Abstract
Tall fescue grass cultivars with or without endophytes were evaluated for their susceptibility to Meloidogyne incognita in the greenhouse. Tall fescue cultivars evaluated included, i) wild-type Jesup (E+, ergot-producing endophyte present), ii) endophyte-free Jesup (E-, no endophyte present), iii) Jesup (Max-Q, non-ergot producing endophyte) and iv) Georgia 5 (E+). Peach was included as the control. Peach supported greater (P ≤ 0.05) reproduction of M. incognita than all tall fescue cultivars. Differences in reproduction were not detected among the tall fescue cultivars and all cultivars were rated as either poor or nonhosts for M. incognita. Suppression of M. incognita reproduction was not influenced by endophyte status. In two other greenhouse experiments, host susceptibility of tall fescue grasses to two M. incognita isolates (BY-peach isolate and GA-peach isolate) did not appear to be related to fungal endophyte strain [i.e., Jesup (Max-Q; nontoxic endophyte strain) vs. Bulldog 51 (toxic endophyte strain)]. Host status of tall fescue varied with species of root-knot nematode. Jesup (Max-Q) was rated as a nonhost for M. incognita (BY-peach isolate and GA-peach isolate) and M. hapla, a poor host for M. javanica and a good host for M. arenaria. Bulldog 51 tall fescue was also a good host for M. arenaria and M. javanica, but not M. incognita. Jesup (Max-Q) tall fescue may have potential as a preplant control strategy for M. incognita and M. hapla in southeastern and northeastern United States, respectively.
Keywords: Endophyte, Festuca arundinacea, host-parasitic relationship, management, Meloidogyne arenaria, Meloidogyne hapla, Meloidogyne incognita, Meloidogyne javanica, resistance, rootknot nematode, Schedonorus arundinaceus, tall fescue grass
Root-knot nematodes are considered to be the most damaging plant-parasitic nematodes in the world and can be found in temperate, tropical and equatorial agricultural producing areas (Lamberti, 1979; Sasser, 1979; Sasser and Freckman, 1987; Nyczepir and Thomas, 2009). Root-knot nematodes reduce fruit production in several economically important Prunus species, including peach. Collectively, these nematodes are more damaging to most crops than most other plant-parasitic nematodes because: i) root-knot nematodes are widely distributed throughout the world; ii) most species complete several generations in a given growing season and have high fecundity; and iii) some species have very wide host ranges. If left unmanaged in peach, root-knot nematodes can cause typical below-ground root galls, associated above-ground stunted growth of young peach trees within the first 2-3 yr following orchard establishment, and even tree death. Additional above-ground symptoms could include reduced tree vigor and early defoliation that ultimately may reduce yield (Nyczepir and Thomas, 2009).
Present management practices in the southeastern United States include preplant fumigation (i.e., Telone II or Vapam) which improves tree establishment and gives control for 2-5 yr, depending on the quality of the fumigation and the nematode involved, and resistant rootstocks (when available) (Nyczepir, 1991; Sharpe et al, 1993). Current research efforts in the Southeast and California have shifted toward various forms of nonchemical nematode control. Emphasis on nonchemical control is partly due to apprehension about the environmental problems associated with soil fumigation with methyl bromide. As a result of its role in ozone depletion, a ban on the importation and manufacture of methyl bromide in the United States took effect in January 2005 (Clean Air Act, 1990), with certain exceptions [i.e., Critical Use Exemption (CUE), quarantine and pre-shipment exemptions, and emergency exemptions]. Therefore, finding an alternative to preplant chemical control is warranted. In Georgia, rotation with Coastal Bermuda grass, which also can be harvested for hay, is recommended for control of Meloidogyne spp., (Nyczepir, 2005). Another potential groundcover rotation crop for nematode management is tall fescue grass.
Tall fescue [Schedonorus arundinaceus (Schreb.) Dumont. = Lolium arundinaceum (Schreb.) Darbysh., formerly Festuca arundinacea Schreb.] is the most widely grown perennial, cool-season turf and forage grass species and it is well-adapted in the upper transition zone between the temperate northeastern and subtropical southeastern United States. The dominant tall fescue variety grown throughout the United States is Kentucky 31 which is planted (by some estimates) on over 16 million ha (Bouton, 2002). Kentucky 31’s popularity among forages is the result of it being vigorous, widely adaptable, able to withstand poor soil conditions, and resistant to pests and drought (Ball et al., 1993). These favorable growth characteristics have been attributed to the symbiotic relationship with the endophytic fungus Neotyphodium coenophialum (Morgan-Jones & W. Gams) Glenn, C. W. Bacon, & Hanlin. The presence of the fungal endophyte in tall fescue also has been reported to confer resistance to some plant-parasitic nematodes (Bernard et al., 1998). For example, populations of Pratylenchus scribneri and Tylenchorhynchus acutus were lower in soil planted to Kentucky 31 tall fescue with the endophyte than without the endophyte (West et al., 1988; Kimmons et al., 1990; Bernard and Gwinn, 1991; Gwinn and Bernard, 1993). Additionally, suppression of Pratylenchus scribneri has also been associated with different strains of Neotyphodium coenophialum and certain tall fescue cultivars (Timper et al., 2005). Populations of P. scribneri were suppressed in cv. Georgia 5 tall fescue which contained the non-ergot endophyte strain AR584, whereas this same endophyte strain did not confer P. scribneri suppression in cv. Jesup. Little research has been reported on the host-parasite relationship between tall fescue and Meloidogyne spp, and what has been published was conflicting. One brief report stated that M. incognita and M. hapla did not reproduce on Kentucky 31 and six other tall fescue cultivars under greenhouse conditions (Chapman, 1973), whereas in another study M. incognita was able to reproduce on tall fescue grass (McGlohon et al., 1961). The presence/absence of N. coenophialum or a specific strain of this endophyte was not reported. Moreover, nematode reproduction of M. marylandi was lower in the presence N. coenophialum-infected (E+) tall fescue than in endophyte-free (E-) tall fescue (Kimmons et al., 1990; Elmi et al., 2000).
