Abstract
The effect of nematode population density at the time of application and formulations of in vitro-produced Pasteuria spp. endospores on the final population density of Belonolaimus longicaudatus was studied in an 84-d-long pot bioassay. The experiment utilized a factorial design consisting of 30 or 300 B. longicaudatus /100 cm3 of sandy soil and three formulations of in vitro-produced Pasteuria spp. endospores (nontreated, granular, or liquid). No differences were observed in percent endospore attachment between nematode inoculum levels during either trial. Granular and liquid formulations of in vitro-produced endospores suppressed nematode population densities by 22% and 59% in the first trial and 20% and 63% in the second, respectively compared with the nontreated control. The liquid formulation increased percent endospore attachment by 147% and 158%, respectively, compared with the granular formulation. The greatest root retention by the host plant was observed at the lower B. longicaudatus inoculation level following application of the liquid formulation. While both the granular and liquid formulations reduced B. longicaudatus population densities in the soil, the liquid spore suspension was most effective.
Keywords: Belonolaimus longicaudatus, biological control, formulation, management, Pasteuria spp., sting nematode, suppression, turfgrass
In recent years, researchers have continued to investigate the usefulness of biocontrol agents for management of plant-parasitic nematodes. ‘Candidatus Pasteuria usgae’ has been recognized as a biological agent that can suppress Belonolaimus longicaudatus in turf (Giblin-Davis, 2000). Previously, ‘Candidatus Pasteuria usgae’ was cultivated on B. longicaudatus grown in aseptic root culture, greenhouse cultures, or was collected from suppressive field sites (Giblin-Davis et al., 1990; Bekal et al., 2001). Recently, Pasteuria Bioscience LLC. developed an in vitro method of culturing Pasteuria spp. that may allow members of this bacterial group to be commercialized as biopesticides.
In vivo-produced Pasteuria spp. endospores have been introduced to soil using various sources of inoculum laden with endospores: ground root material, soil, or second stage juvenile nematodes encumbered with endospores (Stirling and Wachtel, 1980; Dube and Smart, 1987; Chen et al., 1996b; Weibelzahl-Fulton et al., 1996; Giblin-Davis, 2000; Kariuki and Dickson, 2007). Recent research suggests that a liquid spore suspension of in vitro-produced endospores readily enters the turfgrass soil profile, but also that it can be leached below the turfgrass rhizosphere by heavy rainfall or irrigation. Leaching may affect its efficacy and success as an inundative biopesticide on turf (Luc, unpublished). To counteract endospores leaching from the turf rhizosphere it has been proposed that a clay granular formulation might slowly release the propagules so that they persist longer in the turfgrass rhizosphere, although reducing their numbers at a given time.
Belonolaimus longicaudatus and ‘Candidatus Pasteuria usgae’ appear to follow a density dependent relationship (Giblin-Davis, 2000). Field studies with other ecto- or endoparasitic nematode populations naturally infested by different Pasteuria spp. also have density-dependant host and parasite relationships (Ciancio, 1995; Atibalentja et al., 1998; Ciancio and Quénéhervé, 2000). Similarly, studies with in vivo-produced P. penetrans and Meloidogyne arenaria found populations of both organisms fluctuated in the field in a density dependent manner as explained by the Lotka-Volterra model (Orajay, 2009). This model system relies on several factors: host density in soil, host growth rate without limiting factors, percent parasitism of host, host reductions due to parasitism, parasite growth rate influenced by host density, and parasite death rate (Ciancio, 1995). In a previous study, approximately 30 B. longicaudatus were inoculated in 100 cm3 of soil containing 280,000 in vitro-produced Pasteuria spp. endospores/cm3 of soil with about 75% suppression of B. longicaudatus observed after 12 weeks (Luc et al., 2010). While a dose-response relationship was observed in controlled conditions with B. longicaudatus and in vitro-produced endospores, nematode densities were not reduced below initial nematode densities (30 B. longicaudatus/100 cm3 of soil) (Luc et al., 2010). The application of in vitro-produced Pasteuria spp. endospores as an inundative control method raised concern that their effectiveness as a bionematicide might be affected by B. longicaudatus population density at the time of application. The objectives of this research were to determine if the efficacy of in vitro-produced Pasteuria spp. endospores as a biopesticide is affected by B. longicaudatus population density at the time of application, and to compare the efficacy of a clay granule formulation with a liquid spore suspension.
