Abstract
The Mi-1.2 resistance gene in tomato (Solanum lycopersicum) confers resistance against several species of root-knot nematodes (Meloidogyne spp.). This study examined the impact of M. javanica on the reproductive fitness of near-isogenic tomato cultivars with and without Mi-1.2 under field and greenhouse conditions. Surprisingly, neither nematode inoculation or host plant resistance impacted the yield of mature fruits in field microplots (inoculum=8,000 eggs/plant), or fruit or seed production in a follow-up greenhouse bioassay conducted with a higher inoculum level (20,000 eggs/plant). However, under heavy nematode pressure (200,000 eggs/plant), greenhouse-grown plants carrying Mi-1.2 had more than ten-fold greater fruit production than susceptible plants and nearly forty-fold greater estimated lifetime seed production, confirming prior reports of the benefits of Mi-1.2. In all cases Mi-mediated resistance significantly reduced nematode reproduction. These results indicated that tomato can utilize tolerance mechanisms to compensate for moderate levels of nematode infection, but that the Mi-1.2 resistance gene confers a dramatic fitness benefit under heavy nematode pressure. No significant cost of resistance was detected in the absence of nematode infection.
Keywords: costs and benefits of resistance, Meloidogyne javanica, Mi-1, Mi-1.2, nematode resistance, plant reproductive fitness, R gene, root-knot nematode, Solanum lycopersicum, tomato
Plants display an array of sophisticated biochemical and morphological traits that limit attack from a variety of pests, such as pathogens, insects, and other herbivores. Acquired or induced resistance is dependent upon a conglomeration of traits that are upregulated by pest damage, and that can reduce the severity of subsequent assaults from a broad range of potential attackers (Cipollini et al., 2003; Agrawal, 2005). This phenotypic plasticity is presumed to be controlled by many interacting genes in the plant. In contrast, R gene-mediated pest resistance rapidly blocks initial attacks by one or a small number of pest species, and is controlled by simple but highly specific gene-for-gene interactions between the plant and the pest (Dangl and Jones, 2001). According to this model, the presence of a single resistance (R) gene in the plant allows the rapid detection of a corresponding avirulence (Avr) gene in the pest, resulting in incompatibility (Flor, 1971).
Regardless of whether it is acquired or R gene-mediated, any plant trait that blocks pest establishment or limits their proliferation can be considered a source of resistance. However, Karban and Baldwin (1997) propose that the term “plant defense” should be reserved for traits that have also been shown to enhance plant fitness in the presence of pests. Fitness represents the plant’s lifetime reproductive success, and is the critical trait on which natural selection acts. The term “plant defense” implies that the trait in question is an adaptation to pest pressure.
Plant-herbivore interactions have long been used as model systems to study co-evolution (Agrawal, 1998; Baldwin, 1998; De Meaux and Mitchell-Olds, 2003). The theory of co-evolution proposes that reciprocal genetic changes have occurred in plants and their associated herbivores, driven by the costs and benefits of these changes to Darwinian fitness. According to this hypothesis, plants have developed a variety of resistance traits (eg. thorns, trichomes, toxic chemicals, and antinutritive proteins) to combat herbivory and limit its fitness costs (Sagers and Phyllis, 1995; Agrawal et. al., 1999a). Through selection, insects have responded with adaptations to cope with the new plant traits, such as detoxifying enzymes or behavioral avoidance mechanisms (Strauss and Agrawal, 1999; Gardner and Agrawal, 2002). Many of the plant traits that deter herbivores, however, could potentially have developed in response to other selective pressures. For example, leaf trichomes have been identified in biological functions such as toxin removal, UV protection, and water retention (Smith and Hare, 2004). These abiotic fators may aid in the selection of trichome production or trichome density (Karkkainen et al., 2004; Gianoli and Gonzallez-Teuber, 2005). Therefore, before making any inferences about the adaptive significance or evolutionary history of a particular form of resistance, empirical tests are needed to assess the costs and benefits of this trait to plant fitness in the presence and absence of pests.
The majority of studies that have examined the costs and benefits of resistance have focused on traits that contribute to induced insect resistance, such as trichome density in wild radish (Raphanus spp.) and nicotine synthesis in a wild tobacco (Nicotiana attenuata) (Karban et al., 1997; Agrawal, 1999). Fewer research groups have assessed the effects of R genes on plant reproductive success, although dramatic progress has recently been made in studying R gene-mediated bacterial resistance in Arabidopsis thaliana (Tian et al., 2003; Korves and Bergelson, 2004). Data from this system suggests that wild Arabidopsis populations experience intermittent periods of extreme pathogen attack, during which plants that carry RPM1 and other R genes for disease resistance have a strong selective advantage. During periods of low pathogen incidence, however, susceptible plants boast higher fitness than resistant genotypes. Bergelson and coworkers propose that as a result of these trade-offs, both resistant and susceptible alleles of R gene loci are maintained in Arabidopsis populations through balancing selection (Tian et al., 2003; Korves and Bergelson, 2004).