The presence of the endophyte infection is not always beneficial; it has been associated with causing “fescue toxicosis” syndrome (Bacon et al., 1977; Siegel and Bush, 1996). Tall fescue infected with N. coenophialum contains a group of ergot alkaloids produced by this fungus. Fescue toxicosis is sometimes suffered by animals that graze on N. coenophialum-infected grass resulting in elevated body temperature, poor weight gain, and (or) reduced prolactin concentrations (Bouton, 2002). One approach to avoiding fescue toxicosis is to use nontoxic strains of N. coenophialum (i.e., endophyte-friendly) (Bacon and Siegel, 1988). An endophyte-friendly tall fescue provides beneficial effects for the plant without producing the dangerous toxins. One such endophyte-friendly commercial tall fescue variety is Max-Q. Max-Q is the result of a novel, non-toxic endophyte strain (AR542) being inserted in Jesup and Georgia-5 tall fescue; thus providing beef producers the opportunity of avoiding fescue toxicosis (Phillips and Aiken, 2009). Max-Q tall fescue susceptibility to Meloidogyne spp. is unknown. The objective of this research was to evaluate the host susceptibility of endophyte-infected (E+), endophyte-free (E-) and (or) Max-Q tall fescue to Meloidogyne arenaria, M. hapla, M. incognita and M. javanica.
Materials and Methods
Nematode source and inoculum: Two populations of M. incognita (isolates BY-peach and GA-peach) originally isolated from peach in Georgia, and populations of M. arenaria and M. javanica isolated from soybean and peach, respectively, in South Carolina, were all maintained on tomato (Solanum esculentum Mill. cv. ‘Rutgers’) in the greenhouse. A population of M. hapla isolated from clover in MD was maintained on pepper (Capsicum annuum L.) ‘PA 136’ in the greenhouse. Root-knot nematode egg inoculum was extracted from tomato or pepper roots using NaOCl solution (Hussey and Barker, 1973).
Meloidogyne incognita assessment in E+ and E- tall fescue: Four tall fescue cultivars with or without ergot endophytes were evaluated for host susceptibility to M. incognita (GA peach isolate) in the greenhouse. Tall fescue cultivars evaluated included, i) wild-type Jesup (E+, ergot-producing endophyte present), ii) endophyte-free Jesup (E-, no endophyte present), iii) Jesup (Max-Q, non-ergot producing endophyte) and iv) Georgia 5 (E+). Peach (Prunus persica L. Batsch) (susceptible Lovell rootstock) was included as the control. Individual Lovell peach seedlings (94-d-old) or sets of five seeds of each tall fescue cultivar were planted in separate 15 cm diameter plastic pots containing 1,500 cm3 steam pasteurized loamy sand (86% sand, 10% silt, 4% clay; pH 6.1; 0.54% organic matter). Pots without plants were designated as a fallow treatment to compare survival of eggs and J2 in the absence of any plant with survival in soil planted with tall fescue or peach. This information would be useful in determining the presence/absence of a tall fescue root-produced toxic or nemastatic exudate. Approximately 14 d later, the tall fescue seedlings were thinned to one plant per pot and the soil in each treatment pot was infested with 3,000 M. incognita eggs in 40ml water (Nyczepir et al., 1999), which is equivalent to 200 M. incognita eggs/100 cm3 soil. Nematode eggs were extracted from the whole root system of Rutgers tomato with a NaOCl solution as mentioned above. Ten replications of each plant species or cultivar and of the fallow treatment were arranged in randomized complete blocks on benches in an air-conditioned greenhouse (27 ± 5 C). Plants were watered and fertilized with Osmocote (14-14-14) as needed. The experiment was terminated 123 d after inoculation and the following data were collected: number of egg masses per root system (up to 101 egg masses, which is a high rating according to the Taylor and Sasser (1978) egg mass index, number of eggs per root system, number of root galls per root system (also up to 101 galls), and dry root weight (dried at 70 °C in aluminum foil until no more loss in weight occurred). The egg mass index consisted of a 0 to 5 scale, with 0 = no egg masses, 1 = 1 to 2 egg masses, 2 = 3 to 10 egg masses, 3 = 11 to 30 egg masses, 4 = 31 to 100 egg masses, and 5 = > 100 egg masses (Taylor and Sasser, 1978). Host susceptibility was determined according to the egg mass index rating scale as follows: 0 = nonhost (highly resistant), 1-2 = a poor host (resistant), and ≥ 3 = a good host (susceptible). Meloidogyne incognita second-stage juvenile (J2) soil populations were determined by collecting and thoroughly mixing the soil from each pot. Nematodes were extracted from a 100-cm3 subsample by elutriation (Byrd et al., 1976) and centrifugation (Jenkins, 1964) and then counted.
The experiment was repeated one time with minor modifications, which included inoculating 14-d-old established peach and fescue grass seedlings and terminating the study 152 d after inoculation.
Meloidogyne incognita tomato bioassay: Soil remaining after each tall fescue grass experiment was composited by treatment, mixed, and placed into 10-cm-diameter plastic pots. One Rutgers tomato seedling was planted in each pot for a bioassay of M. incognita J2 population levels not detectable by elutriation and centrifugalflotation. Treatments were replicated 10 times, and the number of egg masses per root system, number of eggs per root system, number of root galls per root system, and dry root weight were recorded 90 and 77 d after planting for experiments 1 and 2, respectively.
