Abstract
Organic amendments have been widely used for management of plant-parasitic nematodes. Relatively rapid declines in nematode population levels may occur when decomposing materials release toxic compounds, while longer-term effects might include increases in nematode antagonists. Improved crop nutrition and plant growth following amendment use may lead to tolerance of plant-parasitic nematodes. Results depend on a great variety of factors such as material used, processing/composting of material, application rate, test arena, crop rotation and agronomic practices, soil type, climate, and other environmental factors. Reasons for variable performance and interpretation of results from amendment studies are discussed. Case studies of amendments for nematode management are reviewed from Florida, where composts and crop residues are the most frequently used amendments. Plant growth was often improved by amendment application, free-living nematodes (especially bacterivores) were often stimulated, but suppression of plant-parasitic nematodes was inconsistent. Amendments were generally not as effective as soil fumigation with methyl bromide for managing root-knot nematodes (Meloidogyne spp.), and often population levels or galling of root-knot nematodes in amended plots did not differ from those in non-amended control plots. While amendments may improve plant growth and stimulate soil food webs, additional study and testing are needed before they could be used reliably for management of plant-parasitic nematodes under Florida conditions.
Keywords: biological control, compost, free-living nematodes, mulch, organic agriculture, pest management, soil food web, sustainable agriculture
The use of organic amendments for management of plant-parasitic nematodes has been demonstrated in a large number of studies (Akhtar and Alam, 1993; Akhtar and Malik, 2000; D’Addabbo, 1995; Litterick et al., 2004; Muller and Gooch, 1982; Oka, 2010; Rodriguez-Kabana, 1986; Stirling, 1991; Trivedi and Barker, 1986). Oka (2010) provides a recent overview of the materials that have been most effective for nematode management. However, some controversy still remains about the reliability of organic amendments for nematode management. Numbers of plant-parasitic nematodes are not consistently reduced by addition of organic amendments (Jaffee et al., 1994; McSorley and Gallaher, 1995a), and may be increased in some instances (Kimpinski et al., 2003; Belair and Tremblay, 1995). A review of amendment use in Europe and other temperate locations revealed that plant-parasitic nematode numbers increased with amendment addition in about half of the studies examined, and concluded that “no general nematode suppressiveness could be connected to organic soil amendments” (Thoden et al., 2011).
Despite promising results with amendments in many studies, some recommendations for their use against difficult nematode problems in the United States, such as root-knot nematodes (Meloidogyne spp.) on vegetables, are not very enthusiastic. A recent review (Zasada et al. 2010) on alternatives to methyl bromide for nematode management mentions several chemical nematicides, host plant resistance, crop rotation, and soil solarization, but refers to amendments only in the context of biofumigation. Current recommendations for nematode management on cucurbits in Florida do not mention amendments (Noling, 2009a), and comments about use of amendments on tomato (Solanum lycopersicum), pepper (Capsicum annuum), and eggplant (S. melongena) are not encouraging (Noling, 2009b).
Thus there is some discrepancy between the many favorable reports of efficacy against plant-parasitic nematodes and some of the recent practical recommendations. The objective of this paper is not to provide a comprehensive review of the many different kinds of amendments that have been used against plant-parasitic nematodes, but to examine some of the principles and considerations in using amendments for nematode management and to apply these to some case studies from Florida, where management of plant-parasitic nematodes in the field has been inconsistent.
Mechanisms and Modes of Action
A number of mechanisms have been proposed to explain observed beneficial effects of organic amendments on plants in the presence of nematodes. Most reviewers mention release of nematicidal compounds from decomposing materials, stimulation of natural enemies of nematodes, and improved plant growth and tolerance to nematodes (Akhtar and Malik, 2000; Oka, 2010; Stirling, 1991; Thoden et al., 2011). Multiple mechanisms may operate simultaneously, so it is difficult to distinguish which are most important (Akhtar and Malik, 2000).
Release of materials toxic to nematodes
Plant-specific toxins: Nematicidal compounds have been isolated from a great number of plant species (Ferraz and de Freitas, 2004). Neem (Azadirachta indica) has been widely studied for its nematicidal properties, and has been used as plant extracts, oil cakes, or whole plant materials in a large number of studies, particularly in India (Alam and Malik, 2000; Ferraz and de Freitas, 2004; Oka, 2010; Stirling, 1991). Of 33 studies with neem oil cakes conducted between 1971-1981, 30 (91%) gave positive results (Muller and Gooch, 1982). Neem extracts also enhanced the performance of other organic amendments when used in combination (Oka et al., 2007). Decomposition products from cruciferous plants have shown good activity against nematodes and other plant pathogens (Akhtar and Malik, 2000; Oka, 2010; Zasada and Ferris, 2004). Their most effective applications may be under plastic in biofumigation (Bello, 1998) or biosolarization (Ros et al., 2008), although residues from other crops may be effective with these methods as well (Piedra Buena et al., 2007). Amendments from a number of other plants including castor (Ricinus communis) and velvetbean (Mucuna spp.) may have some potential against nematodes (Oka, 2010; Ritzinger and McSorley, 1998; Stirling, 1991). The suppression of nematodes by marigold (Tagetes spp.) and Crotalaria spp. including sunn hemp (Crotalaria juncea) have been much studied (Hooks et al., 2010; Wang et al., 2001; 2002b). Tannins and phenolic compounds released from some plant residues may be toxic to nematodes (Kokalis-Burelle et al., 1994; Mian and Rodriguez-Kabana, 1982b). However, when plants like castor, velvetbean, marigold, or sunn hemp are grown in the field, effects on nematodes may be difficult to interpret since both cover crop effects and amendment effects may be involved.
Common by-products of decomposition: Many plant residues and other amendments can release nitrogen compounds, organic acids, or other compounds that may have adverse effects on nematodes (Oka, 2010; Thoden et al., 2011). Ammonia is a common and much-studied by-product of decomposition of organic materials (Rodriguez-Kabana, 1986; Rodriguez-Kabana et al., 1987). Measured concentrations of ammonia released from compost in pot experiments were well above the lethal level needed for Meloidogyne javanica suppression (Oka and Yermiyahu, 2002). In examining a range of 15 different amendments, Mian and Rodriguez-Kabana (1982c) found that galling by Meloidogyne arenaria decreased as the %N in the amendments increased. Plant materials with C:N ratios in the range of 15 - 20 were considered most effective. The oil cakes tested had low C:N ratios (C:N = 7.0 - 7.1) and reduced nematode galling, but were also phytotoxic (Mian and Rodriguez-Kabana, 1982a,c). A sewage sludge (very low C:N = 5.8) applied to soil in pots decomposed quickly and released maximum levels of ammoniacal N within 7 days after application (Castagnone-Sereno and Kermarrec, 1991). In general, efficacy against nematodes increases as %N in amendments increases and as C:N ratio decreases (Rodriguez-Kabana et al., 1987). Nematicidal activity usually does not occur from amendments with C:N > 20, possibly due to slow decomposition and inadequate concentrations of released ammonia and other toxins, while materials with low C:N (ca. < 10) can cause phytotoxicity (Rodriguez-Kabana et al., 1987). Rodriguez-Kabana and co-workers pioneered work with mixes of different kinds of amendments to add additional C sources and ameliorate the phytotoxic effects of rapid ammonia release from materials with very low C:N ratios (Mian and Rodriguez-Kabana, 1982a,c; Rodriguez-Kabana and King, 1980; Rodriguez-Kabana et al., 1987).
Urea is a more reliable source of ammonia than various types of amendments; it was more consistent than several plant materials in reducing root-knot nematode numbers and was effective at lower rates (Chavarria-Carvajal and Rodriguez-Kabana, 1998). Urea and ammonia were effective against nematodes at rates as low as 300 – 400 mg/kg soil (0.03 – 0.04%) (Eno et al., 1955; Rodriguez-Kabana et al., 1986; Rodriguez-Kabana et al., 1981; 1989; Rodriguez-Kabana and King, 1980).
Ammonia, NH3, is much more toxic to nematodes than ammonium ion, NH4+ (Oka and Yermiyahu, 2002). Ammonia is ionized to NH4+ under acidic soil conditions (Oka et al., 2007; Rodriguez-Kabana et al., 1989), so increasing soil pH can shift the equilibrium in favor of NH3 and improve activity (Oka, 2010). This may explain the nematode suppression achieved with materials that greatly increased soil pH (Zasada, 2005). Higher concentrations of chitin, an amendment that releases ammonia, were needed in soils with pH 5.5-6.5 in Alabama than in neutral or alkaline soils in Israel (Rodriguez-Kabana et al., 1987). However, ammonia can be lost from soil through nitrification, especially under alkaline conditions (Oka, 2010). In a soil with pH 8.5, mixing amendments with neem extracts prolonged the efficacy of ammonia in soil because the neem inhibited nitrification (Oka et al., 2007).
Stimulation of natural enemies of nematodes: It is well-known that population levels of a wide range of soil organisms may be increased following addition of organic material (Akhtar and Malik, 2000; Chavarria-Carvajal et al., 2001; Oka, 2010; Riegel et al., 1996; Stirling, 1991). For example, adding cover crop residues stimulated fungi that parasitized plant-parasitic nematodes, although effects on nematode population levels were short-lived (Odour-Owino, 2003; Wang et al., 2002a). The idea of adding organic matter to soil to increase biological control of nematodes is not new, and dates back to the 1930s (Linford, 1937; Linford et al., 1938).
The Linford hypothesis: This hypothesis states that addition of organic matter added to soil stimulates natural enemies of nematodes, which in turn attack plant-parasitic nematodes and reduce their numbers (Linford et al., 1938). The idea originated from an earlier study in which Linford (1937) added fresh plant material to soil, increasing free-living nematodes numbers as well as the nematode-antagonistic fungi associated with them. In a separate experiment, he noted reduced galling from root-knot nematodes (Meloidogyne spp.) when pineapple plant material was added to soil, and then linked the two experiments by suggesting that natural enemies may have reduced root-knot numbers in the second experiment (Linford, 1937). Stirling (1991) stated that evidence for the Linford hypothesis is circumstantial, and that addition of organic matter stimulates the entire soil food web. He points out that while there is much evidence that adding organic matter to soil will stimulate a variety of organisms, it is difficult to prove that any of them directly caused observed nematode mortality. Even today, it is difficult to design experiments that can provide definite proof of a direct cause-and-effect sequence of events that result in reduction of a plant-parasitic nematode by a specific predator or parasite as a result of amendment addition.
