Abstract
For this report, we examined the toxic effects of three plant-derived isothiocyanate compounds on second-stage juveniles (J2) of Heterodera glycines. We found significant differences among compounds in the concentration required to affect nematodes, according to mortality and behavioral measurements. The concentrations required to affect behavior were significantly lower than those required for mortality. Both mortality and behavioral measurements were used to investigate whether nematodes in a quiescent state display decreased sensitivity to isothiocyanates compared with actively moving nematodes. Mortality measurements revealed that quiescent nematodes were significantly less sensitive to isothiocyanates than active nematodes. All behavioral measurements following exposure to benzyl- and phenyl isothiocyanate showed significant differences in sensitivity between quiescent and active nematodes. However, significant differences between quiescent and active nematodes were observed in only one of the five behavioral measurements following exposure to allyl isothiocyanate. These results expand the list of plant-derived compounds toxic to H. glycines and illustrate the impact of behavioral quiescence on nematode sensitivity to exogenous toxins.
Keywords: soybean cyst nematode, behavior, toxicology, biofumigation, quiescence, chemical penetration, uptake, glucosinolate
Plant-derived isothiocyanates are lethal to nematodes and are used in biofumigation to manage soil-borne pathogens and pests including nematodes (Chitwood, 2002). Isothiocyanates (-ITC) are formed by catalysis of glucosinolates produced by plants in the Brassicaceae family by the enzyme myrosinase (Lazzeri et al., 2004). Although researchers have tested a range of –ITC compounds on various plant-parasitic nematodes (Lazzeri et al., 1993; Potter et al., 1998; Buskov et al., 2002; Lazzeri et al., 2004; Zasada and Ferris, 2004; Zasada et al., 2009), little is known of the effects of many of these compounds on the infective stage of the soybean cyst nematode, Heterodera glycines. To date, only the effect of allyl isothiocyanate (AITC) on H. glycines mortality has been tested (Yu et al., 2005; Yu et al., 2007; Schroeder and MacGuidwin, 2010).
The traditional measurement of toxicity is the concentration of compound required to kill 50% of the population (LD50). However, sublethal concentrations of a particular toxin may impair activities crucial to an organism's life cycle. These activities are best measured through behavioral assays. For example, researchers determined the ability of plant-parasitic nematodes to migrate through soil columns or infect roots following exposure to potential toxins (Hough and Thomason, 1975; Pree et al., 1989; Gourd et al., 1993; Ibrahim and Haydock, 1999; Zasada and Ferris, 2003). Although these studies may mimic the natural environment, they did not distinguish mortality from altered behavior. Toxins disrupt the movement of nematodes on agar or in solution as measured by the distance traveled and the dimensions of body bends during movement (Nelmes, 1970; Hewlett et al., 1997; Ibrahim and Haydock, 1999; Wuyts et al., 2006; Zasada et al., 2009). Advantages of in vitro assays also include the ability to move nematodes away from the toxin to study brief exposure times or potential recovery following toxin exposure (Faske and Starr, 2006). Quiescent nematodes are less sensitive than actively moving nematodes to exogenously applied toxins, likely due to enhanced exclusion of the toxin from the nematode's body (Freckman et al., 1980; Schroeder and MacGuidwin, 2010). Because quiescence occurs in response to a wide range of triggers (Van Gundy, 1965; Croll, 1970) and affects survival to toxins, it is beneficial to use nematodes in both active and quiescent states in toxicity assays.
We had three objectives for this research: 1) to characterize the lethal concentrations of benzyl (BITC), and phenyl isothiocyanates (PITC) to second-stage juvenile (J2) H. glycines; 2) to compare behavioral measurements to mortality for assessing the sensitivity of J2 H. glycines to AITC, BITC, and PITC; and 3) to examine differences in sensitivity to AITC, BITC, and PITC between quiescent and active nematodes with both mortality and behavioral measurements. By refining the concentrations of isothiocyanates needed to inhibit both active and quiescent H. glycines, this research has practical implications for the control of an important plant-parasitic nematode by means of plant-derived compounds.
Materials and Methods
Nematode inoculum: H. glycines collected from a soybean field in southeastern Wisconsin were maintained in a growth chamber on susceptible soybean cv. McCall at 28°C and 12 hr photoperiod. Cysts were extracted from soil with a procedure modified from Jenkins (1964). H. glycines J2 were obtained from eggs incubated on 25-μm pore filters (Sefar American Inc., Depew, NY) in 3 mM ZnCl2 to induce hatch and the J2 were used within 1 week after hatch (Wong et al., 1993). To ensure activity, only nematodes that moved through a Baermann funnel immediately prior to the experiment were used.
