice (Figure 4D). DFP treatment also diminished the reduction in RPE Rpe65 mRNA levels (Figure 4E). RPE65 is a protein essential for the visual cycle. In addition, mRNA levels of complement factor 3 (C3) were significantly upregulated after light injury, but diminished by DFP treatment (Figure 4C).
Fig. 4.
Graphs showing relative mRNA levels measured by qPCR. DFP decreases oxidative stress and complement markers. Hmox1 and Cp mRNA levels in neural retina (A, B) and Hmox1 mRNA in RPE (D) are significantly upregulated by light exposure; DFP treatment significantly diminishes this upregulation in retina. DFP significantly increases the Rpe65 mRNA level in RPE (E). The increased C3 mRNA level in retina induced by LD is also significantly reduced by DFP treatment (C). LD (n=4), LD+DFP (n=4) and NLD (n=4) retinas are displayed as mean values (±S.E.M). *Significant difference (P<0.05).
DFP inhibited the LD-mediated increase in nitrotyrosine
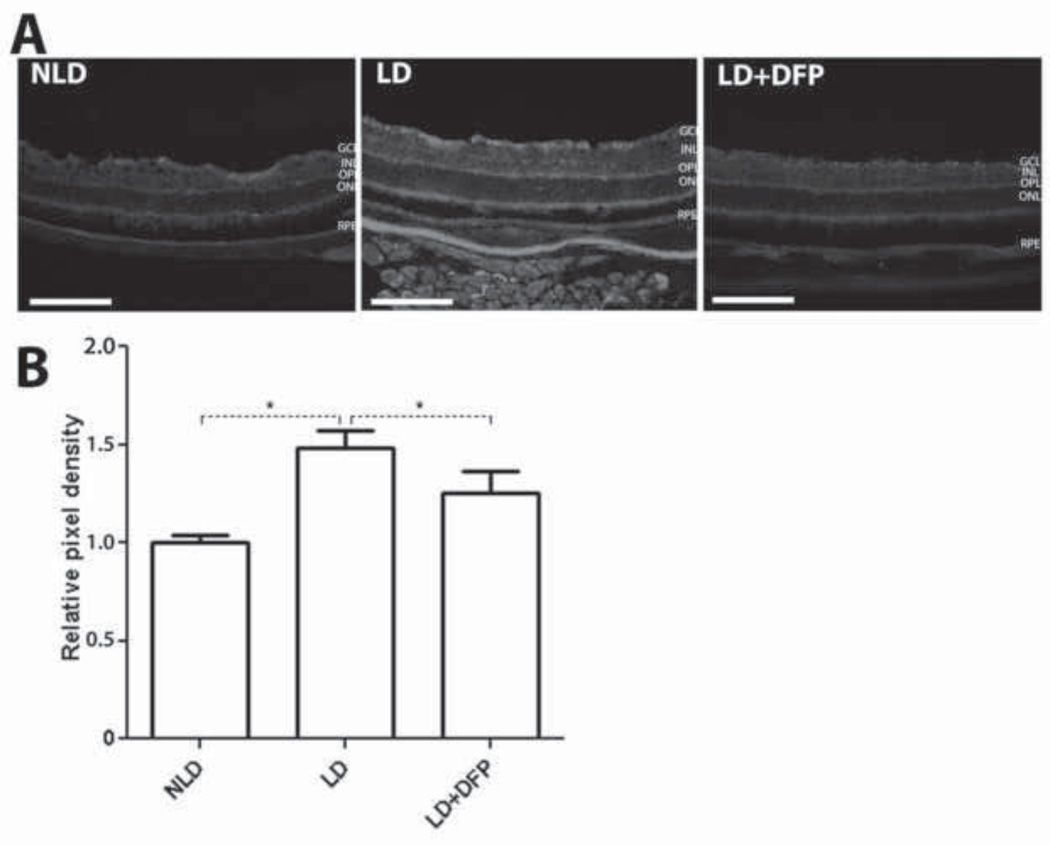
The oxidative marker nitrotyrosine is formed when tyrosine residues are attacked by peroxynitrite, an oxidant formed by the reaction of nitric oxide with superoxide anions that are generated by light exposure [36, 37]. Compared with DFP treated LD retinas, nitrotyrosine immnunoreactivity was increased in non-treated LD retinas (Figure 5A). Quantification of mean pixel density showed that light exposure significantly increased nitrotyrosine levels by 1.5 fold (P=0.0026) compared to NLD mice, and DFP treatment significantly inhibited this increase (P=0.03) (Figure 5B).