Meloidogyne spp. assessment in tall fescue grass: Jesup (Max-Q, non-ergot producing endophyte) and Bulldog 51 (E+) tall fescue grasses were evaluated for host susceptibility to M. arenaria, M. incognita (isolates BY and GA), and M. javanica in the greenhouse. Tomato was included as the control. Individual Rutgers tomato seedlings (36-d-old) or sets of five seeds of each tall fescue cultivar were planted in separate 15-cm-diameter plastic pots as described above. Pots without plants were designated as a fallow treatment. Approximately 13 d later, the tall fescue seedlings were thinned to one plant per pot and the soil in each treatment pot was infested with 3,000 M. arenaria, M. incognita (isolates BY and GA), or M. javanica eggs in 40ml water (Nyczepir et al., 1999). Nematode eggs were extracted from the roots of Rutgers tomato with a NaOCl solution as mentioned above. Eight replications of each plant species or cultivar and of the fallow treatment were arranged in randomized complete blocks on benches in an airconditioned greenhouse (27 + 5 C). Plants were watered and fertilized with Osmocote (14-14-14) as needed. The experiment was terminated 87 d after inoculation and the following data were collected: number of egg masses per root system, number of eggs per root system, number of root galls per root system, and dry root weight. The experiment was repeated one time with minor modifications, which included inoculating 23-d-old and 27-d-old established tomato and fescue grass seedlings, respectively, and terminating the study 91 d after inoculation.
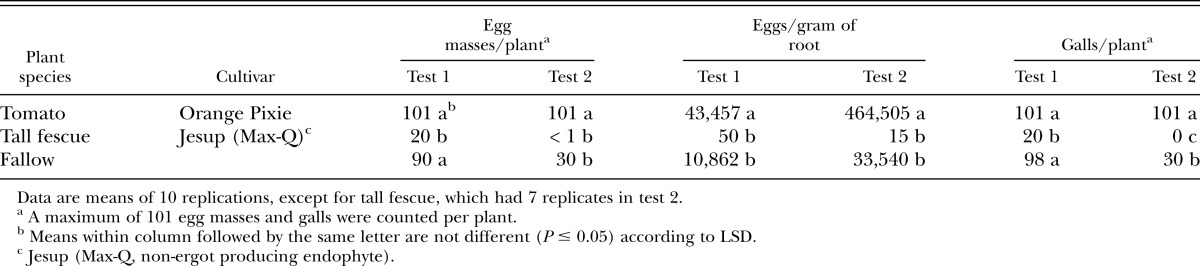
Meloidogyne hapla assessment in tall fescue grass: In a similar greenhouse study at the USDA-ARS Nematology Laboratory in Beltsville, MD, Jesup (Max-Q) tall fescue grass was evaluated for host susceptibility to M. hapla. Procedures were similar to those described for the other nematode/tall fescue combinations, except that tomato cv. ‘Orange Pixie’ was included as the control. Tomato seeds were planted in ProMix seed starting mix (Premier Pro-mix®, Premier Horticulture Inc., Quakertown, PA), and 13 d later, tall fescue was planted into 15-cm-diameter pots (5 seeds per pot) containing loamy sand (composed of 16 sand:9 compost v/v) (83.1% sand, 6.4% silt, 10.5% clay; pH 6.9; 0.8% organic matter) that had been steamed and air dried. Eighteen d after planting, the tall fescue seedlings were thinned to one plant per pot, and the tomato seedlings were transplanted individually into 15-cm-diameter pots containing the same soil. Pots without plants were designated as a fallow treatment. Six d later, all treatments received 3,000 M. hapla eggs/pot in 40 ml water. Ten replications of each plant species and of the fallow treatment were arranged in complete blocks (randomized in test 2 only) on benches in an air-conditioned greenhouse (24 – 29°C). Plants were watered and fertilized with Osmocote (15-9-12) as needed. The experiment was terminated approximately 90 d after inoculation and the following data were collected: number of egg masses per root system (up to 101 egg masses), number of eggs per root system, number of root galls per root system (up to 101 galls), and dry root weight. The experiment was conducted twice.
Meloidogyne spp. tomato bioassay: Soil remaining after each tall fescue experiment was kept separate by treatment and placed back into 15-cm-diameter plastic pots. One Rutgers tomato seedling was planted in each pot for a bioassay to confirm the presence or absence of viable Meloidogyne spp. J2 population levels in soil. In test 1, tomato soil was not included because it was inadvertently discarded. Treatments were replicated eight times, and the same data was recorded as for the above bioassay experiment 76 and 75 d after planting for experiments 1 and 2, respectively.
Meloidogyne hapla tomato bioassay: For M. hapla, the procedures were similar to those already described, with the following exceptions. One ‘Orange Pixie’ tomato seedling (30 to 31-d-old) was transplanted into each 15-cm-diameter pot for the bioassay. All treatments (tomato, tall fescue and fallow) were included in both tests. Ten replications of each treatment (except 7 replicates for tall fescue in test 2) were arranged in a randomized complete block in each test. The M. hapla bioassay was terminated approximately 90 d after tomato transplanting, and the same data was recorded as for the other bioassay experiments. The bioassay was conducted twice.