Mortality study: Mortality assays were done as previously described using nematodes in either an active or quiescent state (Schroeder and MacGuidwin, 2010). Prior to chemical exposure, active J2 (40±4 per treatment) were incubated for 3 hours at room temperature to maintain activity or on ice to induce quiescence. Previous results demonstrated that quiescent nematodes induced by temperature are similar in their reaction to chemicals as quiescent nematodes from other sources (Schroeder and MacGuidwin, 2010) A dilution series of -ITCs were made in methanol and subsequently dissolved in 5 ml 1.5% agarose in glass Petri dishes (6 x 1.5-cm). Control dishes contained 1% methanol in 1.5% agarose. All chemicals were purchased commercially (Sigma Chemical Co., St. Louis, MO) and ≥95% purity. Quiescent nematodes were transferred to the -ITC plates stored at 4°C while active nematodes were transferred to -ITC plates at room temperature. After a 3 hour exposure to -ITC, nematodes were transferred to a 25-μm pore sieve and rinsed thoroughly with water. Nematodes were allowed to recover overnight following exposure to -ITC. The following day, nematodes were observed for movement under a stereomicroscope by a researcher blind to the treatment identity. Nematodes were considered alive if they moved spontaneously or after being mechanically stimulated with a thin wire. Each -ITC was tested at least twice. Data were subjected to probit analysis to determine the LD50 and LD95 with 95% confidence intervals. The z-value was used for comparisons of active and quiescent nematode probit curves. All statistical analyses were accomplished with Minitab Statistical Software (Minitab Inc., State College, PA).
Behavior assay: Quiescent and active nematodes (18±1 per treatment) were exposed to -ITC plates, as described for the mortality assays. Following exposure, washing, and randomization, nematodes were allowed to recover overnight. Each nematode was then placed on the center of its own (6 x 1.5-cm) Petri dish containing 5 ml of 1.5% agarose. Dishes were stored covered at room temperature (20 to 22°C) for 1 hour. Nematodes were removed and the agarose was examined for tracks.
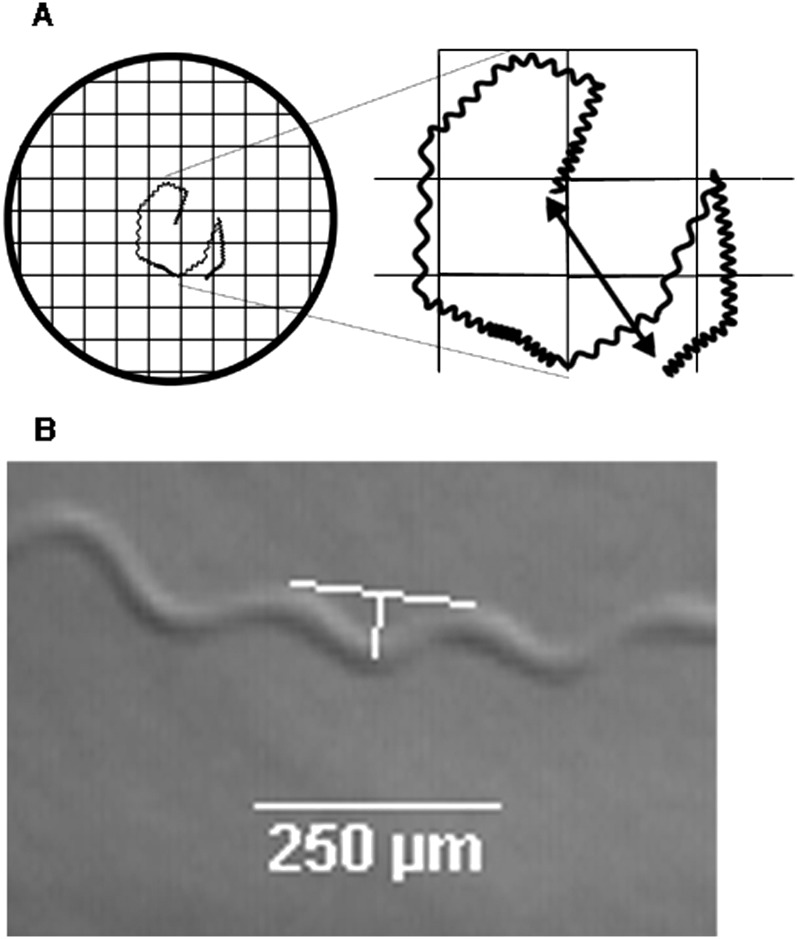
Nematode tracks were examined for five different parameters: evidence of any movement, net distance traveled, quadrats traveled, and wavelength and amplitude of the sinusoidal tracks. All measurements were collected by a researcher blind to the treatment identity. The presence of movement was assessed as a binary function of whether any tracks were produced and analyzed by probit analysis to determine the EC50 and EC95 (the concentrations required to stop movement in 50 and 95% of the test group, respectively). The z-value was used for comparisons of probit curves for active and quiescent nematodes. Net distance was measured as the distance from the start to stop location (Fig. 1a). Quadrats were measured by examining plates with a grid consisting of 1x1 mm squares (Fig. 1a) and counting the number of quadrats that contained nematode tracks. Preliminary data demonstrated this quadrat method to be highly correlated with the total distance traveled using a trace method of tracks with image analysis software (Pearson's correlation coefficient = 0.901, P<0.001). The net distance and quadrat data were transformed by log(x+1) prior to analysis to correct for unequal variance. The wavelength and amplitude of the sinusoidal tracks were calculated from images captured of all tracks on each plate at x35 magnification using a stereomicroscope with attached SONY-CCD camera and Studio v.9 software (Pinnacle Systems Inc., Mountain View, CA). To convert pixels to distance, calibration images of known distances were captured. Track waveforms were subsequently analyzed with WCIF- ImageJ software version 1.37 (http://www.uhnres.utoronto.ca/facilities/wcif/imagej/) (Fig. 1b). Multiple waveforms of the sinusoidal tracks (15 ± 0.3) were examined for each nematode. Nematodes that did not move were counted as zero and included in the analysis. General linear models were fit to the data and ANOVA used to compare treatment effects. Each -ITC was tested at least twice.