Materials and Methods
Two trials were conducted in a growth room at the University of Florida in Gainesville, FL from May to August 2009. These trials utilized a factorial design consisting of two B. longicaudatus inoculum levels: 30 or 300 nematodes/100 cm3 of soil and three formulations of in vitro-produced Pasteuria spp. endospores (nontreated, granular, or liquid) with five replications. Thirty clay pots (10.16-cm-diam.; 10.16-cm-high; 500-cm3-volume) were cleaned, autoclaved, and then filled with 400-cm3 of nematode-free United States Golf Association specification sand (Anonymous, 1993). In vitro-produced endospores were generated from an isolate of Pasteuria sp. that was collected and cultured from B. longicaudatus on turf from Sebring, FL. The propagules were obtained from Pasteuria Bioscience LLC. (Alachua, FL) and kept refrigerated at 4°C for 3 days to quantify the density of endospores/ml and determine their core and sporangia size. Measurements of in vitro-produced endospores indicated that the mean core diam. was consistent with previously published measurements of ‘Candidatus Pasteuria usgae’ (Giblin-Davis et al., 2003), however the mean sporangium diam. was variable. Therefore, until confirmatory studies are completed, we refer to this in vitro-produced isolate(s) as Pasteuria spp. Prior to applying the treatments; the liquid formulation was prepared as a suspension (50 ml) of tap water, growth media, and endospores. The granular formulation was prepared by pipetting 1120 μl of growth media and endospores onto 2 g of a clay blank provided by Pasteuria Bioscience LLC. This process was repeated 10 times to prepare the granular formulation. The mixture of clay, media, and endospores was stirred periodically and allowed to dry at 35 °C for 24 hours. The respective endospore treatments were applied topically to the pots adding 1,380,000 endospores/cm2. Following topical application of the nontreated and granular formulations, 50 ml of water was applied to maintain equal soil moisture between formulations and facilitate endospore release from the granular material. The application of 50 ml of water corresponded to 0.6 cm of irrigation previously shown to move endospores into the top 10 cm of the soil profile. Subsequently, ‘Penncross’ creeping bentgrass (Agrostis palustris) was seeded at 0.08 g/pot (equivalent to 98 kg/ha) and allowed to germinate and establish a root system for 13-dbefore being inoculated with nematodes. Experimental units were kept in a growth room with a light period of 14 hr/d and soil temperature maintained at 24 °C ± 0.5 °C.
Following turf establishment, B. longicaudatus was extracted from pure nematode populations maintained on ‘FX313’ St. Augustine grass (Stenotaphrum secundatum) (Giblin-Davis et al., 1992; Busey et al., 1993) using the sieving and decanting method (Cobb, 1918). Nematode population density was determined by counting the B. longicaudatus in 1-ml aliquots on a counting slide (Hawksley and Sons Limited, Lancing, Sussex, UK) with five replications. The nematode inoculum (mixed-life stages) was pipetted into two holes (1-cm-diam. × 2.5-cm-deep) in the soil at two densities: 120 ± 6 nematodes/pot (30.0 ± 1.5 nematodes/100 cm3 of soil) or 1200 ± 60 nematodes/pot (300.0 ± 15.0 nematodes/100 cm3 of soil).
The turf was watered twice/d with 10 ml of water, and was fertilized every 2-wk with Peters® 20-20-20 (N-P2O5-K2O) fertilizer (United Industries Corp., St. Louis, MO). Nutrient inputs were 12.3 kg/ha N, 5.4 kg/ha P, 10.2 kg/ha K (0.010 g/pot N, 0.004 g/pot P, and 0.008 g/pot K), and trace amounts of essential micronutrients. The turf was trimmed to 3-cm height once/wk.
Nematode population densities and root lengths were assessed with destructive sampling 84-d after nematode inoculation. The entire soil profile of each pot was used to obtain nematode and root samples. Each sample was placed onto a 135-μm sieve, rinsing the roots with water to collect the sand and nematodes. The resulting suspensions were agitated and nematodes were extracted by centrifugal-flotation (Jenkins, 1964) using a 25-μm sieve to catch all the B. longicaudatus vermiform stages present. The nematodes were collected and counted using an Leica DM IL (Leica Microsystems CMS GmbH, Wetzlar, Germany) inverted light microscope ×40 magnification. Subsequently, twenty nematodes were randomly selected from each sample to count the number of endospore attached (Chen et al., 1996a). Roots were collected and then placed into a clear plastic tray and scanned with Epson perfection 4990 photo desktop scanner (Epson, America Inc., Long Beach, CA) to obtain bitmap images of the root system (Bauhus and Messier, 1999). The images were imported into the WinRhizo (Regent Instruments, Chemin Sainte-Foy, Quebec) software program for analysis, to determine root lengths in centimeters. Root lengths are a measure of plant health, successful nematode management usually results in increased root retention by the host plant, but when it fails root losses are observed. All data sets were tested for normality and homoscedasticity. Factorial analysis of variance (ANOVA) and Fisher's LSD was performed to compare counts of B. longicaudatus, percent endospore attachment, and total root lengths for main effects and interactions using SAS (SAS Institute, Cary, NC).