Fig. 5.
Comparison of nitrotyrosine levels in retina by immunolabeling and mean pixel density quantification. Monochromatic fluorescence photomicrographs of LD retinas showing stronger nitrotyrosine immunolabeling than DFP treated LD retinas (A). Immunoreactivity was quantified by measuring the mean pixel intensity within the whole retinas. The increased nitrotyrosine immunoreactivity induced by LD is significantly inhibited by DFP treatment (B). Scale bars=100µm. LD (n=3), LD+DFP (n=3) and NLD (n=3) retinas are displayed as mean values (±S.E.M). Significant difference (*P<0.05, **P<0.005).
Microglial response to light exposure
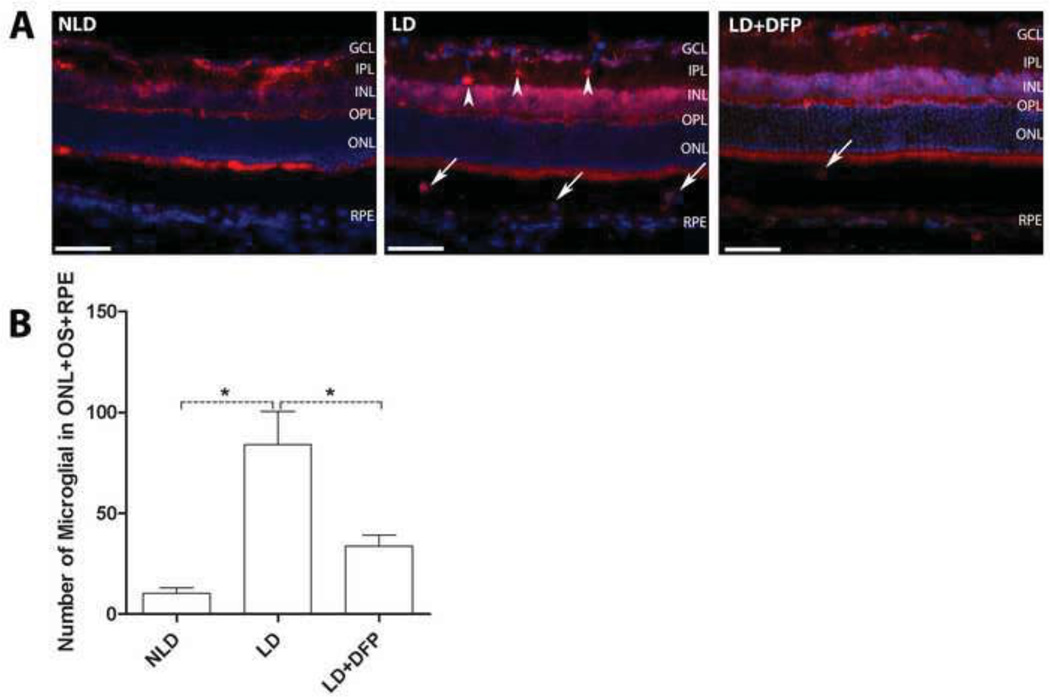
Following light damage, microglial infiltration into the retina has been observed and naloxone treatment to inhibit the infiltration protects against photoreceptor death [38]. In our model, in order to assess potential microglial involvement, we counted microglia/macrophages in the outer retina (Iba1-positive cells in the ONL, outer segment and RPE area) along the full-length of each retinal section in a masked fashion. For each eye, 5 sections with an interval of 900µm including one through ONH were selected for quantification. Microglial cells in the NLD retina are primarily localized to the inner retina including inner plexiform layer (IPL) and ganglion cell layer (GCL), but only a few were observed in outer retina (Fig. 6A). This distribution is consistent with previous reports [39, 40].
Fig. 6.
