Abstract
To reduce the risks associated with global transport of wood infested with pinewood nematode Bursaphelenchus xylophilus, microwave irradiation was tested at 14 temperatures in replicated wood samples to determine the temperature that would kill 99.9968% of nematodes in a sample of ≥ 100,000 organisms, meeting a level of efficacy of Probit 9. Treatment of these heavily infested wood samples (mean of > 1,000 nematodes/g of sapwood) produced 100% mortality at 56 °C and above, held for 1 min. Because this “brute force” approach to Probit 9 treats individual nematodes as the observational unit regardless of the number of wood samples it takes to treat this number of organisms, we also used a modeling approach. The best fit was to a Probit function, which estimated lethal temperature at 62.2 (95% confidence interval 59.0-70.0) °C. This discrepancy between the observed and predicted temperature to achieve Probit 9 efficacy may have been the result of an inherently limited sample size when predicting the true mean from the total population. The rate of temperature increase in the small wood samples (rise time) did not affect final nematode mortality at 56 °C. In addition, microwave treatment of industrial size, infested wood blocks killed 100% of > 200,000 nematodes at ≥ 56 °C held for 1 min in replicated wood samples. The 3rd-stage juvenile (J3) of the nematode, that is resistant to cold temperatures and desiccation, was abundant in our wood samples and did not show any resistance to microwave treatment. Regression analysis of internal wood temperatures as a function of surface temperature produced a regression equation that could be used with a relatively high degree of accuracy to predict internal wood temperatures, under the conditions of this study. These results provide strong evidence of the ability of microwave treatment to successfully eradicate B. xylophilus in infested wood at or above 56 °C held for 1 min.
Keywords: Pinewood nematode, quarantine, microwave, dielectric heating, international trade, embargo, eradication, Probit 9, International Standard of Phytosanitary Measures No. 15
The North American pinewood nematode (PWN), Bursaphelenchus xylophilus [(Steiner & Burhrer) Nickle] (Nematoda: Aphelenchoididae) (formerly B. lignicolus), is the causal agent of pinewilt (Mamiya, 1972). Molecular data support the likelihood that B. xylophilus was introduced from North America to Japan and other countries in Asia (Tares et al., 1992) through infested pine products such as logs, lumber, and/or solid wood packaging materials (Mamiya, 1976). It has also been intercepted in pine shipments from North America to Europe (Tomminen and Lahtinen, 1990), was introduced into Portugal (Mota et al., 1999), and is considered a major threat to other European pine forests (Mota and Vieira, 2008). In Japan losses from pinewilt were ≈2.5 million m3 of pine lumber in just one year (Mamiya, 1983).
The most important vectors of B. xylophilus are cerambycid beetles in the genus Monochamus (Coleoptera: Cerambycidae) (Evans et al., 1993). Hosts of B. xylophilus in nature include various pine species; rarely are conifers other than pine found infested in nature (see review by Dwinell, 1997). In North America, although the nematode has a wide distribution, pinewilt rarely occurs in native pines; instead the disease is largely confined to stressed exotic pines, especially Pinus sylvestris L. (Scotch pine) in the Eastern United States (Wingfield and Blanchette, 1982; Wingfield et al., 1984).
The life cycle of B. xylophilus involves four propagative (J1-4) and four dispersal (JI-IV) juvenile stages (Wingfield et al., 1982). Propagative stages dominate under favorable conditions of sufficient wood moisture, temperature, and nutrient availability. Under these conditions, generation time for B. xylophilus can be as short as three days. In nature, the nematode switches to the dispersal stages as the tree dies from pinewilt. The JIV stage, referred to as dauer larvae, enter healthy trees through Monochamus feeding wounds on young twigs and branches (maturation feeding), or stressed trees and recently cut logs through Monochamus oviposition sites (Mamiya, 1983). Once inside the susceptible host, the dauer larvae molt to adults and feed on living plant tissue and on fungi once the tree dies.
Under unfavorable conditions such as desiccation, low temperatures, or poor nutritional reserves, B. xylophilus will molt from the J2 to the JIII (Ishibashi and Kondo, 1977). Also, in the absence of the beetle vector, the JIII stage accumulates as the wood dries. This stage is considered environmentally resistant to freezing temperatures and desiccation, having the thickest cuticle and largest lipid reserves among life stages (Kondo and Ishibashi, 1978). Whether the JIII stage is resistant to conditions used by commodity treatments to eradicate B. xylophilus from wood has not been well studied. However, in one report resistance of the JIII-stage of B. xylophilus to kiln heating of green lumber did not occur despite predominance of this life stage in the treated boards (Tomminen, 1992).
To reduce the risks associated with global transport of wood infested with B. xylophilus and its associated beetle vectors, comprehensive regulations and embargoes have been put in place by many National Plant Protection Organizations (NPPOs) causing significant economic impact on the North American softwood export trade (Dwinell, 1997). The year following the ban, US softwood exports to Europe declined by $69 million (Hicks, 2001). Subsequently, the European Community Commission enacted regulations, including heat-treatment or kiln-drying requirements for all imported coniferous sawn wood to protect European forests from B. xylophilus and its vectors (Dwinell, 1997). In 2002, the International Standard for Phytosanitary Measures No. 15 (ISPM-15) entitled “Guidelines for Regulating Wood Packaging Material in International Trade” was approved by the International Plant Protection Convention (IPPC) (FAO 2009) (Food and Agriculture Organization, 2009). ISPM-15 currently contains only two phytosanitary treatments approved for use: heat treatment and methyl bromide fumigation; over 173 countries have agreed to comply with this standard. Heat treatment of wood is conventionally done in kilns and requires wood to be heated to 56 °C for 30 min at the core, while fumigation with methyl bromide follows prescribed temperature and chemical concentration dosages for 24 hours to kill B. xylophilus and other pests (Food and Agriculture Organization, 2009).
At the same time that ISPM-15 was approved, the IPPC identified the need to adopt alternative treatments in recognition of restrictions on methyl bromide use as a consequence of the Montreal Protocol (Brent Larson, IPPC Secretariat, personal communication), which limits emissions of methyl bromide into the environment. One such treatment under consideration is dielectric heating by microwaves (MW). Although dielectric irradiation [MW and radio frequency (RF)] can be used to achieve the standard 56 °C for 30 min requirement, it is well established that these approaches heat water-containing materials in a different way than conventional kilns or ovens. Microwaves and RF heat through the profile of the target material simultaneously rather than requiring thermal conduction from the outside of the material to the core. Thus, dielectric heating is far more rapid than kilns or conventional ovens and has been shown to successfully kill several wood pests (Kishi, 1975; Ambrogioni et al., 2004; Fleming et al., 2005; Henin et al., 2008). The purpose of this study was to determine the minimum lethal temperature for MW energy as a new treatment for consideration by the IPPC under ISPM-15.