Results
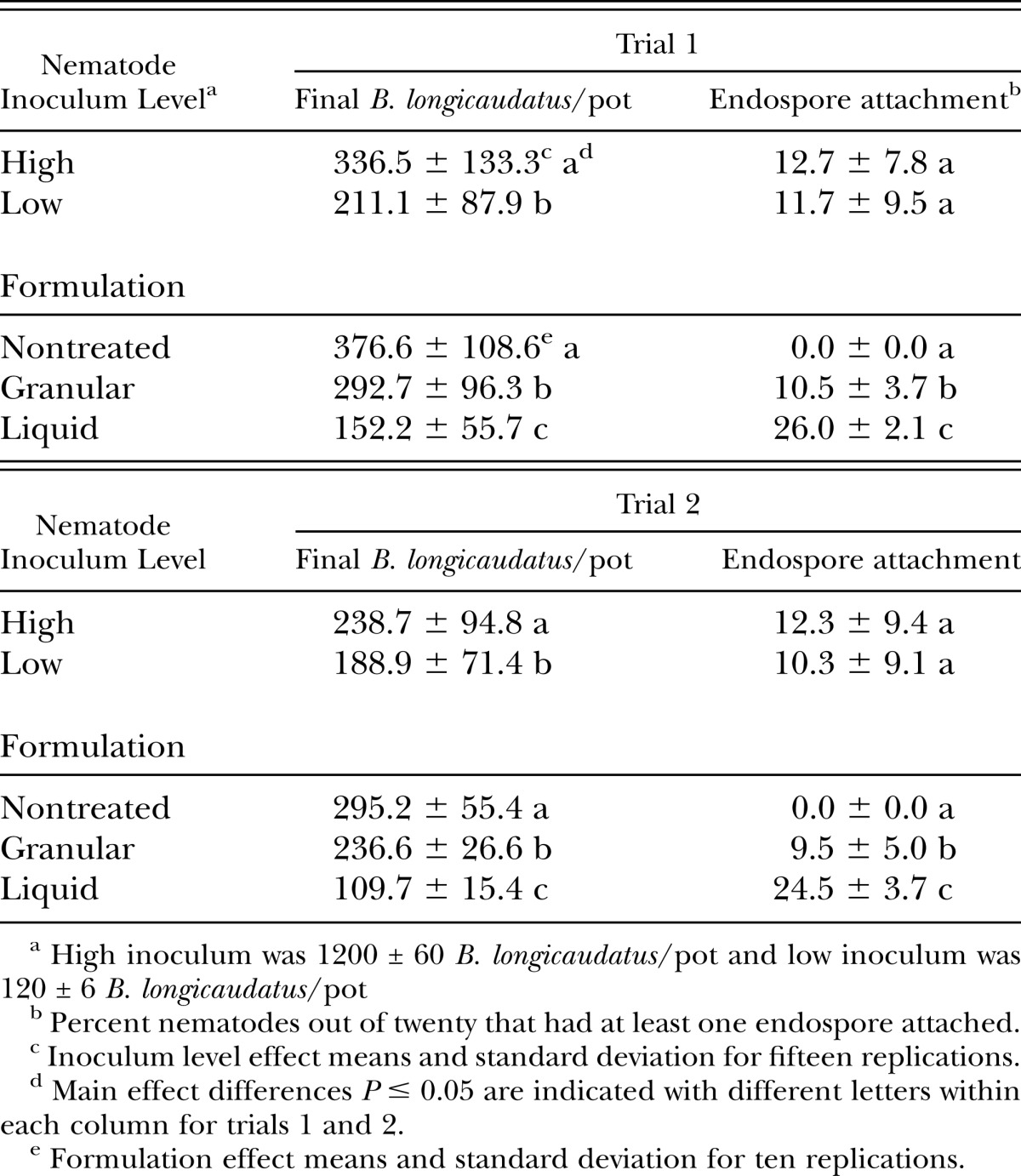
No interaction between B. longicaudatus inoculum levels and formulations of in vitro-produced Pasteuria spp. endospores were observed for B. longicaudatus final population density and percent endospore attachment. Therefore, the data for the formulation comparisons was pooled across inoculum levels (Table 1). A 10-fold increase in nematode inoculum increased (P ≤ 0.05) final population densities of B. longicaudatus per pot by 59% and 26%, respectively for trials 1 and 2 (Table 1). However, no difference was observed in percent endospore attachment between nematode inoculum levels during either trial. Granular and liquid formulations of in vitro-produced Pasteuria spp. endospores suppressed nematode population densities by 22% and 59% during trial one and 20% and 63% during trial two at 84-d after nematode inoculation, respectively compared to the nontreated controls (Table 1). The liquid formulation was more effective than the granular formulation reducing the B. longicaudatus final population density by an additional 37% and 43%, during trial 1 and 2, respectively (Table 1). Similarly, the liquid formulation was more effective than the granular formulation increasing percent endospore attachment by an additional 147% and 158%, during trial 1 and 2, respectively (Table 1).