Fig. 1.
Methodology for behavioral measurements. (A) Cartoon of sample tracks on a Petri dish with grid consisting of 1x1 mm quadrats (left). The number of quadrats with tracks were counted. Enlarged image of tracks with net distance illustrated by double-headed arrow (right). (B) Sample of tracks with drawing (white bars) of wavelength and amplitude measurements. Wavelength was measured as the distance separating the peaks of one waveform. The amplitude was measured as the distance from the trough of one waveform drawn perpendicular to the wavelength line.
Results
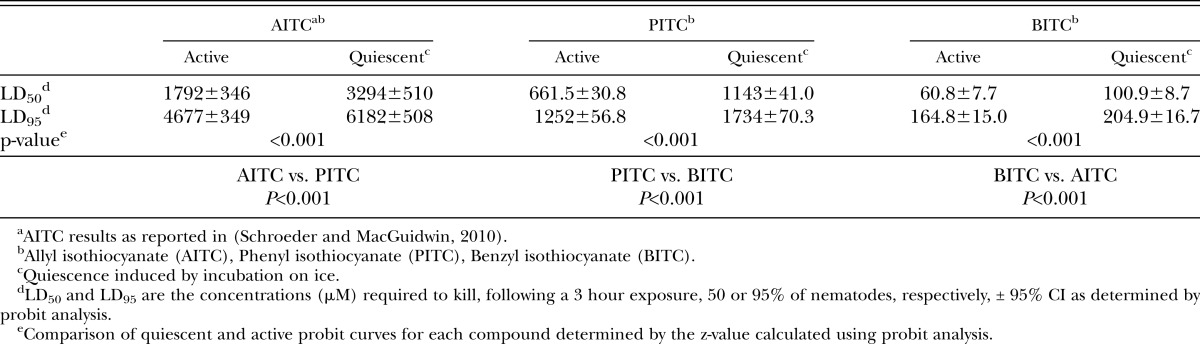
There were significant differences among compounds with mortality as the criterion for toxicity. Benzyl isothiocyanate showed the greatest toxicity followed by PITC and AITC (Table 1). At lethal doses of all -ITCs, nematodes were found in a straight posture, often with obvious disruption of internal structure. At non-lethal concentrations of PITC, but not BITC or AITC, nematodes were often found in a coiled posture.
Table 1.