Evidence from some experiments conflicts with the Linford hypothesis. Jaffee et al. (1994) found that addition of organic amendments increased bacterivorous nematodes, but the nematode-parasitic fungus Hirsutella rhossiliensis decreased. Nematode-trapping fungi may be saprotrophic as well as parasitic on nematodes, so that relationships between numbers of the fungi and their potential nematode prey were inconsistent and did not follow classic predator-prey dynamics (Jaffee, 2006). Furthermore, fungivorous nematodes stimulated by organic matter might exert a negative effect on nematode-trapping fungi (Jaffee, 2006). Nevertheless, stimulation of a wide range of nematode antagonists following addition of organic amendments to soil is well documented (Akhtar and Malik, 2000; Oka, 2010; Riegel et al., 1996; Stirling, 1991; Wang et al., 2001; 2002a), so this remains an important area of exploration and means of enhancing natural enemies of nematodes.
The chitin hypothesis: The utility of chitin as an amendment for nematode control was demonstrated by several key studies in the early 1980s (Godoy et al., 1983; Mian et al., 1982; Rodriguez-Kabana et al., 1983; 1984). One suggested mode of action was that chitin increased levels of chitinolytic fungi in soil, which then parasitized eggs of plant-parasitic nematodes (Rodriguez-Kabana et al., 1983; 1984; 1987). The exact mode of action is unclear (Duncan, 1991), and Stirling (1991) found little evidence that chitinolytic fungi parasitized more nematode eggs when chitin was applied to soil.
However, chitin and other amendments may have multiple modes of action. For example, Kaplan and Noe (1993) attributed reductions in Meloidogyne arenaria levels by chicken litter to ammoniacal nitrogen initially followed by possible suppression by microorganisms. Chitin has a low C:N ratio of 6.4 (Rodriguez-Kabana, 1986), so it decomposes quickly in soil and releases significant amounts of ammonia (Mian et al., 1982). It is interesting to note that while chitinous amendments resulted in impressive reductions in levels of M. arenaria (Godoy et al., 1983; Mian et al., 1982) or Heterodera glycines Rodriguez-Kabana et al., 1984), similar levels of control of these nematodes were achieved under somewhat similar conditions by other materials as well (Kokalis-Burelle et al., 1994). Adding urea with chitin provided additional ammoniacal nitrogen, and the combination with chitin reduced phytotoxicity compared to use of urea alone (Rodriguez-Kabana et al., 1989). Although these observations point more to ammonia release as a mode of action for chitin, the additional possibility of biological control cannot be ruled out, because nematode reductions were observed in a second crop following chitin amendment, long after any short-term effects from ammonia in the first crop would have dissipated (Rodriguez-Kabana et al., 1987).
In field tests in California, amendment of soil with 1-2 mt/ha of a chitin product generally reduced nematode numbers relative to an unamended control, although fumigation with 1,3-D was more effective than chitin in lowering numbers of Heterodera schachtii juveniles in soil (Westerdahl et al., 1992). At the rates tested, the chitin products were considered too expensive for routine use. In an organic soil (80% organic matter) in Canada, chitin amendments did not control Meloidogyne hapla on tomato, but improved plant growth and increased nematode egg numbers (Belair and Tremblay, 1995).
Plant tolerance: Watson (1945) observed that mulched crops were healthy but had as much root-knot galling as unmulched plants: “A puzzling feature of the situation is that plants grown in mulched plots or pots, although healthy, usually have fully as many knot-galls on their roots as do the weaker unmulched control plants.” Regardless of any effects on nematodes, amendments can improve nutrient and water availability which benefit plant health and yield (Akhtar and Malik, 2000; McSorley and Gallaher, 1995a; Noling, 1999). In a 7-year field study with potato (Solanum tuberosum), compost and manure amendments did not reduce plant-parasitic nematode numbers, but yields were increased by an average of 27% (Kimpinski et al., 2003). In another study, crop yields were greatly increased by compost amendments despite high population levels of M. incognita (McSorley and Gallaher, 1995a). Melakeberhan (2006) outlined four scenarios for nematode control relative to host productivity, and these examples of improved plant tolerance from amendments seem to fit the scenario of high efficiency for host productivity but inefficiency for nematode control. Larger plants resulting from amendment provide more roots as a food source and can support higher end-of-season nematode population density and greater carrying capacity for plant-parasitic nematodes (Noling, 1999; Wang et al. 2004a). Thoden et al. (2011) add that nutrient-enriched root systems could improve nematode fecundity as well.
While the above examples illustrate improved plant tolerance to nematodes [as defined by Cook and Evans (1987); i.e., nematode numbers not affected], Stirling (1991) mentioned the possibility that adding amendments with high phenolic levels may improve plant resistance to nematodes, resulting in lower nematode population levels. Certain bacteria, fungi, and plant extracts may induce some degree of nematode resistance in plants (Oka (2010; Thoden et al., 2011), but it is not clear how much this mechanism contributes to nematode suppression by organic amendments.
Habitat modification: Soil pathogens may decrease due to “alteration in soil structure and ecology” (Muller and Gooch, 1982). Adding amendments to soil may alter many factors that affect nematodes directly, including soil structure, particle aggregation, pH, salinity, and levels of carbon dioxide, oxygen, and other chemicals (Oka, 2010). In one study, it was suggested that doubling of soil organic matter content may have contributed to a decrease in Paratrichodorus minor population levels (McSorley and Gallaher, 1996). It is possible to greatly change the nature of the soil and its structure, particularly in greenhouse experiments. For example, very high application rates (50-100%) of composts in pots reduced both root-knot nematode galling and numbers of juveniles (J2) in soil and roots (Nico et al., 2004), but these application levels resulted in pots that contained more compost than soil. Further research is needed to better understand how severe modification of the soil environment affects nematode survival and reproduction, and the consequences these actions also have on plant performance.
Reasons for Variability in Performance
Many factors can affect the performance of organic amendments and the interpretation of experiments involving them. As a result, variable results may be observed, and it can be difficult to make generalizations about performance and effects on nematodes for a variety of reasons.
Many different materials are used: A great range of amendments have been used for nematode management including crop residues, green manures, plant by-products, oil cakes, animal manures and by-products, composts, and industrial or urban wastes (Akhtar and Alam, 1993; Akhtar and Malik, 2000; D’Addabbo, 1995; Muller and Gooch, 1982; Oka, 2010; Rodriguez-Kabana, 1986). Differences in preparation of materials (fresh or dry; composted or not) and application methods may further influence results. Some of the mechanisms and modes of action discussed above may be appropriate with some materials but not applicable with others.
Results vary with nematodes and environmental factors: Effects of the same amendment may differ with nematode species as well as a variety of environmental factors (Oka and Yermiyahu, 2002). Critical environmental parameters may involve chemical properties, physical properties such as soil type, and biological factors such as microorganisms present (Akhtar and Malik, 2000). The latter can be particularly critical since bacteria and fungi affect decomposition pathways and rates (Ferris et al., 2001; Powers and McSorley, 2000). Working with a commercial biosolid amendment across several different soil types and nematode isolates, Mennan and Melakeberhan (2010) found variable results and concluded that performance could be site specific. As a result, it may be expedient to make preliminary tests of new materials or products at the local level before incurring the expenses of large-scale use.
Most organic amendments are fertilizers: In the first paragraph of their review of amendments for nematode control, Muller and Gooch (1982) recognized the importance and historical use of amendments for crop nutrition. Increased organic matter can improve soil properties and the decomposing materials can provide nitrogen and other nutrients that are needed by crops (Powers and McSorley, 2000). A recent review (Cherr et al., 2006) summarized the nitrogen contents, application rates, and management methods for many legumes and nonlegumes that have been used as green manures to provide crop fertility. As a result, observed benefits to crop growth and yield from amendment addition may result from fertility and nutrient benefits, regardless of any effects that amendments may or may not have on nematodes. For example, in a study using chicken litter as a soil amendment (Mian and Rodriguez-Kabana, 1982a), a rate of only 0.5% chicken litter by weight was ineffective in reducing galling caused by M. arenaria, but resulted in a 367% increase in top weight of infested squash (Cucurbita pepo) plants. Improved plant performance following amendment use may be common, but appropriate sampling and data are needed to demonstrate effects on nematodes.
Greenhouse results may differ from field conditions: Materials from many different kinds of plants have been used to suppress nematodes in vitro or in greenhouse experiments, and specific nematicidal compounds have been isolated from about 80 of these (Ferraz and de Freitas, 2004). Many have not been tested under field conditions. In greenhouse tests, very high rates of amendments are sometimes used to demonstrate initial efficacy of materials or to better understand mechanisms (e.g., Barbosa et al., 2004; Castagnone-Serreno and Kermarrec, 1991; Nico et al., 2004; Oka and Yermiyahu, 2002). Unusual methods such as pre-incubation of amendments may affect greenhouse results as well (Morris and Walker, 2002).
Rates may be critical but impractical: Rates for amendments used in greenhouse tests may require very large amounts of material when applied on a field scale. Brady (1974) used an estimate of 2.2 – 2.8 million kg for the weight of 1 ha of soil to a depth of 15 cm. Using the lower end of this range (2200 mt/ha), rates of amendments used in greenhouse tests of 5% and 10% by weight would be equivalent to applications of 110 mt/ha and 220 mt/ha, respectively. Even a lower rate of 1% would still require 22 mt/ha, quite a large amount of material. A few examples of amendment rates used in greenhouse tests include chicken litter at rates equivalent to 10-45 mt/ha (Kaplan and Noe, 1993) or 112 mt/ha (Mian and Rodriguez-Kabana, 1982a), oil cakes at 22 mt/ha (Mian and Rodriguez-Kabana, 1982a), and materials with low C:N ratios at 10 mt/ha (Mian and Rodriguez-Kabana, 1982c). In one field study, yard waste compost was applied at 269 mt/ha, although this application was to small (3.0-m x 4.5-m) plots (McSorley and Gallaher, 1995a). Such high rates have long been recognized as an important limitation to the use of organic amendments (Muller and Gooch, 1982; Rodriguez-Kabana, 1986). This problem may limit economic use of amendments to small sites or to materials that are available in abundant local supply. One way to reduce the large amount of material needed for broadcast application of amendments was to make targeted applications only to the immediate vicinity of plants, so that seedlings develop in a soil environment very rich in the amendment (McSorley et al., 2008).