Statistical analysis: Nematode data were subjected to analysis of variance with the general linear models (GLM) procedure of SAS (SAS Institute, Cary, NC). For the M. incognita (GA-peach isolate) test, appropriate preplanned single-degree-of-freedom comparisons were then used to detect differences between treatment means for Lovell peach vs. combined tall fescue cultivar means and peach vs. fallow means following a significant F-test. Means within the tall fescue cultivars were analyzed using Fishers protected LSD test. For the Meloidogyne spp. test, appropriate preplanned single-degree-of-freedom comparisons were then used to detect differences between treatment means for tomato vs. combined tall fescue cultivars and tomato vs. fallow means following a significant F-test. Means within the tall fescue cultivars were analyzed using ANOVA. For the M. hapla test, treatment means were compared according to ANOVA, whereas in the Meloidogyne spp. and M. hapla resurgence tests, treatment means were compared according to Fishers protected LSD test following a significant F test. Only significant differences (P ≤ 0.05) will be discussed unless stated otherwise.
Results and Discussion
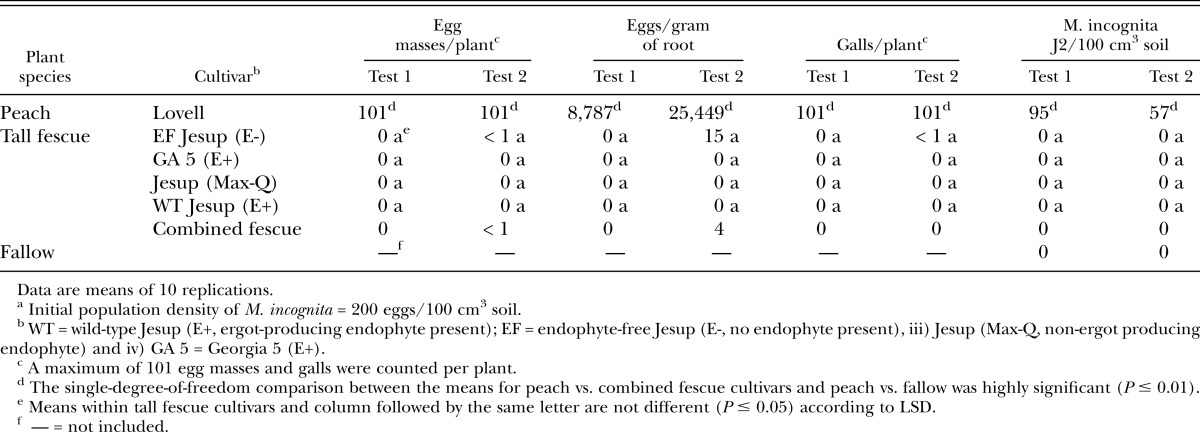
Meloidogyne incognita assessment in E+ and E- tall fescue: In both tests, ‘Lovell’ peach (known susceptible) supported greater (P ≤ 0.05) reproduction of M. incognita (GA-peach isolate) than all tall fescue cultivars combined as indicated by number of egg masses per plant and number of eggs per gram of dry root (Table 1). Similar results were observed for number of root galls per plant and number of M. incognita J2 in the soil. Most individual tall fescue cultivars did not support nematode reproduction and would be rated as highly resistant (nonhost) to M. incognita infection based on the number of egg masses recovered (0 to < 1 egg masses); although EF Jesup (E-) supported very limited reproduction in test 2 alone. Little research has been conducted on host susceptibility of tall fescue to Meloidogyne spp., except for M. marylandi (Kimmons et al., 1990; Gwinn and Bernard, 1993; Elmi et al., 2000). Moreover, studies pertaining to M. incognita are somewhat contradictory. In a brief extension report (no data presented), Chapman (1973) stated that M. incognita was unable to reproduce on seven different tall fescue cultivars (which included endophyte-infected Kentucky 31), whereas in another greenhouse study M. incognita (NC-isolate) reproduction (i.e., egg masses) was detected on Kentucky 31 (McGlohon et al., 1961). Two possible explanations for the varying results in M. incognita reproduction on Kentucky 31 tall fescue may be i) differences in greenhouse conditions under which the respective experiments were conducted and (or) ii) the two nematode isolates had variant host ranges. The current study substantiates the observation made by Chapman (1973) that the tall fescue grass cultivars tested do not support M. incognita reproduction. Results from test 1 and test 2 further indicate that M. incognita reproduction is not significantly affected by the presence of tall fescue endophyte (E+ vs. E-) or whether the endophyte fungus status is toxic [E+, ergot producing endophyte (GA 5 & WT Jesup)] or non-toxic (non-ergot producing endophyte such as in Jesup (Max-Q) (Table 1).
Table 1.
Susceptibility of four tall fescue cultivars and Lovell peach seedlings to Meloidogyne incognita (GA-peach isolate) in the greenhouse 123 (test 1) and 152 (test 2) days after soil infestationa.
Similar observations have been reported for M. naasi, where nematode invasion and development on perennial ryegrass was the same for endophyte-infected (Neotyphodium lolii) and endophyte-free plants (Cook et al., 1991). In contrast, some plant-parasitic nematode populations are affected by endophyte status, such that the presence of the fungal endophyte confers nematode resistance in the plant. For example, populations of P. scribneri were lower in endophyte-infected fescue roots vs. non-infected roots (Bacetty et al., 2009; Timper et al., 2005). It has been hypothesized that the presence of natural tall fescue grass metabolites and (or) fungal endophyte metabolites may be responsible for the nematode suppression observed.