Changes in microglial distribution and number after LD. Fluorescence photomicrographs of Iba1 labeled retinal microglial cells from light-exposed eyes 72hrs following light exposure and counts of these cells in outer retina (ONL+OS+RPE). (A) 72hrs after exposure, labeled microglial were found both in the outer (arrows) and inner (arrow heads) retina. Scale bars=100µm. (B) Quantification of microglial in the outer retina. Compared to NLD control retina, the number of microglia in outer retina increased significantly 72hrs following light exposure, which is significantly diminished by DFP. LD (n=3), LD+DFP (n=3) and NLD (n=3) retinas are displayed as mean values (±S.E.M). *Significant difference (P<0.05).
The number, distribution and morphology of microglia/macrophages in the retina changed rapidly after LD. Three days after light exposure, the number of these cells in the outer retina increased and many were amoeboid in shape (Figure 6A). DFP significantly attenuated the increased number of microglia/macrophages in the outer retina induced by light (P=0.0056) (Figure 6B). Double-labeling for H- or L-ferritin and Iba1, indicated the presence of both forms of ferritin in the microglia, with particularly high levels of the L form (Fig 7).
Fig. 7.
Double-labeling for H/L-ferritin and microglia. Fluorescence photomicrographs for ferritin (green) and microglia (red) in LD retinas: (A, D) Iba1 immunolabeled microglia. (B) H-ferritin immunolabeled cells. (E) L-ferritin immunolabeled cells. (C, F) Merged figures showing co-localization of microglia and ferritins. Scale bars=100µm.
Discussion
In this study, we investigated whether systemic administration of DFP, an oral iron chelator, is an effective prevention for light-induced retinal degeneration. Our data showed that DFP caused a reduction of TUNEL-positive photoreceptors induced by light exposure. Morphological testing by plastic sectioning demonstrated that DFP treatment decreased photoreceptor cell death associated with preservation of photoreceptor morphology compared to untreated mice. We found that the retinal degeneration in our model is more severe in the central retina, especially the superior portion. This focal retinal degeneration also probably explains why we did not observe changes in function of the entire retina measured by electroretinography (data not shown). Furthermore, the number of microglia in the photoreceptor layer increases markedly 72hrs post-light exposure, presumably to phagocytose the dead photoreceptors. These cells may originate from the proliferation of resident microglial or invasion by blood-borne macrophages. The iron storage protein ferritin co-labels with microglia, suggesting that these cells accumulate iron.
The neuroprotective effect of DFP may result from diminished oxidative stress secondary to reduced iron levels in retina, inhibition of microglial activation and iron accumulation, and/or inhibition of the complement cascade. Our qPCR data for C3 mirrors and extends earlier findings by Collier et al [39], who showed that C3 was upregulated in all layers of retinas 2 days following LD. These findings, together with light damage protection by complement factor D knockout [41] suggest that the complement system is also involved in the light-induced retinal degeneration model. DFP’s inhibition of light-induced C3 upregulation may contribute to its retinal protection.
Cp is a ferroxidase, converting the hydroxyl radical-producing ferrous iron to the safer ferric form [42]. Cultured neural cells from ceruloplasmin knockout mice are susceptible to free radical injury, suggesting that Cp may combat oxidative stress [43]. Another marker related to oxidative stress, Hmox1 is the inducible form of the heme oxygenase system, acting as the rate-limiting factor in the catabolism of heme into biliverdin, releasing free iron and carbon monoxide (CO) [44]. Consistent with our previous light damage study [32, 35], both Cp and Hmox1 were upregulated following photo-oxidation of the mouse retina. Based on our qPCR data, DFP treatment significantly attenuated the upregulated expression of Hmox1 and Cp mRNA levels induced by photo-oxidation in the neural retina relative to untreated light damaged retina. The levels of nitrotyrosine were increased by LD and this was inhibited by DFP treatment. The measured nitrotyrosine is likely to result from superoxide produced by light next reacting with nitric oxide to form the oxidant peroxynitrite. Iron can then catalyze the production of nitrotyrosine [45]. These observations suggest that DFP may diminish iron-catalyzed nitrative stress.
