Abstract
Subterranean termites are major global pests of wood structures and wood products. Among the most economically important subterranean termite species in the US are Heterotermes aureus, Reticulitermes flavipes, and Coptotermes formosanus. In prior studies, the entomopathogenic nematode, Steinernema riobrave strain 355, exhibited a high level of virulence to H. aureus compared with other nematode species. However, S. riobrave 355 was reported to be poorly or only moderately virulent to R. flavipes and C. formosanus, respectively. We hypothesized that other strains of S. riobrave may possess a high level of virulence to all three termite species. Under laboratory conditions we compared three novel strains of S. riobrave (3-8b, 7-12, and TP) with the 355 strain for virulence to H. aureus, R. flavipes, and C. formosanus workers. H. aureus was very susceptible to all the S. riobrave strains, and termites in all nematode treatments were dead after 4 d. The TP strain of S. riobrave caused greater mortality in R. flavipes and C. formosanus compared to the other nematode strains. Specifically, the TP strain caused 75% and 91% mortality in R. flavipes and C. formosanus, respectively, which was more than 300% and 70% higher than the mortality caused by other strains. Additional studies are warranted to determine the ability of S. riobrave (TP) to control the targeted termite species under field conditions.
Keywords: Coptotermes formosanus, entomopathogenic nematode, Heterotermes aureus, Reticulitermes flavipes, Steinernema riobrave, termite, biological control
Subterranean termites are major pests of wood structures and wood products in the US and globally, causing billions of dollars in damage (Su and Scheffrahn, 1990). Several of the US pest species belong to the genera Heterotermes, Coptotermes and Reticulitermes (Isoptera: Rhinotermitidae). The key pest species in the desert of southern Arizona, New Mexico, and California is Heterotermes aureus (Snyder), whereas throughout the North America Reticulitermes flavipes Kollar and Coptotermes formosanus Shiraki are among the most economically important species (Su and Scheffrahn, 1990; NPMA, 2003). Current termite control methods include the use of chemical termiticide applications (Su and Scheffrahn, 1990). Due to human safety and environmental concerns, research toward developing alternative control measures is warranted. Entomopathogenic nematodes have shown potential as an alternative control tactic for termites (Poinar 1979; Epsky and Capinera 1988; Wang et al., 2002; Yu et al., 2006).
Entomopathogenic nematodes (genera Steinernema and Heterorhabditis) are biological control agents that kill insects through a mutualistic symbiosis with bacteria; Xenorhabdus spp. are associated with steinernematids and Photorhabdus spp. are associated with heterorhabditids (Poinar, 1990). Infective juveniles (IJ), the only free-living stage, enter hosts through natural openings (mouth, anus and spiracles) or, in some cases, through the cuticle. After entering the host's hemocoel, nematodes release their bacterial symbionts, which are primarily responsible for killing the host in less than 48 hours, defending against secondary invaders and providing the nematodes with nutrition (Dowds and Peters, 2002). The nematodes molt and complete up to three generations within the host, after which IJ exit the cadaver to find new hosts (Kaya and Gaugler, 1993). Entomopathogenic nematodes are used to control a variety of economically important insect pests (Shapiro-Ilan et al., 2002a; Grewal et al., 2005). The level of pest suppression, however, can be highly dependent on matching the optimum nematode species or strain to the particular target pest (Shapiro-Ilan et al., 2002a; Grewal et al., 2005).