For organisms that infest commodities at very high populations, the commonly used level of efficacy is Probit 9, which requires evidence that the treatment kills or sterilizes at least 99.9968% of pests in a test of at least 100,000 individual pests (Baker and Norris, 1968). Several studies (Soma et al., 2001; 2002; 2003) tested methyl bromide fumigation at an efficacy level of Probit 9 as requisite evidence of successful eradication of B. xylophilus in infested wood and wood packaging. To meet Probit 9, our objective was to determine the lethal temperature required to produce mortality at the level of Probit 9 of B. xylophilus in infested wood using MW energy.
Materials and Methods
For all experiments, four different isolates of B. xylophilus, three obtained from the Canadian Forest Service (Q52A, Q1426, Ne15/03) and a fourth from FPInnovations (Vancouver B.C., Canada) were used. Erlenmeyer flasks (500 ml) containing 50 g of whole barley (Pro-Form™ Feeds, Chilliwack, BC Canada) and 50 ml of water were sterilized, inoculated with Botrytis cinerea, plugged with sterile cotton wrapped in gauze, and incubated at 25 °C until the barley was covered in fungal mycelia (approximately 2 - 3 wk). Each flask was then inoculated with nematodes and incubated at 20 °C for a minimum of 3 wk. Prior to inoculation of wood, flasks were rinsed with sterile water to suspend the nematodes in solution. The suspension was collected and the concentration of nematodes determined using a hemacytometer; if needed, adjustments were made to achieve a concentration of approximately 8,000 nematodes/ml.
A two-step approach was used to evaluate the efficacy of MW energy against B. xylophilus as an alternative treatment under ISPM-15. For the first step, we targeted 14 temperatures from ambient (control 20 °C) to 70 °C to determine the temperature that killed 100% of B. xylophilus in small wood samples. From these data, both a “brute force” approach and a modeling approach were used to determine the lethal temperature required to achieve mortality at Probit 9. For the second step, we verified the minimum lethal temperature using wood blocks of industrial scale.
Step 1: Minimum lethal temperature using small wood samples. To prepare small wood samples for inoculation with nematodes, sapwood from healthy, freshly cut lodgepole pine (Pinus contorta var. latifolia Engelm) logs was cut into 2.5 x 3.8 x 0.64 cm samples and sterilized with 25 kiloGrays of ionizing irradiation. These samples were wrapped in two layers of polyethylene during irradiation and storage to maintain high moisture content (≈171%) until they were inoculated with a hyphae/spore mixture of fungi grown for 2 wk on 1% malt extract agar. This fungal mixture included the blue stain fungi Leptographium terebrantis, L. longiclavatum, Ophiostoma montium and O. clavigerum, the wood decay fungi Phellinus chrysoloma and Trichaptum abietinum, and B. cinerea. The wood samples were then placed in 1 liter sterile mason jars lined with water soaked blotting paper and rubber sink matting. Jars were capped with a thick felt fabric and covered by aluminum foil to prevent contamination and moisture loss, while allowing for aeration. All wood samples were incubated at 25 °C for 14 d, after which each sample was inoculated aseptically with 1,000 nematodes in a volume of 100 μl sterile water, and incubated for an additional 35 d.
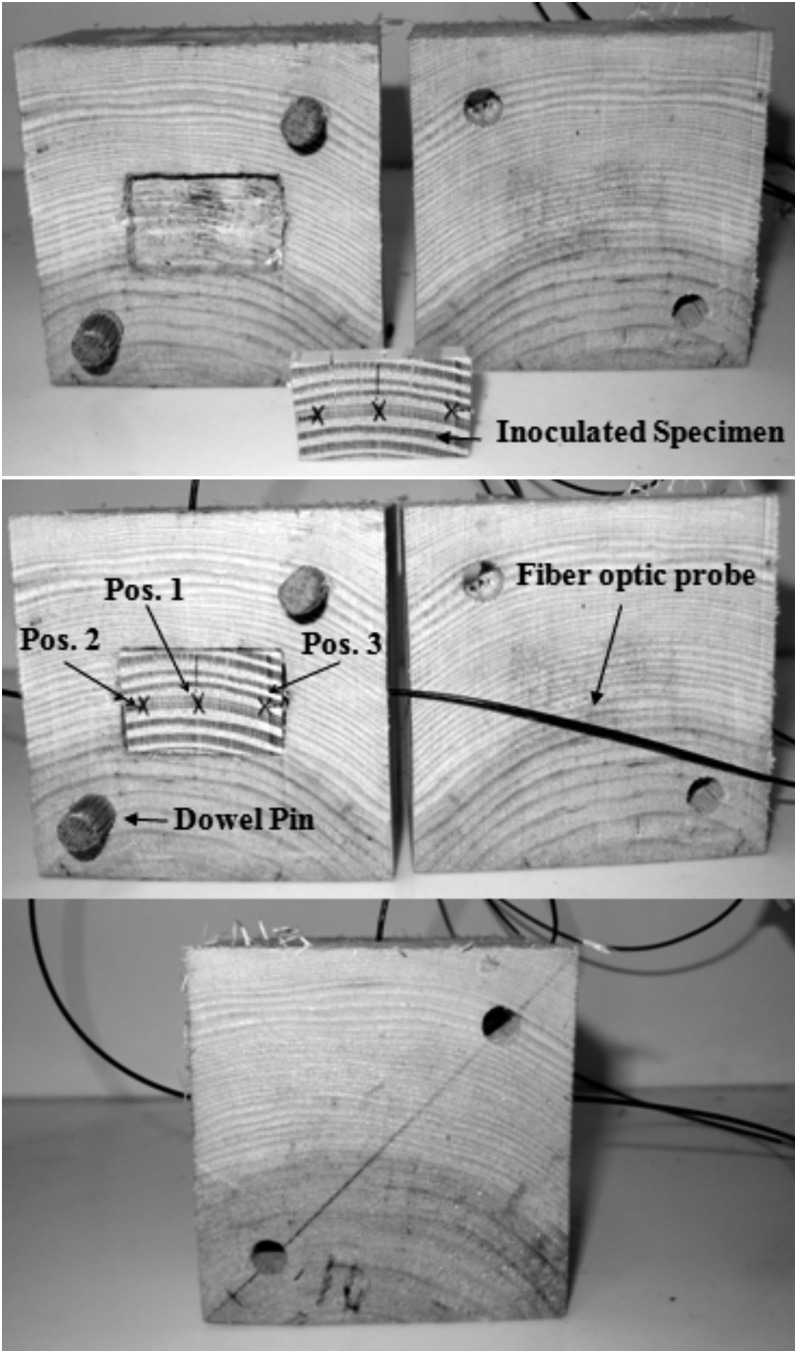
Our use of relatively small wood samples to facilitate detection and counting of live nematodes after treatment created challenges during treatment with respect to rapid heating and evaporative surface cooling rates due to the large surface to volume ratio of the samples. To minimize these effects, the volume of wood material was increased to an industrial scale to reflect a typical, nominal heavy thickness sawn section of lumber. Red pine (Pinus resinosa) blocks (8.9 cm wooden cubes) containing a mixture of heartwood and sapwood were constructed to encapsulate the smaller wood samples (Fig. 1). Pinus resinosa was selected because of its similar green moisture content and density to P. contorta. The cubic blocks were reduced (0.16 cm kerf cut) into two sections, one ≈5.1 cm and the other ≈3.8 cm thick. An insert pocket was routed precisely into the center of the 5.1 cm thick section of the same dimensions as the nematode-infested wood samples, with the center of the block aligning with the center of the sample and the grain orientation matching that of the sample to minimize differences in wood dielectric properties between the two. Pilot holes were drilled for the fiber-optic probes with a #41 index drill bit (0.248 cm OD). Two holes (0.95 cm diam.) were drilled into the faces of opposing corners of the sections, in which 0.95 cm dowel pins were inserted (Fig. 1). These pins provided a tight seal for the faces of the two sections of the cube during MW treatment. The interior of each block was flame sterilized before reuse and care was taken to prevent cross-contamination among samples.