Iron chelators have been used mainly for the treatment of acute iron toxicity and chronic transfusional iron overload in thalassemia and other conditions [46]. Recently, iron-chelating drugs have been tested in additional categories of patients with normal body iron load, such as those with neurodegenerative [30], renal, and infectious diseases [47–49]. Three widely used iron chelators are deferoxamine, deferasirox, and DFP. Deferoxamine has been used for decades as the main iron chelating agent to treat transfusion-related hemosiderosis. It is administered via slow subcutaneous infusion over 8 to 12 hours or intravenously in some patients. Deferoxamine's potential as a therapeutic agent is limited by the route of administration, as well as severe side effects at higher doses such as pigmentary retinopathy [50], bone dysplasia, and auditory toxicity [51]. Retinal toxicity manifesting as a pigmentary retinopathy with altered retinal function documented by ERG has been reported in some patients treated with deferoxamine [50, 52]. The mechanism of deferoxamine’s retinal toxicity has not been explored. Deferasirox received FDA (U.S. Federal Drug Administration) approval in 2005 for treatment of transfusional iron overload, but there is no evidence to date that it can decrease brain or retinal iron levels.
DFP, as a low-molecular-weight iron chelator, has been used for years for patients with transfusional iron overload in Europe and Asia and was recently approved by the FDA. Not only can it decrease liver and cardiac iron levels in patients with transfusional iron overload, DFP can cross the blood–brain barrier [53] and decrease brain iron levels in patients with Friedreich's ataxia [30], associated with improved motor function in some patients. DFP is orally absorbed and binds iron in multiple subcellular and extracellular locations [54, 55]. Unlike deferoxamine, which is a large, positively charged molecule, DFP is only 139 Da and is neutral in the circulation, whether free or bound to iron, and readily penetrates cells [56]. We found previously that DFP does not induce changes in ERG amplitudes or retinal morphology in mice at the doses employed [27].
Our study suggests that DFP may serve as a protective agent against retinal diseases in which oxidative stress has been implicated. The dose of DFP used in this study, 1 mg/ml in drinking water (approximately 250mg/kg/d) plus 75mg/kg by oral gavage, is higher than that recommended dosage in thalassemic patients (75–100 mg/kg/d), but provides proof-of-principle that DFP can be protective in the light induced retinal degeneration model. Approximately 1% to 2% of patients with thalassemia given oral DFP develop reversible agranulocytosis [57], necessitating regular blood cell count monitoring [56, 58]. DFP binds iron with higher affinity than it does copper or zinc, but may diminish levels of these latter metals. Thus levels of iron, copper, and zinc should be monitored in any future clinical trial of DFP for AMD, especially given the evidence that zinc supplementation is protective against AMD [59] and that the elderly may have reduced zinc intake. The safety profile of long-term DFP administration in patients without iron overload requires further investigation. While one patient in a 6mo trial for Friedreich’s ataxia developed reversible agranulocytosis, most tolerated the drug well [30]. Our previous finding that DFP not only protects iron-overloaded retinas of Cp/Heph knockout mice against oxidative stress and retinal degeneration [27] but also extends their lifespan and improves their hematocrit suggests it could have systemic benefits.
This current study provides evidence that DFP can rescue light damage-induced photoreceptor death in which iron dysregulation is not the primary cause of the degeneration. Similarly, a recent study by Obolensky et al [60] demonstrated that treatment with zinc–deferoxamine reduced retinal oxidative stress and enhanced photoreceptor survival, leading to both functional and structural rescue in the rd10 model of retinitis pigmentosa. This rd10 study, together with our light damage study, indicate that iron chelation could protect the retina against a broad range of insults.
Acknowledgements
We acknowledge the help of Dr. Gui-shuang Ying for biostatistics analysis. This work was supported by EY 015240, unrestricted funding from Research to Prevent Blindness, State Scholarship Fund (File No. 2010621116) of China Scholarship Council in Ministry of Education of the P. R. China, the F.M. Kirby Foundation, a gift in memory of Dr. Lee F. Mauger, the Paul and Evanina Bell Mackall Foundation Trust, and an unrestricted grant from ApoPharma, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: Dr. Dunaief and ApoPharma have a patent pending on the use of DFP for AMD, and Dr. Dunaief’s lab receives research funding from ApoPharma.
