Abstract
Physical, chemical, and biological factors of soil may reduce damage caused by plant-parasitic nematodes. Suppression of plant-parasitic nematodes is particularly challenging in soils in which there are short crop sequences, sequential susceptible host crops, or infestations of multiple nematode species. In southern Indiana, a watermelon production system involving rotations with soybean and corn does not suppress Meloidogyne incognita, but several aspects of such systems can be modified to reduce nematode damage in an integrated management approach. Cash crops with resistance to M. incognita can be used to reduce population densities of M. incognita. Small grains as cover crops can be replaced by cover crops with resistance to M. incognita or by crops with biofumigation potential. Mycorrhizal fungal inoculations of potting mixes during transplanting production of watermelon seedlings may improve early crop establishment. Other approaches to nematode management utilize soil suppressiveness. One-year rotations of soybean with corn neither reduced the soil-borne complex of sudden death syndrome (SDS) nor improved soybean root health over that in soybean monoculture. Reduced tillage combined with crop rotation may reduce the activity of soil-borne pathogens in some soils. For example in a long-term trial, numbers of Heterodera glycines and severity of foliar SDS symptoms were reduced under minimum tillage. Thus, sustainable management strategies require holistic approaches that consider entire production systems rather than focus on a single crop in its year of production.
Management of plant-parasitic nematodes is complicated by the complexity of the soil environment (Norton, 1978). Chemical, biological, and cultural methods along with the use of host plant resistance comprise management strategies that have decreased the risk of damage by many nematode species (Hague and Gowen, 1987; Heald, 1987; Kerry, 1987; Halbrendt and LaMondia, 2004; Starr and Roberts, 2004). None of these techniques is without challenges. For example, reliance on chemical control has come into question because chemical pesticides can have negative environmental and human health effects and their usage is under review (Schierow, 2000; Martin, 2003). Usefulness of chemical suppression also can be restricted by regional production conditions. For example, there are only narrow windows of opportunity for pest management inputs if fields are occupied most of the time suitable for plant growth. In particular, narrow crop sequences with host crops of a specific plant-parasitic nematode or infestations with multiple nematode species can complicate the development of management strategies. Integrated methods for the management of plant-parasitic nematodes have been proposed for the production of high and low-value crops (Brown, 1987; McKenry, 1987). These strategies partially benefit from physical, chemical, and biological factors in soil that constrain activities of plant-parasitic nematodes and may reduce their damage potential (Stirling, 1991; Robinson, 2004). An integrated management strategy requires in-depth evaluation of the cropping system. Key questions need to be answered before a successful holistic approach to nematode management is designed. How are plants established? How do vegetation and cropping period overlap? Are there cover crop periods? What is the crop sequence? What is the tillage system? Economical, biological, practical and logistical feasibility of the potential management strategy need to be addressed.
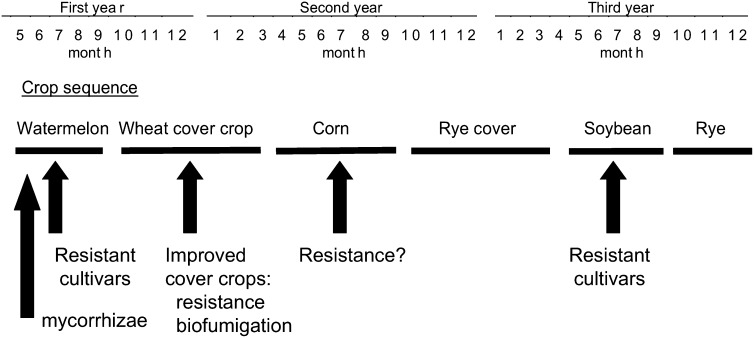
Cropping of high- and low-value Meloidogyne incognita hosts in rotation In southern Indiana, crop sequences of watermelon, soybean and corn, in combination with cereal winter cover crops (Fig. 1), do not suppress Meloidogyne incognita because all of these crops are hosts to Meloidogyne incognita. This nematode is widely distributed in the watermelon production area (Kruger et al., 2007). Availability of resistance in the high-value, M. incognita-susceptible watermelon is limited, although some recent advances in identifying host plant resistance have been made (Thies and Levi, 2007). Probably because of the difficulty of obtaining host plant resistance, the alternative strategy of grafting desired watermelon scions on root knot-nematode resistant rootstocks is currently explored (Thies et al., 2010). The production system is restricted by the limited availability of days suitable for soil fumigation, bare fallow, or summer cover cropping of nematode-antagonistic plants because a crop occupies the field most of the time when soil temperatures are suitable. A holistic view of this cropping sequence must be taken to design an integrated management system by modifying incremental portions of the system to reduce population densities of M. incognita throughout the cropping sequence rather than single crops.