Rotation effects and amendment effects not easily separated: Residues from previous crops are convenient to use as amendments. Low nematode population levels may follow crops such as sunn hemp or marigold because they are poor or non-hosts of some nematodes (Hooks et al., 2010; McSorley, 2011; Wang et al., 2002b). In these cases, both rotation and amendment effects on nematodes may occur but they may be difficult to distinguish. If there were substantial amendment effects from crop residues, these should be evident after a rotation crop is turned into the soil. Rotation with a non-host or poor host crop would be expected to provide a reduction in nematode population levels similar to that achieved through clean fallow. Suppressive effects from decomposing crop residues might be expected to cause additional nematode mortality, resulting in even lower nematode numbers than those following fallow. However many examples suggest that effective rotation crops are generally incapable of reducing nematode numbers below those achieved following fallow (Bhan et al., 2009; Hooks et al., 2010; McSorley, 2011), suggesting that any effect beyond starvation on a non-host is relatively insignificant. Marigold, for example, is often used in rotations for managing nematode populations (Hooks et al., 2010), but its effect as a soil amendment compared to other materials has been relatively small (Ploeg, 2000) or insignificant (Ko and Schmitt, 1996).
It is possible to design experiments in which rotation and amendment effects can be separated, such as Wang et al. (2003, 2008) in which residues from rotation crops were cut and moved to other plots in a factorial design, or Wang et al. (2007) in which residues were either maintained or removed from greenhouse pots in which cover crops had grown. In the latter study, no differences between pots with residues and pots without residues were observed for 3-4 plant-parasitic nematode genera (including Meloidogyne) in four tests (Wang et al., 2007).
Amendment effects not limited to plant-parasitic nematodes: One of the most consistent effects observed following the addition of organic amendments to soil is the increase in numbers of free-living nematodes (Akhtar and Malik, 2000; Bulluck et al., 2002; Ettema and Bongers, 1993; Linford, 1937; Rodriguez-Kabana, 1986; Stirling, 1991; Thoden et al., 2011). A predictable sequence of nematode dynamics occurs following soil enrichment by organic matter, generally with initial increase in numbers of bacterivores, followed by funigivores, and later by omnivores and predators (Ferris et al., 2001; Ferris and Matute, 2003; Georgieva et al., 2005b; Koenning and Barker, 2004; McSorley and Frederick, 1999; Wang et al., 2004c; Wasilewska and Bienkowski, 1985). These events and successions are so well recognized that distinct guilds have been developed for free-living nematodes (Bongers and Bongers, 1998; Ferris et al., 2001). Rhabditid bacterivores typically respond very quickly to amendment addition, followed later by Cephalobidae bacterivores (Ferris and Matute, 2003; Georgieva et al., 2005b). Successions of fungivores occur as well; Brzeski and Szczech (1999) observed that Aphelenchoides spp. increased first, later followed by Ditylenchus spp. and finally Filenchus spp. Quality of crop residue affects the timing of these events, which proceed more quickly if C:N ratios are low and decomposition is fast (Georgieva et al., 2005a; McSorley and Frederick, 1999). Bacterivorous nematodes are especially quick to recover from severe disturbances once their food sources return (Ferris et al., 2001), and can build their population levels quickly even after events such as fumigation (Wang et al., 2006a) or addition of ammonia (Rodriguez-Kabana, 1986).
It has been suggested that increased crop yields observed with amendments are due to activities of free-living nematodes, especially bacterivores (Thoden et al., 2011). While these nematodes are of great benefit in mineralization, it is not clear how much their activity contributes quantitatively to yield effects. Increased numbers of bacterivores and increased plant yields are likely correlated in many amendment studies, but both nematode reproduction and plant growth may benefit directly from the nutrients supplied by the amendments. Designing experiments to elucidate cause-and-effect contributions of free-living nematodes to crop yields is challenging.
Case Studies
Florida scenario: Climate in Florida ranges from warm temperate to subtropical (Myers and Ewel, 1990). Many crops are produced on very sandy soils (> 90% sand) with low pH (often 5.5 - 6.5) and low (< 2%) organic matter. Problems from Meloidogyne spp. are common on these soils, with Belonolaimus longicaudatus or Paratrichodorus spp. in some locations (McSorley, 1996; Perry and Rhoades, 1982; Rhoades and Forbes, 1986). In extreme southern Florida, many crops have been produced on an unusual rocky soil (Krome very gravelly loam, a loamy skeletal carbonatic hyperthermic lithic Rendoll; Noble et al., 1996) with alkaline pH usually 7.2 - 8.0, and problems caused by M. incognita and Rotylenchulus reniformis are important.
Most Florida growers typically would not have access to large quantities of neem or chitin. In a greenhouse study, a rate of 0.05% crab chitin was more effective than a 20% application rate of crab compost for suppressing M. javanica (Rich and Hodge, 1993). Although rarely used for this purpose today, the efficacy of anhydrous ammonia for nematode control was demonstrated in an early study in Florida (Eno et al., 1955). However, the high rates of ammonia used changed soil pH from <5.5 to >8.0. Crop residues and composts are the most frequently used organic amendments. The urban areas of the state produce large quantities of biodegradable organic material and composts are produced by a large number of private and public facilities (Li et al., 2000).
Regardless of their effects on nematodes, most applications of amendments to Florida vegetable crops typically result in positive yield responses (Li et al., 2000).
Approach: Data from a number of experiments were examined to evaluate overall effects of amendments on nematodes under Florida conditions. When appropriate, statistical analyses by the original authors are used to indicate differences among treatments. Crop yield data from individual experiments are expressed as percents, where 100% is the yield level of a nematode-susceptible crop in a non-amended control treatment, and yield of the crop following an amendment treatment is expressed as a percent of the control yield. Nematode data were similarly expressed as percents, where 100% is the number of nematodes in the control treatment, and nematode numbers in amendment or other treatments are expressed as percents of the control treatment. This approach was developed to compare diverse types of nematode data sets, because many papers reported nematode numbers in soil but some reported only galling indices.
Effects of amendments on nematode numbers and plant performance: Meloidogyne spp. are key pests on many Florida crops (McSorley, 1996; 2001). Management of root-knot nematodes on agricultural crops can be especially challenging due to their unusually high growth rates on favorable hosts (Rodriguez-Kabana et al., 1986; Wang et al., 2003). Several authors (Csizinszky, 1999; Noling, 1999; Watson, 1945) observed that composts or mulches did not suppress root-knot nematodes under Florida conditions. However despite lack of nematode control, tomato yield in compost-amended plots was 196% of the yield in control plots (Noling, 1999).
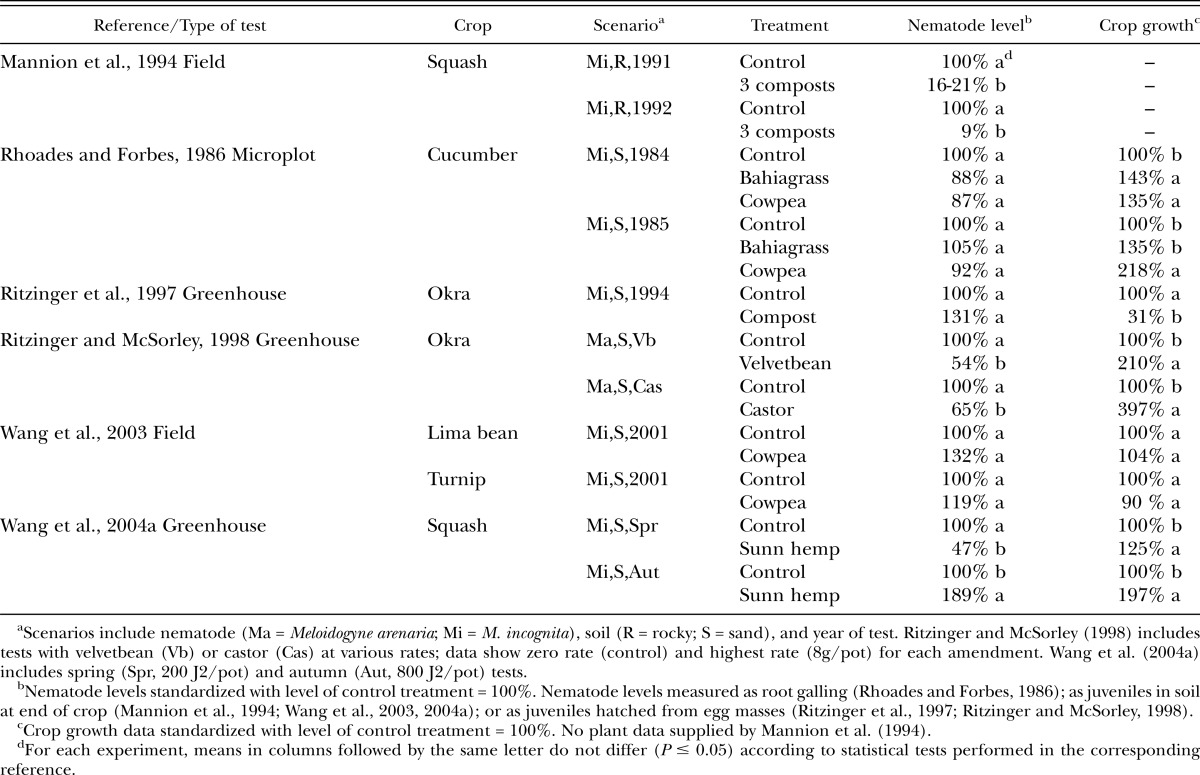
Data from six very different kinds of studies with root-knot nematodes are summarized (Table 1). Root-knot nematode levels were not suppressed if the amendments were used as mulch (Rhoades and Forbes, 1986) or had a high (>30:1) C:N ratio (Ritzinger et al., 1997). In contrast, amendments of composted municipal solid waste (Mannion et al., 1994) reduced Meloidogyne spp. levels, while crop residues were either effective (Ritzinger and McSorley, 1998; Wang et al., 2004a) or had no effect (Wang et al., 2003) (Table 1). Crop growth benefitted from amendment in many cases, but a notable exception was the study by Ritzinger et al. (1997) in which high rates of a material with a high C:N ratio likely affected availability of nutrients to the crop. Results from the study of Wang et al. (2004a) were particularly interesting for several reasons. Biological control was implicated because in some of the soils used in this study, increased levels of nematode-trapping fungi were recovered in amended pots, and differences in suppression were observed in microwaved soil (to kill fungi) vs control soil. In addition, the sunn hemp amendment was effective against M. incognita when the nematode population level was low (spring test, final nematode population (Pf) in control = 15/100 cm3 soil) but not when it was higher (autumn test, Pf = 184/100cm3) and seemed to overwhelm any potential beneficial effect against the nematode (although plant growth was excellent).