Fig. 1.
Treatment configuration of lodgepole pine (Pinus contorta) small wood samples used to determine minimum lethal temperature using microwave irradiation. Due to the high surface area to volume ratio of the small wood samples, each sample was encased in a red pine (Pinus resinosa) cube for insulation to prevent evaporative cooling during the one-min hold time. Arrows indicate the fiber optic probe positions (Pos. 1-3) in the small wood samples.
Before testing and between each reuse, all P. resinosa blocks were submerged in water to ensure that the moisture content remained above the fiber saturation point (FSP) (28-30% moisture content). This practice limited drying of the test sample during treatment and served to reduce any variability in heating that might occur from a moisture gradient between the sample and the block that encapsulated the sample.
To obtain an initial estimate of lethal temperature, we first conducted an exploratory experiment by treating a minimum of 10 small wood samples (prepared as described above) with MW irradiation at each of 14 different temperatures: 28 (exception, N=5), 40, 48, 52, 54, 56, 58, 60, 62, 63, 65, 67, 70 °C, and an ambient control (≈20 °C). The control was handled in the same way as treated samples but the MW was not turned on. After determining the minimum treatment temperature that produced 100% mortality we bracketed this temperature and tested additional replicates at the lowest lethal temperature (56 °C), 1 step below (54 °C), 1 step above (58 °C), and the ambient control. Thus, at 20 (ambient control), 54, 56, and 58 °C, sample sizes were 20, 27, 29, and 29, respectively. Each wood sample was weighed before and after MW treatment to the nearest 0.1 mg to check for significance of wood water loss due to MW heating. Moisture loss from the wood samples after MW treatment never exceeded 1%. Percentage moisture content of the samples was determined immediately following MW treatment by the standard method of oven dry weight (American Society for Testing and Materials, 1996).
For all experiments a MRI (Microwave Research and Applications, Inc., Laurel, MD) Model BP-211: 2.45 GHz, 3.2kW MW oven with an internal unit chamber size of 0.045 m3 designed for research was used. The MRI is designed to provide a uniform spatial delivery of MW energy within the heating chamber. During MW treatment, temperatures were continuously monitored at three locations in all wood samples (Fig. 1) using a computer recording system with multiple fiber-optic probes (Model STM-2; Luxtron, Santa Clara, CA) to record thermal elevation responses at 1-sec intervals with a reported uncertainty of ± 0.5°C. Multiple probes were interfaced with a computer through a four-channel data acquisition unit (FOT Labkit; Luxtron, Santa Clara, CA). Before testing, the sensors were calibrated in a 70 °C water bath. One sensor was placed in the middle of the sample (hereafter Position 1, Fig. 1), and the other two were approximately 0.3 cm from each long end, at the midpoint of both the width (1.25 cm) and thickness (0.3 cm) of the sample (Positions 2 and 3, Fig. 1). The MW heating cycle was terminated when the last of the three fiber optic sensors reached the set point temperature, and then the samples were kept in the microwave for a 1 min “hold time”. Immediately after the hold time, the encapsulation blocks were opened and infrared (IR) images of the sample were taken using a FLIR Thermacam E65 IR camera (FLIR, Wilsonville, OR). A type-K thermocouple was used to measure the temperature of the wood surface and to determine the emissivity (1.0) prior to image analysis.
Prior to treatment, we pre-assessed nematode populations using standard sterile techniques and Baermann funnels. Fifty wood samples were randomly selected, weighed, and 1/3 of the block was chiseled into matchstick size pieces to determine the mean number of nematodes/g of wet wood weight. Of these 50 blocks, the remaining thirds were used for MW treatment and post-treatment assessment. In addition, using the Oil Red O staining procedure described by Stamps and Linit (1995), we determined that at least 50% of the nematode populations in our samples were JIII . This observation is consistent with previous reports (Tomminen and Nuorteva, 1992; Soma et al., 2003) in which JIII averaged ≈90% of the B. xylophilus population in naturally-infested pine wood.
For post-treatment assessment, 1/3 of each wood sample was tested for survival of nematodes at Day 0 if the sample was negative for live nematodes, the remaining 1/3 of the sample was retested at 21 d post-treatment. These samples were held in a clean bag under humid conditions at 25 °C. Percentage mortality, using each sample as an individual replicate, and mean nematode population for each treatment temperature were calculated. The number of live nematodes/g was multiplied by the wet weight of the wood sample before treatment to determine the number of nematodes treated in that sample.
As described above, two approaches (a “brute force” and a modeling approach) were used to determine lethal temperature at Probit 9. The “brute force” approach is used to determine the dose (temperature) that kills at least 99.9968% of a minimum of 100,000 nematodes regardless of how many individual wood samples are needed to achieve this nematode population. For the “brute force” approach, we used pre- and post-assessment of nematode numbers to ensure that we treated at least 100,000 nematodes at the minimum temperature that produced 100% mortality.
The modeling approach designated each wood sample as an individual observation with temperature as a fixed factor and success/failure as the outcome variable, using the same data set as the “brute force” approach. If even one nematode survived in a sample during either the Day 0 or Day 21 post-treatment assessment, this sample was considered a failure. This creates a binomial (success or failure) analysis used to obtain the model that best fits the data to estimate the minimum temperature required to obtain Probit 9 efficacy (POLOPLUS v.2, LeOre Software, Berkeley, CA).
Step 2: Verification of minimum lethal temperature using industrial scale, large wood blocks. Large wood blocks (10.2 x 10.2 x 25.4 cm) were prepared from the same group of freshly felled P. contorta logs cut at the same time as the small wood blocks. Each log was stripped until about 50% of the bark was removed to facilitate colonization of the sapwood by fungi. Then logs were sprayed with a fine mist of a 0.04% Tween 80 solution (Sigma-Aldrich, St. Louis, MO) containing a mixture of macerated mycelium from the same six species of fungi as described above for the small wood samples. The ends of the logs were painted with an epoxy seal (Intergard 740; International Paint, Houston, TX) to prevent moisture loss and then suspended above a pool of water. Logs were covered with a protective lumber wrap and incubated outdoors, with occasional watering to maintain high moisture content suitable for maximum fungal growth.