Fig. 1.
Example of watermelon-soybean-corn sequence in South Indiana with possible modification options.
Three approaches will be discussed, whereby integrated management could be applied to decrease nematode damage. In the first approach, susceptible cash crops could be replaced with cultivars with resistance to M. incognita. This principle has been used to protect susceptible crops with few or no resistant cultivars in a sequence by planting them after resistant cash crops (Westphal and Scott, 2005; Koenning and Edmisten, 2008). For example in cotton production, population densities of Rotylenchulus reniformis were reduced under R. reniformis-resistant soybean to such levels that the following susceptible cotton was protected from extensive nematode damage (Westphal and Scott, 2005). In the current project, eight cultivars were planted at early and late planting dates into soil infested with both M. incognita and Heterodera glycines, and two cultivars were planted in control plots fumigated with 1,3-dichloropropene. Some of the tested cultivars suppressed M. incognita following early and late planting. Some cultivars with resistance to both nematodes produced competitive yields. Some cultivars were resistant only to M. incognita but not to the widely distributed H. glycines (Kruger et al., 2008). This indicated that infestations of multiple nematodes must be monitored for optimal crop production (Weaver et al., 1995; Kruger et al., 2008). Long-term, such management approach may be at risk for the development of populations that can overcome the resistance incorporated in the cultivars because of the high level of selection pressure (Young, 1992; Roberts, 2002). The risk for such development may be mitigated if a gain in virulence is associated with a loss in fitness as reported in tomato. In that system, populations of M. incognita virulent on Mi-carrying tomato were compromised in their fitness on susceptible tomato and thus could potentially be counter-selected against when susceptible cultivars would be grown (Castagnone-Sereno et al., 2007). If these concepts would apply to root-knot nematode on soybean is mostly unknown. Meanwhile, new resistance sources against root-knot nematodes in soybean are discovered (Harris et al., 2003; Shannon et al., 2009).
As the second part of the integrated management approach, the choice of winter cover crops, typically rye and wheat in southwest Indiana, for reducing of wind and water erosion may be improved. Rye and wheat are hosts of M. incognita (Ibrahim et al., 1993: Kaloshian et al., 1989) and nematode galling has been observed on rye roots when it is grown as a winter cover crop (Westphal, unpub. data). Several Brassica cover crops have shown potential as substitutes, which either are resistant to M. incognita, e.g., ‘Boss’ (Westphal et al., 2006) or have biofumigation activity (Kirkegaard and Sarwar, 1998). The use of cover crops that are not only resistant to M. incognita but also produce biofumigants appear the most suited for replacing cereals as winter crops. In a microplot trial, four treatments: fallow; M. incognita-resistant oilseed radish, Raphanus sativus conv. oleiformis, ‘Boss’; oilseed radish, susceptible to M. incognita, ‘Siletina’; and rye. Secale cereale were established in two M. incognita-infested, sandy loam soils in September. In the following February, galling on Boss was much less than on Siletina. At planting of watermelon in May, numbers of M. incognita second-stage juveniles in soil were lowest after bare fallow (i.e, unacceptable for erosion control considerations), and were lower under Boss than under Siletina and rye. At watermelon harvest, nematode numbers in all treatments were similar (Westphal et al., 2006). Although Boss, which is not winterhardy, suppressed M. incognita, further improvements may be obtained by incorporating Brassica cover crops such as canola (Brassica napus) that can overwinter in Indiana. Such cover crops could be established in the fall after harvest of the precrop to watermelon, establish root systems while reducing M. incognita, as a trap crop, and continue producing biomass in the following early spring. As a second benefit, residual canola biomass in the spring would provide a substrate for biofumigation.