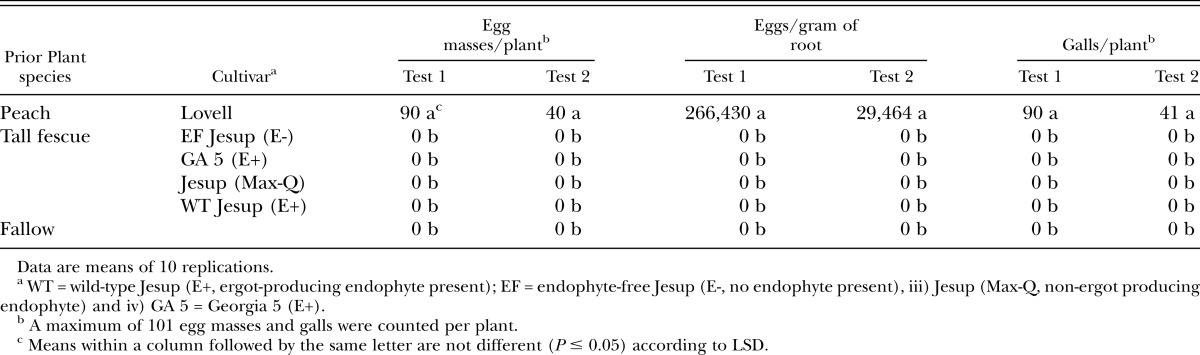
Meloidogyne incognita tomato bioassay: Meloidogyne incognita reproduction was greatest (P ≤ 0.05) on tomato in soil previously planted to peach than in soil previously planted to all tall fescue cultivars and in fallow soil (Table 2). No nematode reproduction occurred on tomato grown in soil in which WT Jesup (E+), EF Jesup (E-), Jesup (Max-Q), and GA 5 (E+) had previously grown or in fallow soil. It appears that all tall fescue cultivars tested are nonhosts for M. incognita. The mode of action of nematode suppression in these tall fescue cultivars was not addressed in this study, but two possible mechanisms of nematode suppression may be i) the inability of M. incognita to complete its life cycle and (or) ii) the occurrence of natural plant metabolites in tall fescue grass regardless of the presence/absence of the endophyte fungus. Several ergot alkaloids (e.g., ergovaline and α-ergocryptine) and polyphenolic compounds (e.g., chlorogenic acid) have been identified in tall fescue roots as being nematicidal or nematistatic to P. scribneri, respectively (Bacetty et al., 2009). However, only the polyphenolic compounds were not correlated with endophyte status. Possibly the polyphenols or other natural product(s) are involved with suppression of M. incognita in tall fescue grass and warrant further investigation.
Table 2.
Resurgence in Meloidogyne incognita population density on Rutgers tomato in soil that had been previously planted to tall fescue or peach, or left fallow in the greenhouse after 90 (test 1) and 77 (test 2) days.
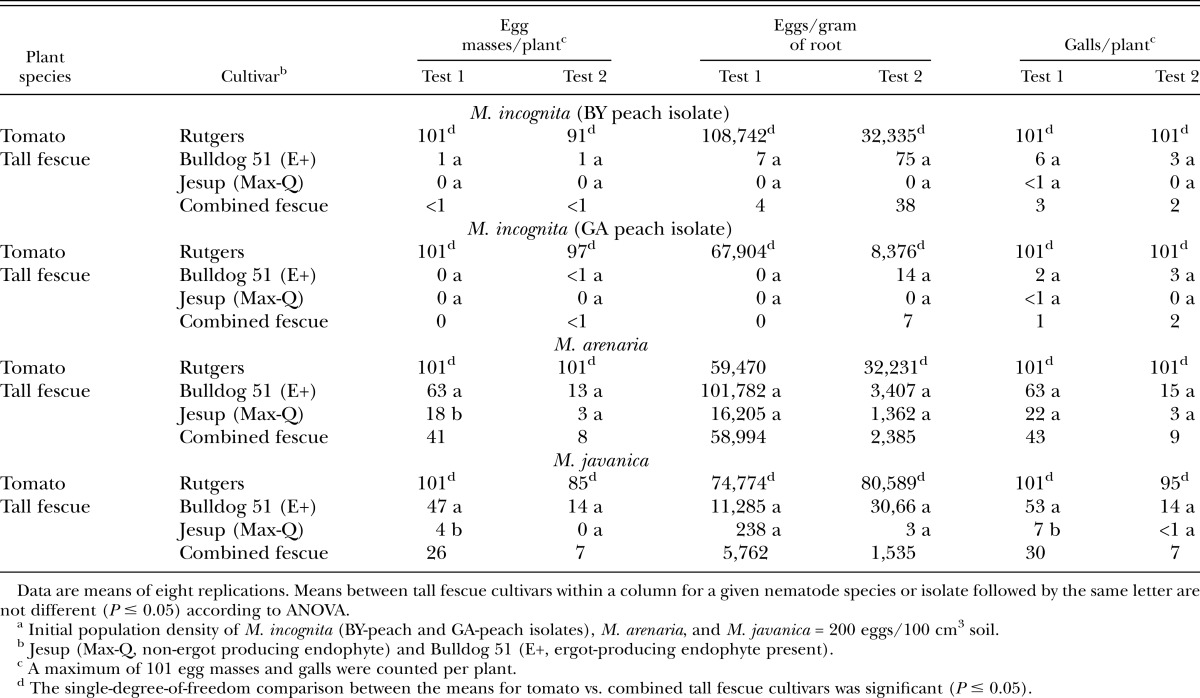
Meloidogyne spp. assessment in tall fescue grass: In both tests, tomato (known susceptible) supported greater (P ≤ 0.05) reproduction of M. incognita GA-peach, M. incognita BY-peach isolate, and M. javanica than both tall fescue cultivars combined as indicated by number of egg masses per plant and number of eggs per gram of dry root (Table 3). Comparable results were also observed for number of root galls per plant. Reproduction of M. arenaria on tomato and tall fescue varied between test 1 and test 2 for some parameters. In both tests, tomato supported greater (P ≤ 0.05) reproduction of M. arenaria than both tall fescue cultivars combined as indicated by number of egg masses per plant. However, there were no differences in M. arenaria reproduction as measured by number of eggs per gram of dry root in test 1, but were different in test 2 (Table 3). Tomato supported greater (P ≤ 0.05) reproduction of M. hapla than Jesup (Max-Q) tall fescue as indicated by number of egg masses per plant and number of eggs per gram of dry root in both tests (Table 4).