Prior studies reported variable efficacy and virulence of entomopathogenic nematodes versus subterranean termites. For example, Epsky and Capinera (1988) concluded that Steinernema carpocapsae (Weiser) showed potential for control of Reticulitermes tibialis (Banks) in laboratory and field trials. In contrast, Mauldin and Bea1 (1989) reported that S. carpocapsae, Steinernema feltiae (Filipjev), and Heterorhabditis bacteriophora (Poinar) failed to control R. flavipes in simulated field trials. In terms of the potential control of termite species that are of particular importance to the southwest US, laboratory screening indicated superior virulence in the 355 (=TX) strain of Steinernema riobrave (Cabanillas, Poinar and Raulston) to H. aureus compared with the virulence of S. carpocapsae, S. feltiae, and H. bacteriophora (Yu et al., 2006). Thus, S. riobrave (355) may have substantial potential to be developed as a biocontrol agent for H. aureus due to its high level of virulence to H. aureus as well as its ability to withstand relatively high temperatures that may be encountered during application (Grewal et al., 1994; Yu et al., 2006). However, S. riobrave (355) does not possess a high level of virulence to R. flavipes or C. formosanus (Wang et al., 2002; Yu et al., 2006). Reticulitermes flavipes and C. formosanus are important subterranean termites in the US although they do not occur in the desert southwest.
To broaden the potential of S. riobrave as a biocontrol agent for termites, it would be useful if other important termite species beyond H. aureus could be targeted. In 2001, a number of novel strains of S. riobrave were discovered in Texas and Mexico; compared with the commercially available strain (355), several of the novel strains were found to possess superior virulence to the citrus weevil, Diaprepes abbreviatus (L.) (Stuart et al., 2004). The superior virulence in the new strains relative to the 355 strain may have been due to deterioration of the latter based on excessive culturing (Shapiro-Ilan et al., 1996; Bilgrami et al., 2006). Therefore, we hypothesized that the novel S. riobrave strains would also have higher virulence to other pests including termite species. In this paper we compared S. riobrave (355) to three novel S. riobrave strains (3-8b, 7-12, and TP) for virulence to H. aureus, R. flavipes, and C. formosanus.
Materials and Methods
Insects and nematodes: H. aureus were collected from the University of Arizona Santa Rita Experimental Range located near Green Valley, Arizona, and were maintained in the laboratory in corrugated cardboard rolls in complete darkness at 22oC and 90% relative humidity (Yu et al., 2006). R. flavipes were collected from Whitehall Forest in Athens, Georgia, and C. formosanus were collected from field sites in central Alabama (Auburn and Opelika, Lee County). R. flavipes and C. formosanus were maintained in clear plastic rectangular culture boxes (27cm x 20cm x 10cm) in complete darkness at 25oC and 75-80% relative humidity (Yu et al., 2006).
All entomopathogenic nematode strains were cultured in last instar greater wax moth larvae, Galleria mellonella (L.), according to procedures described by Kaya and Stock (1997). The S. riobrave strains included in this study were the commercially available 355 (=TX) strain as well as 3-8b, 7-12, and TP (= #3 in Stuart et al. [2004]). The three novel strains (3-8b, 7-12, and TP) were chosen because, they were found to possess superior virulence to D. abbreviatus relative to the 355 strain and caused numerically the highest D. abbreviatus mortality among all strains tested (Stuart et al., 2004). Nematodes were maintained in the laboratory in distilled water suspension at 15°C. Prior to use in bioassays, the nematodes were acclimated for 4 h at room temperature (ca. 22oC).
Bioassays: Nematode virulence was studied in sand-filled Petri dishes based on procedures described by Yu et al. (2006). Ten grams of dry, autoclaved sand was placed in each Petri dish. All sand used in the experiment (QUIKRETE® Premium Play Sand® [No. 1113], Atlanta, GA, US) was sifted to 0.2 to 0.5 mm particle size. Approximately 800 IJs were applied to each Petri dish in 1 ml of distilled water. Control dishes received only distilled water. Ten worker termites were introduced into the Petri dish after nematode applications. Thus, there were 15 treatments and controls total (four nematode strains + control x 3 termite species). Termite mortality was recorded daily for 7 d. All termite-nematode combinations were replicated 5 times and the experiment was repeated twice.
Data analysis: Corrected mortality was calculated based on Abbott (1925). Significant treatment effects 7 d post-treatment were determined using analysis of variance (ANOVA). Mortality data were transformed (arcsine of the square root) prior to ANOVA. The Student-Newman-Keuls' test was used to further elucidate treatment differences when a significant F value was detected by ANOVA. The statistical software program CoStat (Version 6.311) with an alpha level set at 0.05 was used for statistical analyses.