Table 1.
Effect of inoculum level of Belonolaimus longicaudatus and formulations of in vitro-produced Pasteuria sp. on Belonolaimus longicaudatus final population densities and percent endospore attachment in pots planted with ‘Penncross’ creeping bentgrass and grown in a growth room for 84-days after nematode inoculation. Data were pooled across main effects because nematode inoculum level and formulation interaction was not significant (P≤ 0.05).
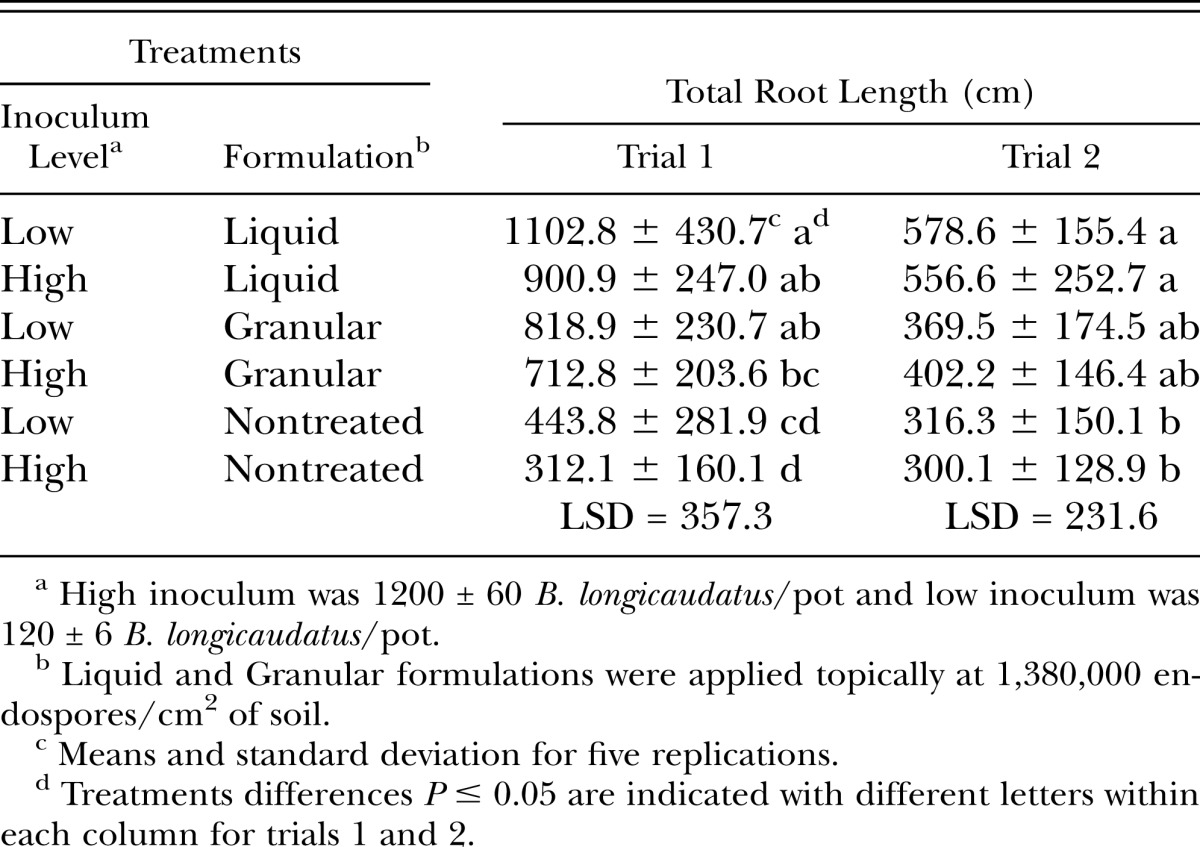
An interaction between B. longicaudatus inoculum levels and formulations of in vitro-produced Pasteuria spp. endospores was observed for total root lengths. The greatest root retention was observed with the combination of a low B. longicaudatus inoculum level and the application of a liquid formulation of in vitro-produced Pasteuria spp. endospores. Conversely, the greatest root losses were observed when a high inoculum level of B. longicaudatus was applied in the nontreated control (Table 2).