Table 3.
Susceptibility of Bulldog 51 and Jesup (Max-Q) tall fescue cultivars and Rutgers tomato seedlings to Meloidogyne incognita (BY-peach and GA-peach isolates), M. arenaria and M. javanica in the greenhouse 87 (test 1) and 91 (test 2) days after soil infestationa.
Table 4.
Susceptibility of tall fescue grass and tomato seedlings to Meloidogyne hapla in the greenhouse 90 days after soil infestationa.
Host susceptibility based on egg mass index differed between the individual tall fescue cultivars. Jesup (Max-Q) would be rated as highly resistant (nonhost) to both M. incognita peach isolates and M. hapla infection based on the number of egg masses recovered (0 egg masses) (Tables 3 & 4). However, Jesup (Max-Q) would be rated a poor host for M. javanica (between 0 and 4 egg masses) and a good host for M. arenaria (≥ 3 egg masses) (Table 3). Bulldog 51 would be rated a poor host for both M. incognita peach isolates (≤1 egg masses), and a good host for both M. javanica and M. arenaria. It is evident from this study that Jesup (Max-Q) and Bulldog 51 tall fescue cultivars differed in ability to suppress reproduction among the Meloidogyne spp. Bulldog 51 generally supported greater reproduction than Jesup (Max-Q) for all species tested (Table 3). Two possible explanations for the differences in nematode reproduction observed between Bulldog 51 and Jesup (Max-Q) may be due to the strain of fungal endophyte present or host plant resistance. Both cultivars have the fungal endophyte (E), but where they differ is that Bulldog 51 contains the ergot-producing endophyte (i.e., E+, toxic endophyte) and Jesup (Max-Q) the novel non-ergot producing endophyte (i.e., endophyte-friendly). The advantage of an endophyte-friendly tall fescue such as Jesup (Max-Q) is that it provides all the beneficial effects for the plant without producing the dangerous toxins that affect grazing cattle. However, the differences in suppression of root-knot nematode reproduction appear to be more associated with nematode species than tall fescue cultivar or endophyte status. Therefore, when considering tall fescue grass as a preplant rotation for managing root-knot nematode prior to establishing a peach orchard, for optimum results it is important to i) to know which root-knot nematode species is present in soil and ii) select the appropriate tall fescue cultivar if grazing cattle prior to orchard establishment is an option.
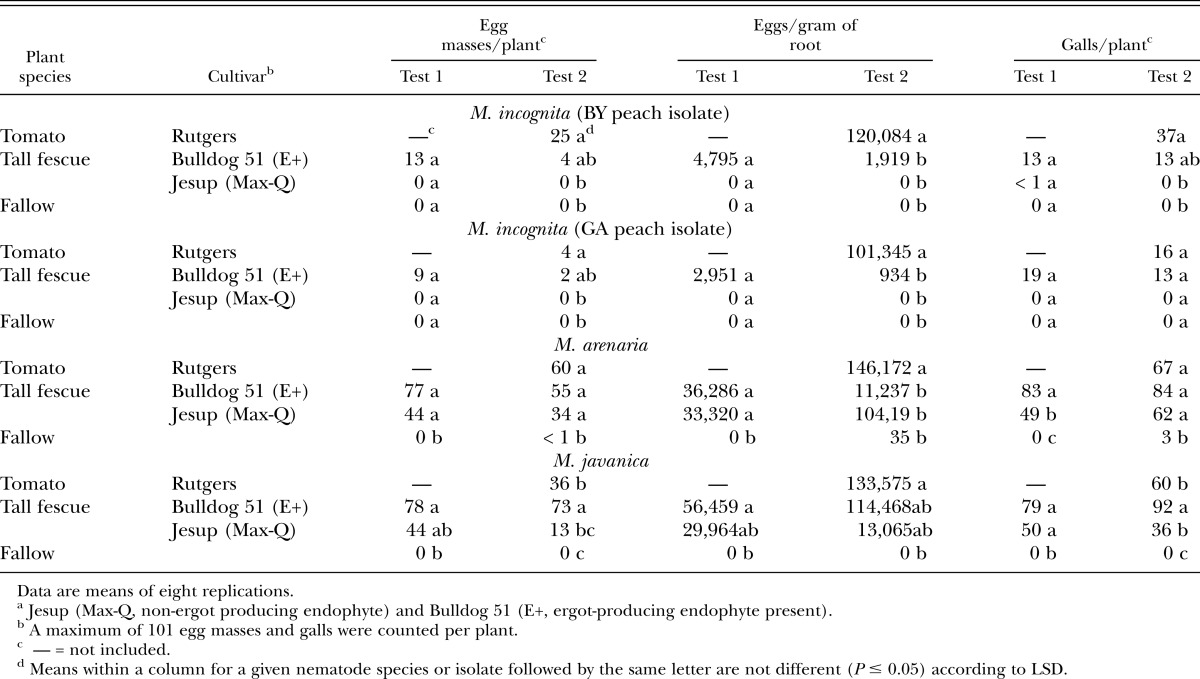
Meloidogyne spp. tomato bioassay: No Meloidogyne incognita (BY-peach isolate and GA-peach isolate) reproduction was detected on tomato grown in soil in which Jesup (Max-Q) tall fescue had previously grown, or in fallow soil, as indicated by number of egg masses per plant and number of eggs per gram of dry root in both tests (Table 5). These results substantiate the previous M. incognita study above in that Jesup (Max-Q) tall fescue is a nonhost to M. incognita regardless of which peach isolate was used. However, reproduction of both M. incognita isolates increased on tomato in soil previously planted to Bulldog 51 tall fescue (tests 1 and 2) and tomato (test 2 only), even though differences among plant species cultivars and fallow were not always significant (P > 0.05).