Several survival parameters were measured to analyze the relationships between risk of mortality due to nematode infection, and nematode species. These parameters included the time course of survival (the survival distribution), nematode strain and the relative hazard ratios of death. The statistical comparison of the time course of mortality within and between treatments was analyzed using the Breslow statistic (Kaplan-Meier survival test; SPSS 16.0.0, 2007). In addition, a Cox proportional regression analysis was performed on termite survival over time by including in the model the variable nematode strains.
Results
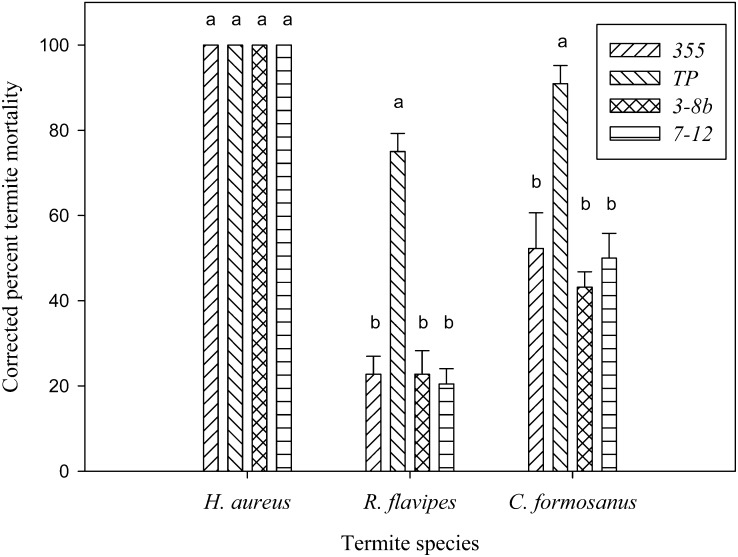
An overall analysis of corrected mortality indicated a significant interaction between S. riobrave strains and termite species (F = 9.57; df = 6, 48; P < 0.001). Therefore, the mortality data for each individual termite species and nematode strain were analyzed separately. After 7 d of exposure, H. aureus exhibited a high level of susceptibility to all the S. riobrave strains, and termites in all nematode treatments were dead after 4 d (Figs. 1 & 2). R. flavipes mortality data indicated significant differences among nematode strains (F = 27.4; df = 3, 16; P < 0.001); the TP strain caused significantly higher mortality compared to the other three strains (which were not different from each other) (Fig. 1). Similarly, TP strain also caused significantly higher mortality in C. formosanus (F = 14.4; df = 3, 16; P < 0.001) (Fig. 1).
Fig. 1.
Mean (± SEM) corrected percentage termite mortality after exposure to different strains of Steinernema riobrave (355, TP, 3-8b, or 7-12) for 7 d. Within each termite species, different letters above bars indicate statistical significance (P ≤ 0.05, SNK test).