Concentration of isothiocyanates (μM) necessary for mortality in J2 H. glycines as determined by probit analysis
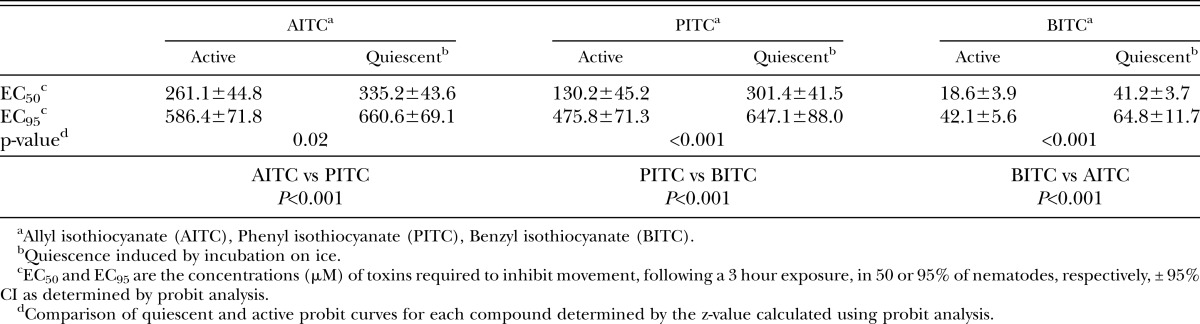
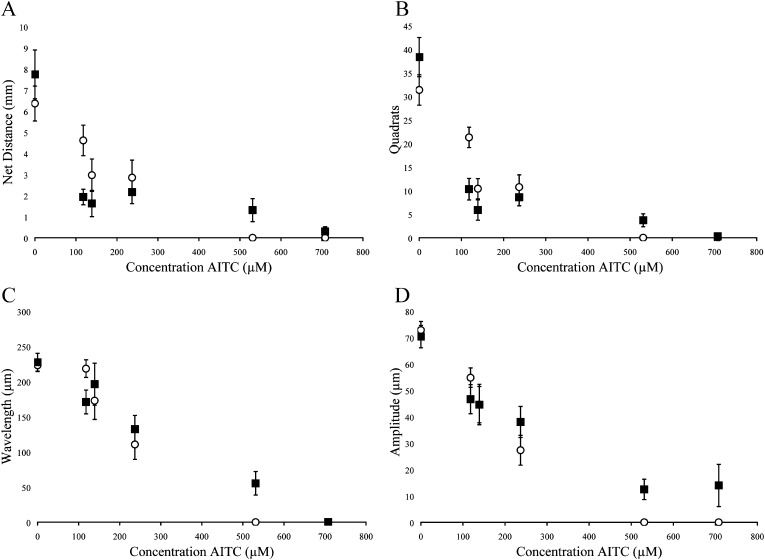
Concentrations necessary to modify normal motility were consistently lower than concentrations necessary to kill nematodes. The LD50 for active nematodes was approximately 7-, 5-, and 3-fold greater than the EC50 for AITC, PITC, and BITC, respectively (Table 1,2). Similar to the mortality data, the presence of movement behavioral data showed the compounds differed significantly in toxicity with BITC as the most effective followed by PITC and AITC (Table 2). The criteria of net distance traveled, quadrats traveled, and wavelength and amplitude of the sinusoidal tracks also showed decreasing values with increasing -ITC concentrations (Fig. 2-4; Table 3). At high concentrations of –ITCs much of the decrease seen in these behavioral measurements was due to the absence of any movement in nematodes.
Table 2.
Concentration of isothiocyanate (μM) required to inhibit spontaneous movement in J2 H. glycines as determined by the presence of tracks on agarose
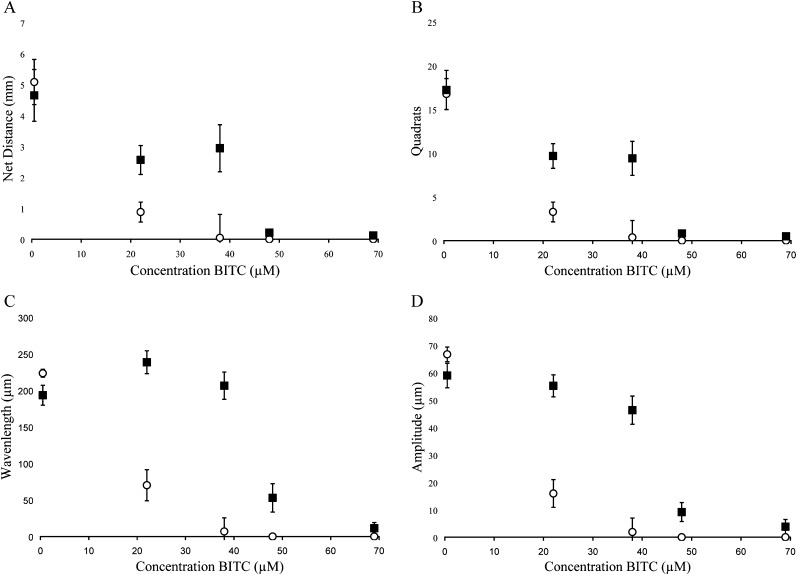
Fig. 2.
Behavioral measurements following exposure of quiescent (▪) and active (○) J2 H. glycines to allyl isothiocyanate (AITC). Data were recorded by examining tracks for (A) net distance, (B) quadrats traveled, (C) wavelength of sinusoidal tracks, (D) amplitude of sinusoidal tracks. Each point represents the mean ± SEM for pooled data representing 36±4 nematodes. Statistical analysis is presented in Table 3.
Fig. 3.
Behavioral measurements following exposure of quiescent (▪) and active (○) J2 H. glycines to benzyl isothiocyanate (BITC). Data were recorded by examining tracks for (A) net distance, (B) quadrats traveled, (C) wavelength of sinusoidal tracks, (D) amplitude of sinusoidal tracks. Each point represents the mean ±SEM for pooled data representing 32 nematodes. Statistical analysis is presented in Table 3.