Table 5.
Resurgence in Meloidogyne spp. population density on Rutgers tomato in soil previously planted to tall fescue grass or tomato, or left fallow in the greenhouse after 76 (test 1) and 75 (test 2) days.
Reproduction of M. arenaria, M. javanica, and M. hapla was detected on tomato grown in soil in which Jesup (Max-Q) tall fescue, Bulldog 51 tall fescue or tomato had previously grown as indicated by number of egg masses per plant and number of eggs per gram of dry root in both tests (Table 5 & 6). Differences in nematode reproduction among plant cultivars and fallow for M. arenaria and M. javanica were not always significant (P > 0.05) (Table 5). However, reproduction of M. hapla was greatest (P ≤ 0.05) on tomato in soil previously planted to tomato than in soil previously planted to Jesup (Max-Q) or in fallow soil for both tests, except in test 1 for number of egg masses per plant in fallow soil (Table 6). No differences in M. hapla reproduction were detected on tomato in soil previously planted to Jesup (Max-Q) or fallow in both tests, except for number of egg masses per plant in test 1, and galls per plant in both tests.
Table 6.
Resurgence in Meloidogyne hapla population density on Orange Pixie tomato in soil previously planted to tall fescue grass or tomato, or left fallow in the greenhouse after 90 days.
The tomato bioassay results indicate that Jesup (Max-Q) tall fescue soil suppressed the resurgence in M. incognita (i.e., BY-peach and GA-peach isolates) infection on tomato roots, but not M. arenaria, M. javanica, and M. hapla. However, even though Jesup (Max-Q) soil did not completely suppress the resurgence in M hapla infection on tomato roots like for M. incognita, M. hapla reproduction was generally lower (P ≤ 0.05) on tomato planted into Jesup (Max-Q) soil than tomato soil as measured by number of egg masses per plant and number of eggs per gram of dry root in both tests (Table 6). It appears that M. hapla inoculum may be able to survive longer in the soil in the presence of a nonhost or the absence of a host [i.e., Jesup (Max-Q) and fallow treatments] than M. incognita inoculum. It is not certain why M. incognita resurgence was suppressed more than the other three Meloidogyne spp., but one explanation may be associated with host resistance. Since Jesup (Max-Q) is a nonhost for M. incognita and that no reproduction occurred, then no viable residual nematode inoculum would remain in the soil to infect the replanted tomato. The nonhost factor(s) associated with Jesup (Max-Q) was not investigated in this study, but may include the associated natural tall fescue grass metabolites or some unknown nematoxic compound in the roots that affect some Meloidogyne spp. more than others. Further investigations into these areas are warranted, because if a plant chemical is identified, this could possibly be utilized as a potential biobased compound against M. incognita and contribute to the understanding associated with tall fescue resistance toward certain root-knot nematode species.
In summary, we demonstrated that the commercial tall fescue cultivar, Jesup (Max-Q), is a nonhost for M. incognita and M. hapla, a poor host for M. javanica, and a good host for M. arenaria. The host resistance in Jesup to M. incognita is not related to the fungal endophyte status, because endophyte-free plants were also resistant. Jesup (Max-Q) tall fescue may have potential as a preplant control strategy for M. incognita and M. hapla in southeastern and northeastern United States, respectively. However, it is important that preplant nematode samples be collected and root-knot nematodes be identified to species prior to orchard or field plot establishment. Additional field testing of Jesup (Max-Q) as an alternative to preplant chemical control of M. incognita and M. hapla is warranted.
Footnotes
The authors thank M. A. Bacon, P. N. Hall, P. Crowley, S. Rupprecht, E. Brinker and W. Villiard for technical assistance and Dr. J. Bouton and D. Wood from The University of Georgia for providing the tall fescue seed used in this work.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
The paper was edited by Nancy Kokalis-Burelle.