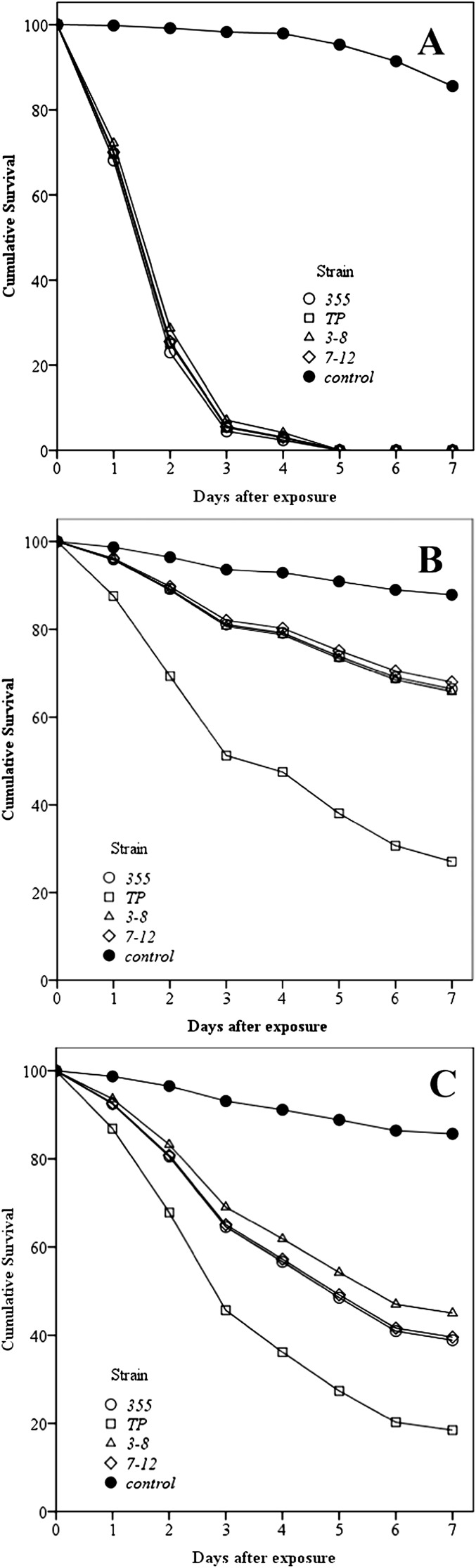
Fig. 2.
Cumulative percentage survival over time (based on Cox proportional regression analysis) of Heterotermes aureus (A), Reticulitermes flavipes (B), and Coptotermes formosanus (C) following exposure to Steinernema riobrave strains (355, TP, 3-8b, 7-12) or a non-treated control.
All four S. riobrave strains caused significantly different mortality levels among termite species. H. aureus was the most susceptible, followed by C. formosanus, and R. flavipes was the least susceptible among the three (F = 88.7; df = 2, 12; P < 0.001 for the 355 strain), (F = 15.7; df = 2, 12; P < 0.001 for the TP strain), (F = 172.9; df = 2, 12; P < 0.001 for the 3-8b strain), and (F = 172.6; df = 2, 12; P < 0.001 for the 7-12 strain).
The relationships between time and insect survival following exposure to nematodes are depicted in Fig. 2 for each termite species. A Cox proportional regression model showed that S. riobrave strain was a significant and independent predicator of termite survival. Different termite species showed significant variation of susceptibility to S. riobrave. For H. aureus, nematode strain was the most important predicator of termite survival (Wald statistic = 26.1, df = 4, P < 0.001). Termites exposed to 355, TP, 3-8b and 7-12 strains of S. riobrave had 176.94 (Wald statistic = 25.8, df = 1, P < 0.001); 166.3 (Wald statistic = 25.3, df = 1, P < 0.001); 150.4 (Wald statistic = 24.2, df = 1, P < 0.001); and 164.2 (Wald statistic = 25.0, df = 1, P < 0.001) times the hazard ratio of death compared with the control, respectively.
Similarly, nematode strain was the most important predicator of R. flavipes survival (Wald statistic = 43.6, df = 4, P < 0.001). Termites exposed to 355, TP, 3-8b and 7-12 strains of S. riobrave had 3.16 (Wald statistic = 5.8, df = 1, P = 0.016); 10.11 (Wald statistic = 27.5, df = 1, P < 0.001); 3.2 (Wald statistic = 6.0, df = 1, P = 0.014); and 3.0 (Wald statistic = 5.1, df = 1, P = 0.023) times the hazard ratio of death compared with the control, respectively. The Wald statistic tests the null hypothesis whenever a relationship within or between data items can be expressed as a statistical model, with parameters to be estimated from a sample.
Nematode strain was the most important predicator of C. formosanus survival (Wald statistic = 38.0, df = 4, P < 0.001). Termites exposed to 355, TP, 3-8b and 7-12 strains of S. riobrave had 6.1 (Wald statistic = 18.4 df = 1, P < 0.001); 10.9 (Wald statistic = 24.4, df = 1, P < 0.001); 2.2 (Wald statistic = 14.7, df = 1, P < 0.001); and 6.0 (Wald statistic = 17.9, df = 1, P < 0.001) times the hazard ratio of death compared with the control, respectively.