References
- 1.A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor HR. Ultraviolet radiation and the eye: an epidemiologic study. Trans Am Ophthalmol Soc. 1989;87:802–853. [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor HR, Munoz B, West S, Bressler NM, Bressler SB, Rosenthal FS. Visible light and risk of age-related macular degeneration. Trans Am Ophthalmol Soc. 1990;88:163–173. discussion 173–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Marlor RL, Blais BR, Preston FR, Boyden DG. Foveomacular retinitis, an important problem in military medicine: epidemiology. Invest Ophthalmol. 1973;12:5–16. [PubMed] [Google Scholar]
- 5.Young RW. Solar radiation and age-related macular degeneration. Surv Ophthalmol. 1988;32:252–269. doi: 10.1016/0039-6257(88)90174-9. [DOI] [PubMed] [Google Scholar]
- 6.Noell WK, Walker VS, Kang BS, Berman S. Retinal damage by light in rats. Invest Ophthalmol. 1966;5:450–473. [PubMed] [Google Scholar]
- 7.Shahinfar S, Edward DP, Tso MO. A pathologic study of photoreceptor cell death in retinal photic injury. Curr Eye Res. 1991;10:47–59. doi: 10.3109/02713689109007610. [DOI] [PubMed] [Google Scholar]
- 8.Marc RE, Jones BW, Watt CB, Vazquez-Chona F, Vaughan DK, Organisciak DT. Extreme retinal remodeling triggered by light damage: implications for age related macular degeneration. Mol Vis. 2008;14:782–806. [PMC free article] [PubMed] [Google Scholar]
- 9.Organisciak DT, Darrow RM, Jiang YI, Marak GE, Blanks JC. Protection by dimethylthiourea against retinal light damage in rats. Invest Ophthalmol Vis Sci. 1992;33:1599–1609. [PubMed] [Google Scholar]
- 10.Ranchon I, Chen S, Alvarez K, Anderson RE. Systemic administration of phenyl-N-tert-butylnitrone protects the retina from light damage. Invest Ophthalmol Vis Sci. 2001;42:1375–1379. [PubMed] [Google Scholar]
- 11.Li CM, Chung BH, Presley JB, Malek G, Zhang X, Dashti N, Li L, Chen J, Bradley K, Kruth HS, Curcio CA. Lipoprotein-like particles and cholesteryl esters in human Bruch's membrane: initial characterization. Invest Ophthalmol Vis Sci. 2005;46:2576–2586. doi: 10.1167/iovs.05-0034. [DOI] [PubMed] [Google Scholar]
- 12.Takeda A, Baffi JZ, Kleinman ME, Cho WG, Nozaki M, Yamada K, Kaneko H, Albuquerque RJ, Dridi S, Saito K, Raisler BJ, Budd SJ, Geisen P, Munitz A, Ambati BK, Green MG, Ishibashi T, Wright JD, Humbles AA, Gerard CJ, Ogura Y, Pan Y, Smith JR, Grisanti S, Hartnett ME, Rothenberg ME, Ambati J. CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature. 2009;460:225–230. doi: 10.1038/nature08151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cousins SW, Espinosa-Heidmann DG, Csaky KG. Monocyte activation in patients with age-related macular degeneration: a biomarker of risk for choroidal neovascularization. Arch Ophthalmol. 2004;122:1013–1018. doi: 10.1001/archopht.122.7.1013. [DOI] [PubMed] [Google Scholar]
- 14.Spraul CW, Lang GE, Grossniklaus HE. Morphometric analysis of the choroid, Bruch's membrane, and retinal pigment epithelium in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37:2724–2735. [PubMed] [Google Scholar]