After 25 d of incubation, logs were inoculated with the four isolates of B. xylophilus. Ten holes (10 mm diam. x 2 cm deep) were drilled on each side of the logs in a spiral pattern 20 cm apart. Each hole was injected with 1 ml of sterile water containing approximately 8,000 nematodes. Holes were plugged with wooden dowels and sealed with waterproof wood glue (Titebond III; Franklin International, Columbus, OH). Incubation continued for seven more wk. Immediately prior to experimental treatment, logs were cut into approximately 10 x 10 x 25 cm blocks from along the log lengths, incorporating as much sapwood as possible.
For pre- and post-treatment assessment of nematode populations in the large wood blocks, small sections of 2.5 cm thick were cut from both ends of each block. Two 3 to 6 g subsamples were chiseled out of each section, chipped into match size pieces and processed using the Baermann funnel method. Since nematodes are primarily found in sapwood, we estimated the mean number of nematodes/g of wet weight in the sapwood by separating the 2.5 cm thick sections into sapwood and heartwood, weighing them separately, and calculating the proportion that constituted sapwood in the block. Then each large block was weighed and the percent sapwood used to estimate the amount of sapwood in the block. Two similar sized samples of both the sapwood and heartwood from each block were weighed, dried overnight at 105 °C and reweighed to calculate moisture content. For post-treatment assessments of the nematode population, two 3 to 6 g samples were removed from the sapwood of each block using a hand drill fitted with an auger hole-boring bit. Shavings were weighed, and processed using the Baermann funnel method.
Sample sizes were 10 at 56 °C, 8 at 58 °C, and 10 ambient controls. Temperature was monitored on three faces (top and both sides). Holes were drilled in the center of each face 2.54 cm below the surface for placement of thermal sensors. Temperature monitoring was conducted before and after treatment, and the same MW and sensor equipment was used, as described above. After the 1 min hold time, the treated block was removed from the MW chamber and thermal images were taken of all wood surfaces to provide a permanent map of surface temperature elevation. All temperature data were used in regression analysis to determine if surface temperature was a significant predictor of internal wood temperature.
Temperature rise time effects on nematode mortality: To determine if different power densities influence B. xylophilus mortality during MW treatment at the same target temperature, we treated an additional 10 small wood samples with high and low power (≈3.2 and 1.0 kW, respectively) at 56 °C. Samples were assessed for survival of nematodes at Day 0 and Day 21 post-treatment as described above. We calculated percentage mortality using each sample as an individual replicate. Temperature rise time was determined by subtracting the final wood temperature from the initial temperature, as determined by the mean temperature of the three fiber optic probes in the sample during treatment, divided by the treatment time. Contingency analysis was conducted to compare percentage mortality among power levels (JMP, SAS Institute, v.7, Cary, NC).
Results
Step 1: Minimum lethal temperature using small wood samples. In a pre-treatment subsample of 50 small wood samples used to determine the lethal temperature of MW irradiation (using 1/3 of the sample), there was an average of 2,125 ± 216 (standard error of the mean; SEM) nematodes/g of test wood (range: 375 to 7,215 nematodes/g). Based on the wet weight of the wood samples, each sample was infested with an average of 10,826 ± 918 nematodes (range: 2,321 to 32,801 nematodes). Thus, at each test temperature, an average of approximately 100,000 nematodes were tested (except at 28 °C for which 5 samples were used instead of 10, which is an average of 54,130 nematodes).
At 56 °C and above held for 1 min, 100% of the total nematode population was killed in all samples. For example, of the total 222,673 nematodes treated at 56 °C (N=29), there were no survivors. Thus 56 °C for 1 min using MW irradiation was the minimum treatment required to achieve efficacy of Probit 9. At oven shut off when the lowest reading among the three fiber optic probes had reached the target temperature of 56 °C, the mean recorded temperatures were 56.1 ± 0.60, 71.3 ± 0.91, and 60.8 ± 0.63 °C at Positions 1 (mid-point of sample), 2 and 3 (1.25 cm from the ends and 0.3 cm deep), respectively (Fig. 1). Readings at 1 min following termination of the treatment cycle increased to maximum temperatures of 62 ± 0.56, 72 ± 1.34, and 66 ± 1.14 °C at Positions 1, 2, and 3, respectively.
Post-treatment assessment of controls showed recovery of live nematodes from all samples at both 1 wk following pre-assessment when all MW treatments had been completed and at 21 d post-treatment. The total population of nematodes recovered from controls was 173,385 (1,646 ± 411 nematodes/g, N=20 wood samples), which represents control mortality of 3.76% of the total control nematode population and 0% on a per sample basis since every control sample still contained live nematodes.
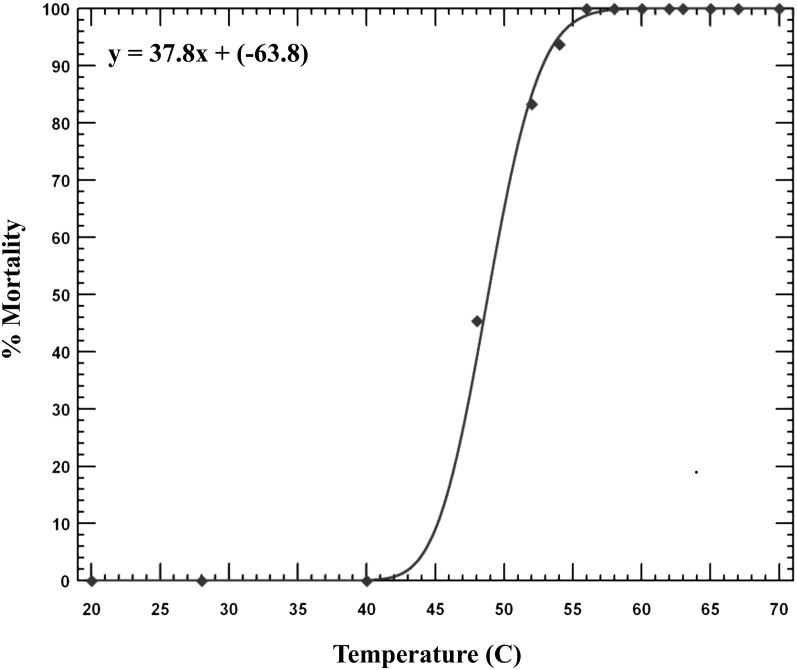
When we modeled lethal temperatures using each wood sample as one replicate, Probit analysis estimated the temperature required to produce Probit 9 efficacy at 62.2 (95% CI 59.0-70.0) °C (Fig. 2). The probability that the data fit a probit distribution was 99% and the heterogeneity was 0.096 (heterogeneity < 1 represents a good fit).
Fig. 2.
Modeling of the dose-response of small wood samples infested with Bursaphelenchus xylophilus and treated with microwave irradiation at 14 different temperatures with a one-min hold time. Each wood sample represented one observation. Each data point represents the percentage mortality at that temperature. The distribution was determined by best fit of the data. Probit model Chi-square Goodness of Fit test = 1.15, df = 12, heterogeneity (h) = 0.096 (h < 1 represents a good fit of the data).