The goal of the present study was to use tomato as a model system to measure the fitness costs and benefits of R gene-mediated herbivore resistance. The Mi-1.2 gene is present in many tomato cultivars and confers resistance to three common root-knot nematode species (Meloidogyne incognita, M. javanica, M. arenaria), as well as three insect species (the potato aphid, Macrosiphum euphorbiae; the sweetpotato whitefly, Bemisia tabaci; and the tomato psyllid, Bactericerca cockerelli) (Milligan et al., 1998; Rossi et al., 1998; Nombela et al., 2003; Casteel et al., 2006). Our study focused on the effects of Mi-1.2 on root-knot nematodes, which are the most damaging of these herbivores on tomato. Root-knot nematodes disrupt the vascular system of their host plant causing symptoms that include stunted plant growth, chlorosis or premature death, and increase susceptibility to drought and other pathogens (Jenkins and Taylor, 1967). These endoparasites cause severe yield reductions in agricultural crops including cultivated tomato, and Mi-1.2 is the only known source of root-knot nematode resistance in cultivated tomato (Williamson, 1998). In plants that carry Mi-1.2, a hypersensitive reaction (HR), which involves rapid localized cell death, stops the nematode from establishing a feeding site (Dropkin, 1969; Williamson, 1998). Many studies of artificially-inoculated plants have shown that Mi-mediated resistance can dramatically reduce root-knot nematode survival, reproduction and gall induction (eg. Gilbert and McGuire, 1956; Barham and Winstead, 1957; Dropkin, 1969; Milligan et al., 1998; Goggin et al. 2004), but fewer studies have quantified the effects of resistance on fruit production by the host plant (Sorribas et al., 2005; Lopez-Perez et al., 2006). Furthermore, previous studies have not examined the impact of Mi-1.2 on seed production, a key measure of plant fitness that would strongly influence the adaptive value of resistance in an evolutionary context. The objectives of this study were to examine the potential fitness costs and benefits of the R gene-mediated herbivore resistance, and to explore the role of nematodes as a selection pressure favoring plants that carry Mi-1.2. Fruit production was compared under field conditions in near-isogenic tomato cultivars with and without Mi-1.2 challenged with nematodes or mock-inoculated with water. In addition, fruit and seed production were measured in greenhouse assays of the same design performed at two inoculum levels (moderate and high nematode pressure).
Materials and Methods
Nematode cultures and inoculation: Root-knot nematodes, Meloidogyne javanica (VW4 isolate), were obtained from Dr. V. M. Williamson (University of California, Davis). Nematodes were maintained on susceptible tomato plants (cv. Moneymaker) under greenhouse conditions (∼24°C-27°C; 16:8 L:D photoperiod). Nematode eggs were collected from plants inoculated at least seven weeks prior to collection. Eggs were extracted from infected root systems using a 10% solution of commercial bleach, and were resuspended in water and quantified by examining serial dilutions with a light microscope (Hussey and Barker, 1973). Experimental plants were inoculated with 8,000 (microplot assay), 20,000 (greenhouse assay 1) or 200,000 (greenhouse assay 2) nematode eggs via pipette on each side of the root crown, while control plants were mock-inoculated with a comparable volume of water.
Microplot Field Trial: Two indeterminate, nearly isogenic tomato cultivars with (cv Motelle) and without (cv. Moneymaker) the Mi-1.2 resistance gene were germinated in sand (Play Sand, Quikrete, Atlanta, GA) in the greenhouse. When seedlings had 5-6 true leaves, seedlings were transplanted to the field in a randomized design in individual microplots of pasteurized sandy loam (each 46 X 46 X 61 cm deep, 1 plant per microplot) embedded in the earth on the grounds of the Agricultural Experiment Station (planting date: 5/18/05), and assigned to four treatment groups (susceptible inoculated, susceptible control, resistant inoculated, resistant control; 8 plants/ treatment). Control plants were mock-inoculated with water, whereas plants assigned to the nematode treatment were inoculated with 8,000 eggs/plant). Plants were irrigated as needed and fertilized every two weeks with Expert Gardener Plant Food (Chemsico, St. Louis, MO. NPK ratio: 15-30-15) according to the manufacturer’s instructions for tomato. Tomatoes were picked at the red-ripe stage approximately every 3 days during the fruit production period (11 harvests from 7/15 to 8/17). Data from all harvest dates were pooled to calculate total yield per plant. At the end of peak fruit production (8/17), all remaining immature green fruits were collected separately, and whole root systems were dug up and sent to a nematology diagnostics facility (Dr. Kirkpatrick, University of Arkansas, Hope, AR) for nematode egg quantification. Whole root systems were dried and weighed after nematode extraction to calculate the number of eggs per gram of dry root tissue.
Greenhouse Assays: The near-isogenic tomato cultivars Castlerock II (Mi-1.2-, susceptible) and Sun 6082 (Mi-1.2+, resistant) were used for greenhouse assays because their determinate growth habit was better suited to the space constraints of the greenhouse than the indeterminate cultivars Moneymaker and Motelle. All plants were grown in 11-liter plastic pots with ∼10-liters autoclaved sand (Play Sand, Quikrete, Atlanta, GA) under stable greenhouse conditions (∼24°C-27°C; 16:8 L:D photoperiod). Tomatoes were watered three times daily with a nutrient solution containing 1000 mg/L CaNO (Hydro Agri North America, Tampa, FL), 500 mg/L MgSO (Giles Chemical Corp, Waynesville, NC), and 500 mg/L Hydroponic 4-18-38 Growmore fertilizer (Growmore, Gardena, CA).
Four treatments of tomato plants (susceptible inoculated, susceptible control, resistant inoculated, resistant control; 8 plants/ treatment) were grown and allowed to fruit. Assay 1 utilized a moderate inoculum level (20,000 eggs/ plant), and assay 2 used a high inoculum level (200,000 eggs/plant). The fruit was collected and weighed as it became ripe. Red-ripe fruit was collected twice a week until the end of peak fruit production (10 weeks for assay 1; 18 weeks for assay 2). At the end of the experiment, as fruit production was waning, all remaining green fruit was collected and scored separately. Three ripe tomatoes of average size were chosen from each plant for seed extraction. Seeds were counted and weighed (AG285, Mettler Toledo, Columbus, OH) for analysis. Total foliar biomass (stems and leaves) was collected at the end of the experiment, after fruit production began to wane, and was dried for five days at 26°C before being weighed. Whole root systems were collected and sent to the nematology diagnostics facility (University of Arkansas, Hope, AR) for nematode egg quantification. Whole root systems were dried and weighed after nematode extraction. Germination rates of seeds were measured from a sub-sample of the seeds collected (10 seeds/plant; 8 plants/treatment group). Seeds were sown in 1 cup plastic square pots of vermiculite (Vermiculite, Schultz, Atlanta, GA) and grown in greenhouse conditions same as above, and watered daily with tap water. Germination rates were recorded every two days for ten days after planting.