- 15.Donoso LA, Kim D, Frost A, Callahan A, Hageman G. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2006;51:137–152. doi: 10.1016/j.survophthal.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y, Yanase E, Feng X, Siegel MM, Sparrow JR. Structural characterization of bisretinoid A2E photocleavage products and implications for age-related macular degeneration. Proc Natl Acad Sci U S A. 2010;107:7275–7280. doi: 10.1073/pnas.0913112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGeer EG, Klegeris A, McGeer PL. Inflammation, the complement system and the diseases of aging. Neurobiol Aging. 2005;26(Suppl 1):94–97. doi: 10.1016/j.neurobiolaging.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14:194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai J, Nelson KC, Wu M, Sternberg P, Jr, Jones DP. Oxidative damage and protection of the RPE. Prog Retin Eye Res. 2000;19:205–221. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 20.Cano M, Thimmalappula R, Fujihara M, Nagai N, Sporn M, Wang AL, Neufeld AH, Biswal S, Handa JT. Cigarette smoking, oxidative stress, the anti-oxidant response through Nrf2 signaling, and Age-related Macular Degeneration. Vision Res. 2010;50:652–664. doi: 10.1016/j.visres.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Age-Related Eye Disease Study: a clinical trial of zinc and antioxidants--Age-Related Eye Disease Study Report No. 2. J Nutr. 2000;130:1516S–1519S. doi: 10.1093/jn/130.5.1516S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen JK, Dong A, Hackett SF, Bell WR, Green WR, Campochiaro PA. Oxidative damage in age-related macular degeneration. Histol Histopathol. 2007;22:1301–1308. doi: 10.14670/HH-22.1301. [DOI] [PubMed] [Google Scholar]
- 23.Hadziahmetovic M, Dentchev T, Song Y, Haddad N, He X, Hahn P, Pratico D, Wen R, Harris ZL, Lambris JD, Beard J, Dunaief JL. Ceruloplasmin/hephaestin knockout mice model morphologic and molecular features of AMD. Invest Ophthalmol Vis Sci. 2008;49:2728–2736. doi: 10.1167/iovs.07-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunaief JL. Iron induced oxidative damage as a potential factor in age-related macular degeneration: the Cogan Lecture. Invest Ophthalmol Vis Sci. 2006;47:4660–4664. doi: 10.1167/iovs.06-0568. [DOI] [PubMed] [Google Scholar]
- 25.Hahn P, Milam AH, Dunaief JL. Maculas affected by age-related macular degeneration contain increased chelatable iron in the retinal pigment epithelium and Bruch's membrane. Arch Ophthalmol. 2003;121:1099–1105. doi: 10.1001/archopht.121.8.1099. [DOI] [PubMed] [Google Scholar]
- 26.Wong RW, Richa DC, Hahn P, Green WR, Dunaief JL. Iron toxicity as a potential factor in AMD. Retina. 2007;27:997–1003. doi: 10.1097/IAE.0b013e318074c290. [DOI] [PubMed] [Google Scholar]
- 27.Hadziahmetovic M, Song Y, Wolkow N, Iacovelli J, Grieco S, Lee J, Lyubarsky A, Pratico D, Connelly J, Spino M, Harris ZL, Dunaief JL. The oral iron chelator deferiprone protects against iron overload-induced retinal degeneration. Invest Ophthalmol Vis Sci. 2011;52:959–968. doi: 10.1167/iovs.10-6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li ZL, Lam S, Tso MO. Desferrioxamine ameliorates retinal photic injury in albino rats. Curr Eye Res. 1991;10:133–144. doi: 10.3109/02713689109001741. [DOI] [PubMed] [Google Scholar]
- 29.Lakhanpal V, Schocket SS, Jiji R. Deferoxamine (Desferal)-induced toxic retinal pigmentary degeneration and presumed optic neuropathy. Ophthalmology. 1984;91:443–451. doi: 10.1016/s0161-6420(84)34267-1. [DOI] [PubMed] [Google Scholar]