Table 1.
Effect of amendment treatments on root-knot nematode (Meloidogyne spp.) levels and plant weights of susceptible vegetable crops in Florida.
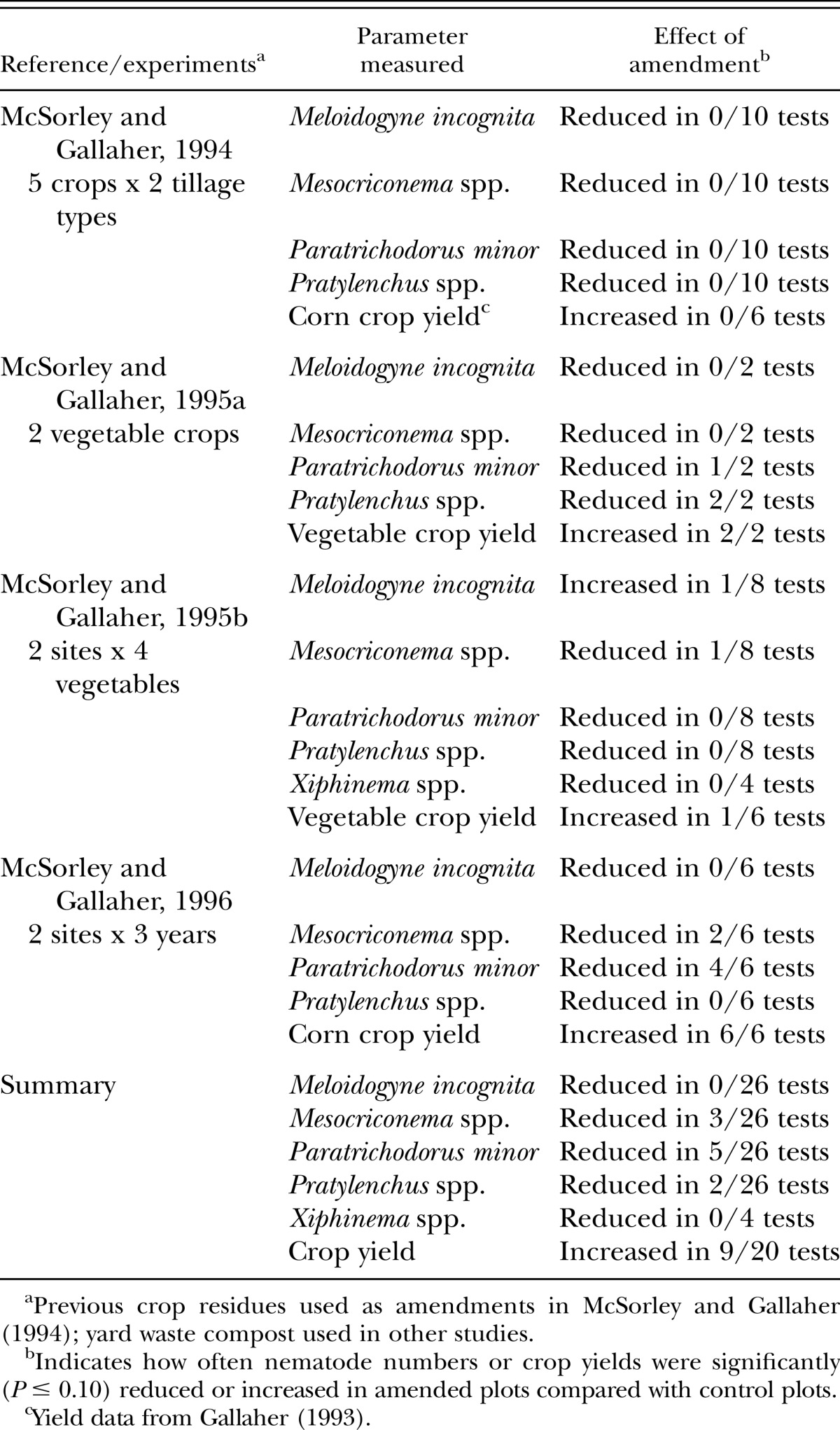
A number of field experiments were conducted in the 1990s on sandy soils (92-94% sand) with acidic pH (5.2-5.8) and low organic matter (1.5-2.6%) in north Florida (McSorley and Gallaher, 1994; 1995a,b; 1996). One of these studies (McSorley and Gallaher,1994) used green manure residues from previous crops as amendments, while the others used a yard waste compost (C:N 34:1 to 46:1, 269 mt/ha application rate). Root-knot nematode population levels were never reduced by these amendments, and other plant-parasitic nematodes were not consistently affected (Table 2). The data shown (Table 2) are from treatments where amendments were incorporated into soil; parallel treatments in which the same amendments were used as mulch gave nearly identical results (McSorley and Gallaher, 1995a,b; 1996). Due to the high C:N ratio of the yard waste compost, release of nematicidal levels of nitrogen compounds would not be expected (Rodriguez-Kabana, 1986; Rodriguez-Kabana et al., 1987). However, crop yields increased as a result of amendment addition in about half of these tests. The amendments released nutrients upon decomposition, and they also greatly improved the water-holding capacity of these sandy soils (McSorley and Gallaher, 1995a; 1996). With the high rates of yard waste compost added over time, soil organic matter increased from 1.6% to > 3.1% at one site (McSorley and Gallaher, 1996). Paratrichodorus minor was affected often by compost in one of these studies (McSorley and Gallaher, 1996), but rarely in the others (Table 2). This study differed from the others because it involved multiple additions of the compost over time that altered soil organic matter content. It was suggested that this habitat change was detrimental to P. minor (McSorley and Gallaher, 1996), since trichodorids typically prefer sandy soils with low organic matter in Florida (Perry and Rhoades, 1982).
Table 2.
Effect of amendment treatments on plant-parasitic nematode numbers and crop yields in experiments on sandy soils in north Florida.