Statistics: For each assay, tomato yield, seed count and weight, root and foliar dry weight, and nematode reproduction were compared on our 4 treatment groups using full factorial 2-way analysis of variance (ANOVA) and Student’s t-test (JMP version 5.01, SAS Institute, Cary, NC). For any measurements that included subreplication, averages are reported ± the standard error of the means (SEM). All other measurements for which we have only one estimate per replicate are reported ± the standard deviation of the mean (SD).
Results
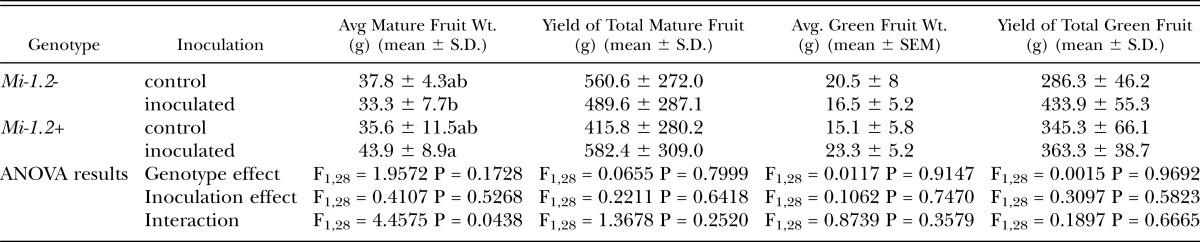
Microplot Assay: In field-grown resistant (Mi-1.2+) and susceptible (Mi-1.2-) plants inoculated with 8,000 nematode eggs or mock-inoculated with water, the total yield of mature fruits was not significantly impacted by plant genotype or nematode inoculation, although when all immature fruits were collected after peak fruit production was over, resistant plants inoculated with nematodes yield significantly more green fruits than susceptible, inoculated plants (Table 1). Dry root weight (averages: Mi-1.2-, control = 0.92 ± 0.45g, Mi-1.2-, inoculated 1.40 = ± 0.48g, Mi-1.2+, control = 0.96 ± 0.83g, Mi-1.2+, inoculated = 1.01 ± 0.31g) was not significantly influenced by cultivar (F1,28= 0.8050, P=0.3772), inoculation (F1,28=1.8243, P=0.1876), or the interaction between the two (F1,28=1.1855, P=0.2855). Nematode reproduction, as measured by the number of eggs per gram of dry root weight, was significantly lower on the resistant cultivar Motelle (2,800 eggs/g) than on the susceptible cultivar Moneymaker (85,260 eggs/g) (F1,14=30.09, P < 0.0001).
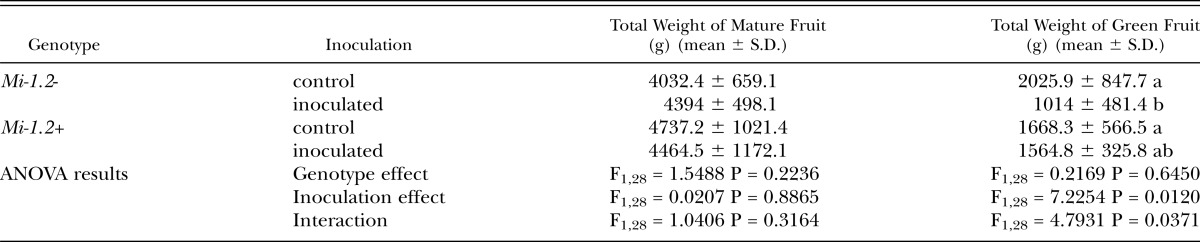
Table 1.
Fruit production from field-grown plants inoculated with 8,000 nematode eggs. Red-ripe fruits were harvested twice a week for 11 weeks, and then, as fruit production was waning, all remaining green fruits were collected at the end of the assay. Data on total mature and immature fruit weights were analyzed by Two-way ANOVA. Where there was a significant interaction between treatment and genotype, mean separations were also performed using Student’s t tests (values followed by the same letter are not significantly different from each other at α=0.05) Neither nematode inoculation nor plant genotype had a significant effect on the number or total weight of mature fruits collected, although, in the presence of nematodes, the yield of immature fruits at the end of the season was higher for resistant plants than susceptible plants.
Greenhouse Bioassays: To follow up on the microplot assay, two greenhouse assays were performed to measure the impact of Mi-1.2 and of nematode infection at two inoculum levels (assay 1: 20,000 eggs/plant; assay 2: 200,000 eggs/plant) on fruit and seed production, nematode infection, and above and below-ground biomass.
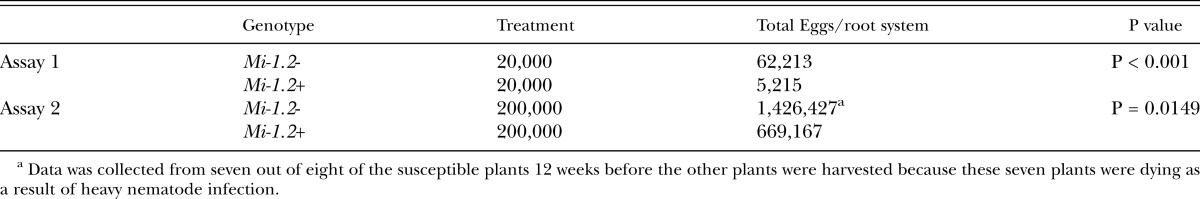
Nematode infection: Nematode data was transformed for analysis using the formula ‘log +1’ to normalize the variances. Mi-mediated resistance significantly reduced nematode reproduction for both inoculum levels, as measured by the number of egg masses per root system (Table 2).
Table 2.