Step 2: Verification of minimum lethal temperature using industrial scale, large wood blocks. The moisture content of sapwood and heartwood pre-treatment was 148.6 ± 3.75 and 47.3 ± 1.07%, respectively. Pre-treatment weights were 1,602 ± 29.7 g/block, with mean sapwood weight of 950 ± 80.8 g/block. Pre-treatment nematode populations averaged 25 ± 8 nematodes/g of wet weight of sapwood in treated blocks and 19 ± 5 nematodes/g in controls. Because nematode numbers were very low in heartwood, the weight of heartwood was not included in the estimate of the number of nematodes in treated or control blocks. Using wet weight of sapwood only in each block, a total of 266,688 and 90,273 nematodes were treated at 56 and 58 °C, respectively. Pre-treatment assessment of controls indicated a population of 17,983 ± 4,720 nematodes in sapwood (N = 10).
At 56 °C, one of the 10 treated blocks had some survivors. In this block, mortality was 98.7% of nematodes pre-assessed at Day 0 and 99.3% at Day 21 post-treatment. All other blocks had 100% mortality at both 56 and 58 °C. To ascertain the possible reason for failure in one of the blocks treated at 56 °C, we examined the temperature data for this block and discovered that although the center probe recorded 57 °C for a few seconds after oven shut-off, during the 1 min hold time the temperature of this block decreased to 55.6 °C. Because lethal temperature had not been maintained for 1 min in this block, in contrast to all other treated blocks and the protocol for treatment, this data point was omitted from our analysis. For all treated blocks, the rate of temperature increase from a starting temperature of 18-19 °C was 8.5-9.5 °C/min.
During post-treatment assessment of nematode populations, we did not observe any trend in the proportion of survivors as a function of life stage, despite the fact that >50% of the observed nematodes were JIII. In control blocks, nematode populations increased during the 21 d post-treatment before final assessment to 52 ± 12 nematodes/g, which was a 50% increase in population (total population in sapwood of 10 blocks after 21 days was 49,507 ± 11,709).
Temperature rise time effects on nematode mortality. Regardless of the rate of temperature increase using high or low power, nematode mortality was 100% as long as the minimum temperature of 56 °C was reached in the wood samples.
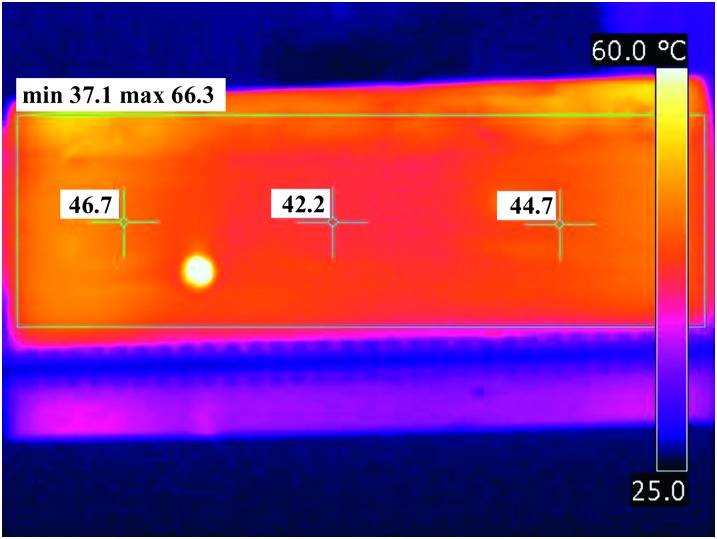
Post-diagnostic use of surface infared images. Since the fiber optic probe recordings of temperature were limited to finite locations in the samples, we used our thermal IR images to supplement the probe readings to examine any unexpected observed results. For example, one wood block treated to 56 °C had nematode survivors when no others did (described above). In addition to the failure to hold fiber optic probe readings of at least 56 °C for 1 min, a cold spot was detected in the surface IR image with a low temperature of 37 °C, which was well below the desired temperature (Fig. 3).
Fig. 3.
Thermo-graphic image of the industrial scale wood sample in which there was a small number of Bursaphelenchus xylophilus that survived treatment at an internal wood temperature of 56 °C. The center cross-hair represents the temperature on the surface above one of the fiber optic probe readings. The other two temperatures show some of the variability in temperatures across the face of the sample. The scale bar on the right indicates the temperature range, from 25-60 °C. Note that the minimum temperature reading, indicated at the top left, was 37.1 °C.
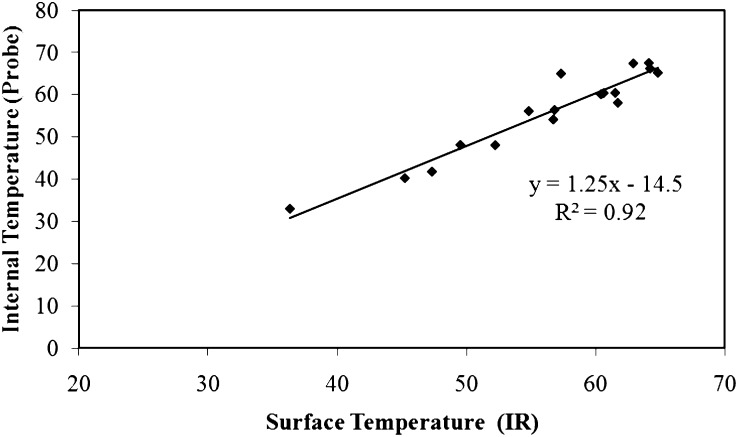
Prediction of internal temperature from infared surface readings: Prediction of internal temperatures was made by constructing a calibration line drawn from a plot of the internal temperature (fiber optic probe measurement) against the temperature on the surface above the probe (IR measurement). The regression curve used to predict internal temperatures in the small wood samples from the IR data is shown in Fig. 4 (R2 = 0.92).
Fig. 4.
Regression analysis of internal wood temperatures as a function of surface temperatures taken with a thermal imaging camera. A regression equation was used to predict the internal temperaures of samples (at 0.15 cm) from the surface infared measurements.