Table 2.
Interaction effect of inoculum level of Belonolaimus longicaudatus and formulation of in vitro-produced Pasteuria sp. endospores on total root length of ‘Penncross’ creeping bentgrass at 84-days after nematode inoculation.
Discussion
Increasing the B. longicaudatus inoculum levels increased B. longicaudatus densities per pot. However, increased B. longicaudatus densities did not increase the attachment rates of the in vitro-produced Pasteuria spp. endospores. Data suggest that while Belonolaimus longicaudatus and ‘Candidatus Pasteuria usgae’ form a density dependent relationship in natural soil environments (Giblin-Davis, 2000), the inundative application of in vitro-produced Pasteuria spp. endospores as a biopesticide reduces B. longicaudatus population densities equally at high or low nematode population densities. However, these experiments were not run sufficiently long to observe whether density dependent recycling (classical biological control) was indeed possible.
Both formulations of in vitro-produced Pasteuria spp. endospores suppressed nematode population densities compared with the nontreated control, indicating both formulations were effective. However, the liquid formulation was more effective at suppressing B. longicaudatus final population densities and exhibited increased percent endospore attachment compared with the granular formulation. Previous research has shown that 0.6 cm of irrigation (50 ml/pot) was sufficient to move a liquid formulation of in vitro-produced Pasteuria spp. endospores to a soil depth of 10 cm (Luc, unpublished). However, 0.6 cm of irrigation may not have been enough water to release a majority of the in vitro-produced Pasteuria spp. endospores from the clay substrate and then move them into the soil profile, resulting in reduced nematode suppression and decreased endospore attachment. Furthermore, the liquid formulation provided increased root abundance relative to the nontreated formulation in both trials.
In conclusion, these experiments indicate that B. longicaudatus levels in the soil at time of application does not affect efficacy of in vitro-produced Pasteuria spp. endospores to suppress B. longicaudatus in short term experiments. Furthermore, while the granular formulation reduced B. longicaudatus population densities, it was not as effective as the liquid formulation. Further research studying spore release rate from clay and quantifying the number of in vitro-produced Pasteuria spp. endospores/g soil in the turfgrass rhizosphere over time with increasing irrigation rates would be very helpful in predicting efficacy in the field. Inundative biopesticides using in vitro-produced Pasteuria spp. endospores may be an important component of integrated pest management for B. longicaudatus in the future.
Footnotes
A portion of the Doctor of Philosophy dissertation by the first author.