Effects of Mi-mediated resistance on nematode reproduction in greenhouse bioassays. Plants were inoculated with nematode eggs at moderate (20,000 eggs/plant) and high (20,000 eggs/plant) inoculum levels under greenhouse conditions. After peak fruit production was over, root systems were harvested, nematode eggs were extracted from the roots, and egg numbers on inoculated plants were compared by One-way ANOVA. At both inoculum levels, resistant (Mi-1.2+) plants had significantly lower eggs/ gram root than susceptible (Mi-1.2-) plants.
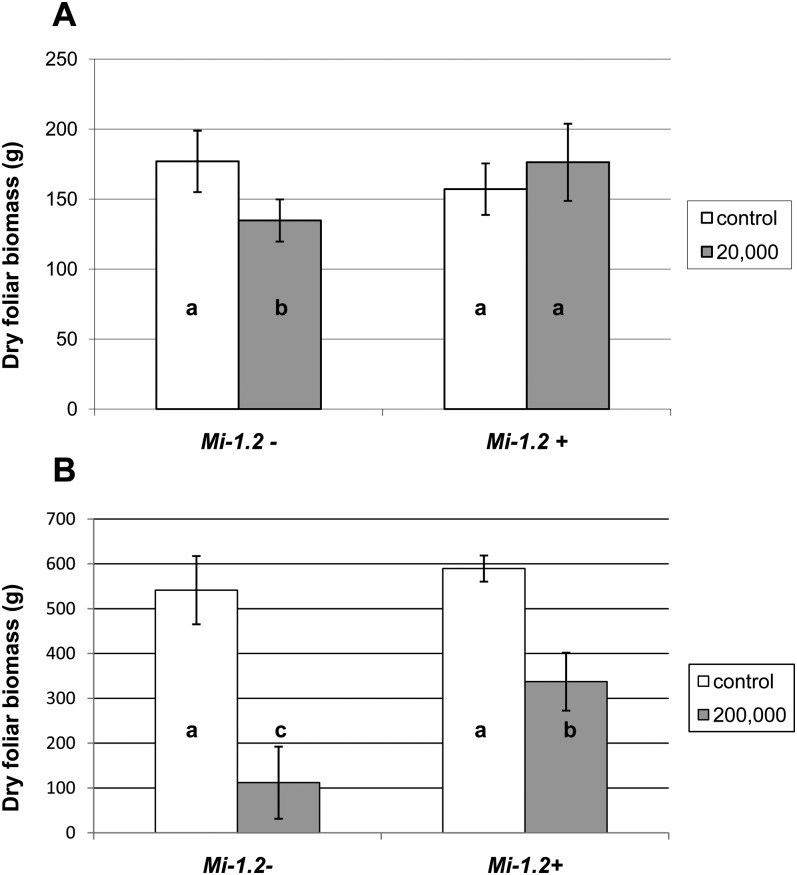
Foliar Biomass: When plants were inoculated with 20,000 nematode eggs, there was a significant interaction between the treatment (control or inoculated) and the plant genotype (P < 0.05), because nematode inoculation significantly reduced foliar dry weight of the susceptible (Mi-1.2-) genotype, but not the resistant (Mi-1.2+) cultivar (Fig. 1 A). At the higher (200,000 eggs/plant) inoculum level, foliar dry weight was significantly impacted by treatment, genotype, and the interaction between these factors (P < 0.001) (Fig. 1 B). Both genotypes suffered a reduction in foliar biomass, but this reduction was significantly greater in the susceptible cultivar (P < 0.05).
Fig. 1.
Effects of Mi-mediated resistance and nematode inoculation on dry foliar weight (±S.D.) of tomato plants. Plants were inoculated with 20,000 (A) and 200,000 (B) nematode eggs, while control plants were mock-inoculated with water. Foliar dry weight was analyzed by Two-way ANOVA. Where there was a significant interaction between treatment and genotype, mean separations were also performed using Student’s t-tests (values followed by the same letter are not significantly different from each other at α=0.05). (A) When plants were inoculated with 20,000 nematode eggs, nematode inoculation significantly reduced foliar dry weight of the susceptible (Mi-1.2-) genotype, but not the resistant (Mi-1.2+) cultivar. (B) At the higher inoculum level, both genotypes suffered a reduction in foliar biomass, but this reduction was significantly greater in the susceptible cultivar. Seven of eight susceptible plants inoculated with nematodes were harvested about 3 months earlier than other plants because they were succumbing to nematode infection.
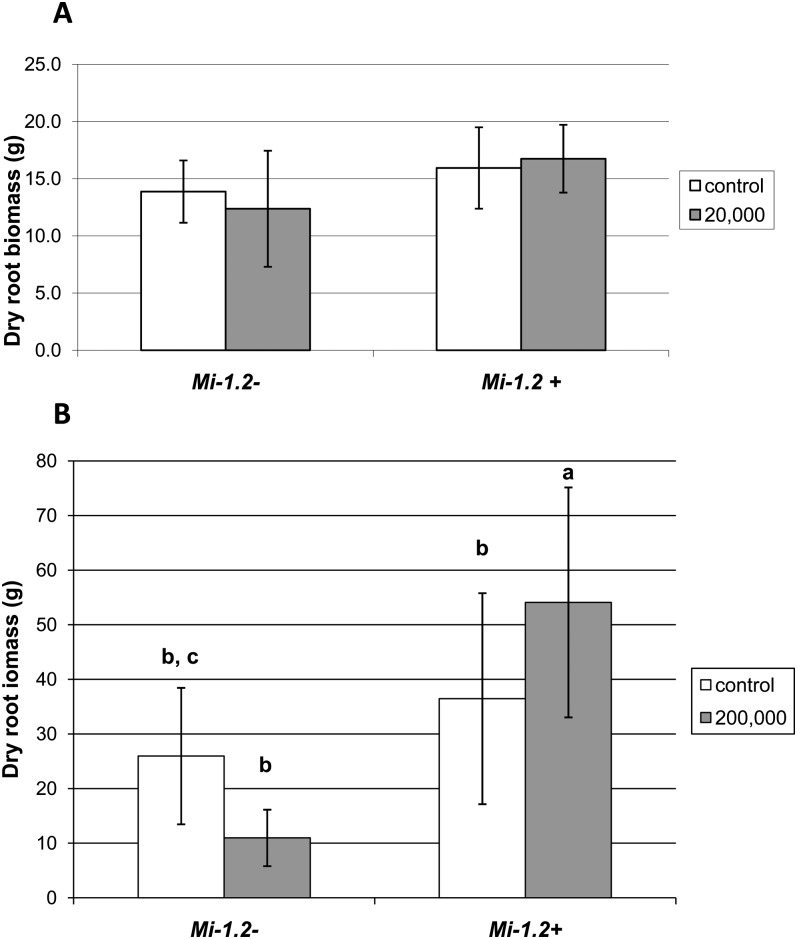
Root biomass: At the lower inoculum level, nematode infestation did not have a significant effect on root weights, nor was there a significant interaction between the treatment and genotype (P > 0.10), although overall root weights were higher in the resistant genotype than in the susceptible cultivar (P = 0.02) (Fig. 2 A). At the higher inoculum level, there was a significant interaction between treatment and genotype (P = 0.007), because nematode infection increased the root biomass of the resistant genotype (Mi-1.2+) compared to the uninoculated resistant plants (Figure 2 B). Nematode challenge may have stimulated the root growth, or the increase may have been due to modest gall formation.