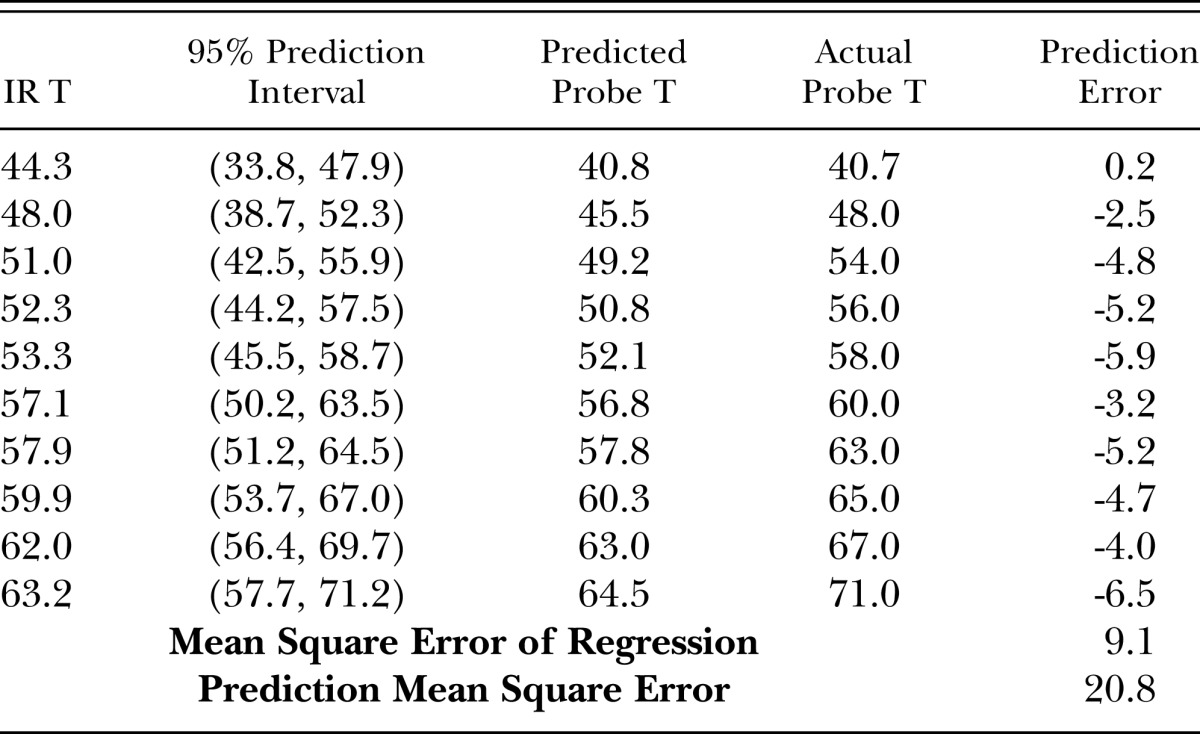
The regression function was then used to predict the internal temperature of other samples (Table 1). The predictive capacity of the regression was evaluated in two ways. The first was a 95% prediction interval (PI), which is an estimation of an interval where 95% of predictions fall. The prediction intervals showed good agreement with the actual values measured by the probe, since they all fell within the 95% prediction intervals. The second method of evaluating the prediction capacity of the regression was to compare the predicted values based on the regression to actual values measured by the probe. As seen in Table 1, the predictions consistently under-estimated the actual temperature, indicating that the regression equation made conservative predictions of the internal temperature, with the maximum discrepancy between predicted and actual values of 6.5 °C.
Table 1.
Prediction performance of the internal temperature from infrared surface temperature (IR T). Values are in °C.
Discussion
Using MW irradiation, the minimum temperature required to produce greater than 99.99638% mortality of a population of nematodes > 100,000 (Probit 9) was 56 °C held for 1 min, both in small wood samples and in commercial-sized wood blocks. We found no evidence of B. xylophilus JIII resistance to MW heating. This result is consistent with another study in which the JIII was equally susceptible to mortality using a conventional kiln to heat green lumber (Tomminen and Nuorteva, 1992). Although our goal was to determine the treatment that produced efficacy of Probit 9, this approach would not necessarily be appropriate for other organisms that occur in commodities at much lower population densities or present a lower relative risk due to their biological or ecological characteristics.
The “brute force” approach we used to establish lethal temperature depends upon the total number of organisms treated regardless of the number of individual pieces of the commodity required to obtain the requisite number of organisms. However, when we modeled lethal temperature using Probit analysis and each wood sample was treated as an individual observation, the model estimated a higher temperature than that using the “brute force”approach. The difference in minimum lethal temperature was not due to a poor fit of the data to the Probit distribution (Fig. 2). The difference in the observed temperature and the Probit 9 model predicted temperature may be inherent in the potential differences between a sample of nematodes and the whole nematode population. As more and more samples of a population are taken over time, the estimated temperature would get closer and closer to the true mean (regressing to the mean), which is an inherent limitation of modeling. A similar discrepancy occurred in another study. Tasked with addressing concerns of the European Union about the potential for introduction of B. xylophilus in infested lumber, Canadian researchers conducted a study of conventional heat treatment using different temperature/time combinations to obtain a dose response (Smith, 1991). In their study, the “brute force” approach estimated Probit 9 at 52 °C for 30 min, but the model estimated Probit 9 at 56 °C. In both cases the difference between the observed and predicted temperature was about 4 °C, however, the Canadian study did not include post-treatment assessment of nematodes 21 d following treatment, as was done in our study.
Although the minimum lethal temperature of 56 °C using MW treatment was the same as for conventional heat treatment, the difference between the two modalities is in the time required to produce 100% mortality. Microwave energy required only 1 min of exposure at this temperature. This is likely because dielectric applications such as MW heat through the profile of the treated material simultaneously, which is referred to as volumetric heating (Ramaswamy and Tang, 2008), rather than relying on heat transfer of thermal energy from the surface to the core.
A number of other methods have been investigated for eradicating B. xylophilus in coniferous wood products, including fumigation (Leesch et al., 1989), radiation (Eichholz et al., 1991), heat treatments (Dwinell, 1990; Smith, 1991), kiln- or vacuum-drying (Dwinell, 1990; Tomminen and Nuorteva, 1992; Dwinell et al., 1994), and two preliminary studies using MW irradiation (Ambrogioni, 2004; Fleming et al., 2005). Fleming et al. (2005) examined the use of MW energy to kill B. xylophilus, but the wood samples were artificially infested by introducing live nematodes into holes drilled into the wood and containing them in situ during treatment by a wood plug rather than using wood naturally infested with B. xylophilus. A study by Ambrogioni et al. (2004) suggested that MW energy was effective at killing a number of nematode species in naturally infested pine, including B. mucronatus, but their data are difficult to interpret due to lack of replication and imprecision in temperature monitoring.
The time required to reach target temperature when the MW was set at different power levels (rise times) did not significantly affect nematode mortality at a given final temperature under our experimental conditions, although the slowest rise time we observed at the lowest power was 4.5 °C/sec. Rise time depends not just on power input but also on the size of the load being treated. The statistical non-significance of rise time as a factor in mortality at 56 °C suggests that commercial operators can devise a MW system to heat wood in the most efficient manner possible without concern for heating rates.
Analysis of IR images for the industrial-sized block that failed to kill 100% of nematodes at 56 °C revealed cold spots on the surface. In addition, the fiber optic probe temperatures for this block did not maintain our target of 56 °C for 1 min; thus, this sample was omitted from our data set. However, there were other large wood blocks with cold spots that did not yield viable nematodes during post-treatment assessment. In these cases, it is possible that there were no nematodes in the wood at these cold spots. These findings indicate that surface imaging would be most useful as a diagnostic tool for ensuring minimum lethal temperatures are reached, particularly in larger wood pieces.
