Abstract
Rotylenchulus reniformis is one of 10 described species of reniform nematodes and is considered the most economically significant pest within the genus, parasitizing a variety of important agricultural crops. Rotylenchulus reniformis collected from cotton fields in the Southeastern US were observed to have the nematode parasitic bacterium Pasteuria attached to their cuticles. Challenge with a Pasteuria-specific monoclonal antibody in live immuno-fluorescent assay (IFA) confirmed the discovery of Pasteuria infecting R. reniformis. Scanning and transmission electron microscopy were employed to observe endospore ultrastructure and sporogenesis within the host. Pasteuria were observed to infect and complete their life-cycle in juvenile, male and female R. reniformis. Molecular analysis using Pasteuria species-specific and degenerate primers for 16s rRNA and spoII, and subsequent phylogenetic assessment, placed the Pasteuria associated with R. reniformis in a distinct clade within established assemblages for the Pasteuria infecting phytopathogenic nematodes. A global phylogenetic assessment of Pasteuria 16s rDNA using the Neighbor-Joining method resulted in a clear branch with 100% boot-strap support that effectively partitioned the Pasteuria infecting phytopathogenic nematodes from the Pasteuria associated with bacterivorous nematodes. Phylogenetic analysis of the R. reniformis Pasteuria and Pasteuria spp. parasitizing a number of economically important plant parasitic nematodes revealed that Pasteuria with different host specificities are closely related and likely constitute biotypes of the same species. This suggests host preference, and thus effective differentiation and classification are most likely predicated by an influential virulence determinant(s) that has yet to be elucidated. Pasteuria Pr3 endospores produced by in vitro fermentation demonstrated efficacy as a commercial bionematicide to control R. reniformis on cotton in pot tests, when applied as a seed treatment and in a granular formulation. Population control was comparable to a seed-applied nematicide/insecticide (thiodicarb/imidacloprid) at a seed coating application rate of 1.0 x 108 spores/seed.
Keywords: biological control, cotton, reniform nematode, endospore, Gossypium hirsutum, molecular biology, morphometrics, Pasteuria spp., phylogenetics, Rotylenchulus reniformis, ultrastructure
Rotylenchulus reniformis (Linford and Olivera, 1940) is one of 10 described species of reniform nematodes and is the most economically significant, having the largest geographical distribution (Robinson et al., 1997). Rotylenchulus reniformis is a semi-endoparasitic nematode occurring in tropical and subtropical regions, where it parasitizes a wide variety of crops, including cotton, vegetable crops, and several tropical fruit species, including pineapple (McSorley 1980; McSorley et al. 1981, 1982; Robinson et al., 1997; Wang and Hooks, 2009). Rotylenchulus reniformis is an important nematode pest of cotton (Gossypium hirsutum L). in the southern United States, supplanting Southern root-knot nematode (Meloidogyne incognita) as the major nematode pest of cotton in Mississippi, Louisiana, and Alabama (Heald and Robinson 1990; Koenning et al., 2004). Losses in annual United States cotton production attributed to reniform nematode parasitism are estimated to exceed $100M (Blasingame, 2006; Robinson et al., 2008), with losses attributed primarily to a delay in fruit set and reduction in nutrient uptake (Robinson et al., 2005). Means commonly employed to mitigate crop damage caused by reniform nematodes include crop rotation and applying soil fumigants.
Pasteuria spp. are today recognized as ubiquitous soil-borne, Gram-positive, endospore-forming bacterial endoparasites of numerous plant parasitic nematodes. Pasteuria ramosa first described by Metchnikoff (1888), as a parasite of water fleas (Cladocera; Daphinidae), and named for his mentor Louis Pasteur, represents the type species for the genus (Sayre et al., 1979). Sayre and Wergin (1977), were the first to establish the relationship between P. ramosa (Ebert et al., 1996), and a bacterial parasite of the root-knot nematode Meloidogyne sp. (Pasteuria penetrans). Pasteuria ssp. are benign to soil microflora and vertebrates but have reported associations with more than 100 genera, comprising 323 species of soilborne nematodes worldwide, including many economically important phytopathogenic nematodes (Chen and Dickson, 1998).
Pasteuria spp. occur in nature as obligate parasites, relying upon exponential growth and sporulation within the body cavity of a host nematode. Infection and proliferation of Pasteuria spp. are universal in all nematode hosts. The life cycle is characterized by initial recognition and attachment of the endospore to the host cuticle, endospore germination, and infection through development of a ‘penetration tube’. Subsequent vegetative growth and proliferation within the host pseudocoelom proceeds through development of mycelial-like microcolonies. The onset of sporulation is observed by the formation of tetrad and diad clusters of cells, followed by completion of differentiation into infective saucer-shaped endospores enveloped by a labile exosporium. The genetic basis of sporogenesis in Pasteuria spp. mirrors the well described process for the closely related genus Bacillus (Doi, 1989).