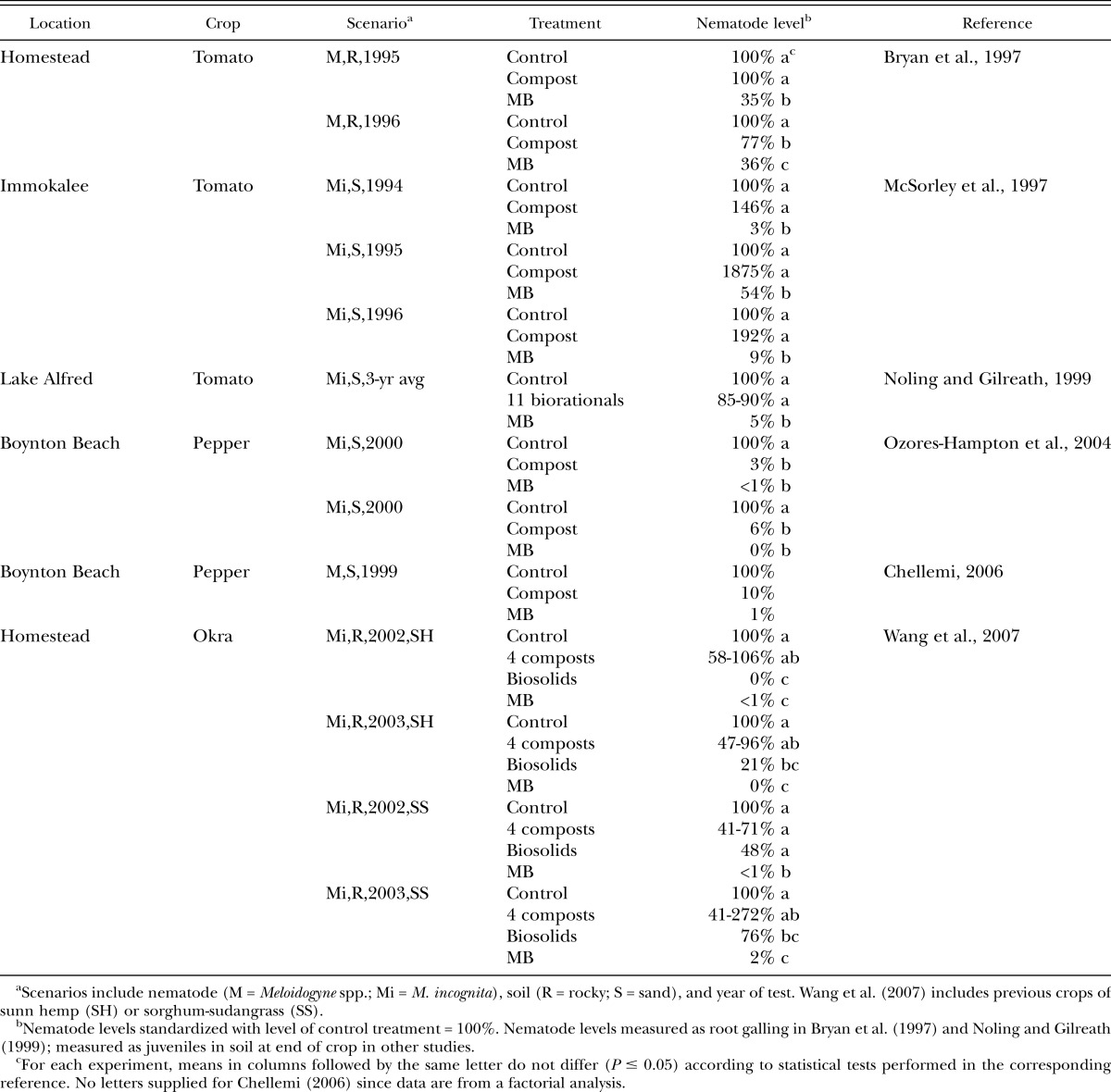
Comparisons to soil fumigation: Several studies have compared organic amendments and soil fumigation with methyl bromide to each other for managing root-knot nematodes in Florida (Table 3). Four of these were field tests, but Noling and Gilreath (1999) was a field microplot study and Wang et al. (2007) was conducted in pots. Five of these studies utilized various types of composts, usually biosolids or plant debris (yard waste) from urban sources, or a combination of composted biosolids plus yard waste. In contrast, Noling and Gilreath (1999) examined 11 different biorational amendments, including various plant oils and extracts, neem products, ground sesame (Sesamum indicum), rapeseed meal, seaweed extract, and rhizobacteria. These amendments did not reduce levels of M. incognita relative to an untreated control (Table 3), and tomato yields following these amendments were lower (P < 0.05) and generally only 50-70% of the yield obtained in fumigated plots (Noling and Gilreath, 1999). The study by Ozores-Hampton et al. (2004) combined the use of compost amendments with soil solarization, so this was really a biosolarization study rather than simply an evaluation of amendments alone, and the biosolarization treatment performed as well as methyl bromide (Table 3). These results support the well-known idea that biosolarization and biofumigation are among the most effective uses of organic amendments for nematode management (Bello, 1998; Ros et al., 2008; Zasada et al., 2010). In the other four studies that utilized composts, more suppression of root-knot nematodes was usually obtained by fumigation than by the amendments (Table 3). The exception was the biosolid amendment used by Wang et al. (2007), which resulted in nematode population levels similar to methyl bromide in 3 of 4 tests (Table 3). The reason for the favorable performance of the biosolid amendments was not clear, although this material contained much higher N levels than the other 4 amendments used (Wang et al., 2007). Apart from this biosolid amendment and the biosolarization study of Ozores-Hampton et al., (2004), it was evident that amendments used in these examples could not match the efficacy of methyl bromide in reducing nematode levels. Crow (2005) compared a variety of commercial amendment products to the nonfumigant nematicide fenamiphos for management of Belonolaimus longicaudatus, Hoplolaimus galeatus, and Trichodorus obtusus on turfgrass, but none of the products (including fenamiphos) were effective in reducing nematode population levels relative to non-treated control plots.
Table 3.
Effect of amendment treatments and fumigation with methyl bromide (MB) on root-knot nematode (Meloidogyne spp.) levels on susceptible vegetable crops at various locations in Florida.
Comparisons to solarization: Mixed results were obtained when comparing solarization with organic or nitrogen amendments in Florida. Application of inorganic ammonium amendments suppressed numbers of B. longicaudatus at 3 wk after treatment application in one field, whereas solarization suppressed 6 of 7 plant-parasitic nematodes (including M. incognita) in one field and 5 of 7 nematodes in a second field (McSorley and McGovern, 2000). Compared to a compost of horticultural waste and chicken litter, solarization reduced numbers of M. incognita, P. minor, and Mesocriconema spp., but numbers of all three nematodes recovered (similar to control) in subsequent susceptible vegetable crops (McSorley et al., 1999). However in another study, neither solarization nor amendment with sunn hemp crop residue had much impact on plant-parasitic nematode levels (McSorley et al., 2008). An amendment of urban plant debris reduced numbers of Paratrichodorus spp. relative to solarization, while numbers of Helicotylenchus spp. and Tylenchorhynchus spp. were similar in both treatments (Chellemi, 2006). Resurgence of Paratrichodorus spp. after solarization appears to be a common occurrence (McSorley et al., 1999; McSorley and McGovern, 2000).
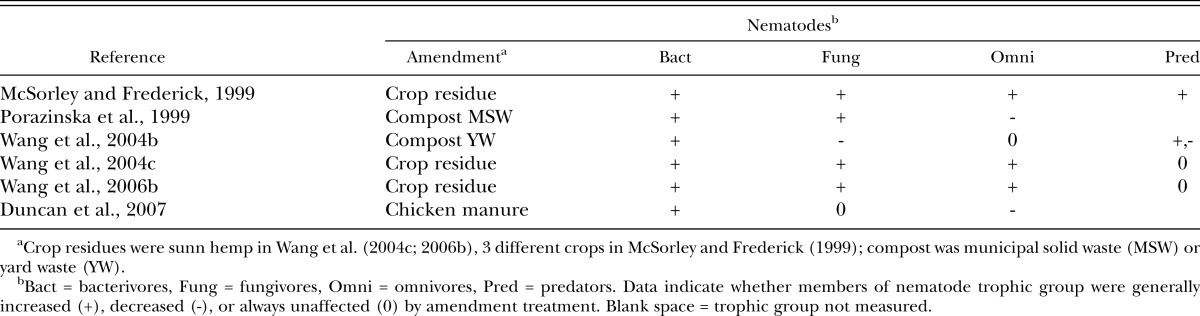
Amendments and free-living nematodes in Florida: Several studies in Florida have provided data on the effects of amendments on genera and trophic groups of free-living nematodes (Table 4). Stimulation of bacterivores following amendment addition was observed in all of these studies. Successions of free-living nematodes were observed in two of the Florida studies, with early buildup of bacterivores and fungivores followed by later increases in the omnivore genera Eudorylaimus and Mesodorylaimus (McSorley and Frederick, 1999; Wang et al., 2004c). In these studies, the rapid buildup of Rhabditidae in response to amendment application followed by peaks in Cephalobidae are in agreement with observations from other regions (Ferris and Matute, 2003; Georgieva et al., 2005b), and provide additional data that support the nematode guild structure (based on succession and trophic groups) proposed by other authors (Bongers and Bongers, 1998; Ferris et al., 2001). Successions of fungivores occurred as well, with increases in Aphelenchus and Aphelenchoides at 14 days, and tylenchid fungivores (Filenchus, Tylenchus) at 56 days (Wang et al., 2004c), similar to the successions of these genera observed in Europe (Brzeski and Szczech, 1999; Georgieva et al., 2005b). In one Florida study, enchytraeid worms dominated the fungivore niche at 28-42 days (Wang et al., 2004c).
Table 4.
Effect of amendment treatments on nematode trophic groups in experiments on sandy soils in Florida.
However, addition of amendments did not stimulate all nematode trophic groups in some of the Florida studies (Table 4). In one study (Wang et al., 2004b), application of yard waste compost decreased the fungivorous dorylaim Diphtherophora and the predator Mononchus, but increased another predator, Tobrilus. Omnivorous nematodes were suppressed by a municipal solid waste compost that contained relatively high concentrations of copper (Porazinska et al., 1999), which can adversely affect omnivorous and predatory nematodes (Korthals et al., 1996). Omnivorous nematodes were also suppressed by chicken manure, but this amendment affected a wide range of organisms including entomopathogenic nematodes, nematode-trapping fungi, and microarthropods (Duncan et al., 2007). Entomopathogenic nematodes recovered quickly but nematode-trapping fungi did not, which probably contributed to increased suppression of the target insect by these nematodes. Such examples illustrate the complex responses of soil food webs to amendment addition, as well as the varied effects from different types of amendments.
Assessment and Future Direction
From the above examples, it is evident that effects of organic amendments in the field in Florida were quite variable, although improvement of plant growth and stimulation of bacterivorous nematodes are frequent benefits. At present, it appears that the amendments used are not reliable alternatives to methyl bromide for management of Meloidogyne spp. and other important plant-parasitic nematodes in Florida, and that additional research will be needed to improve their efficacy and consistency for this purpose. One of the first questions to consider is whether a rapid reduction in nematode population levels is desired or whether long-term effects are more important. To kill nematodes quickly with ammonia and other nitrogenous compounds, materials with very low C:N ratios are needed; crop residues and most composts made from yard waste or other woody plant material are insufficient. Greatest efficacy against nematodes has occurred from amendments with C:N < 10, but such materials are usually phytotoxic as well (Rodriguez-Kabana, 1986; Rodriguez-Kabana et al., 1987), so they are best used prior to planting. If they are locally available, then materials that release nematode-specific toxins may be a possibility, but extensive testing will be needed to demonstrate consistent efficacy. As modes of action become better understood, it may be easier to utilize specific amendments more effectively (Oka, 2010). Several commercial products have been tested under Florida conditions, but were ineffective (Crow, 2005; Noling and Gilreath, 1999). At present, biosolarization may be the most effective use of organic amendments against plant-parasitic nematodes (Ozores-Hampton et al., 2004; Ros et al., 2008).
If organic amendments enhance biological control organisms, their effects are expected to occur over the longer term, not immediately after amendment application (Rodriguez-Kabana et al., 1987; Stirling, 1991). Although lacking definitive proof, the Linford (1937) hypothesis remains an attractive approach for attempting to manipulate naturally-occurring biological control of nematodes. Organic amendments stimulate a broad range of organisms in the soil food web, many of which are potential predators or parasites of plant-parasitic nematodes (Akhtar and Malik, 2000; Ferris et al., 2001; Ferris and Matute, 2003; Oka, 2010; Riegel et al., 1996; Stirling, 1991). But maintaining a sustained response of trophic groups beyond bacterivores and fungivores may be difficult. In the examples presented from Florida (Table 4), many bacterivores responded to soil amendments, but positive responses from higher nematode trophic levels (omnivores, predators) were less frequent.