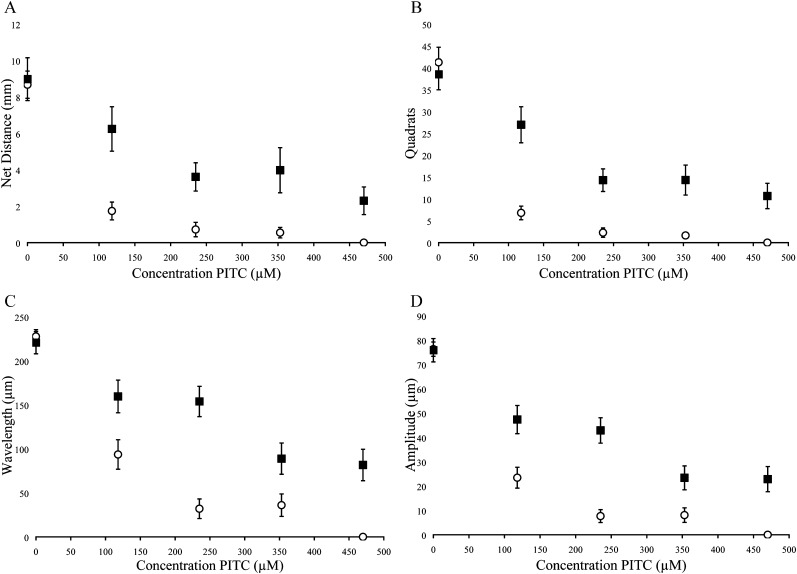
Fig. 4.
Behavioral measurements following exposure of quiescent (▪) and active (○) J2 H. glycines to phenyl isothiocyanate (PITC). Data were recorded by examining tracks for (A) net distance, (B) quadrats traveled, (C) wavelength of sinusoidal tracks, (D) amplitude of sinusoidal tracks. Each point represents the mean ± SEM for pooled data representing 40 nematodes. Statistical analysis is presented in Table 3.
Table 3.
Statistical summary of general linear models with ANOVA for behavioral data following exposure of J2 H. glycines to -ITC toxins as presented in figures 2-4.
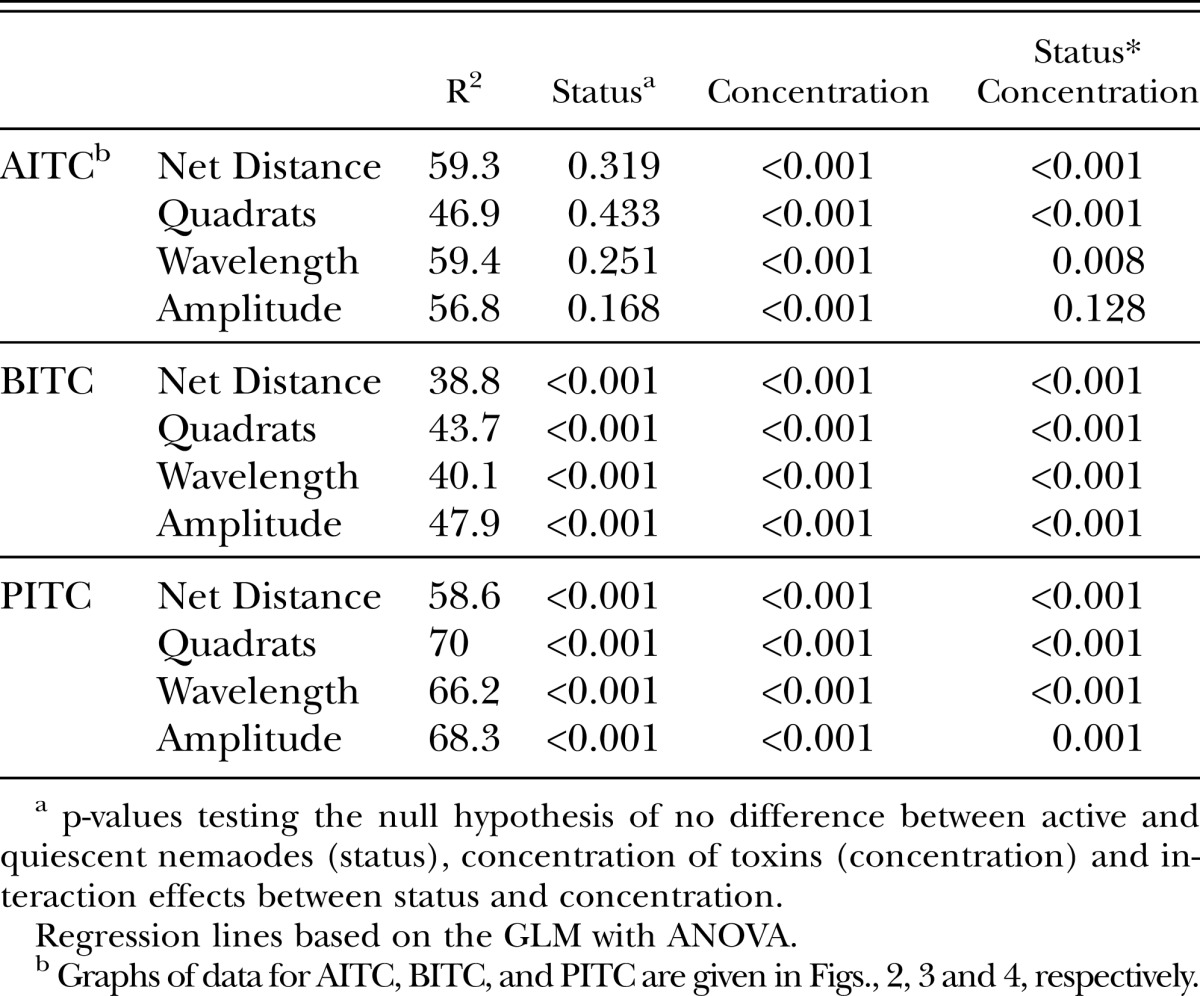
Quiescent nematodes required 84, 73 and 66% greater concentrations of AITC, PITC and BITC, respectively, to kill 50% of the test group compared with active nematodes (Table 1). Differences in sensitivity to -ITCs were also found between active and quiescent nematodes according to behavioral measurements (Figs. 2-4 and Tables 2,3). Active and quiescent nematodes differed in their sensitivity to BITC and PITC concentrations regardless of the criteria used to ascertain toxicity (Tables 1-3). For the AITC behavioral data, only the binary presence of movement measurement showed a significant difference between active and quiescent nematodes (P=0.02) (Tables 2,3; Fig. 2). Significant interaction effects between concentration and status (active vs. quiescent) were found for the majority of the continuous behavioral data for all of the chemicals tested (Table 3).
Discussion
Motility is a sensitive and useful measure for studying the response of J2 H. glycines to toxins. Behavioral assays, used to assess toxin efficacy for microbivorous (Opperman and Chang, 1991; Dhawan et al., 1999), animal- (Ishibashi and Takii, 1993; Patel and Wright, 1996), and plant-parasitic (Nelmes, 1970; Hough and Thomason, 1975; Ibrahim and Haydock, 1999) nematodes, have the advantage of identifying multiple responses in addition to morbidity. Motility is essential for infection, mating, and other life sustaining activities so it is a relevant proxy for evaluating the effects of toxins. A motility assay was similar to a reproductive assay for evaluating the sensitivity of C. elegans to ethanol (Dhawan et al., 1999), supporting the utility of behavioral metrics for identifying compounds likely to reduce nematode population densities.