This paper was edited by Nancy Kokalis-Burelle
Literature Cited
- Anonymous. USGA recommendation for a method of putting green construction: The 1993 revision. USGA Green Section Record. 1993;31:1–3. [Google Scholar]
- Atibalentja N, Noel GR, Liao TF, Gertner GZ. Population changes in Heterodera glycines and Its Bacterial Parasite Pasteuria sp. in Naturally Infest Soil. Journal of Nematology. 1998;30(1):81–92. [PMC free article] [PubMed] [Google Scholar]
- Bauhus J, Messier C. Evaluation of fine root length and diameter measurements obtained using RHIZO image analysis. Agronomy Journal. 1999;91:142–147. [Google Scholar]
- Bekal S, Borneman J, Springer MS, Giblin-Davies RM, Becker JO. Phenotypic and molecular analysis of a Pasteuria strain parasitic to the sting nematode. Journal of Nematology. 2001;33:110–115. [PMC free article] [PubMed] [Google Scholar]
- Busey P, Giblin-Davis RM, Center BJ. Resistance in Stenotaphrum to the sting nematode. Crop Science. 1993;33:1066–1070. [Google Scholar]
- Chen ZX, Dickson DW, Hewlett TE. Quantification of endospore concentrations of Pasteuria penetrans in tomato root material. Journal of Nematology. 1996a;28:50–55. [PMC free article] [PubMed] [Google Scholar]
- Chen ZX, Dickson DW, McSorley R, Mitchell DJ, Hewlett TE. Suppression of Meloidogyne arenaria race 1 by soil application of endospores of Pasteuria penetrans. Journal of Nematology. 1996b;28:159–168. [PMC free article] [PubMed] [Google Scholar]
- Cobb NA. Washington D.C: Government Printing Office; 1918. Estimating the nema population of soil with special reference to the sugar beet and root gall nematodes Heterodera schachtii Schmidt and Heterodera radicicola (Greef) Müller, and with a description of Tylencholaimus aequalis n. sp. [Google Scholar]
- Ciancio A. Density-Dependant Parasitism of Xiphinema diversicaudatum by Pasteuria penetrans in a Naturally Infested Field. Phytopathology. 1995;85:144–149. [Google Scholar]
- Ciancio A, Quénéhervé P. Popularion dynamics of Meloidogyne incognita and infestion levels by Pasteuria penetrans in a naturally infested field in Martinique. Nematropica. 2000;30:77–86. [Google Scholar]
- Dube B, Smart GC., Jr Biocontrol of Meloidogyne incognita by Paecilomyces lilacinus and Pasteuria penetrans. Journal of Nematology. 1987;19:222–227. [PMC free article] [PubMed] [Google Scholar]
- Giblin-Davis RM. Pasteuria sp. for biological control of the sting nematode, Belonolaimus longicaudatus, in turfgrass. In: Clark JM, Kenna MP, editors. American Chemical Society Symposium Series No. 743: Fate and Management of Turfgrass Chemicals. New York: Oxford Press; 2000. pp. 408–426. [Google Scholar]
- Giblin-Davis RM, McDaniel LL, Bilz FG. Isolates of Pasteuria penetrans group from phytoparasitic nematodes in bermudagrass turf. Journal of Nematology. 1990;22:750–762. [PMC free article] [PubMed] [Google Scholar]
- Giblin-Davis RM, Cisar JL, Bilz FG, Williams KE. Host status of different bermudagrasses (Cynodon spp.) for the sting nematode, Belonolaimus longicaudatus. Journal of Nematology. 1992;24(4S):749–756. [PMC free article] [PubMed] [Google Scholar]
- Giblin-Davis RM, Williams DS, Bekal S, Dickson DW, Becker JO, Preston JF. ‘Candidatus Pasteuria usgae’ sp. nov., an obligate endoparasite of the phytoparasitic nematode, Belonolaimus longicaudatus. International Journal of systematic and evolutionary microbiology. 2003;53:197–200. doi: 10.1099/ijs.0.02292-0. [DOI] [PubMed] [Google Scholar]
- Jenkins WR. A rapid centrifugal-floatation technique for separating nematodes from soil. Plant Disease Reporter. 1964;48:692. [Google Scholar]
- Kariuki GM, Dickson DW. Transfer and development of Pasteuria penetrans. Journal of Nematology. 2007;39(1):55–61. [PMC free article] [PubMed] [Google Scholar]
- Luc JE, Crow WT, McSorley R, Giblin-Davis RM. Supression of Belonolaimus longicaudatus with in vitro-produced Pasteuria sp. Endospores. Nematropica. 2010;40((2)) In Press. [PMC free article] [PubMed] [Google Scholar]
- Orajay JI. (Dissertation) University of Florida: Gainesville,FL; 2009. Population dynamics of Pasteuria penetrans in a peanut field and description of a Pasteuria isolate infecting Mesocriconema ornata. [Google Scholar]
- Stirling GR, Wachtel MF. Mass production of Bacillus penetrans for the biological control of root-knot nematodes. Nematologica. 1980;26:308–312. [Google Scholar]
- Weibelzahl-Fulton E, Dickson DW, Whitty EB. Suppression of Meloidogyne incognita and M. javanica by Pasteuria penetrans in field soil. Journal of Nematology. 1996;28:43–49. [PMC free article] [PubMed] [Google Scholar]