Fig. 2.
Effects of Mi-mediated resistance and nematode inoculation on root weight (±S.D.) of tomato plants. Plants were inoculated with 20,000 (A) and 200,000 (B) nematode eggs, while control plants were mock-inoculated with water. Nematode infection did not alter root biomass at the lower inoculum level (A), whereas the higher inoculum level caused an increase in the root biomass of resistant (Mi-1.2-) plants.
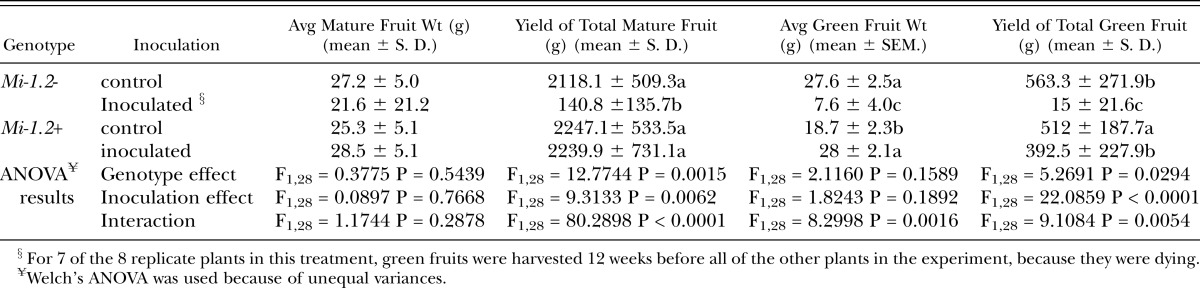
Fruit production: In assay 1, in which plants were inoculated with 20,000 eggs, neither nematode inoculation nor plant genotype had a significant effect on the number or total yield (in grams) of mature fruits collected (Table 3). However, in the presence of nematodes, resistant plants produced significantly larger fruits than susceptible plants. There was no significant difference among treatments in the number, total weight, or average weight of green fruits collected from the plants at the termination of the experiment, when fruit production was waning. In assay 2, in which plants were inoculated with 200,000 nematode eggs, nematode infection dramatically reduced the number and total weight of mature fruits produced by susceptible plants, but had no effect on the fruit production of resistant plants, which was comparable to that of uninoculated controls (Table 4). Compared to susceptible plants challenged with nematodes, inoculated resistant plants also bore more green fruits at the end of the experiment. These dramatic differences in yield resulted from the fact that all but one of the susceptible inoculated plants died before the end of the experiment.
Table 3.
Fruit production from greenhouse-grown plants inoculated with 20,000 nematode eggs. Red-ripe fruits were harvested twice a week for 10 weeks, and all remaining green fruits were harvested as fruit production was waning, at the end of the assay. Data on fruit number, average and total fruit weight was analyzed by Two-way ANOVA. Where there was a significant interaction between treatment and genotype, mean separations were also performed using Student’s t tests (values followed by the same letter are not significantly different from each other at α=0.05). Neither nematode inoculation nor plant genotype had a significant effect on the number or total weight of mature fruits collected, but in the presence of nematodes, resistant (Mi-1.2+) plants produced significantly larger fruits than susceptible (Mi-1.2-) plants. There was no significant difference among treatments in the number, total weight, or average weight of green fruits collected from the plants at the termination of the experiment.
Table 4.
Fruit production of greenhouse-grown plants inoculated with 200,000 nematode eggs. Red-ripe fruits were harvested twice a week for 18 weeks, and all remaining green fruits were harvested as fruit production was waning, at the end of the assay. Data on fruit number, average and total fruit weight was analyzed by Two-way ANOVA. Where there was a significant interaction between treatment and genotype, mean separations were also performed using Student’s t tests (values followed by the same letter are not significantly different from each other at α=0.05). Nematode infection dramatically reduced mature fruit yield in the susceptible (Mi-1.2-) but not the resistant cultivar (Mi-1.2+). Nematode inoculation reduced the remaining green fruits at the end of the season in both genotypes, but the magnitude of this effect was greater in the susceptible cultivar.