Discussion
The utility of entomopathogenic nematodes in pest management has been greatly enhanced through the discovery of new entomopathogenic nematode species or strains that possess superior biocontrol traits. For example, the discovery of S. scapterisci led to substantial progress in commercial applications for the control of mole crickets, Scapteriscus spp. (Orthoptera: Gryllotalpidae) (Shapiro-Ilan et al., 2002a), and the relatively recently discovered GPS11 strain of H. bacteriophora has shown superior potential for control of white grubs Popillia japonica Newman and Cyclocephala borealis Arrow (Coleoptera: Scarabaeidae) (Grewal et al., 2004) and the grape root borer, Vitacea polistiformis (Harris) (Williams et al., 2010). Here, we observed superior virulence in the novel TP strain of S. riobrave versus two termite species. Relative to the other S. riobrave strains tested, the TP strain caused an increase in R. flavipes mortality more than 3-fold, and an increase of more than 70% in C. formosanus. Our hypothesis, that some of the more recently isolated S. riobrave strains that showed higher virulence to D. abbreviatus (Stuart et al., 2004) would also exhibit higher virulence to certain termite species, was supported.
A high level of virulence to H. aureus was established previously using the 355 strain of S. riobrave (Yu et al., 2006). Yet, prior studies also indicated that the S. riobrave (355) strain does not possess a high level of virulence to other important subterranean termites such as R. flavipes or C. formosanus (Wang et al., 2002; Yu et al., 2006); and our data further support those findings. Therefore, our finding regarding the high virulence of S. riobrave (TP) on all the termite species tested, enhances the biocontrol potential for control of subterranean termites using entomopathogenic nematodes. All of the S. riobrave strains tested in our study exhibited a high level of virulence to H. aureus without any detectable differences among them; possibly, additional screening using lower rates of application would indicate some separation among the strains.
The basis for the virulence differences we observed among S. riobrave strains is not clear. All of the S. riobrave were originally isolated from the Rio Grande Valley near Weslaco, Texas or Reynosa, Mexico, and the TP strain is from the same collection site as 3-8b. However, the insect mortality caused by these strains was significantly different. This confirms the theory that local populations of entomopathogenic nematodes can be genetically heterogeneous and variable for important biological traits (Somasekhar et al., 2002). Given that the S. riobrave (355) has been commercialized and has been in culture substantially longer than the other strains, it is conceivable that some of the lower virulence observed in this strain is due to trait deterioration. For example, it has been reported that during repeated culturing of entomopathogenic nematodes in laboratory or industrial settings, beneficial traits (such as virulence, fecundity, environmental tolerance) can diminish due to genetic or non-genetic processes (Shapiro et al., 1996; Wang and Grewal, 2002; Bilgrami et al., 2006; Adhikari et al., 2010). Alternatively, the novel strains may simply possess innate virulence factors (e.g., in their bacterial strains) that are broadly effective to a wide host range.
Laboratory screening for virulence among entomopathogenic nematode strains has been useful in selecting suitable candidates for biological control (Shapiro and McCoy, 2000; Shapiro-Ilan et al., 2002a). However, nematode species or strains that exhibit the highest virulence for a particular pest in the laboratory may not necessarily prove to be the most efficacious under field conditions. For example, when comparing six different nematode species, S. feltiae caused the highest mortality in larvae of the plum curculio, Conotrachelus nenuphar (Herbst) under laboratory conditions (Shapiro-Ilan et al., 2002b), but failed to cause significant mortality when applied in a peach orchard (whereas another nematode, S. riobrave, caused high levels of mortality in the laboratory and field) (Shapiro-Ilan et al., 2004). Beyond innate virulence, a variety of biotic and abiotic factors can contribute to field efficacy (Shapiro-Ilan et al., 2006). Therefore, the biocontrol potential of S. riobrave (TP) indicated in the laboratory must be confirmed in studies conducted under field conditions. In addition to being compatible with environmental conditions associated with the target pest habitat (which is a requirement for all biocontrol applications), particular challenges for control of subterranean termites include the ability to infect multiple stages (Mankowski et al., 2005) and overcome the colony's defensive behaviors (Wilson-Rich et al., 2007).