Effectively modulating host populations via an unknown mechanism resulting in loss of fecundity (sterility), Pasteuria spp. play an ecological role as a morbus agent and a castrating parasite. Disease presents universally, but differs in growth stage and sex of the members of the population that are infective amongst the ectoparasitic and endoparasitic nematodes. Disease manifests in the death of free-living juveniles, presumably, as a result of spore encumbrance and proliferation, and induces a loss of fecundity in mature individuals. The epidemiological model for Pasteuria spp., as studied in the cladoceran parasite P. ramosa, shows that infection largely follows the law of mass action, where the infection rate is linearly correlated with the densities of the host and the parasite (Regoes et al., 2003). Ciancio (1995), reported a temporal, density-dependent relationship between Pasteuria spp. and Xiphinema diversicaudatum occurring in naturally infested fields, supporting the concept of applying Pasteuria spp. as an amendment to the soil ecosystem to dramatically reduce nematode host populations. Numerous studies have established the causal effect of Pasteuria spp. in reducing plant parasitic nematode populations and increasing crop yields (Mankau, 1980; Bird and Brisbane, 1988; Sayre and Starr, 1988; Ciancio, 1995; Chen et al., 1996; Weibelzahl-Fulton et al., 1996; Chen et al., 1997; Chen and Dickson, 1998; Kariuki and Dickson, 2007).
Not surprisingly, Pasteuria spp. have gained prominence as natural biocontrol agents for plant parasitic nematodes. Pasteuria spp. have been rigorously described for the following significantly destructive phytopathogenic nematode pests: Pasteuria penetrans on Meloidogyne spp. (root-knot nematodes) (Sayre and Starr, 1985); Pasteuria nishizawae on Heterodera spp. and Globodera spp. (cyst nematodes) (Sayre et al., 1991); ‘Candidatus Pasteuria usage' on Belonolaimus longicaudatus (sting nematode) (Giblin-Davis et al., 2003); and Pasteuria thornei on Pratylenchus spp. (lesion nematodes) (Starr and Sayre, 1988). Host specificity of spore attachment varies in robustness within Pasteuria spp. populations, ranging from cross-genera to race-specific (Chen and Dickson, 1998). However, for many reported cases of multiple host affinities, development within the host was either not observed, or was not pursued. This suggests the existence of some degree of plasticity in host recognition, and the presence of mechanisms aside from host recognition and attachment promulgate infection. Therefore, it is necessary to evaluate development within the host as a requisite to assigning host parasite relationships with new species, strains ‘biotypes” of Pasteuria.
We introduce and describe a newly isolated Pasteuria spp. parasitizing Rotylenchulus reniformis, collected from cotton fields in and around Huxford, AL, and Quincy, FL. Here we examine endospore morphometrics, ultrastructure, development, molecular genetics, and the biocontrol capabilities of this important new biocontrol agent of reniform nematodes.
Materials and Methods
Pasteuria sp. isolates and cultivation: Rotylenchulus reniformis infected with Pasteuria spp. was obtained from soil collected at a farm near Huxford, AL (Pasteuria spp. isolate Pr3) and from Quincy, FL (isolate Pr4) (gift from Jimmy Rich; North Florida Research and Education Center, University of Florida, IFAS). Spore-filled specimens were extracted from the soil using the sugar flotation centrifugation technique (Jenkins, 1964). Infected juveniles, males and females were observed microscopically at 600X and collected from a counting dish using a glass pipette. Infection with in vivo or in vitro (produced in fermentation by Pasteuria Bioscience, Inc.) endospores was initiated by comingling nematodes and endospores in water with attachment aided by centrifugation (Hewlett and Dickson, 1993). Infected nematodes where cultivated in a greenhouse on cotton (Gossypium hirsutum cv. Deltapine 444 BG RR).
DNA isolation: Endospore genomic DNA was extracted from Pasteuria spp. infected nematode cadavers using a modification of the QIAamp DNA stool mini kit (Qiagen cat # 51504). The spore pellet containing ∼1.0 M spores was washed once in water and suspended in 49 μl of TE buffer pH 8.0. To this was added 1 μl of 10 mg/ml Lysozyme (Sigma-Aldrich, St. Louis, MO), the sample was vortexed and incubated at 37 oC for 30 min with intermittent vortexing. ASL lysis buffer was added to the sample and the sample was heated at 95 oC for 5 min, and vortexed for 15 sec. The sample was then subjected to two freeze/thaw cycles by immersion in an ethanol/dry ice bath and a 65 oC water bath prior to continuing with the manufacturer's protocol. gDNA extracts were subjected to whole genome amplification using Φ29 DNA polymerase multiple-strand displacement amplification (MDA) (Genomiphi V2, GE Healthcare, Piscataway, NJ) (Nong et al., 2007). MDA products were utilized in downstream applications to increase the concentration of DNA template, for example in PCR of single copy genes.
Gene amplification: PCR was performed using Easy-A High Fidelity PCR Cloning Enzyme (Stratagene - Agilent Technologies, Santa Clara, CA). Degenerate and non-generate primers were used to obtain the following partial coding sequence (cds) of Pasteuria spp. genes: 16s rDNA gene, LMS_F39-GCGGCGTGCCTAMTACA (modified from Atibalentja et al., 2000), and LMS_R1239-CGACTTCGCYTCCCTCTGTAACGG (this study); and spoIIAA_AB, LMS_F-AGGTTGTTGATGTGGTGTT and LMS_R-TTTCCCTGCTGGCTTTCT. PCR was performed on a BioRad iCycler thermocycler (Hercules, CA) using the following reaction conditions: 95 oC (1 min); 40 cycles of 95 oC (1 min), 61oC (16s rDNA) or 50 oC (spoII) (30 sec), 72 oC (1.5 min); 72 oC (7 min); 4 oC (hold).