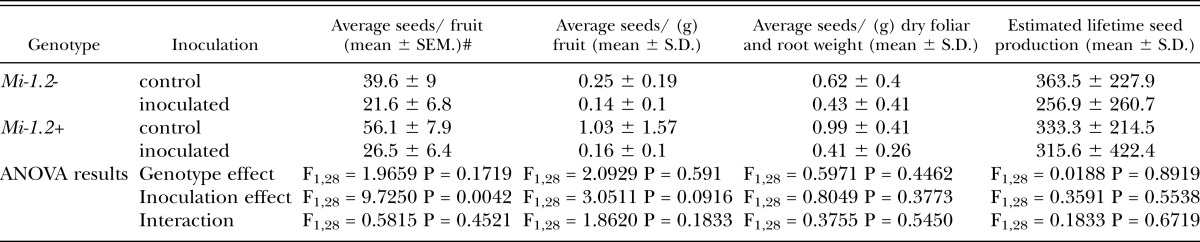
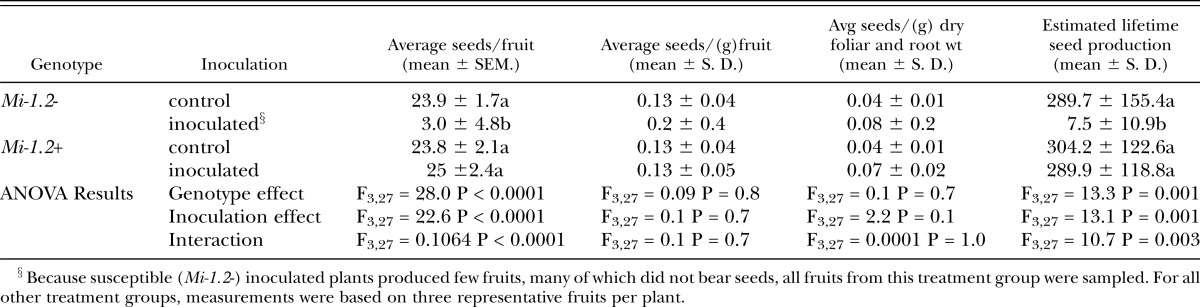
Seed production: At the lower inoculum level (20,000 eggs), nematode challenge caused a decrease in the average number of seeds per mature fruit from both cultivars, but did not significantly reduce the estimated lifetime seed production of either genotype (Table 5). Because nematode infection did not have a marked impact on seed production in this assay, we did not discern a fitness benefit associated with Mi-mediated resistance at this inoculum level. At the higher inoculum level (200,000 eggs), however, nematode infection dramatically reduced seed production in the susceptible line (Table 6), and the majority of fruits produced by infected susceptible plants bore no seeds. In contrast, the seed production of inoculated resistant plants was comparable to that of uninoculated controls, indicating that resistance provided a dramatic fitness benefit. The average weights of individual seeds as well as their germination rates were also measured, but nematode infection and Mi-mediated resistance did not impact these parameters (data not shown).
Table 5.
Seed production of plants inoculated with 20,000 nematode eggs. Seeds were harvested from three representative fruits per plant. The lifetime seed production was estimated by multiplying the total number of grams of ripe fruit produced by the average number of seeds per gram for the tomatoes sampled. All parameters were analyzed by Two-Way ANOVA. Nematode infection reduced the number of seeds per fruit for both cultivars, but all other parameters were unaffected by nematode challenge or by genotype.
Table 6.
Seed production of plants inoculated with 200,000 nematode eggs. Seeds were harvested from a cross-sample of fruits§, and the estimated lifetime seed production was estimated by multiplying the total number of grams of ripe fruit produced by the average number of seeds per gram for the tomatoes sampled. All parameters were analyzed by Two-Way ANOVA. Where there was a significant interaction between treatment and genotype, mean separations were also performed using Student’s t tests (values followed by the same letter are not significantly different from each other at α=0.05). Nematode infection reduced the number of seeds per fruit and the estimated lifetime seed production of susceptible (Mi-1.2-) but not resistant (Mi-1.2+) plants.
Discussion
When inoculated with 200,000 nematode eggs, the tomato cultivar that carries the Mi-1.2 resistance gene had significantly greater foliar biomass, root mass, fruit and seed production compared to inoculated susceptible plants (Mi-1.2-). Whereas the resistant plants survived and reproduced, all but one of the susceptible inoculated plants died before the end of the experiment and produced few if any seeds. Therefore, at this inoculum level, Mi-mediated resistance provided a dramatic fitness benefit to the plants. In contrast, at a lower inoculum level (20,000 eggs/plant), Mi-mediated resistance had no major impact on estimated lifetime seed production, although it reduced nematode numbers and conferred a modest benefit for fruit size and vegetative (root and foliar) biomass. We were not able to detect an advantage of Mi-mediated resistance at this inoculum level in large part because nematode infection did not significantly reduce the reproductive success of the susceptible cultivar. Similarly, when we compared the yields of a different set of near-isogenic tomato cultivars (Mi-1.2+ and Mi-1.2-) that were field-grown in microplots and inoculated with 8,000 eggs/plant or mock-inoculated with water, we did not observe any reductions in mature fruit yield as a result of nematode infection. These results indicate that the tomato plants were able to tolerate or compensate for moderate levels of nematode infection. This finding is consistent with data presented in two recent studies of nematode infestation on tomato. Lopez-Perez et al. (2006) inoculated greenhouse-grown plants with 0, 102, 103, 104, and 105 root-knot nematode eggs, and found that only the highest inoculum level significantly reduced the total fruit mass produced by a susceptible (Mi-1.2-) tomato cultivar. In a field study, Sorribas et al. (2005) grew a susceptible cultivar in naturally-infested soil for three consecutive years and observed abundant galls and nematode eggs on the roots (∼40,000 – 50,000 eggs/ gram root mass). Although this study concluded that total yield over three years was lower in infested versus fumigated soil, a year-by-year analysis of the data shows that nematodes significantly reduced yield in only one out of three field seasons.