Footnotes
The authors thank Dr. James Hagler (USDA-ARS, ALARC) for critical review of this paper.
Current Address Hao Yu: Guardian Pest Solutions, 701 E 4th St., Duluth, MN 55805.
This paper was edited by Kris Lambert.
Literature Cited
- Abbott WS. A method for computing the effectiveness of an insecticide. Journal of Economic Entomology. 1925;18:265–267. [Google Scholar]
- Adhikari BN, Chin-Yo L, Xiaodong B, Ciche TA, Grewal PS, Dillman AR, Chaston JM, Shapiro-Ilan DI, Bilgrami AL, Gaugler R, Sternberg PW, Adams BJ. Transcriptional profiling of trait deterioration in the insect pathogenic nematode Heterorhabditis bacteriophora. BMC Genomics. 10:609. doi: 10.1186/1471-2164-10-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgrami AL, Gaugler R, Shapiro-Ilan DI, Adams BJ. Source of trait deterioration in entomopathogenic nematodes Heterorhabditis bacteriophora and Steinernema carpocapsae during in vivo culture. Nematology. 2006;8:397–409. [Google Scholar]
- Dowds BCA, Peters A. Virulence mechanisms. In: Gaugler R, editor. Entomopathogenic Nematology. New York: CABI; 2002. pp. 79–98. [Google Scholar]
- Epsky ND, Capinera JL. Efficacy of the entomogenous nematode Steinernema feltiae against a subterranean termite, Reticulitermes tibialis (Isoptera, Rhinotermitidae) Journal of Economic Entomology. 1988;81:1313–1317. [Google Scholar]
- Grewal PS, Selvan S, Gaugler R. Thermal adaptation of entomopathogenic nematodes – niche breadth for infection, establishment and reproduction. Journal of Thermal Biology. 1994;19:245–253. [Google Scholar]
- Grewal PS, Power KT, Grewal SK, Suggars A, Haupricht S. Enhanced consistency in biological control of white grubs (Coleoptera: Scarabaeidae) with new strains of entomopathogenic nematodes. Biological Control. 2004;30:73–82. [Google Scholar]
- Grewal PS, Ehlers R-U, Shapiro-Ilan DI. Wallingford, UK: CABI Publishing; 2005. Nematodes as Biocontrol Agents. [Google Scholar]
- Kaya HK, Gaugler R. Entomopathogenic nematodes. Annual Review of Entomology. 1993;38:181–206. [Google Scholar]
- Kaya HK, Stock SP. Techniques in insect nematology. In: Lacey LA, editor. Manual of Techniques in Insect Pathology. San Diego, CA: Academic Press; 1997. pp. 281–324. [Google Scholar]
- Mauldin JK, Beal RH. Entomogenous nematodes for control of subterranean termites, Reticulitermes Spp (Isoptera, Rhinotermitidae) Journal of Economic Entomology. 1989;82:1638–1642. [Google Scholar]
- Mankowski ME, Kaya HK, Grace JK, Sipes B. Differential susceptibility of subterranean termite castes to entomopathogenic nematodes. Biocontrol Science and Technology. 2005;15:367–377. [Google Scholar]
- [NPMA] National Pest Management Association. National Pest Management Association Web page. 2003. http://65.217.229.171/media/art_protect_your_home.asp.