- 30.Boddaert N, Le QSKH, Rotig A, Leroy-Willig A, Gallet S, Brunelle F, Sidi D, Thalabard JC, Munnich A, Cabantchik ZI. Selective iron chelation in Friedreich ataxia: biologic and clinical implications. Blood. 2007;110:401–408. doi: 10.1182/blood-2006-12-065433. [DOI] [PubMed] [Google Scholar]
- 31.Shah SV, Rajapurkar MM. The role of labile iron in kidney disease and treatment with chelation. Hemoglobin. 2009;33:378–385. doi: 10.3109/03630260903212233. [DOI] [PubMed] [Google Scholar]
- 32.Chen L, Wu W, Dentchev T, Zeng Y, Wang J, Tsui I, Tobias JW, Bennett J, Baldwin D, Dunaief JL. Light damage induced changes in mouse retinal gene expression. Exp Eye Res. 2004;79:239–247. doi: 10.1016/j.exer.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 34.Dunaief JL, Dentchev T, Ying GS, Milam AH. The role of apoptosis in age-related macular degeneration. Arch Ophthalmol. 2002;120:1435–1442. doi: 10.1001/archopht.120.11.1435. [DOI] [PubMed] [Google Scholar]
- 35.Chen L, Dentchev T, Wong R, Hahn P, Wen R, Bennett J, Dunaief JL. Increased expression of ceruloplasmin in the retina following photic injury. Mol Vis. 2003;9:151–158. [PubMed] [Google Scholar]
- 36.Mittag TW, Bayer AU, La VAIL MM. Light-induced retinal damage in mice carrying a mutated SOD I gene. Exp Eye Res. 1999;69:677–683. doi: 10.1006/exer.1999.0748. [DOI] [PubMed] [Google Scholar]
- 37.Wang M, Lam TT, Fu J, Tso MO. TEMPOL, a superoxide dismutase mimic, ameliorates light-induced retinal degeneration. Res Commun Mol Pathol Pharmacol. 1995;89:291–305. [PubMed] [Google Scholar]
- 38.Ni YQ, Xu GZ, Hu WZ, Shi L, Qin YW, Da CD. Neuroprotective effects of naloxone against light-induced photoreceptor degeneration through inhibiting retinal microglial activation. Invest Ophthalmol Vis Sci. 2008;49:2589–2598. doi: 10.1167/iovs.07-1173. [DOI] [PubMed] [Google Scholar]
- 39.Collier RJ, Wang Y, Smith SS, Martin E, Ornberg R, Rhoades K, Romano C. Complement Deposition and Microglial Activation in the Outer Retina in Light-Induced Retinopathy: Inhibition by a 5-HT1A agonist. Invest Ophthalmol Vis Sci. 2011 doi: 10.1167/iovs.10-6418. [DOI] [PubMed] [Google Scholar]
- 40.Zhang C, Shen JK, Lam TT, Zeng HY, Chiang SK, Yang F, Tso MO. Activation of microglia and chemokines in light-induced retinal degeneration. Mol Vis. 2005;11:887–895. [PubMed] [Google Scholar]
- 41.Rohrer B, Guo Y, Kunchithapautham K, Gilkeson GS. Eliminating complement factor D reduces photoreceptor susceptibility to light-induced damage. Invest Ophthalmol Vis Sci. 2007;48:5282–5289. doi: 10.1167/iovs.07-0282. [DOI] [PubMed] [Google Scholar]
- 42.Osaki S, Johnson DA, Frieden E. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J Biol Chem. 1966;241:2746–2751. [PubMed] [Google Scholar]
- 43.Patel BN, Dunn RJ, Jeong SY, Zhu Q, Julien JP, David S. Ceruloplasmin regulates iron levels in the CNS and prevents free radical injury. J Neurosci. 2002;22:6578–6586. doi: 10.1523/JNEUROSCI.22-15-06578.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beckman JS, Ischiropoulos H, Zhu L, der Woerd M v, Smith C, Chen J, Harrison J, Martin JC, Tsai M. Kinetics of superoxide dismutase- and iron-catalyzed nitration of phenolics by peroxynitrite. Arch Biochem Biophys. 1992;298:438–445. doi: 10.1016/0003-9861(92)90432-v. [DOI] [PubMed] [Google Scholar]
- 46.Kontoghiorghes GJ, Efstathiou A, Kleanthous M, Michaelides Y, Kolnagou A. Risk/benefit assessment, advantages over other drugs and targeting methods in the use of deferiprone as a pharmaceutical antioxidant in iron loading and non iron loading conditions. Hemoglobin. 2009;33:386–397. doi: 10.3109/03630260903217141. [DOI] [PubMed] [Google Scholar]