Therefore, tomato appears to utilize tolerance as well as resistance to minimize the impact of nematodes on seed production, and the relative importance of these two adaptations varies depending upon the intensity of the nematode pressure. In contrast to resistance, defined as any trait that reduces pest infestations, tolerance reduces the impact of infestations on plant fitness (Restif and Koella, 2004). The physiological and molecular mechanisms underlying tolerance are not thoroughly understood, but are thought to involve relocation of resources such as photoassimilates to less vulnerable parts of the plant (Agrawal et al., 1999b). Plants display varying degrees of tolerance against biotic and abiotic stresses, and selection for tolerance in crop plants has been a goal of agricultural breeding for decades. More recently, experiments to determine how plants use tolerance are increasing. A study by Schwachtje and coworkers showed that Nicotiana attenuata relocates sugars to roots in response to simulated herbivore attack, for storage and future regrowth (Schwachtje et al., 2006). Further work is needed to explore mechanisms of plant tolerance to nematode infection, and also to study tolerance under field conditions. The plants in this experiment were grown with abundant irrigation and fertilization, whereas plants grown under natural conditions may have more limited water and nutrients to allocate for growth and reproduction. Furthermore, plants in this study were grown in autoclaved sand, absent of root pathogens and other pests that might otherwise attack plants predisposed to stress by nematode infestation. Therefore, the threshold level of nematode pressure at which the plant’s tolerance mechanisms become inadequate and resistance becomes beneficial is likely to be lower under natural conditions than under the optimal growth conditions of an experimental trial.
The strength of the selection pressure posed by nematodes is also highly variable over space and time. Naturally-occurring infection rates for the root-knot nematode are influenced by many factors such as soil type, climate, and the availability of other host plants over the course of the year (Jenkins and Taylor, 1967). As a result of this and of the limited mobility of root-knot nematodes, nematode distribution even within a single field can be extremely patchy. It is therefore impossible to estimate “average” infestation levels for this pest. However, in a study of Georgia field plots in which tomato was grown in rotation with other crops, Johnson and Campbell (1980) found that juvenile root-knot nematode populations ranged from 0-13 per cm3 of soil, and surveys of agricultural and uncultivated soils in Florida and tomato fields in California yielded similar ranges (Ploeg, 2002; McSorley et al., 2007). Given that the sodium hypochlorite egg extraction method that we used to collect our inoculum typically results in an egg hatch rate of 20-50 percent (Hussey and Barker, 1973; Dr. Terrance Kirkpatrick; personal communication), and that our plants were grown in 10 L of sand, we estimate that our higher inoculum level (200,000 eggs/plant) would fall between 4 and 10 juveniles per cm3 of soil, well within naturally-occurring ranges. Thus, naturally-occurring nematode infestations could act as a selection pressure favoring plants that carry Mi-1.2. It is also worth noting that we did not observe any costs associated with Mi-mediated resistance in the absence of nematode infection, in contrast to other forms of R-gene mediated pathogen resistance that are associated with a metabolic cost to the plant (Korves and Bergelson, 2004). This lack of costs may contribute to the maintenance of nematode resistance in plant populations even when the occurrence of damaging levels of nematode infection is spotty. Furthermore, Mi-mediated resistance also deters aphids, whiteflies, and psyllids, all of which could have an additive effect on plant fitness. This study clearly demonstrates the fitness advantage provided by Mi-1.2 in response to nematode challenge, and future work is merited to assess the benefits of Mi-1.2 in response to multiple biotic challenges.
LITERATURE CITED
- Agrawal AA, Tuzun S, Bent E. 1999a. Induced plant defense: Induced plant defenses against pathogens and herbivores: biochemistry, ecology, and agriculture. APS Press, St. Paul, Minn; pp. 251–268. [Google Scholar]
- Agrawal AA. Induced responses to herbivory in wild radish: Effects on several herbivores and plant fitness. Ecology. 1999;80:1713–1723. [Google Scholar]
- Agrawal AA, Strauss S, Stout MJ. Costs of induced responses and tolerance to herbivory in male and female fitness components of wild radish. Evolution. 1999b;53:1093–1104. doi: 10.1111/j.1558-5646.1999.tb04524.x. [DOI] [PubMed] [Google Scholar]
- Agrawal AA. Future directions in the study of induced plant responses to herbivory. Entomologia Experimentalis et Applicata. 2005;115:97–105. [Google Scholar]
- Agrawal AA. Induced responses to herbivory and increased plant performance. Science. 1998;279(5354):1201–1202. doi: 10.1126/science.279.5354.1201. [DOI] [PubMed] [Google Scholar]
- Agrawal AA. Overcompensation of plants in response to herbivory and the by-product benefits of mutualism. Trends in Plant Science. 2000;5:309–313. doi: 10.1016/s1360-1385(00)01679-4. [DOI] [PubMed] [Google Scholar]
- Baldwin IT. Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(14):8113–8118. doi: 10.1073/pnas.95.14.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barham WS, Winstead NN. Inheritance of resistance to root knot nematodes in tomatoes. Proceedings of the American Society of Horticultural Science. 1957;69:372–377. [Google Scholar]
- Casteel CL, Walling LL, Paine TD. Behavior and biology of the tomato psyllid, Bactericerca cockerelli, in response to the Mi-1.2 gene. Entomologia Experimentalis et Applicata. 2006;121:67–72. [Google Scholar]
- Cipollini D, Purrington CB, Bergelson J. Costs of induced responses in plants. Basic and Applied Ecology. 2003;4:79–89. [Google Scholar]