Cloning and sequencing: Positive PCR reactions as needed were extended using Crimson Taq DNA polymerase (New England Biolabs, Inc.), cloned into pCR®4.0-TOPO® vector and transformed into One Shot® chemically competent cells (Invitrogen). Transformed cells were plated on Luria broth agar amended with Kanamycin (50 μg/ml) (LB/KN) agar plates. Positive transformants were grown overnight in LB/KN broth and plasmids were extracted using Zyppy Plasmid Miniprep Kit (Zymo Research Corp.). Plasmids were screened for positive transformants by restriction digestion using EcoRI and StuI (Duan et al., 2003) restriction enzymes (New England Biolabs, Inc.) in a double digest. Sanger sequencing was performed by the University of Florida Interdisciplinary Center for Biotechnology Research (ICBR). Multiple clones were sequenced to obtain consensus sequence for a given coding sequence (cds).
Phylogenetic analysis: Partial cds were aligned using CLUSTAL W2 (Thompson et al., 1994) using default settings. Phylogenetic analysis was performed using MEGA 4.1 (Tamura et al., 2007). The evolutionary history was inferred using the Neighbor-Joining method (Saitou and Nei, 1987). The percentage of replicate trees in which the associated taxa clustered together where assessed in the bootstrap test (1000 replicates) (Felsenstein, 1985). The evolutionary distances were computed using the Maximum Composite Likelihood method (Tamura et al., 2004) and are in the units of the number of base substitutions per site. All positions containing gaps and missing data were eliminated from the dataset (Complete deletion option). Pairwise identity was performed using EMBL-EBI EMBOSS Pairwise alignment (www.ebi.ac.uk/Tools/emboss/align/) (Rice et al., 2000) using the following method settings: Needleman-Wunsch algorithm (global) (Needleman and Wunsch, 1970); Gap opening 10.0; Gap extend 0.5; Matrix DNAfull.
Immunofluorescent assay: Vermiform R. reniformis were exposed to in vitro produced Pasteuria sp. endospores (Pasteuria Bioscience, Inc.). Spore attachment was facilitated by slow speed centrifugation, and the second stage juveniles (J2) were cultivated on cotton (cv. Deltapine 555 BG/RR) in a greenhouse. Plants were harvested at 28 days and females were removed from roots with the aid of a dissecting microscope. Pasteuria sp. infected cadavers were hand-picked with a pipette, transferred to an Alcian blue coated circular cover slip (12 mm, #1 thickness) and ruptured using a wire dental cleaning tool and dried under a lamp at room temperature, and processed as described previously (Schmidt et al., 2003). Mounted specimens where challenged with anti-Pasteuria monoclonal antibody MAb2A41D10 (Schmidt et al., 2003) in PBS containing 1% BSA or 0.2 % Tween 20, and detected using anti-mouse μ-chain specific secondary Ab with an FITC conjugate (Sigma). Preparations were stored in the dark and examined using a Leica DM2000 epifluorescent microscope.
Developmental windows within the host: Rotylenchulus reniformis from pot culture were attached with in vitro produced Pasteuria spp. Pr3 endospores using a centrifuge attachment technique that resulted in 40-50% of the vermiform nematodes with spores adhering to their cuticles. Attached nematodes were placed into small plastic cups filled with 100 cm3 of an autoclaved mixture of 3 parts sand and 1 part sandy loam soil. Cups with or without a single cotton plant (Gossypium hirsutum cv. Deltapine 444 BG/RR) were established at two-day intervals for 25 days, and placed in a growth chamber at 28 oC. At the end of the experiment, ten nematodes observed with Pasteuria spp. infection were selected from cups representing each time interval for observation. Nematodes were crushed and observed at 600X for the presence of bacterial cells and sporulating structures. This experiment was conducted in replicate and the data were combined for analysis.
Transmission (TEM) and Scanning Electron Microscopy (SEM): Specimens for TEM were fixed in 4% paraformadehyde, 2.5% glutaraldehyde in 0.1M sodium cacodylate buffer, pH 7.24. Nematodes were processed with the aid of a Pelco BioWave laboratory microwave (Ted Pella, Redding, CA). The samples were washed in 0.1M sodium cacodylate pH 7.24, pelleted by microcentrifugation and encapsulated in 6% low-temperature gelling agarose type VII (Sigma- Aldrich, St. Louis MO). Encapsulated nematodes were post fixed with 2% buffered OsO4, water washed and dehydrated in a graded ethanol series 25%, 50%, 75%, 95%, 100%, followed by 100% acetone. Dehydrated samples were infiltrated in a graded infiltration series of acetone/Spurrs epoxy resin (Ellis, 2006), 30%, 50%, 70%, 100% and cured at 60 oC. Cured resin blocks were trimmed, thin sectioned and collected on Formvar copper slot grids, post-stained with 2% uranyl acetate and Reynold's lead citrate. Sections were examined with a Hitachi H-7000 TEM (Hitachi High Technologies America, Inc., Schaumburg, IL) and digital images acquired with a Veleta 2k x 2k camera and iTEM software (Olympus Soft-Imaging Solutions Corp, Lakewood, CO). Specimens prepared for SEM were fixed and dehydrated as above, followed by critical point drying (Bal-Tec CPD030, Leica Microsystems, Bannockburn, IL). Specimens were mounted on carbon adhesive tabs on an aluminum specimen mount, Au/Pd sputter coated (DeskII, Denton Vacuum, Moorestown, NJ) and examined using a field-emission scanning electron microscope (S-4000, Hitachi High Technologies America, Inc., Schaumburg, IL). High-resolution digital micrographs were acquired using PCI Quartz software (Quartz Imaging Corporation, Vancouver, BC, Canada).