As expected we found that a higher concentration of -ITCs was required to kill rather than alter behavior of J2 H. glycines. Recent work has examined the effect of BITC on the behavior of Meloidogyne incognita (Zasada et al., 2009). The concentration of BITC needed to affect H. glycines movement was similar to their results. It is likely that if we had assessed behavior during exposure, there would be an even greater difference in concentrations required for mortality versus alterations in nematode behavior. All behavioral measurements for a given compound resulted in similar estimates of –ITC concentrations needed to achieve the EC50. Given this agreement, the presence of movement, net distance, and quadrats were the easiest and most straightforward methods for measuring behavior.
We were interested in testing whether differences in sensitivity are found between active and quiescent nematodes as previously described for AITC (Schroeder and MacGuidwin, 2010) and if the behavioral measurements provided additional information to describe toxin encounters. Both mortality and behavioral measurements showed altered sensitivity to toxins between quiescent and active nematodes. Nematodes that were active immediately before exposure were completely immobilized at concentrations of BITC and PITC that only impaired the motility of nematodes that were quiescent when exposure began. The enhanced performance of biofumigation in warm moist soils (Ploeg and Stapleton, 2001; Oka, 2010) might occur, in part, because these conditions encourage nematode activity. The lack of a relationship between the activity status of nematodes at the time of exposure and sensitivity to AITC using behavioral measurements is also interesting and indicates the importance of including behavioral measures in toxicity assays designed to compare compounds for their effects under field conditions.