- 47.Kontoghiorghes GJ, Kolnagou A, Peng CT, Shah SV, Aessopos A. Safety issues of iron chelation therapy in patients with normal range iron stores including thalassaemia, neurodegenerative, renal and infectious diseases. Expert Opin Drug Saf. 2010;9:201–206. doi: 10.1517/14740330903535845. [DOI] [PubMed] [Google Scholar]
- 48.Liu G, Men P, Perry G, Smith MA. Nanoparticle and iron chelators as a potential novel Alzheimer therapy. Methods Mol Biol. 2010;610:123–144. doi: 10.1007/978-1-60327-029-8_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boelaert JR, Weinberg GA, Weinberg ED. Altered iron metabolism in HIV infection: mechanisms, possible consequences, and proposals for management. Infect Agents Dis. 1996;5:36–46. [PubMed] [Google Scholar]
- 50.Hidajat RR, McLay JL, Goode DH, Spearing RL. EOG as a monitor of desferrioxamine retinal toxicity. Doc Ophthalmol. 2004;109:273–278. doi: 10.1007/s10633-005-1336-9. [DOI] [PubMed] [Google Scholar]
- 51.Karimi M, Asadi-Pooya AA, Khademi B, Asadi-Pooya K, Yarmohammadi H. Evaluation of the incidence of sensorineural hearing loss in beta-thalassemia major patients under regular chelation therapy with desferrioxamine. Acta Haematol. 2002;108:79–83. doi: 10.1159/000064748. [DOI] [PubMed] [Google Scholar]
- 52.Baath JS, Lam WC, Kirby M, Chun A. Deferoxamine-related ocular toxicity: incidence and outcome in a pediatric population. Retina. 2008;28:894–899. doi: 10.1097/IAE.0b013e3181679f67. [DOI] [PubMed] [Google Scholar]
- 53.Fredenburg AM, Sethi RK, Allen DD, Yokel RA. The pharmacokinetics and blood-brain barrier permeation of the chelators 1,2 dimethly-, 1,2 diethyl-, and 1-[ethan-1'ol]-2-methyl-3-hydroxypyridin-4-one in the rat. Toxicology. 1996;108:191–199. doi: 10.1016/0300-483x(95)03301-u. [DOI] [PubMed] [Google Scholar]
- 54.Kontoghiorghes GJ, Neocleous K, Kolnagou A. Benefits and risks of deferiprone in iron overload in Thalassaemia and other conditions: comparison of epidemiological and therapeutic aspects with deferoxamine. Drug Saf. 2003;26:553–584. doi: 10.2165/00002018-200326080-00003. [DOI] [PubMed] [Google Scholar]
- 55.Sohn YS, Breuer W, Munnich A, Cabantchik ZI. Redistribution of accumulated cell iron: a modality of chelation with therapeutic implications. Blood. 2008;111:1690–1699. doi: 10.1182/blood-2007-07-102335. [DOI] [PubMed] [Google Scholar]
- 56.Cappellini MD, Pattoneri P. Oral iron chelators. Annu Rev Med. 2009;60:25–38. doi: 10.1146/annurev.med.60.041807.123243. [DOI] [PubMed] [Google Scholar]
- 57.Galanello R, Campus S. Deferiprone chelation therapy for thalassemia major. Acta Haematol. 2009;122:155–164. doi: 10.1159/000243800. [DOI] [PubMed] [Google Scholar]
- 58.Cappellini MD, Piga A. Current status in iron chelation in hemoglobinopathies. Curr Mol Med. 2008;8:663–674. doi: 10.2174/156652408786241438. [DOI] [PubMed] [Google Scholar]
- 59.A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Obolensky A, Berenshtein E, Lederman M, Bulvik B, Alper-Pinus R, Yaul R, Deleon E, Chowers I, Chevion M, Banin E. Zinc-desferrioxamine attenuates retinal degeneration in the rd10 mouse model of retinitis pigmentosa. Free Radic Biol Med. 2011 doi: 10.1016/j.freeradbiomed.2011.07.014. [DOI] [PubMed] [Google Scholar]