Biocontrol tests: Greenhouse trials were conducted to evaluate the efficacy of Pasteuria spp. Pr3 endospores produced by in vitro fermentation for control of reniform nematodes on cotton. Granular formulation trial: 5000 R. reniformis eggs were inoculated into 960 ml styrofoam cups containing 500 cm3 of sandy-loam soil. Two cotton seeds (cv. Deltapine 555 BG/RR) were planted into the center of each pot. A granular fired-clay formulation was prepared that contained approximately 5.0 x 108 endospores/gram of formulation. Six replicate pots were surface inoculated with the granular formulation at the following endospore loading rates: 2.0 x 107, 4.0 x 107, 6.0 x 107, 8.0 x 107, 1.2 x 108 spores/500 cm3 of soil, and an untreated control. Pots were placed in a growth chamber holding a constant temperature of 26 oC and test was taken down after 20 days. Plant height, wet root weight and number of female reniform were recorded.
Seed treatment trial: Soil infested with R. reniformis, classified as a clay loam (pH 6.6; CEC = 4.6 - 9.0 cmolckg-1) was collected from Huxford, AL. The soil was processed through a 1 cm mesh sieve and subsequently mixed 1:1 with sand. Five cotton seeds (cv. DP0912 B2RF Acceleron) were planted in 1 kg of soil in 10 cm diam. polyvinyl chloride (PVC) pots with screen bottoms. The seeds were pre-treated with one of each of the following: 1.0 x 107 Pasteuria spp. spores/seed, 1.0 x 108 Pasteuria spp. spores/seed, or Aeris™ (Thiodicarb (24%); Dimethyl N,N' [thiobis[methylimino)carbonyloxy]]bis[ethanimidotioate;Imidacloprid (24%) 1-[(6-Chloro-3-pyridinyl)methyl]-N-nitro-2-imidazolidinimine])(Bayer CropScience, Inc.), and an untreated control. Pots were left uncovered during the study and maintained under greenhouse conditions. Cotton plants were hand watered twice daily and received no further treatments. Pots were arranged in a randomized complete block design with seven replications per treatment. The tests were taken down at 2, 4, and 8 weeks after planting (WAP). Upon completion of each trial, the following data were collected: number of plants, weight of fresh shoots (g), weight of fresh roots (g), plant shoot height (cm), and R. reniformis enumeration from soil and roots. Nematodes were extracted from soil and roots (Rodríguez-Kábana and Pope, 1981). Nematodes were retained on a 25-μm pore sieve and transferred to a petri-dish for identification and enumeration using a compound microscope. Analysis of variance (ANOVA) and Duncan's New MRT were performed using ARM8 (Gylling Data Management, Inc.). Means followed by the same letter are not significantly different at P ≤ 0.05. Mean comparisons were performed only when ANOVA Treatment P(F) was significant at the mean comparison observed significance level.
Results
Live Immuno-fluorescent Assay (IFA): Rotylenchulus reniformis in soil collected from a cotton field in Huxford, AL, were observed to have putative Pasteuria spp. (Pr3) endospores attached to their cuticles and mature endospores present within the body (Figs. 1). Live immunofluorescent assay analysis of Pasteuria spp. endospore-filled R. reniformis cadavers, infected by in vitro fermentation produced Pr3 endospores cultured from source cadavers (Pasteuria Bioscience, Inc.), displayed immunofluorescence upon challenge with anti-Pasteuria monoclonal antibody MAb2A41D10. MAb affinity for an epitope presented on the endospore surface confirmed the presence of Pasteuria spp. in the native field population (data not shown) and within hosts inoculated with in vitro produced Pr3 endospores (Figs. 2).
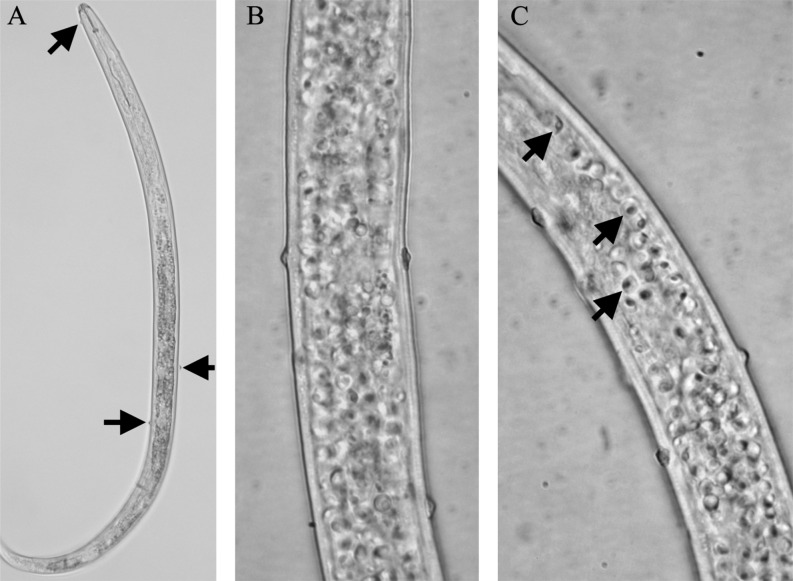
Fig. 1.