Literature Cited
- Bacetty AA, Snook ME, Glenn AE, Noe JP, Hill N, Culbreath A, Timper P, Nagabhyru P, Bacon CW. Toxicity of endophyte-infected tall fescue alkaloids and grass metabolites on Pratylenchus scribneri. Phytopathology. 2009;99:1336–1345. doi: 10.1094/PHYTO-99-12-1336. [DOI] [PubMed] [Google Scholar]
- Bacon CW, Porter JK, Robbins JD, Luttrell ES. Epichloe typhina from toxic tall fescue grasses. Applied Environmental Microbiology. 1977;34:576–581. doi: 10.1128/aem.34.5.576-581.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball DM, Pedersen JF, Lacefield GD. The tall-fescue endophyte. American Scientist. 1993;81:370–379. [Google Scholar]
- Bacon CW, Siegel MR. Endophyte parasitism of tall fescue. Journal of Production Agriculture. 1988;1:45–55. [Google Scholar]
- Bernard EC, Gwinn KD. Behavior and reproduction of Meloidogyne marylandi and Pratylenchus scribneri in roots and rhizosphere of endophyte-infected tall fescue. Journal of Nematology. 1991;23:520. (Abstr.) [Google Scholar]
- Bernard EC, Gwinn KD, Griffin GD. Forage Grasses. In: Barker KR, Pederson GA, Windham GL, editors. Plant nematode interactions, American Society of Agronomy Monograph Series, No. 36. Wisconsin: American Society of Agronomy; 1998. pp. 427–454. [Google Scholar]
- Bouton J. Tall fescue toxicity leads to the development of ‘Max-Q’. Ag News and Views, 20 (8/August), 5. 2002 [Google Scholar]
- Byrd DW, Jr, Barker KR, Ferris H, Nusbaum CJ, Griffin WE, Small RH, Stone CA. Two semi-automatic elutriators for extracting nematodes and certain fungi from soil. Journal of Nematology. 1976;8:206–212. [PMC free article] [PubMed] [Google Scholar]
- Chapman RA. Southern and northern root-knot nematodes did not reproduce on tall fescue. 85th Annual Report University of Kentucky Agricultural Experiment Station. 1973;85:94. [Google Scholar]
- Clean Air Act. Washington, DC: U.S. Congress; 1990. Title VI. Stratospheric Ozone Protection Pub L. 101–549, Section 6001. [Google Scholar]
- Cook R, Lewis GC, Mizen KA. Effects on plant-parasitic nematodes of infection of perennial ryegrass, Lolium perenne, by the endophytic fungus, Acremonium lolii. Crop Protection. 1991;10:403–407. [Google Scholar]
- Elmi AA, West CP, Robbins RT, Kirkpatrick TL. Endophyte effects on reproduction of a root-knot nematode (Meloidogyne marylandi) and osmotic adjustment in tall fescue. Grass and Forage Science. 2000;55:166–172. [Google Scholar]
- Gwinn KD, Bernard EC. Interactions of endophyte-infected grasses with the nematodes Meloidogyne marylandi and Pratylenchus scribneri. In: Hume DE, Latch GCM, Easton HS, editors. Proceedings of the second international symposium on Acremonium/grass interactions. Palmerston North, New Zealand: AgResearch; 1993. pp. 156–160. [Google Scholar]
- Hussey RS, Barker KR. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Disease Reporter. 1973;57:1025–1028. [Google Scholar]
- Jenkins WR. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Disease Reporter. 1964;48:692. [Google Scholar]
- Kimmons CA, Gwinn KD, Bernard EC. Nematode reproduction on endophyte-infected and endophyte-free tall fescue. Plant Disease. 1990;74:757–761. [Google Scholar]
- Lamberti F, Bleve-Zacheo T. Studies on Xiphinema americanum sensu lato with description of fifteen new species (Nematoda, Longidoridae) Nematologia Mediterranea. 1979;7:51–106. [Google Scholar]
- McGlohon NE, Sasser JN, Sherwood RT. Investigations of plant-parasitic nematodes associated with forage crops in North Carolina. North Carolina Sate University Experiment Statuin Technical Bulletin. 1961;148:1–39. [Google Scholar]
- Nyczepir AP. Nematode management strategies in stone fruits in the United States. Journal of Nematology. 1991;23:334–341. [PMC free article] [PubMed] [Google Scholar]
- Nyczepir AP. Nematodes. In: Horton D, Johnson D, editors. Southeastern peach growers’. handbook, Georgia: University of Georgia, Cooperative Extension Service; 2005. pp. 191–198. [Google Scholar]
- Nyczepir AP, Beckman TG, Reighard GL. Reproduction and development of Meloidogyne incognita and M. javanica on Guardian peach rootstock. Journal of Nematology. 1999;31:334–340. [PMC free article] [PubMed] [Google Scholar]
- Nyczepir AP, Thomas SH. Current and future management strategies in intensive crop production systems. In: Perry RN, Moens M, Starr JL, editors. Root-knot nematodes. United Kingdom: CABI; 2009. pp. 412–443. [Google Scholar]
- Phillips TD, Aiken GE. Online Forage and Grazinglands doi:10.1094/FG-2009-1102-01-RV; 2009. Novel-endophyte-infected tall fescues. [Google Scholar]
- Sasser JN. Pathogenicity, host ranges and variability in Meloidogyne species. In: Lamberti F, Taylor CE, editors. Root-Knot Nematodes (Meloidogyne Species): Systematics, Biology and Control. New York: Academic Press; 1979. pp. 257–268. [Google Scholar]
- Sasser JN, Freckman DW. A world perspective in nematology: The role of the society. In: Veech JA, Dickson DW, editors. Vistas on Nematology. Maryland: Society of Nematologists, Inc; 1987. pp. 7–14. [Google Scholar]
- Sharpe RR, Pusey PL, Nyczepir AP, Florkowski WJ. Yield and economics of intervention with peach tree short life disease. Journal of Production Agriculture. 1993;6:241–244. [Google Scholar]
- Siegel MR, Bush LP. Defensive chemicals in grass-fungal endophyte associations. In: Romeo JT, Saunders JA, Barbosa P, editors. Phytochemical diversity and redundancy in ecological interactions. New York: Plenum Press; 1996. pp. 81–119. [Google Scholar]
- Taylor AL, Sasser JN. Raleigh, NC: North Carolina State University Graphics; 1978. Biology, identification, and control of root-knot nematode (Meloidogyne species) [Google Scholar]
- Timper P, Gates RN, Bouton JH. Response of Pratylenchus spp. in tall fescue infected with different strains of the fungal endophyte Neotyphodium coenophialum. Nematology. 2005;7:105–110. [Google Scholar]
- West CP, Izekor E, Oosterhuis DM, Robbins RT. The effect of Acremonium coenophialum on the growth and nematode infestation of tall fescue. Plant and Soil. 1988;112:3–6. [Google Scholar]