While the primary function of the IR analyses was for diagnostic purposes, we had sufficient IR data to perform a preliminary assessment of its capacity to predict internal temperatures. Accurate and precise prediction of internal temperatures from the surface IR data could be extremely useful to help resolve limitations of the fiber optic measurements. IR technology provides fast temperature observations of an entire surface of a sample, yielding a more comprehensive picture of temperature profiles compared to the fiber optic probes, which measure temperature at only a few points in the sample. IR technology is limited, however, to the surface of the observed object, giving no indication of the temperature below the surface.
Regression of internal temperature as a function of surface temperature produced an equation that we used to predict internal temperatures (Fig. 4), but our predictions consistently under-estimated the actual temperature, indicating that the equation was conservative (Table 1). Furthermore, confidence intervals for predicting temperature, or the under-estimate, could be used to determine the set-point temperature to account for the level of uncertainty in predicting minimum lethal temperature. For example, if the lethal temperature is considered 56 °C, our equation predicts a surface temperature of 62 °C will ensure that internal lethal temperatures are reached. In practice, conservative predictions ensure that the desired temperature is achieved, but will not guarantee that resources are not lost by overheating. Further analysis combined with proper economic and logistic analysis can be used to assess the feasibility of such over-estimates in treatment temperature. It is not likely that such an overshoot would be acceptable, considering that the current treatment is not conventionally “value-added.”
To minimize overshoot, we investigated possible reasons for divergence from predicted temperatures. Prediction error may occur from the regression equation not being a proper fit, or from variability in the data used to create the regression. The former was examined by comparing the mean squared prediction error (MSPR) to the mean squared error (MSE) of the regression (Neter et al., 1990) (see bottom of Table 1). The difference between MSPR (20.8) compared to MSE (9.1) was relatively small, as a certain amount of variability is inherent in prediction; thus the regression equation does not introduce a large amount of error when making predictions. Accordingly, we suggest that the divergence between actual and predicted temperatures is a consequence of variability in the data from which the regression was created. The complex conditions that exist during MW treatment of water-containing materials affect heating and heat transfer in the system. Energy loss from the phase change of water from liquid to gas at the wood surface causes evaporative cooling; thus, surface temperature can be significantly cooler than just below the surface of the material. Evaporative cooling has been observed in several studies where dielectric heat was applied to wood materials (Antti and Perre, 1999; Zieloka and Gierlik, 1999).
Equivalence testing was used for a more complete evaluation of prediction performance. The most appropriate validation of our model using MW treatment is to show its equivalence to existing treatments with a low risk of B. xylophilus survival. The typical null hypothesis of no difference (the models are similar) is not appropriate for two reasons. If the null hypothesis is accepted, the appropriate conclusion is there are insufficient data to reject the equivalence of the model, not that the models differ. Additionally, the risk of a Type II error, accepting the null hypothesis that the models are similar when they are not, is high when the sample size is too small. The power (1-β) of a test is low when β is high. Equivalence testing reverses the typical null hypotheses by using a hypothesis of non-similarity; thus the Type I error of accepting the alternative hypothesis of similarity when it is false can be set by the researcher to be small (Probit 9) while the effect of a Type II error, due to an insufficient sample size or other causes, will result in the acceptance of the null hypothesis that the models are not equivalent. The viability of validating models with equivalence testing has been studied by Robinson and Froese (2004). The limitation of the analysis is that while it provides the investigator with an absolute determination, the region in which the model is evaluated is arbitrary. For example, in the current study, the equivalence test was evaluated for regions of dissimilarity of 2 to 20 °C, i.e., the acceptable disparity between the model and actual values was ± 1 to ± 10 °C.
The equivalence test revealed that for α = 0.05, the region of dissimilarity must be 12 °C or larger for the model to be considered equivalent to the measured data. We recognized as discussed above that the prediction was conservative, which translates to an overestimate of 6 °C. Because phytosanitary treatment is subject to very stringent standards, we also evaluated α (Type I error) = 0.001, revealing a region of dissimilarity of 16 °C (8 °C overestimate) or larger. A power analysis was also performed, and at all α values, the power was greater than 0.999 at the required regions of dissimilarity, so the probability of a Type II error was acceptably small. As before, it is difficult to conclusively determine if a 6-8 °C overshoot would be acceptable without a proper economic analysis and feasibility study, but this analysis provides a useful starting point.
Based on the results reported here, dielectric heating appears to be more rapid and equally as effective as any treatment investigated to date for eradicating B. xylophilus in infested wood. In practice, predicting internal temperature from surface temperature requires further study. The regression relationship reported here is not necessarily applicable beyond the conditions of these experiments. While the prediction results display sufficient accuracy to support the merits of forecasting internal temperatures from IR readings, a more in-depth study focused primarily on prediction is required to explore its full potential.
This study has several implications. These data support consideration of MW energy as an alternative treatment of wood packaging material under ISPM-15. In addition our results have the potential to be used to develop an industrial scale treatment schedule for wood infested with B. xylophilus.
Acknowledgments
We are grateful to John Tsirogotis and Katie Mulfinger for experimental assistance and Scott Myers of USDA, APHIS for consulting on statistical analysis. Funding for this project was provided by USDA CSREES Grant No. 2005-51102-03287. A portion of this project was conducted at FPInnovations and financially supported by the Canadian Forest Service under the existing Contribution Agreement between the Government of Canada and FPInnovations.