Brightfield micrographs of Pasteuria spp. Pr3 endospores attached to and infecting Rotylenchulus reniformis recovered from field soil in Huxford, AL. A) Arrows indicate endospores attached to the cuticle surface. B and C) Show endospores attached to the cuticle and the presence of mature endospores within the body cavity (indicated by arrows in C).
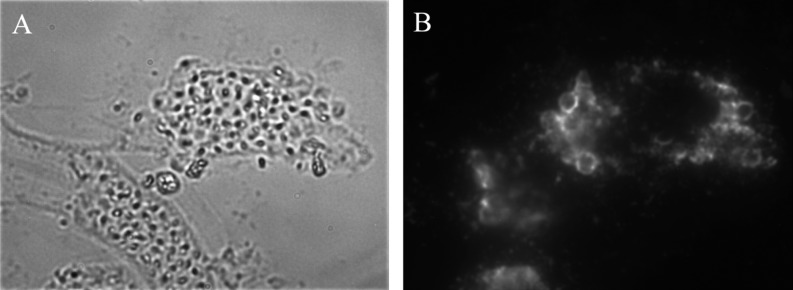
Fig. 2.
Immunofluorescent micrograph showing detection of Rotylenchulus reniformis Pasteuria spp. Pr3 in situ in a mechanically ruptured specimen using monoclonal antibody MAb2A41D10 and visualized with fluorescein-conjugated anti-mouse IgM μ-chain specific secondary antibody. A) Brightfield 40X objective under epifluorescence with a 495 nm excitation filter showing an epitope on the surface of R. reniformis Pasteuria spp. that is reactive with this Pasteuria-specific MAb. B) Same as in A) with fluorescence excitation.
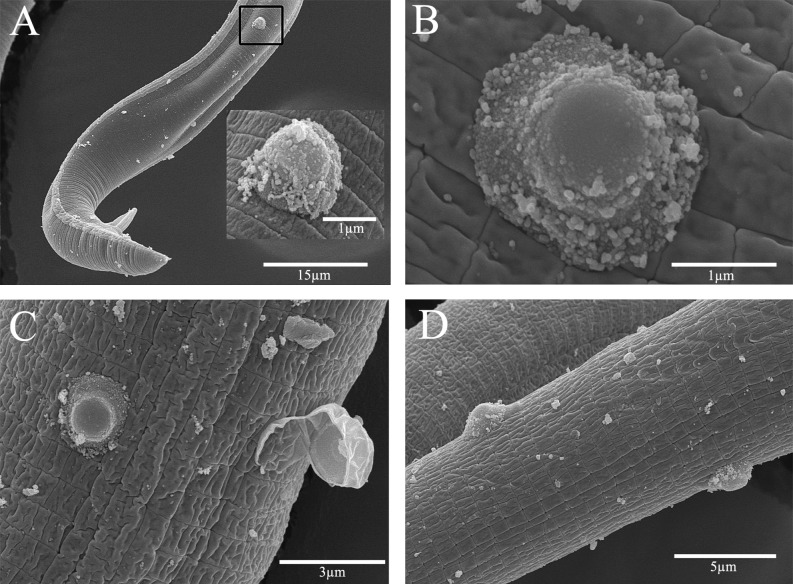
Spore morphometrics: Scanning electron microscopy was performed on Pr3 (native and fermentation produced) endospores (Figs. 3 and Figs. 4, respectively), and native Pr4 endospores (Figs. 5) attached to R. reniformis hosts. Rotylenchulus reniformis Pasteuria spp. endospores are distinctive, presenting as highly domed-shaped (vertically elongated), and having a predominant and horizontally elliptical central body (spore core). The endospore surface was granular in appearance, with evidence of sloughing of a loosely attached, fragmented exosporium. Measurements were obtained of endospores attached to the host to obtain representative measurements of field isolates. Pr4 endospores displayed an average height of 1.29 μM (+/- 0.150) and a width of 2.26 μM (+/- 0.206), reporting an average height to width ratio of 0.57. The average height and width of Pr3 endospores are 1.24 μM (+/- 0.144) and 1.98 μM (+/- 0.228), respectively. The average height to width ratio is 0.63. Additionally, the height and width of the spore core was 0.43 μM (+/- 0.042) and 0.962 μM (+/- 0.107), respectively.