Previous results demonstrated that quiescence was correlated with reduced exogenous chemical penetration of J2 H. glycines, suggesting a mechanism for the decreased sensitivity to toxins shown by quiescent nematodes (Schroeder and MacGuidwin, 2010). One factor affecting the penetration and bioavailability of compounds is hydrophobicity (Castro and Thomason, 1973; Thompson et al., 1993). The octanol-water partition coefficient (log-P) is a standard measurement of hydrophobicity. Based on computer modeling of partition coefficients, BITC and PITC are 6 and 12 times more hydrophobic than AITC, respectively (log-P= 2.21(AITC), 3.01(BITC), 3.30(PITC)). This difference in hydrophobicity may account for the differences seen between compounds. However, further testing with additional compounds would be required to confirm this hypothesis. An additional factor affecting results is the mode of induction for quiescence. We previously showed that quiescent nematodes induced by temperature react similarly to quiescent nematodes induced by CO2 (Schroeder and MacGuidwin, 2010). However, we cannot completely rule out the direct effect of temperature on chemical dynamics.
Our study expanded the list of –ITCs characterized for toxicity to H. glycines and confirmed studies showing differential sensitivity of nematodes to specific –ITCs (Zasada and Ferris, 2003). Our data showing BITC to be more toxic than AITC are in agreement with these authors. In contrast, we found PITC also to be more toxic than AITC. The difference in results may reflect species-specific reactions as noted by Zasada and Ferris (2003) comparing the response of Tylenchulus semipenetrans and Meloidogyne incognita to –ITCs or by Faske and Starr (2006) comparing the response of Meloigodyne incognita and Rotylenchulus reniformis to abamectin. Our study showed a higher LD50 for H. glycines exposed to AITC than reported by Yu et al. (2005), but our shorter exposure time most likely accounts for the discrepancy.
Plant-parasitic nematodes must not only survive a chemically adverse environment, they must be competent for all activities essential to perpetuate the population. Inhibition of any essential behaviors due to toxins will prevent nematode development. Our study shows the importance of characterizing a range of measurements that reflect interruption of a nematodes life-cycle. Our research has expanded the list of compounds toxic to J2 H. glycines. Our behavioral analysis refines the minimum concentrations needed to affect the nematode. It has also illustrated the important role quiescence plays in the sensitivity of nematodes to toxins.
Literature Cited
- Buskov S, Serra B, Rosa E, Sorensen H, Sorensen JC. Effects of intact glucosinolates and products produced from glucosinolates in myrosinase-catalyzed hydrolysis on the potato cyst nematode (Globodera rostochiensis Cv. Woll) Journal of Agricultural and Food Chemistry. 2002;50:690–695. doi: 10.1021/jf010470s. [DOI] [PubMed] [Google Scholar]
- Castro CE, Thomason IJ. Permeation dynamics and osmoregulation in. Aphelenchus avenae. 1973;19:100–108. [Google Scholar]
- Chitwood DJ. Phytochemical based strategies for nematode control. Annual Review of Phytopathology. 2002;40:221–249. doi: 10.1146/annurev.phyto.40.032602.130045. [DOI] [PubMed] [Google Scholar]
- Croll NA. The behaviour of nematodes. Edward Arnold: London; 1970. [Google Scholar]
- Dhawan R, Dusenbery DB, Williams PL. Comparison of lethality, reproduction, and behavior as toxicological endpoints in the nematode Caenorhabditis elegans. Journal of Toxicology and Environmental Health. Part A. 1999;58:451–462. doi: 10.1080/009841099157179. [DOI] [PubMed] [Google Scholar]
- Faske TR, Starr JL. Sensitivity of Meloidogyne incognita and Rotylenchulus reniformis to Abamectin. Journal of Nematology. 2006;38:240–244. [PMC free article] [PubMed] [Google Scholar]
- Freckman DW, Demeure Y, Munnecke D, Van Gundy SD. Resistance of anhydrobiotic Aphelenchus avenae to methyl bromide fumigation. Journal of Nematology. 1980;12:19–22. [PMC free article] [PubMed] [Google Scholar]
- Gourd TR, Schmitt DP, Barker KR. Differential sensitivity of Meloidogyne spp. and Heterodera glycines to selected nematicides. Journal of Nematology. 1993;25:746–751. [PMC free article] [PubMed] [Google Scholar]
- Hewlett TE, Hewlett EM, Dickson DW. Response of Meloidogyne spp., Heterodera glycines, and Radopholus similis to tannic acid. Journal of Nematology. 1997;29:737–741. [PMC free article] [PubMed] [Google Scholar]