Footnotes
This paper was edited by Inga Zasada
Literature Cited
- Ambrogioni L, Cavalli M, Coniglio D, Caroppo S, Roversi PF. Microwave treatment for eliminating nematodes associated with conifer and broad-leaf tree wood. Redia. 2004;87:129–138. [Google Scholar]
- American Society for Testing and Materials, A. West Conshohocken, PA: ASTM; 1996. Standard test for specific gravity of wood and wood-based materials. D. 2395. [Google Scholar]
- Antti AL, Perre P. A microwave applicator for on line wood drying: temperature and moisture distribution in wood. Wood Science and Technology. 1999;33:123–138. [Google Scholar]
- Baker JM, Norris DM. A complex of fungi mutualistically involved in the nutrition of the ambrosia beetle Xyleborus ferrugineus. Journal of Invertebrate Pathology. 1968;11:246–250. [Google Scholar]
- Dwinell LD. Heat-treating and drying southern pine lumber infested with pinewood nematodes. Forest Products Journal. 1990;40:11–12. [Google Scholar]
- Dwinell LD. The pinewood nematode: regulation and mitigation. Annual Review of Phytopathology. 1997;35:153–166. doi: 10.1146/annurev.phyto.35.1.153. [DOI] [PubMed] [Google Scholar]
- Dwinell LD, Avramidis S, Clark JE. Evaluation of radiofrequency dryer for eradicating the pinewood nematode in green sawn wood. Forest Products Journal. 1994;44:19–24. [Google Scholar]
- Eichholz GC, Bogdanov A, Dwinell L. Radiation sensitivity of pinewood nematodes in wood chips. Applied Radiation and Isotopes. 1991;42:177–179. [Google Scholar]
- Evans H, Hart-Tree M, Kubicek Q. Risk of transmission of pinewood nematode, its vectors, and pine wilt to EC forests. 1993:25. Rep. EC/Canada/USA Technical Team. [Google Scholar]
- Fleming MR, Janowiak JJ, Halbrendt JM, Bauer LS, Miller DL, Hoover K. Feasibility of eradicating cerambycid larvae and pinewood nematodes infesting lumber with commerical 2.45 GHz microwave equipment. Forest Products Journal. 2005;55:227–232. [Google Scholar]
- Food and Agriculture Organization, F. Rome: Food and Agriculture Organization; 2009. International Standards for Phytosanitary Measures: Guidelines for regulating wood packaging material in international trade. [Google Scholar]
- Henin J-M, Charron S, Luypaert PJ, Jourez B, Hebert J. Strategy to control the effectiveness of microwave treatment of wood in the framework of the implementation of ISPM 15. Forest Products Journal. 2008;58:75–81. [Google Scholar]
- Hicks C. St. Paul: American Phytopathological Society; 2001. Exotic Pests and International Trade. Exotic Forest Pests Online Symposium. [Google Scholar]
- Ishibashi N, Kondo E. Ecological significance of dormancy in plant parasitic nematodes. 5. Occurrence and survival of dispersal forms of pine wood nematode, Bursaphelenchus-lignicolus. Applied Entomology and Zoology. 1977;12:93–302. [Google Scholar]
- Kishi Y. Effects of microwave irradiation on Monochamus alternatus Hope (Coleoptera: Cerambycidae) and Bursaphelenchus lignicolus M. & K. (Nematoda: Aphelenchoididae) Japanese Journal of Applied Entomology and Zoology. 1975;19:290–291. [Google Scholar]
- Kondo E, Ishibashi N. Ecological significance of dormancy in plant parasitic nematodes. 7. Ultrastructural differences between propagative and dispersal forms in pine wood nematode, Bursaphelenchus-lignicolus, with reference to survival. Applied Entomology and Zoology. 1978;13:1–11. [Google Scholar]
- Leesch J, Davis R, Simonaitis R, Dwinell LD. In transit shipboard fumigation of pinewood chips to control Bursaphelenchus xylophilus. EPPO Bulletin. 1989;19:173–181. [Google Scholar]
- Mamiya Y. Pine wood nematode, Bursaphelenchus lignicolus Mamiya and Kiyohara, as a causal agent of pine wilting disease. Review of Plant Protection Research. 1972;5:46–60. [Google Scholar]
- Mamiya Y. Pine wilting disease caused by the pine wood nematode, Bursaphelenchus lignicolus, in Japan. Jarq. 1976;10:207–211. [Google Scholar]
- Mamiya Y. Pathology of the pine wilt disease caused by Bursaphelenchus xylophilus. Annual Review of Phytopathology. 1983;21:201–220. doi: 10.1146/annurev.py.21.090183.001221. [DOI] [PubMed] [Google Scholar]
- Mota M, Vieira P. Netherlands: Springer; 2008. Pine Wilt Disease: A Worldwide Threat to Forest Ecosystems. [Google Scholar]
- Mota MM, Penas AC, Bravo MA, Sousa E, Braasch H. First report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology. 1999;1:727–734. [Google Scholar]
- Neter J, Wasserman W, Kutner MH. Homewood, IL: Richard D. Irwin, Inc; 1990. Applied Linear Statistical Models: regression, analysis of variance, and experimental designs. [Google Scholar]
- Ramaswamy H, Tang J. Microwave and radio frequency heating. Food Science and Technology International. 2008;14:423–427. [Google Scholar]
- Robinson AP, Froese RE. Model validation using equivalence tests. Ecological Modeling. 2004;176:349–358. [Google Scholar]
- Smith RS. Vancouver, BC: Forintek Canada Corp; 1991. The use of heat treatment in the eradication of the pinewood nematode and its vectors in softwood lumber. [Google Scholar]
- Soma Y, Goto M, Naito H, Ogawa N, Kawakami F. Effects of some fumigants on mortality of pine wood nematode, Bursaphelenchus xylophilus infecting wooden packages. Research Bulletin of the Plant Protection Service Japan. 2003;39:7–14. [Google Scholar]
- Soma Y, Naito H, Misuma T, Mizobuchi M, Tsuchiya Y, Matsuoka I, Kawakami F. Effects of some fumigants on pine wood nematode, Bursaphelenchus xylophilus infecting wooden packages 1. Susceptibility of pine wood nematode to methyl bromide, sulfuryl fluoride and methyl isothiocyanate. Research Bulletin of the Plant Protection Service Japan. 2001;37:19–26. [Google Scholar]
- Soma Y, Naito H, Misumi T, Tsuchiya Y, Mitsobuchi M, Matsuoka I, Kawakami F, Hirata K, Komatsu H. Effects of some fumigants on pine wood nematode, Bursaphelenchus xylophilus infecting wooden packages. 2. Mortality of pine wood nematode by methyl bromide tent fumigation. Research Bulletin of the Plant Protection Service Japan. 2002;38:13–19. [Google Scholar]
- Stamps WT, Linit MJ. A rapid and simple method for staining lipid in fixed nematodes. Journal of Nematology. 1995;27:244–247. [PMC free article] [PubMed] [Google Scholar]
- Tares S, Abad P, Bruguier N, Deguiran G. Identification and evidence for relationships among geographical isolates of Bursaphelenchus spp (pinewood nematode) using homologous DNA probes. Heredity. 1992;68:157–164. doi: 10.1038/hdy.1992.24. [DOI] [PubMed] [Google Scholar]
- Tomminen J. The effects of beetles on the dispersal stages of Bursaphelenchus mucronatus Mamiya & Enda (Nematoda: Aphelenchoididae) in wood chips of Pinus sylvestris L. Entomologica Fennica. 1992;3:195–203. [Google Scholar]
- Tomminen J, Lahtinen J. Interception of the pinewood nematode, Bursaphelnechus xylophilus, in green lumber imported to Finland from Canada. Nematologica. 1990;36:397. [Google Scholar]
- Tomminen J, Nuorteva M. Pinewood nematode, Bursaphelenchus xylophilus in commercial sawn wood and its control by kiln-heating. Scandinavian Journal of Forest Research. 1992;7:113–120. [Google Scholar]
- Wingfield MJ, Blanchette RA. The pine wood nematode associated with stressed trees and cut timber in Minnesota and Wisconsin. Phytopathology. 1982;72:1141–1141. [Google Scholar]
- Wingfield MJ, Blanchette RA, Nicholls TH. Is the pine wood nematode an important pathogen in the United States? Journal of Forestry. 1984;82:232–235. [Google Scholar]
- Wingfield MJ, Blanchette RA, Nicholls TH, Robbins K. The pinewood nematode - A comparison of the situation in the United States and Japan. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestiere. 1982;12:71–75. [Google Scholar]
- Zieloka P, Gierlik E. Temperature distribution during conventional and microwave wood heating. Holz Roh Werkst. 1999;57:247–249. [Google Scholar]