Some of the more positive results in this area have resulted from increased incidence and levels of nematode-antagonistic fungi following amendment application. Application of sunn hemp crop residues decreased population levels of R. reniformis and increased levels of nematode-trapping fungi in soil (Wang et al., 2001; 2002a). Suppression of M. incognita was documented in an amended soil, and increases in nematode-trapping fungi were consistent with the observed suppression (Wang et al., 2004a). However, abundance of fungi and presumably their impact vary depending on nematode and fungal populations levels initially present in the soil (Wang et al., 2004a) as well as a variety of agricultural practices (Wang et al., 2001; 2002a). Furthermore, there is no certainty that nematode antagonistic fungi will increase following amendment addition, as the opposite was observed by Duncan et al. (2007) and by Jaffee et al. (1994). Interactions of nematodes and fungal antagonists may not follow classical models (especially with fungivorous nematodes!), underscoring the complexity and difficulty of predicting these interactions in soil ecosystems (Jaffee, 2006).
Large differences in suppression of M. incognita have been observed in predator-rich soils from natural areas compared to adjacent agricultural soils in areas with warm climates such as Florida (McSorley and Wang, 2009) and California (Sanchez-Moreno and Ferris, 2007). We may aspire to manipulate agricultural soils to resemble “natural” soils, but this remains a great challenge. Some indication of changes that may occur from long-term manipulation of agricultural soils has come from studies of transition to organic agriculture, which limits use of agrichemicals and utilizes organic materials as nutrient sources (Briar et al., 2007; Litterick et al., 2004; Neher, 1999). In organic agriculture sites that relied on long-term inputs of amendments as fertilizer sources, initial buildup of bacterivores was observed, but changes in higher nematode taxa were not maintained and reductions of plant-parasitic nematodes were not consistently obtained (Briar et al., 2007; Bulluck et al., 2002; Hu and Qi, 2010; Neher, 1999). An increase in omnivore levels at one of these organic sites dissipated after the first year, presumably due to excessive tillage (Briar et al., 2007). In another study, increased levels of omnivorous and predatory nematodes following compost amendment were apparent one month after planting a corn crop, but effects disappeared after 2 months (Hu and Qi, 2010). Increased levels of omnivores were maintained in a long-term organic site in Switzerland, but plant parasites remained high as well (Birkhofer et al., 2008). Of course, amendment addition may impact many potential predators and parasites other than omnivorous nematodes, but these examples as well as results from Florida (Table 4) illustrate the difficulty and inconsistency of manipulating and maintaining one beneficial group. Lower levels of plant disease are reported in fields with long-term organic management compared to conventional fields (Litterick et al., 2004). However, more plant-parasitic nematodes were found in organic sites than conventional sites (Birkhofer et al., 2008; Hu and Qi, 2010; Neher, 1999). Therefore, long-term use of amendments to build suppressive elements of the soil food web remains an elusive goal that continues to require additional study. But regardless of their effects on plant-parasitic nematodes, organic amendments can consistently provide important benefits to soil food webs and agroecosystems, especially in improving crop fertility and soil structure.
Literature Cited
- Akhtar M, Alam MM. Utilization of waste materials in nematode control: A review. Bioresource Technology. 1993;45:1–7. [Google Scholar]
- Akhtar M, Malik A. Roles of organic soil amendments and soil organisms in the biological control of plant-parasitic nematodes: A review. Bioresource Technology. 2000;74:35–47. [Google Scholar]
- Barbosa GM, de C, Mendes ML, Tavares-Filho J, Rodriguez PBN, Vizoni E. Effect of sewage sludge compost on Meloidogyne javanica on tomato. Nematropica. 2004;34:13–21. [Google Scholar]
- Belair G, Tremblay N. The influence of chitin-urea amendments applied to an organic soil on a Meloidogyne hapla population and on the growth of greenhouse tomato. Phytoprotection. 1995;76:75–80. [Google Scholar]
- Bello A. 1998. Biofumigation and integrated crop management. Pp. 99–126 in A. Bello, J.A. Gonzalez, M. Arias, and R. Rodriguez-Kabana, eds. Alternatives to Methyl Bromide for the Southern European Countries. Valencia, Spain: Graficas Papallona S.C.V. [Google Scholar]
- Bhan M, McSorley R, Chase CA. Effect of cropping system complexity non plant-parasitic nematodes associated with organically grown vegetables in Florida. Nematropica. 2010;40:53–70. [Google Scholar]
- Birkhofer K, Bezemer TM, Bloem J, Bonkowski M, Christensen S, Dubois D, Ekelund F, Fliessbach A, Gunst L, Hedlund K, Mader P, Mikola J, Robin C, Setala H, Tatin-Froux F, Van der Putten WH, Scheu S. Long-term organic farming fosters below and aboveground biota: Implications for soil quality, biological control and productivity. Soil Biology and Biochemistry. 2008;40:2297–2308. [Google Scholar]
- Bongers T, Bongers M. Functional diversity of nematodes. Applied Soil Ecology. 1998;10:239–251. [Google Scholar]
- Brady NC. 1974. The nature and properties of soils. 8th Ed. New York: Macmillan Publishing Co., Inc. [Google Scholar]
- Briar SS, Grewal PS, Somasekhar N, Stinner D, Miller SA. Soil nematode community, organic matter, microbial biomass and nitrogen dynamics in field plots transitioning from conventional to organic management. Applied Soil Ecology. 2007;37:256–266. [Google Scholar]
- Bryan HH, Ramos LJ, Codallo MM, Scott JW. Effects of soil fumigation, compost, and non-fumigation on the yield, fruit quality, disease incidence, and other variables of tomato cultivars. Proceedings of the Florida State Horticultural Society. 1997;110:269–272. [Google Scholar]
- Brzeski MW, Szczech M. Effect of continuous soil amendment with coniferous sawdust on nematodes and microorganisms. Nematologia Mediterranea. 1999;27:159–166. [Google Scholar]
- Bulluck LR, III, Barker KR, Ristaino JB. Influences of organic and synthetic soil fertility amendments on nematode trophic groups and community dynamics under tomatoes. Applied Soil Ecology. 2002;21:233–250. [Google Scholar]
- Castagnone-Sereno P, Kermarrec A. Invasion of tomato roots and reproduction of Meloidogyne incognita as affected by raw sewage sludge. Supplement to the Journal of Nematology. 1991;23:724–728. [PMC free article] [PubMed] [Google Scholar]
- Chavarria-Carvajal JA, Rodriguez-Kabana R. Changes in soil enzymatic activity and control of Meloidogyne incognita using four organic amendments. Nematropica. 1998;28:7–18. [Google Scholar]
- Chavarria-Carvajal JA, Rodriguez-Kabana R, Kloepper JW, Morgan-Jones G. Changes in populations of microorganisms associated with organic amendments and benzaldehyde to control plant-parasitic nematodes. Nematropica. 2001;31:165–180. [Google Scholar]
- Chellemi DO. Effect of urban plant debris and soil management practices on plant parasitic nematodes, Phytophthora blight and Pythium root rot of bell pepper. Crop Protection. 2006;25:1109–1116. [Google Scholar]
- Cherr CM, Scholberg JMS, McSorley R. Green manure approaches to crop production: a synthesis. Agronomy Journal. 2006;98:302–319. [Google Scholar]
- Cook R, Evans K. 1987. Resistance and tolerance. Pp. 179–231 in R.H. Brown and B.R. Kerry, eds. Principles and Practice of Nematode Control in Crops. Sydney: Academic Press. [Google Scholar]
- Crow WT. Alternatives to fenamiphos for management of plant-parasitic nematodes on bermudagrass. Journal of Nematology. 2005;37:477–482. [PMC free article] [PubMed] [Google Scholar]
- Csizinszky AA. Yield and nutrient uptake of ‘Capistrano’ bell peppers in compost-amended sandy soil. Proceedings of the Florida State Horticultural Society. 1999;112:333–337. [Google Scholar]
- D’Addabbo, T The nematicidal effect of organic amendments: A review of the literature, 1982-1994. Nematologia Mediterranea. 1995;23:299–305. [Google Scholar]
- Duncan LW. Current options for nematode management. Annual Review of Phytopathology. 1991;29:469–490. doi: 10.1146/annurev.py.29.090191.002345. [DOI] [PubMed] [Google Scholar]
- Duncan LW, Graham JH, Zellers J, Bright D, Dunn DC, El-Borai FE, Porazinska DL. Food web responses to augmenting the entomopathogenic nematodes in bare and animal manure-mulched soil. Journal of Nematology. 2007;39:176–189. [PMC free article] [PubMed] [Google Scholar]
- Eno CF, Blue WG, Good JM., Jr The effect of anhydrous ammonia on nematodes, fungi, bacteria, and nitrification in some Florida soils. Proceedings of the Soil Science Society of America. 1955;19:55–58. [Google Scholar]
- Ettema CH, Bongers T. Characterization of nematode colonization and succession in disturbed soil using the maturity index. Biology and Fertility of Soils. 1993;16:79–85. [Google Scholar]
- Ferraz S, de Freitas LG. 2004. Use of antagonistic plants and natural products. Pp. 931–977 in Z.X. Chen, S.Y. Chen, and D.W. Dickson, eds. Nematology Advances and Perspectives. Beijing, China: Tsinghua University Press. [Google Scholar]
- Ferris H, Bongers T, de Goede RGM. A framework for soil food web diagnostics: extension of the nematode faunal analysis concept. Applied Soil Ecology. 2001;18:13–29. [Google Scholar]