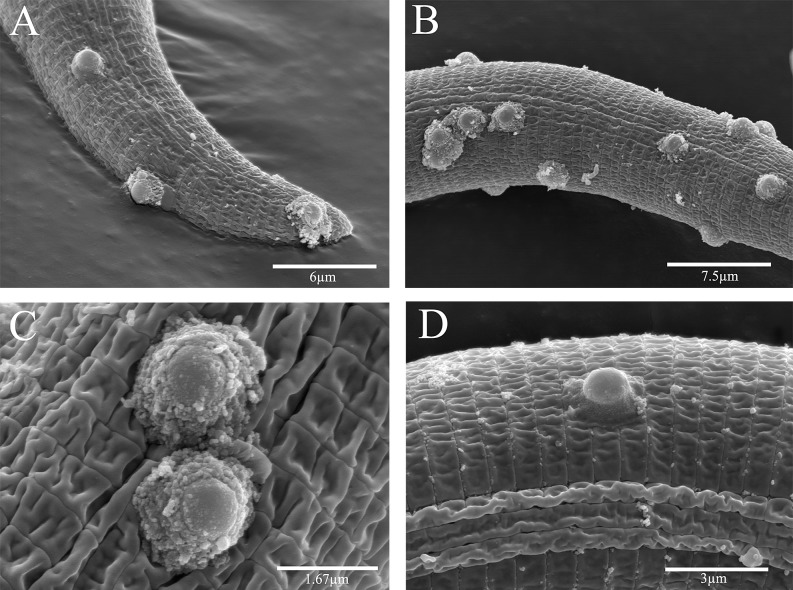
Fig. 3.
Scanning electron photomicrographs of Rotylenchulus reniformis Pasteuria spp. Pr3 isolated from a cotton field in Huxford, AL. A-D) Showing Pasteuria Pr3 endospore morphology as recovered and observed on a field population of R. reniformis.
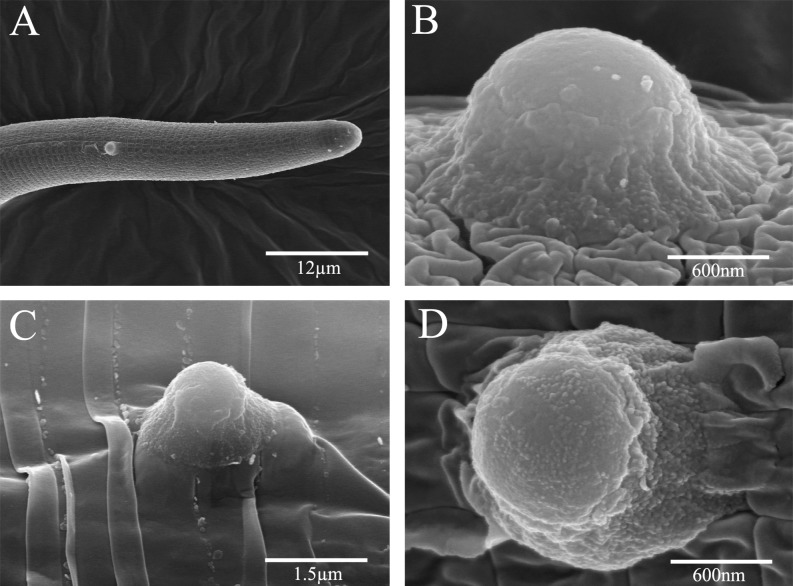
Fig. 4.
Scanning electron photomicrographs of Rotylenchulus reniformis Pasteuria spp. Pr3 endospores produced by in vitro fermentation. A-D) Pasteuria Pr3 endospores attached to the cuticle of R. reniformis demonstrating endospore morphological characteristics. Note in C) the endospore attached to an R. reniformis specimen in the process of molting.
Fig. 5.
Scanning electron photomicrographs of Rotylenchulus reniformis Pasteuria spp. Pr4 isolated from a cotton field in Quincy, FL. A) Male R. reniformis with a Pasteuria spp. endospore attached. B-D) Showing morphology of the Pr4 isolate on several specimens. Note in C) the presence of a non-germinated endospore releasing and partially encapsulated by epidermal tissue following a recent molt.
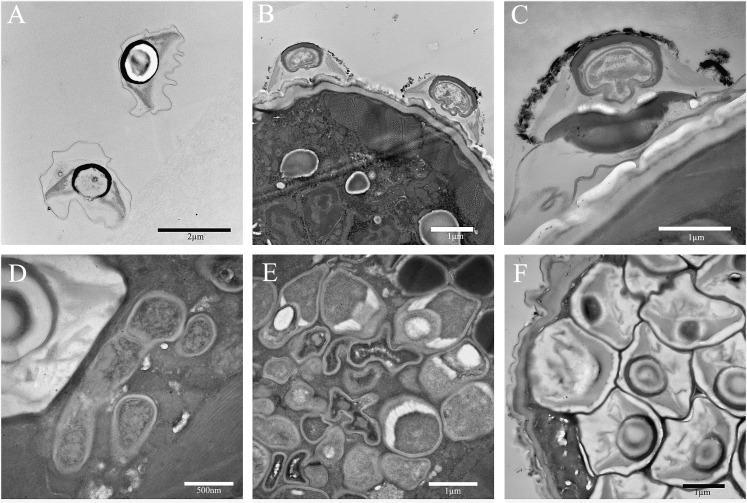
Transmission electron micrographs show the developmental stages of Pr3 display the typical sporulation typology documented for the Pasteuria spp.. The various morphologies associated with Stages I-VII of sporulation in the Pasteuria associated with R. reniformis are presented in Figs. 6. The outer spore coat of the mature endospore is observed to be asymmetrical and is thickest at the apex; gradually thinning towards the basal germinal pore. The parasporial fibers emerge from mid-lateral of the central body, along the full lateral extent of the central body. The central body is minimally covered apically by the sporangial sack.