- Cooper WR, Jia L, Goggin F. Effects of jasmonate-induced defenses on root-knot nematode infection of resistant and susceptible tomato cultivars. Journal of Chemical Ecology. 2005;31(9):1953–1967. doi: 10.1007/s10886-005-6070-y. [DOI] [PubMed] [Google Scholar]
- Dangl JL, Jones JD. Plant pathogens and integrated defense responses to infection. Nature. 2001;411:826–833. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- De Meaux J, Mitchell-Olds T. Evolution of plant resistance at the molecular level: Ecological context of species interactions. Heredity. 2003;91:345–352. doi: 10.1038/sj.hdy.6800342. [DOI] [PubMed] [Google Scholar]
- Dropkin VH. The necrotic reaction of tomatoes and other hosts resistant to Meloidogyne: reversal by temperature. Phytopathology. 1969;59:1632–1637. [Google Scholar]
- Flor H. Current status of gene-for-gene concept. Annual Review of Phytopathology. 1971;9:275–296. [Google Scholar]
- Gardner SN, Agrawal AA. Induced plant defense and the evolution of counter-defenses in herbivores. Evolutionary Ecology Research. 2002;4:1131–1151. [Google Scholar]
- Gianoli E, Gonzallez-Teuber M. Environmental heterogeneity and population differentiation in plasticity to drought in Convolvulus chilensis (Convolvulaceae) Evolutionary Ecology. 2005;19:603–613. [Google Scholar]
- Gilbert JC, McGuire DC. Inheritance of resistance to severe root knot from Meloidogyne incognita in commercial type tomatoes. Proceedings of the American Society of Horticultural Science. 1956;68:437–442. [Google Scholar]
- Goggin FL, Shah G, Williamson VW, Ullman DE. Instability of Mi-mediated resistance in transgenic tomato plants. Molecular Breeding. 2004;13:357–364. [Google Scholar]
- Hiel M. Ecological costs of induced resistance. Current Opinion in Plant Biology. 2002;5:1–6. doi: 10.1016/s1369-5266(02)00267-4. [DOI] [PubMed] [Google Scholar]
- Hussey RS, Barker KH. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Disease Reporter. 1973;57:1025Y1028. [Google Scholar]
- Jenkins WR, Taylor DP. 1967 Plant Nematology. New York, NY: Reinhold Publishing Corporation. [Google Scholar]
- Johnson AW, Campbell GM. Managing nematode populations densities on tomato transplants using crop rotation and a nematicide. Journal of Nematology. 1980;12(1):6–19. [PMC free article] [PubMed] [Google Scholar]
- Karban R, Agrawal AA, Mangel M. The benefits of induced defenses against herbivores. Ecology. 1997;5:1351–1355. [Google Scholar]
- Karkkainen K, Loe G, Agren J. Population structure in Arabidopsis lyrata: evidence for divergent selection on trichome production. Evolution. 2004;58:2831–2836. [PubMed] [Google Scholar]
- Korves T, Bergelson J. A novel cost of R gene resistance in the presence of disease. The American Naturalist. 2004;163:489–504. doi: 10.1086/382552. [DOI] [PubMed] [Google Scholar]
- Lopez-Perez JA, Le Strange M, Kaloshian I, Ploeg AT. Differential response of Mi gene-resistant tomato rootstocks to root-knot nematodes (Meloidogyne incognita) Crop Protection. 2006;25:382–388. [Google Scholar]
- Milligan SB, Bodeau J, Yaghoobi J, Kaloshian I, Zabel P, Williamson VM. The root knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell. 1998;10:1307–1319. doi: 10.1105/tpc.10.8.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSorley R, Wang K-H, Church G. Suppression of root-knot nematodes in natural and agricultural soils. Applied Soil Ecology. 2008;39:291–298. [Google Scholar]
- Nombela G, Williamson VM, Muniz M. The root-knot nematode resistance gene Mi-1.2 of tomato is responsible for resistance against the whitefly Bemisia tabaci. Molecular Plant-Microbe Interactions. 2003;16:645–649. doi: 10.1094/MPMI.2003.16.7.645. [DOI] [PubMed] [Google Scholar]
- Ploeg AT. Effects of selected Marigold varieties on root-knot nematodes and tomato and melon yields. Plant Disease. 2002;86:505–508. doi: 10.1094/PDIS.2002.86.5.505. [DOI] [PubMed] [Google Scholar]
- Restif O, Koella J. Concurrent evolution of resistance and tolerance to pathogens. American Naturalist. 2004;164:90–102. doi: 10.1086/423713. [DOI] [PubMed] [Google Scholar]
- Rossi M, Goggin FL, Milligan SB, Kaloshian I, Ullman DE, Williamson VM. The nematode resistance gene Mi of tomato confers resistance against the potato aphid. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9750–9754. doi: 10.1073/pnas.95.17.9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagers CL, Phyllis CD. Benefits and costs of defense in a neotropical shrub. Ecology. 1995;76:1835–1843. [Google Scholar]
- Schwachtje J, Minchin PEH, Jahnke S, Van Dongen JT, Schittko U, Baldwin IT. SNF1-related kinases allow plants to tolerate herbivory by allocating carbon to roots. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12935–12940. doi: 10.1073/pnas.0602316103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PG. Embryo culture of a tomato species hybrid. Proceedings of the American Society for Horticultural Science. 1944;44:413–416. [Google Scholar]
- Smith JL, Hare JD. Spectral properties, gas exchange, and water potential of leaves of grandular and nongrandular trichome types in Datura wrightii (Solanaceae) Functional Plant Biology. 2004;31:267–273. doi: 10.1071/FP03178. [DOI] [PubMed] [Google Scholar]
- Sorribas FJ, Ornat C, Verdejo-Lucas S, Aleano M, Valero J. Effectiveness and profitability of the Mi-resistant tomatoes to control root-knot nematodes. European Journal of Plant Pathology. 2005;111:29–38. [Google Scholar]
- Strauss S, Agrawal AA. The ecology and evolution of plant tolerance to herbivory. Tree. 1999;14:179–185. doi: 10.1016/s0169-5347(98)01576-6. [DOI] [PubMed] [Google Scholar]
- Tian D, Traw MB, Chen JQ, Kreitman M, Bergelson J. Fitness costs of R gene-mediated resistance in Arabidopsis thaliana. Nature. 2003;423:74–77. doi: 10.1038/nature01588. [DOI] [PubMed] [Google Scholar]
- Williamson VM. Root-knot nematode resistance genes in tomato and their potential for future use. Annual Review of Phytopathology. 1998;36:277–293. doi: 10.1146/annurev.phyto.36.1.277. [DOI] [PubMed] [Google Scholar]