- Ferris H, Matute MM. Structural and functional succession in the nematode fauna of a soil food web. Applied Soil Ecology. 2003;23:93–110. [Google Scholar]
- Gallaher RN. 1993. Cover crops and nitrogen management for no-tillage corn. Pp. 81–84 in P.K. Bollich, ed. Proceedings of the 1993 Southern Conservation Tillage Conference for Sustainable Agriculture. Louisiana Agricultural Experiment Station, Monroe, LA. [Google Scholar]
- Georgieva S, Christensen S, Petersen H, Gjelstrup P, Thorup-Kristensen K. Early decomposer assemblages of soil organisms in litterbags with vetch and rye roots. Soil Biology and Biochemistry. 2005a;37:1145–1155. [Google Scholar]
- Georgieva S, Christensen S, Stevnbak K. Nematode succession and microfauna-microorganism interactions during root residue decomposition. Soil Biology and Biochemistry. 2005b;37:1763–1774. [Google Scholar]
- Godoy G, Rodriguez-Kabana R, Shelby RA, Morgan-Jones G. Chitin amendments for control of Meloidogyne arenaria in infested soil. II. Effects on microbial population. Nematropica. 1983;13:63–74. [Google Scholar]
- Hooks CRR, Wang K-H, Ploeg A, McSorley R. Using marigold (Tagetes spp.) as a cover crop to protect crops from plant-parasitic nematodes. Applied Soil Ecology. 2010;46:307–320. [Google Scholar]
- Hu C, Qi Y. Effect of compost and chemical fertilizer on soil nematode community in a Chinese maize field. European Journal of Soil Biology. 2010;46:230–236. [Google Scholar]
- Jaffee BA. Interactions among a soil organic amendment, nematodes, and the nematode-trapping fungus Dactylellina candidum. Phytopathology. 2006;96:1388–1396. doi: 10.1094/PHYTO-96-1388. [DOI] [PubMed] [Google Scholar]
- Jaffee BA, Ferris H, Stapleton JJ, Norton MVK, Muldoon AE. Parasitism of nematodes by the fungus Hirsutella rhossiliensis as affected by certain organic amendments. Journal of Nematology. 1994;26:152–161. [PMC free article] [PubMed] [Google Scholar]
- Kaplan M, Noe JP. Effects of chicken-excrement amendments on Meloidogyne arenaria. Journal of Nematology. 1993;25:71–77. [PMC free article] [PubMed] [Google Scholar]
- Kimpinski J, Gallant CF, Henry R, Macleod JA, Sanderson JB, Sturz AV. Effect of compost and manure soil amendments on nematodes and yields of potato and barley: a 7-year study. Journal of Nematology. 2003;35:289–293. [PMC free article] [PubMed] [Google Scholar]
- Ko MP, Schmitt DP. Changes in plant-parasitic nematode populations in pineapple fields following inter-cycle cover crops. Journal of Nematology. 1996;28:546–556. [PMC free article] [PubMed] [Google Scholar]
- Koenning SR, Barker KR. Influence of poultry litter applications on nematode communities in cotton agroecosystems. Journal of Nematology. 2004;36:524–533. [PMC free article] [PubMed] [Google Scholar]
- Kokalis-Burelle N, Rodriguez-Kabana R, Weaver CF, King PS. Evaluation of powdered pine bark for control of Meloidogyne arenaria and Heterodera glycines on soybean. Plant and Soil. 1994;162:163–168. [Google Scholar]
- Korthals GW, van de Ende A, van Megen H, Lexmond TM, Kammenga JE, Bongers T. Short-term effects of cadmium, copper, nickel and zinc on soil nematodes from different feeding and life-history strategy groups. Applied Soil Ecology. 1996;4:107–117. [Google Scholar]
- Li YC, Stofella PJ, Brayn HH. Management of organic amendments in vegetable crop production systems in Florida. Soil and Crop Science Society of Florida Proceedings. 2000;59:17–21. [Google Scholar]
- Linford MB. Stimulated activity of natural enemies of nematodes. Science. 1937;85:123–124. doi: 10.1126/science.85.2196.123. [DOI] [PubMed] [Google Scholar]
- Linford MB, Yap F, Oliveira JM. Reduction of soil populations of the root-knot nematode during decomposition of organic matter. Soil Science. 1938;45:127–141. [Google Scholar]
- Litterick AM, Harrier L, Wallace P, Watson CA, Wood M. The role of uncomposted materials, composts, manures, and compost extracts in reducing pest and disease incidence and severity in sustainable temperate agricultural and horticultural crop production – A review. Critical Reviews in Plant Sciences. 2004;23:453–479. [Google Scholar]
- Mannion CM, Schaffer B, Ozores-Hampton M, Bryan HH, McSorley R. Nematode population dynamics in municipal solid waste-amended soil during tomato and squash cultivation. Nematropica. 1994;24:17–24. [Google Scholar]
- McSorley R. Impact of crop management practices on soil nematode populations. Soil and Crop Science Society of Florida Proceedings. 1996;55:63–66. [Google Scholar]
- McSorley R. Multiple cropping systems for nematode management: A review. Soil and Crop Science Society of Florida Proceedings. 2001;60:132–142. [Google Scholar]
- McSorley R. 2011. Assessment of rotation crops and cover crops for management of root-knot nematodes (Meloidogyne spp.) in the southeastern United States. Nematropica 41:200–214. [Google Scholar]
- McSorley R, Frederick JJ. Nematode population fluctuations during decomposition of specific organic amendments. Journal of Nematology. 1999;31:37–44. [PMC free article] [PubMed] [Google Scholar]
- McSorley R, Gallaher RN. Effect of tillage and crop residue management on nematode densities on corn. Journal of Nematology. 1994;26:669–674. [PMC free article] [PubMed] [Google Scholar]
- McSorley R, Gallaher RN. Cultural practices improve crop tolerance to nematodes. Nematropica. 1995a;25:53–60. [Google Scholar]
- McSorley R, Gallaher RN. Effect of yard waste compost on plant-parasitic nematode densities in vegetable crops. Journal of Nematology. 1995b;27:545–549. [PMC free article] [PubMed] [Google Scholar]
- McSorley R, Gallaher RN. Effect of yard waste compost on nematode densities and maize yield. Journal of Nematology. 1996;28:655–660. [PMC free article] [PubMed] [Google Scholar]
- McSorley R, McGovern RJ. Effects of solarization and ammonium amendments on plant-parasitic nematodes. Journal of Nematology. 2000;32:537–541. [PMC free article] [PubMed] [Google Scholar]
- McSorley R, Ozores-Hampton M, Stansly PA, Conner JM. Nematode management, soil fertility, and yield in organic vegetable production. Nematropica. 1999;29:205–213. [Google Scholar]
- McSorley R, Stansly PA, Noling JW, Obreza TA, Conner JM. Impact of organic soil amendments and fumigation on plant-parasitic nematodes in a southwest Florida vegetable field. Nematropica. 1997;27:181–189. [Google Scholar]
- McSorley R, Wang K-H. Possibilities for biological control of root-knot nematodes by natural predators in Florida soils. Proceedings of the Florida State Horticultural Society. 2009;122:421–425. [Google Scholar]
- McSorley R, Wang K-H, Frederick JJ. Integrated effects of solarization, sunn hemp cover crop, and amendment on nematodes, weeds, and pepper yields. Nematropica. 2008;38:115–125. [Google Scholar]
- Melakeberhan H. Fertiliser use efficiency of soybean cultivars infected with Meloidogyne incognita and Pratylenchus penetrans. Nematology. 2006;8:129–137. [Google Scholar]
- Mennan S, Melakeberhan H. Effects of biosolid amendment on populations of Meloidogyne hapla and soils with different textures and pHs. Bioresource Technology. 2010;101:7158–7164. doi: 10.1016/j.biortech.2010.04.023. [DOI] [PubMed] [Google Scholar]
- Mian IH, Godoy G, Shelby RA, Rodriguez-Kabana R, Morgan-Jones G. Chitin amendments for control of Meloidogyne arenaria in infested soil. Nematropica. 1982;12:71–84. [Google Scholar]
- Mian IH, Rodriguez-Kabana R. Soil amendments with oil cakes and chicken litter for control of Meloidogyne arenaria. Nematropica. 1982a;12:205–220. [Google Scholar]
- Mian IH, Rodriguez-Kabana R. Organic amendments with high tannin and phenolic contents for control of Meloidogyne arenaria in infested soil. Nematropica. 1982b;12:221–234. [Google Scholar]
- Mian IH, Rodriguez-Kabana R. Survey of the nematicidal properties of some organic materials available in Alabama as amendments to soil for control of Meloidogyne arenaria. Nematropica. 1982c;12:235–246. [Google Scholar]
- Morris JB, Walker JT. Non-traditional legumes as potential soil amendments for nematode control. Journal of Nematology. 2002;34:358–361. [PMC free article] [PubMed] [Google Scholar]
- Muller R, Gooch PS. Organic amendments in nematode control: An examination of the literature. Nematropica. 1982;12:319–326. [Google Scholar]
- Myers RL, Ewel JJ. 1990. Ecosystems of Florida. Orlando, FL: University of Central Florida Press. [Google Scholar]
- Neher DA. Nematode communities in organically and conventionally managed agricultural soils. Journal of Nematology. 1999;31:142–154. [PMC free article] [PubMed] [Google Scholar]
- Nico AI, Rafael RM, Jimenez-Diaz M, Castillo P. Control of root-knot nematodes by composted agro-industrial wastes in potting mixtures. Crop Protection. 2004;23:581–587. [Google Scholar]
- Noble CV, Drew RW, Slabaugh JD. 1996. Soil survey of Dade County Area, Florida. Natural Resource Conservation Service, United States Department of Agriculture, Washington, DC. [Google Scholar]
- Noling JW. 1999. Plant resistance and soil amendments in Florida tomato and pepper. Pp. 34–1 to 34-4 in G.L. Obenauf, ed. 2002 Annual Research Conference on Methyl Bromide Alternatives and Emissions Reductions. Fresno, CA: Methyl Bromide Alternatives Outreach. [Google Scholar]
- Noling JW. 2009a. Nematode management in cucurbits (cucumber, melons, squash). ENY-025, Florida Cooperative Extension Service, University of Florida, Gainesville, FL. http://edis.ifas.ufl.edu/ng025.
- Noling JW. 2009b. Nematode management in tomatoes, pepper, and eggplant. ENY-032, Florida Cooperative Extension Service, University of Florida, Gainesville, FL. http://edis.ifas.ufl.edu/ng032.