In a third management approach, planting plug amendments, applied during seeding of watermelon seeds into soil-less potting mix in seedling trays, are considered. Transplanting to plastic-mulched beds is standard practice in many watermelon production areas (Hochmuth et al., 2001). In this transplanting system, watermelon roots often appear less vigorous than those of direct-seeded plants (Egel et al., 2008). Mycorrhizal associations benefit vegetable root function (Linderman, 1988) and may improve watermelon seedlings in this system. In the current project, watermelon was tested as a host for vesicular arbuscular mycorrhizal fungi. The colonization potentials of three commercial formulations of mycorrhizal inocula on watermelon were compared to a non-inoculated control. Such seedlings were planted to M. incognita-infested field plots or in a trial with two different P fertilizer levels. Seedlings with amendments of mycorrhizal fungi with one type of mycorrhizal fungus amendment produced increased early vine length and another type of inoculum or a mycorrhizal colonization-enhancing treatment increased early fruit yield (Westphal et al., 2008). While the process of amending the potting mix with the mycorrhizal fungi inoculum may create only limited expense, the utility of this method will depend on product cost of the inoculum. Assuming the cost of a single (seedless watermelon) transplant of $0.28 (Taylor et al., 2008), the product cost depending on formulation may vary between a few cents to close to the cost of the transplant itself. As outlined by Taylor and coworkers (2008) for the economics of using grafted watermelon transplants (estimated cost: $0.75 each) in the presence of Fusarium wilt, the feasibility of transplant treatments depends on the risk for the soil-borne problem to occur and how much yield protection can be realized. Although the amendment of planting plugs with mycorrhizal fungi may not become a standard practice, it may be beneficial in some instances to improve plant establishment and early fruit yield (Westphal et al., 2008).
In summary, watermelon production in southern Indiana fields where M. incognita is present was improved by implementing several low-cost production modifications. Soybean cultivars with resistance to both nematodes, M. incognita and H. glycines, were identified and shown to suppress nematode populations. Covercropping with Brassica species with resistance to M. incognita in some cases may offer the added benefit of biofumigation activity. Finally, amendment of transplant potting mixes with mycorrhizal fungi may improve watermelon seedling vigor.
Effects of cropping systems on the severity of soil-borne diseases of soybean: Cropping systems of low-value, large-acre crops, e.g., soybean-corn production systems, face different management challenges than high value crops (Brown, 1987). In the Midwest, soybean and corn are rotated in alternate years assuming that one year of the non-host reduces soil-borne problems of the other crop. In crop rotation trials at two locations in Indiana, soybean root health and severity of foliar sudden death syndrome (SDS) symptoms for monoculture soybean were compared with soybean after either one-year of fallow (either non-treated or treated with the biocide dazomet) or one year of one of four corn hybrids (Xing and Westphal, 2009). At both locations, external root necrosis was reduced after fallow, and even more so after biocide-treated fallow compared to plots with a crop in the previous year. At one location, severity of foliar symptoms of SDS in the soybean year in fallow treatments was lower than in other treatments; the SDS foliar rating for monoculture soybean was less than corn rotation for two hybrids. At the second location, however, soybean in fallow plots had higher foliar SDS ratings than corn treatment of soybean plots; soybean monoculture had higher SDS ratings than two of the corn hybrid treatments. In this study, corn did not improve root health as well as a fallow or biocide-treated fallow, suggesting that the soybean-corn crop sequence leads to poor soybean root health (Xing and Westphal, 2009).
Although effects of tillage practices on certain physical and biological properties of soil are well understood (Kladivko, 2001), effects on soil-borne pathogens are not. A long-term tillage trial in a mollisol infested with both H. glycines and the SDS-pathogen, examined the following continuous tillage treatments (listed in decreasing order of soil disturbance): (I) plow + secondary tillage; (II) chisel plow + secondary tillage; (III) ridge tillage; and (IV) no tillage. Suppression of the two pathogens is urgently needed because they cause concomittantly increase damage on susceptible soybean (Xing and Westphal, 2006). Tillage treatments were compared in a corn-soybean rotation and in soybean monoculture. The results showed that population densities of H. glycines in the rotation were positively correlated with soil disturbance level, whereas in soybean monoculture population densities were consistently high and unrelated to tillage (Westphal et al., 2009). Foliar SDS symptoms, like nematodes, were decreased by decreasing tillage (Westphal, unpub. data). Thus for some reason, decreasing tillage appeared to increase pathogen suppression in soil where crops were rotated.
Conclusions: Current cropping systems are often vulnerable to serious problems with plant-parasitic nematodes. Ecological and human health concerns, and economic considerations require a holistic approach to a truly integrated pest management system. Detailed evaluation of the entire cropping system, though more cumbersome than single crop-approaches, is essential to integrate novel tools and strategies successfully into these systems. “Silver-bullet” approaches must be replaced by the combination of partial management tools for reducing the risk of damage by plant-parasitic nematodes. The benefits of novel management tools and agronomic practices in improving sustainable agricultural production require further exploitation to realize the goal of sustainable production with minimal environmental impacts.