- Poinar GO., Jr . Boca Raton, FL: CRC Press; 1979. Nematodes for Biological Control of Insects. [Google Scholar]
- Poinar GO., Jr . Biology and taxonomy of Steinernematidae and Heterorhabditidae. In: Gaugler R, Kaya HK, editors. Entomopathogenic Nematodes in Biological Control. Boca Raton, FL: CRC Press; 1990. pp. 23–62. [Google Scholar]
- Shapiro DI, McCoy CW. Effect of culture method and formulation on the virulence of Steinernema riobrave (Rhabditida: Steinernematidae) to Diaprepes abbreviatus (Curculionidae) Journal of Nematology. 2000;32:281–288. [PMC free article] [PubMed] [Google Scholar]
- Shapiro DI, Glazer I, Segal D. Trait stability in and fitness of the heat tolerant entomopathogenic nematode Heterorhabditis bacteriophora IS5 strain. Biological Control. 1996;6:238–244. [Google Scholar]
- Shapiro-Ilan DI, Gouge DH, Koppenhöfer AM. Factors affecting commercial success: Case studies in cotton, turf and citrus. In: Gaugler R, editor. Entomopathogenic Nematology. New York: CABI; 2002a. pp. 333–356. [Google Scholar]
- Shapiro-Ilan DI, Mizell RF, J F. Susceptibility of the Plum Curculio, Conotrachelus nenuphar, to Entomopathogenic Nematodes. Journal of Nematology. 2002b;34:246–249. [PMC free article] [PubMed] [Google Scholar]
- Shapiro-Ilan DI, Mizell RF, Cottrell TE, Horton DL. Measuring field efficacy of Steinernema feltiae and Steinernema riobrave for suppression of plum curculio, Conotrachelus nenuphar, larvae. Biological Control. 2004;30:496–503. [Google Scholar]
- Shapiro-Ilan DI, Gouge DH, Piggott SJ, Patterson Fife J. Application technology and environmental considerations for use of entomopathogenic nematodes in biological control. Biological Control. 2006;38:124–33. [Google Scholar]
- Somasekhar N, Grewal PS, Klein MG. Genetic variability in stress tolerance and fitness among natural populations of Steinernema carpocapsae. Biological Control. 2002;23:303–310. [Google Scholar]
- Stuart RJ, Shapiro-Ilan DI, James RR, Nguyen KB, McCoy CW. Virulence of new and mixed strains of the entomopathogenic nematode Steinernema riobrave to larvae of the citrus root weevil Diaprepes abbreviatus. Biological Control. 2004;30:439–445. [Google Scholar]
- Su NY, Scheffrahn RH. Economically Important Termites in the United-States and Their Control. Sociobiology. 1990;17:77–94. [Google Scholar]
- Wang X, Grewal PS. Rapid genetic deterioration of environmental tolerance and reproductive potential of an entomopathogenic nematode during laboratory maintenance. Biological Control. 2002;23:71–78. [Google Scholar]
- Wang CL, Powell JE, Nguyen K. Laboratory evaluations of four entomopathogenic nematodes for control of subterranean termites (Isoptera: Rhinotermitidae) Environmental Entomology. 2002;31:381–387. [Google Scholar]
- Williams RN, Fickle DS, Grewal PS, Dutcher J. Field efficacy against the grape root borer Vitacea Polistiformis (Lepidoptera: Sesiidae) and persistence of Heterorhabditis zealandica and H. bacteriophora (Nematoda: Heterorhabditidae) in vineyards. Biological Control. 2010;53:86–91. [Google Scholar]
- Wilson-Rich N, Stuart RJ, Rosengaus RB. Susceptibility and behavioral responses of the dampwood termite Zootermopsis angusticollis to the entomopathogenic nematode Steinernema carpocapsae. Journal of Invertebrate Pathology. 2007;95:17–25. doi: 10.1016/j.jip.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Yu H, Gouge DH, Baker P. Parasitism of subterranean termites (Isoptera: Rhinotermitidae: Termitidae) by entomopathogenic nematodes (Rhabditida: Steinernematidae: Heterorhabditidae) Journal of Economic Entomology. 2006;99:1112–1119. doi: 10.1603/0022-0493-99.4.1112. [DOI] [PubMed] [Google Scholar]