Fig. 6.
Transmission electron photomicrographs of Rotylenchulus reniformis Pasteuria Pr3 parasitizing its host. A) Free endospores. B-C) Pasteuria endospores bound to host cuticle in the first stage of infection. C) Shows development of the germ (penetration) tube at the base of the attached endospore indicating germination. D) Stage I of sporulation (mycelial stage) of development (center right) and a mature endospore (upper left) in the pseudocoelom of a single specimen. E) Stages I-V of sporulation. F) Fully mature Stage VII Pasteuria endospores.
Developmental windows: Rotylenchulus reniformis Pasteuria spp. were observed to infect and complete their life-cycle in juvenile, male and female reniform nematodes. Pasteuria spp. germination occurred rapidly after attachment on day 1, with and without cotton plants. Mycelial structures and thalli were present by day 3 in soil with cotton plants and by day 7 in soil alone. Mature Pasteuria endospores were formed by day 15 in soil with cotton plants and by day 23 in soil alone.
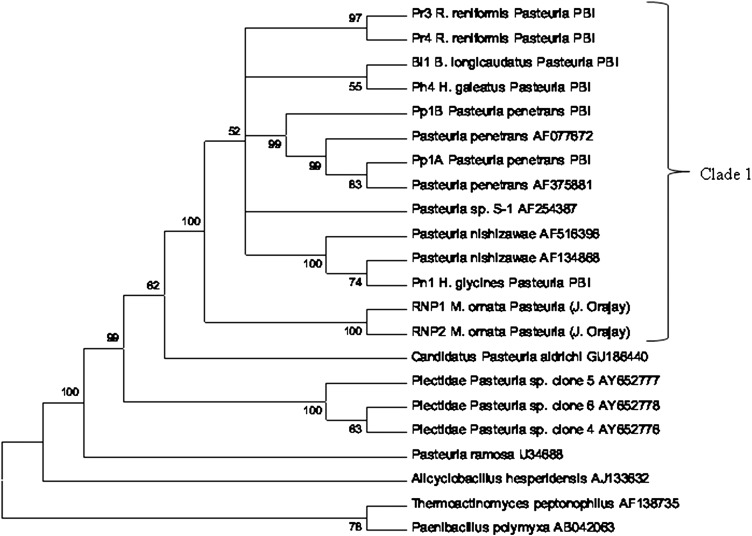
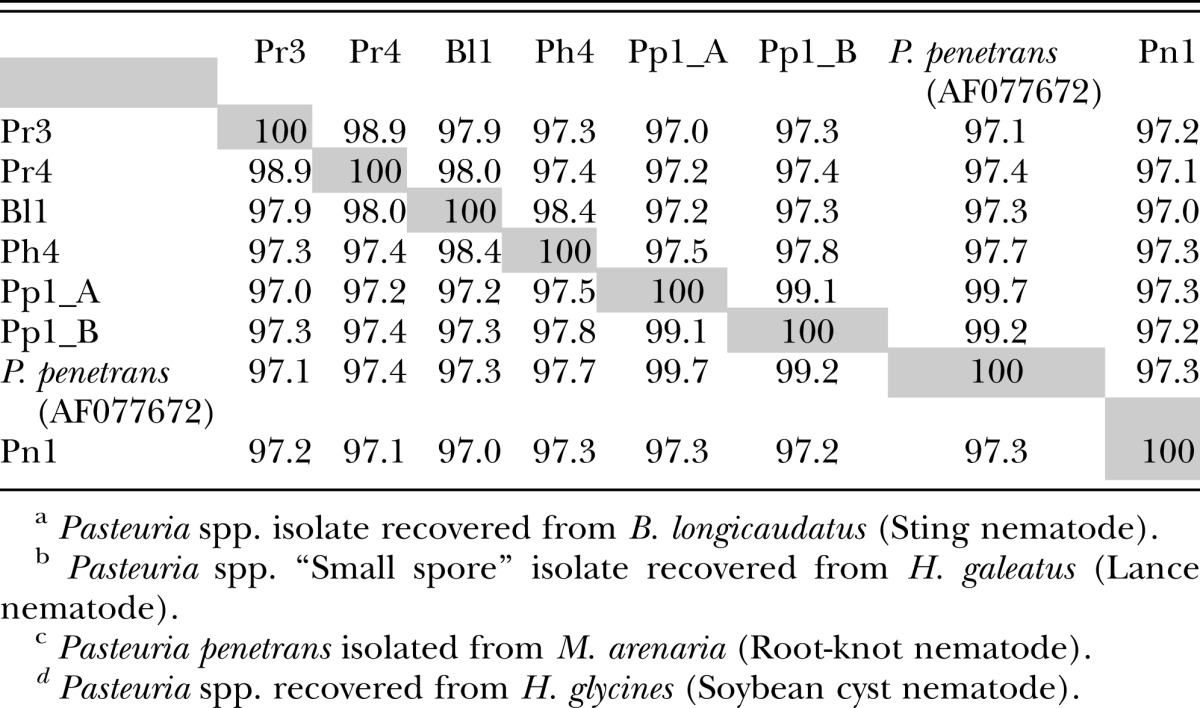
Phylogenetic analysis: PCR amplification using Pasteuria species specific degenerate primers selectively amplified Pasteuria spp. 16s rDNA in heterogeneous gDNA extracts. Phylogenetic analysis of the partial cds of 16s rDNA using the neighbor-joining method and boot strap test of reliability (1000 replications) places Rotylenchulus reniformis Pasteuria spp. congeneric with Pasteuria spp.. Based upon 16s rDNA, R. reniformis Pasteuria isolates Pr3 and Pr4 share close homology to two other newly sequenced isolates, one from Belonolaimus longicaudatus (Bl1), and a “small-spore” Pasteuria (Giblin-Davis et al., 1990) from Hoplolaimus galeatus (Ph4) (Figs. 7). The Pasteuria isolated from plant parasitic nematodes are observed to form a “super” monophyletic clade with 100% boot strap support, distinguishing them from Pasteuria associated with the bacteriophagous nematodes. Distinctive sub-clades within the phytopathogenic nematode Pasteuria are comprised of the P. penetrans group (Meloidogyne sp.), with 99% boot strap support, Pasteuria isolated from Heterodera glycines with 100% boot strap support, and the Mesocriconema ornatum (Orajay, 2009) Pasteuria with 100% boot strap support. Phylogenetic analysis of a 778 bp sequence of the combined portion of spoIIAA and spoIIAB place R. reniformis Pasteuria spp. isolates congeneric within the nematode