- Hough A, Thomason IJ. Effects of aldicarb on the behavior of Heterodera schachtii and Meloidogyne javanica. Journal of Nematology. 1975;7:221–229. [PMC free article] [PubMed] [Google Scholar]
- Ibrahim SK, Haydock PP. Cadusafos inhibits hatching, invasion, and movement of the Potato Cyst Nematode Globodera pallida. Journal of Nematology. 1999;31:201–206. [PMC free article] [PubMed] [Google Scholar]
- Ishibashi N, Takii S. Effects of insecticides on movement, nictation, and infectivity of Steinernema carpocapsae. Journal of Nematology. 1993;25:204–213. [PMC free article] [PubMed] [Google Scholar]
- Jenkins WR, A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Disease. 1964;48:692. [Google Scholar]
- Lazzeri L, Curto G, Leoni O, Dallavalle E. Effects of glucosinolates and their enzymatic hydrolysis products via myrosinase on the root-knot nematode Meloidogyne incognita (Kofoid et White) Chitw. Journal of Agricultural and Food Chemistry. 2004;52:6703–6707. doi: 10.1021/jf030776u. [DOI] [PubMed] [Google Scholar]
- Lazzeri L, Tacconi R, Palmieri S. Invitro activity of some glucosinolates and their reaction-products toward a population of the nematode Heterodera schachtii. Journal of Agricultural and Food Chemistry. 1993;41:825–829. [Google Scholar]
- Nelmes AJ. Behavioral responses of Heterodera rostochiensis larvae to aldicarb and its sulfoxide and sulfone. Journal of Nematology. 1970;2:223–227. [PMC free article] [PubMed] [Google Scholar]
- Oka Y. Mechanisms of nematode suppression by organic soil amendments - A review. Applied Soil Ecology. 2010;44:101–115. [Google Scholar]
- Opperman CH, Chang S. Effects of aldicarb and fenamiphos on acetylcholinesterase and motility of Caenorhabditis elegans. Journal of Nematology. 1991;23:20–27. [PMC free article] [PubMed] [Google Scholar]
- Patel MN, Wright DJ. The influence of neuroactive pesticides on the behaviour of entomopathogenic nematodes. Journal of Helminthology. 1996;70:53–61. [Google Scholar]
- Ploeg AT, Stapleton JJ. Glasshouse studies on the effects of time, temperature and amendment of soil with broccoli plant residues on the infestation of melon plants by Meloidogyne incognita and M. javanica. Nematology. 2001;3:855–861. [Google Scholar]
- Potter MJ, Davies K, Rathjen AJ. Suppressive impact of glucosinolates in Brassica vegetative tissues on root lesion nematode Pratylenchus neglectus. Journal of Chemical Ecology. 1998;24:67–80. [Google Scholar]
- Pree DJ, Townshend JL, Archibald DE. Carbamate and organophosphorus nematicides: Acetylcholinesterase inhibition and effects on dispersal. Journal of Nematology. 1989;21:483–489. [PMC free article] [PubMed] [Google Scholar]
- Schroeder NE, MacGuidwin AE. Behavioral quiescence reduces the penetration and toxicity of exogenous compounds in second-stage juveniles of Heterodera glycines. Nematology. 2010;12:277–287. [Google Scholar]
- Thompson DP, Ho NF, Sims SM, Geary TG. Mechanistic approaches to quantitate anthelmintic absorption by gastrointestinal nematodes. Parasitology Today (Personal ed.) 1993;9:31–35. doi: 10.1016/0169-4758(93)90162-9. [DOI] [PubMed] [Google Scholar]
- Van Gundy SD, Factors in Survival of Nematodes. Annu.Rev.Phytopathol. 1965;3:46–68. [Google Scholar]
- Wong AT, Tylka GL, Hartzler RG. Effects of eight herbicides on in vitro hatching of Heterodera glycines. Journal of Nematology. 1993;25:578–584. [PMC free article] [PubMed] [Google Scholar]
- Wuyts N, Swennen R, De Waele D. Effects of plant phenylpropanoid pathway products and selected terpenoids and alkaloids on the behaviour of the plant-parasitic nematodes Radopholus similis, Pratylenchus penetrans and Meloidogyne incognita. Nematology. 2006;8:89–101. [Google Scholar]
- Yu Q, Tsao R, Chiba M, Potter J. Selective nematicidal activity of allyl isothiocyanate. Journal of Food Agriculture and Environment. 2005;3:218–221. [Google Scholar]
- Yu Q, Tsao R, Chiba M, Potter J. Elucidation of the nematicidal activity of bran and seed meal of oriental mustard (Brassica juncea) under controlled conditions. Journal of Food Agriculture & Environment. 2007;5:374–379. [Google Scholar]
- Zasada IA, Ferris H. Sensitivity of Meloidogyne javanica and Tylenchulus semipenetrans to isothiocyanates in laboratory assays. Phytopathology. 2003;93:747–750. doi: 10.1094/PHYTO.2003.93.6.747. [DOI] [PubMed] [Google Scholar]
- Zasada IA, Ferris H. Nematode suppression with brassicaceous amendments: application based upon glucosinolate profiles. Soil Biology & Biochemistry. 2004;36:1017–1024. [Google Scholar]
- Zasada IA, Masler EP, Rogers ST, Halbrendt JM. Behavioral response of Meloidogyne incognita to benzyl isothiocyanate. Nematology. 2009;11:603–610. [Google Scholar]