- Noling JW, Gilreath JP. 1999. Propargyl bromide, biorationals, and other fumigants for nematode control. Pp. 33–1 to 33-4 in G.L. Obenauf, Ed. 2002 Annual Research Conference on Methyl Bromide Alternatives and Emissions Reductions. Fresno, CA: Methyl Bromide Alternatives Outreach. [Google Scholar]
- Odour-Owino P. Integrated management of root-knot nematodes using agro-chemicals, organic matter and the antagonistic fungus, Paecilomyces lilacinus in natural field soil. Nematologia Mediterranea. 2003;31:121–123. [Google Scholar]
- Oka Y. Mechanisms of nematode suppression by organic soil amendments – A review. Applied Soil Ecology. 2010;44:101–115. [Google Scholar]
- Oka Y, Tkachi N, Shuker S, Yermiyahu U. Enhanced nematicidal activity of organic and inorganic ammonia-releasing amendments by Azadirachta indica extracts. Journal of Nematiology. 2007;39:9–16. [PMC free article] [PubMed] [Google Scholar]
- Oka Y, Yermiyahu U. Suppressive effects of composts against the root-knot nematode Meloidogyne javanica on tomato. Nematology. 2002;4:891–898. [Google Scholar]
- Ozores-Hampton M, Stansly PA, McSorley R, Roe NE, Chellemi DO. Long term large scale soil solarization as a low-input production system for Florida vegetables. Acta Horticulturae. 2004;638:177–188. [Google Scholar]
- Perry VG, Rhoades HL. 1982. The trichodorid nematodes. Pp. 183–186 in R.D. Riggs, ed. Nematology in the Southern Region of the United States. Southern Cooperative Series Bulletin 276. Fayetteville, AR: Arkansas Agricultural Experiment Station. [Google Scholar]
- Piedra Buena A, Garcia-Alvarez A, Diez-Rojo MA, Ros C, Fernandez P, Lacasa A, Bello A. Use of pepper crop residues for the control of root-knot nematodes. Bioresource Technology. 2007;98:2846–2851. doi: 10.1016/j.biortech.2006.09.042. [DOI] [PubMed] [Google Scholar]
- Ploeg AT. Effects of amending soil with Tagetes patula cv. Single Gold on Meloidogyne incognita infestation of tomato. Nematology. 2000;2:489–493. [Google Scholar]
- Porazinska DL, Duncan LW, McSorley R, Graham JH. Nematode communities as indicators of status and processes of a soil ecosystem influenced by agricultural management practices. Applied Soil Ecology. 1999;13:69–86. [Google Scholar]
- Powers LE, McSorley R. 2000. Ecological principles of agriculture. Albany, NY: Delmar Thomson Learning. [Google Scholar]
- Rhoades HL, Forbes RB. Effects of fallow, cover crops, organic mulches, and fenamiphos on nematode populations, soil nutrients, and subsequent crop growth. Nematropica. 1986;16:141–151. [Google Scholar]
- Rich JR, Hodge CH. Utilization of blue crab scrap compost to suppress Meloidogyne javanica on tomato. Nematropica. 1993;23:1–5. [Google Scholar]
- Riegel C, Fernandez FA, Noe JP. Meloidogyne incognita infested soil amended with chicken litter. Journal of Nematology. 1996;28:369–378. [PMC free article] [PubMed] [Google Scholar]
- Ritzinger CHSP, McSorley R. Effect of castor and velvetbean organic amendments on Meloidogyne arenaria in greenhouse experiments. Journal of Nematology. 1998;30:624–631. [PMC free article] [PubMed] [Google Scholar]
- Ritzinger CHSP, McSorley R, Gallaher RN. Effect of organic amendment placement and inoculum density of Meloidogyne incognita on okra seedlings. Soil and Crop Science Society of Florida Proceedings. 1997;56:28–31. [Google Scholar]
- Rodriguez-Kabana R. Organic and inorganic nitrogen amendments to soil as nematode suppressants. Journal of Nematology. 1986;18:129–135. [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Kabana R, Boube D, Young RW. Chitinous materials from blue crab for control of root-knot nematode. I. Effect of urea and enzymatic studies. Nematropica. 1989;19:53–74. [Google Scholar]
- Rodriguez-Kabana R, Godoy G, Morgan-Jones G, Shelby RA. The determination of soil chitinase activity: Conditions for assay and ecological studies. Plant and Soil. 1983;75:95–106. [Google Scholar]
- Rodriguez-Kabana R, King PS. Use of mixture of urea and blackstrap molasses for control of root-knot nematodes in soil. Nematropica. 1980;10:38–44. [Google Scholar]
- Rodriguez-Kabana R, King PS, Pope MH. Combinations of anhydrous ammonia and ethylene dibromide for control of nematodes parasitic of soybeans. Nematropica. 1981;11:27–41. [Google Scholar]
- Rodriguez-Kabana R, Morgan-Jones G, Chet I. Biological control of nematodes: Soil amendments and microbial antagonists. Plant and Soil. 1987;100:237–247. [Google Scholar]
- Rodriguez-Kabana R, Morgan-Jones G, Ownley-Gintis B. Effects of chitin amendments to soil on Heterodera glycines, microbial populations, and colonization of cysts by fungi. Nematropica. 1984;14:10–25. [Google Scholar]
- Rodriguez-Kabana R, Weaver CF, Robertson DG, Snoddy EL. Population dynamics of Meloidogyne arenaria juveniles in a field with ‘Florunner’ peanut. Nematropica. 1986;16:185–196. [Google Scholar]
- Ros M, Garcia C, Hernandez MT, Lacasa A, Fernandez P, Pascual JA. Effects of biosolarization as methyl bromide alternative for Meloidogyne incognita control on quality of soil under pepper. Biology and Fertility of Soils. 2008;45:37–44. [Google Scholar]
- Sanchez-Moreno S, Ferris H. Suppressive service of the soil food web: effects of environmental management. Agriculture. Ecosystems and Environment. 2007;119:75–87. [Google Scholar]
- Stirling GR. 1991. Biological control of plant-parasitic nematodes. Wallingford, UK: CAB International. [Google Scholar]
- Thoden TC, Korthals GW, Termorshuizen AJ. Organic amendments and their influences on plant-parasitic and free-living nematodes: a promising method for nematode management? Nematology. 2011;13:133–153. [Google Scholar]
- Trivedi PC, Barker KR. Management of nematodes by cultural practices. Nematropica. 1986;16:213–236. [Google Scholar]
- Wang K-H, McSorley R, Gallaher RN. Host status and amendment effects of cowpea on Meloidogyne incognita in vegetable cropping systems. Nematropica. 2003;33:215–224. [Google Scholar]
- Wang K-H, McSorley R, Gallaher RN. Effect of Crotalaria juncea amendment on squash infected with Meloidogyne incognita. Journal of Nematology. 2004a;36:290–296. [PMC free article] [PubMed] [Google Scholar]
- Wang K-H, McSorley R, Gallaher RN. Relationship of soil management history and nutrient status to nematode community structure. Nematropica. 2004b;34:83–95. [Google Scholar]
- Wang K-H, McSorley R, Gallaher RN, Kokalis-Burelle N. Cover crops and organic mulches for nematode, weed, and plant health management. Nematology. 2008;10:231–242. [Google Scholar]
- Wang K-H, McSorley R, Kokalis-Burelle N. Effects of cover cropping, solarization, and soil fumigation on nematode communities. Plant and Soil. 2006a;286:229–243. [Google Scholar]
- Wang K-H, McSorley R, Marshall AJ, Gallaher RN. Nematode community changes associated with decomposition of Crotalaria juncea amendment in litterbags. Applied Soil Ecology. 2004c;27:31–45. [Google Scholar]
- Wang K-H, McSorley R, Marshall AJ, Gallaher RN. Influence of organic Crotalaria juncea hay and ammonium nitrate fertilizers on soil nematode communities. Applied Soil Ecology. 2006b;31:186–198. [Google Scholar]
- Wang K-H, Sipes BS, Schmitt DP. Suppression of Rotylenchulus reniformis by Crotalaria juncea, Brassica napus, and Tagetes erecta. Nematropica. 2001;31:237–251. [Google Scholar]
- Wang K-H, Sipes BS, Schmitt DP. Management of Rotylenchulus reniformis in pineapple, Ananas comosus, by intercycle cover crops. Journal of Nematology. 2002a;34:106–114. [PMC free article] [PubMed] [Google Scholar]
- Wang K-H, Sipes BS, Schmitt DP. Crotalaria as a cover crop for nematode management: a review. Nematropica. 2002b;32:35–57. [Google Scholar]
- Wang Q, Li Y, Klassen W, Handoo Z. Influence of cover crops and soil amendments on okra (Abelmoschus esculentus L.) production and soil nematodes. Renewable Agriculture and Food Systems. 2007;22:41–53. [Google Scholar]
- Wasilewska L, Bienkowski P. Experimental study of the occurrence and activity of soil nematodes in decomposition of plant material. Pedobiologia. 1985;28:41–57. [Google Scholar]
- Watson JR. Mulches to control root-knot. Proceedings of the Florida Academy of Sciences. 1945;7:151–153. [Google Scholar]
- Westerdahl BB, Carlson HL, Grant J, Radewald JD, Welch N, Anderson CA, Darso J, Kirby D, Shibuya F. Management of plant-parasitic nematodes with a chitin-urea soil amendment and other materials. Supplement to Journal of Nematology. 1992;24:669–680. [PMC free article] [PubMed] [Google Scholar]
- Zasada I. Factors affecting the suppression of Heterodera glycines by N-Viro Soil. Journal of Nematology. 2005;37:220–225. [PMC free article] [PubMed] [Google Scholar]
- Zasada IA, Ferris H. Nematode suppression with brassicaceous amendments: application based upon glucosinolate profiles. Soil Biology and Biochemistry. 2004;36:1017–1024. [Google Scholar]
- Zasada IA, Halbrendt JM, Kokalis-Burelle N, LaMondia J, McKenry MV, Noling JW. Managing nematodes without methyl bromide. Annual Review of Phytopathology. 2010;48:311–328. doi: 10.1146/annurev-phyto-073009-114425. [DOI] [PubMed] [Google Scholar]