LITERATURE CITED
- Brown RH. 1987. Control strategies in low-value crops. Pp. 351–388 in R. H. Brown and B. R. Kerry, eds. Principles and Practice of Nematode Control in Crops. Sydney, Australia: Academic Press. [Google Scholar]
- Castagnone-Sereno P, Bongiovanni M, Wajnberg E. Selection and parasite evolution: a reproductive fitness cost associated with virulence in the parthenogenic nematode Meloidogyne incognita. Evolutionary Ecology. 2007;21:259–270. [Google Scholar]
- Egel DS, Martyn R, Gunter C. Planting method, plastic mulch, and fumigation influence growth, yield, and root structure of watermelon. HortScience. 2008;43:1410–1414. [Google Scholar]
- Hague NGM, Gowen SR. 1987. Chemical control of nematodes. Pp. 131–178 in R. H. Brown and B. R. Kerry, eds. Principles and Practice of Nematode Control in Crops. Sydney, Australia: Academic Press. [Google Scholar]
- Halbrendt JM, LaMondia JA. 2004. Crop rotation and other cultural practices. Pp. 909–930 in Z. X. Chen, S. Y. Chen, and D. W. Dickson, eds. Nematology Advances and Perspectives: Vol. II Nematode Management and Utilization. Oxfordshire, UK: CAB International. [Google Scholar]
- Harris DK, Boerma HR, Hussey RS, Finnerty SL. Additional sources of soybean germplasm resistant to two species of root-knot nematode. Crop Science. 2003;43:1848–1851. [Google Scholar]
- Heald CM. 1987. Classical nematode management practices. Pp. 100–105 in J. A. Veech and D. W. Dickson, eds. Vistas on Nematology. Hyatssville, MD: Society of Nematologists. [Google Scholar]
- Hochmuth GJ, Kee E, Hartz TK, Dainello FJ, Motes JE. 2001. Cultural Management. Pp. 78–97 in D. N. Maynard, ed. Watermelons: Characteristics, Production and Marketing. Alexandria, VA: ASHS Horticulture Crop Production Series, ASHS Press. [Google Scholar]
- Ibrahim IKA, Lewis SA, Harshman DC. Host suitability of graminaceous crop cultivars for isolates of Meloidogyne arenaria and M. incognita. Supplement Journal of Nematology. 1993;25:858–862. [PMC free article] [PubMed] [Google Scholar]
- Kaloshian I, Roberts PA, Thomason IJ. Resistance to Meloidogyne spp. in allohexaploid wheat derived from Triticum turgidum and Aegilops squarrosa. Journal of Nematology. 1989;21:42–47. [PMC free article] [PubMed] [Google Scholar]
- Kerry BR. 1987. Biological control. Pp. 223–263 in R. H. Brown and B. R. Kerry, eds. Principles and Practice of Nematode Control in Crops. Sydney, Australia: Academic Press. [Google Scholar]
- Kirkegaard JA, Sarwar M. Biofumigation potential of Brassicas. I. Variation in glucosinolate profiles of diverse field-grown Brassicas. Plant and Soil. 1998;201:71–89. [Google Scholar]
- Kladivko E. Tillage systems and soil ecology. Soil and Tillage Research. 2001;61:61–76. [Google Scholar]
- Koenning SR, Edmisten KL. Rotation with corn and soybean for management of Meloidogyne incognita in cotton. Journal of Nematology. 2008;40:258–265. [Google Scholar]
- Kruger GR, Xing LJ, LeRoy A, Westphal A. Meloidogyne incognita resistance in soybean under Midwest conditions. Crop Science. 2008;48:716–726. [Google Scholar]
- Kruger GR, Xing LJ, Santini J, Westphal A. Distribution and damage caused by root-knot nematodes on soybean in southwest Indiana. 2007 Plant Health Progress, Online, doi:10.1094/PHP-2007-1031-01-RS. [Google Scholar]
- Linderman RG. Mycorrhizal interactions with the rhizosphere microflora: The mycorrhizosphere effect. Phytopathology. 1988;78:366–371. [Google Scholar]
- Martin FN. Development of alternative strategies for management of soilborne pathogens currently controlled with methyl bromide. Annual Review of Phytopathology. 2003;41:325–350. doi: 10.1146/annurev.phyto.41.052002.095514. [DOI] [PubMed] [Google Scholar]
- McKenry MV. 1987. Control strategies in high-value crops. Pp. 330–349 in R. H. Brown and B. R. Kerry, eds. Principles and Practice of Nematode Control in Crops. Sydney, Australia: Academic Press. [Google Scholar]
- Norton DC. 1978. Ecology of Plant-parasitic Nematodes. New York, NY: John Wiley & Sons, Pp. 268. [Google Scholar]
- Roberts PA. 2002. Concepts and consequences of resistance. Pp. 23–41 in J. L. Starr, R. Cook, and J. Bridge, eds. Plant Resistance to Parasitic Nematodes. London, UK: CAB International. [Google Scholar]
- Robinson AF. 2004. Nematode behavior and migrations through soil and host tissue. Pp. 330–405 in Z. X. Chen, S. Y. Chen, and D. W. Dickson, eds. Nematology Advances and Perspectives: Vol. I Nematode Morphology, Physiology, and Ecology. Oxfordshire, UK: CAB International. [Google Scholar]
- Schierow L-J. FQPA: Origin and outcome. Choices: The Magazine of Food, Farm & Resource Issues. 2000;15:18–21. [Google Scholar]
- Shannon JG, Lee JD, Wrather JA, Sleper DA, Mian MAR, Bond JP, Robbins RT. Registration of SS99-2281 soybean germplasm line with resistance to frogeye leaf spot and three nematode species. Journal of Plant Registrations. 2009;3:94–98. [Google Scholar]
- Starr JL, Roberts PA. 2004. Resistance to plant-parasitic nematodes. Pp. 879–907 in Z. X. Chen, S. Y. Chen, and D. W. Dickson, eds. Nematology Advances and Perspectives: Vol. II Nematode Management and Utilization. Oxfordshire, UK: CAB International. [Google Scholar]
- Stirling GR. 1991. Biological Control of Plant-parasitic Nematodes: Progress, Problems, and Prospects. Wallingford, UK: CAB International, Pp. 282. [Google Scholar]
- Taylor M, Bruton B, Fish W, Roberts W. 2008. Cost benefit analyses of using grafted watermelon transplants for Fusarium wilt disease control. Pp. 343–350 in D. I. Leskovar ed. Proceeding of the IVth IS on Seed, Transplant and Stand Establishment of Hort Crops, Acta Horticulturae 782, Leuven, Belgium: International Society of Horticultural Science. [Google Scholar]
- Thies JA, Levi A. Characterization of watermelon (Citrullus lanatus var. citroides) germplasm for resistance to root-knot nematodes. HortScience. 2007;42:1530–1533. [Google Scholar]
- Thies JA, Ariss JJ, Hassell RL, Olson S, Kousik CS, Levi A. Grafting for management of southern root-knot nematode, Meloidogyne incognita, in watermelon. Plant Disease. 2010;94:1195–1199. doi: 10.1094/PDIS-09-09-0640. [DOI] [PubMed] [Google Scholar]
- Weaver DB, Rodriguez-Kabana R, Carden EL. Comparison of crop rotation and fallow for the management of Heterodera glycines and Meloidogyne spp. in soybean. Journal of Nematology. 1995;27S:585–591. [PMC free article] [PubMed] [Google Scholar]
- Westphal A, Scott AW. Implementation of soybean in cotton cropping sequences for management of Rotylenchulus reniformis in South Texas. Crop Science. 2005;45:233–239. [Google Scholar]
- Westphal A, Xing LJ, Egel DS. 2006. Use of cover crops for management of root knot nematodes in cucurbits. Annual International Research Conference Methyl Bromide Alternatives Emission Reduction, Orlando, FL, Nov. 6-9, 2006. (abstract) 22-1-3. [Google Scholar]
- Westphal A, Camberato JJ, Snyder NL, Xing LJ. Effects of inoculations with mycorrhizal fungi of soil-less potting mixes during transplant production on watermelon growth and early fruit yield. HortScience. 2008;43:354–360. [Google Scholar]
- Westphal A, Xing LJ, Pillsbury R, Vyn TJ. Effects of tillage on population densities of Heterodera glycines. Field Crops Research. 2009;113:218–226. [Google Scholar]
- Xing LJ, Westphal A. Interaction of Fusarium solani f. sp. glycines and Heterodera glycines in sudden death syndrome of soybean. Phytopathology. 2006;96:763–770. doi: 10.1094/PHYTO-96-0763. [DOI] [PubMed] [Google Scholar]
- Xing LJ, Westphal A. Effects of crop rotation of soybean with corn on severity of sudden death syndrome and population densities of Heterodera glycines in naturally infested soil. Field Crops Research. 2009;112:107–117. [Google Scholar]
- Young LD. Problems and strategies associated with long-term use of nematode resistant cultivars. Journal of Nematology. 1992;24:228–233. [PMC free article] [PubMed] [Google Scholar]