Pasteuria spp. with 100% boot strap support (data not shown). Results of percent identity analysis of the 16s rDNA partial cds using EMBOSS (Rice et al., 2000) show that R. reniformis Pasteuria spp and several newly screened Pasteuria spp. isolates from Belonolaimus longicaudatus and Hoplolaimus galeatus share ≥ 97% identity over the 1170 Bp 5'-biased end of the 16 rDNA molecule used for analysis (Tables 1).
Fig. 7.
Phylogenetic relationship of Rotylenchulus reniformis Pasteuria (Pr3 and Pr4) to other Pasteuria spp. associated with plant parasitic nematodes (Clade 1), bacterivorous nematodes, and the Cladoceran (Daphnia magna) based on 16s rDNA sequences. The tree was constructed using 1170 bp alignable sequences with a total of 1126 positions in the final data set, and Thermoactinomyces peptonophilus and Paenibacillus polymyxa serving as out groups. The evolutionary history was inferred using the Neighbor-Joining method. The evolutionary distances were computed using the Maximum Composite Likelihood method. All positions containing gaps and missing data were eliminated from the dataset (Complete deletion option). The boot strap consensus tree inferred from 1000 replicates is taken to represent the evolutionary history of the taxa analyzed using MEGA 4.1 (Tamura et al., 2007). Branches corresponding to partitions reproduced in the in less than 50% bootstrap replicates are collapsed.
Table 1.
Pairwise identity matrix compiled using the 1170 bp 5”-biased 16s rDNA cds of Pasteuria spp. isolates using EMBOSS (EMBL-EBI) Needle-Wunsch (global) algorithm with the following methods settings: Matrix DNA full; Gap opening 10.0; and Gap extend 0.5.
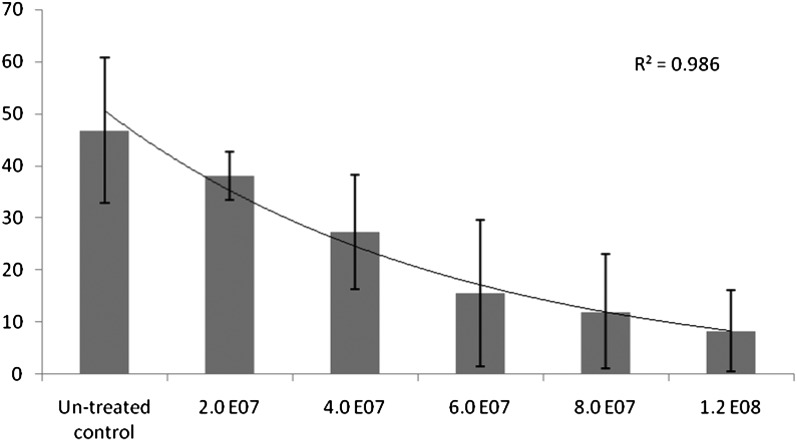
Biocontrol efficacy tests: A topical granular formation and a seed coat treatment application of Pr3 endospores was tested in separate trials. Application of a granular clay formulation comprising 2.0 x 107 to 1.2 x 108 spores per 500 cm3 soil compared with an untreated control (Figs. 8) showed a inverse linear relationship between spore loading rate and number of female reniform nematodes producing a theoretical line fit of R2 = 0.986. The maximum soil spore loading rate of 1.2 x 108 spores/500 cm3 reduced females by 82.3% as compared to the untreated control.
Fig. 8.
Mean number of Rotylenchulus reniformis females on roots following a 20 day greenhouse cotton test showing dose response of varying application rates of Pasteuria Pr3 endospores applied as a top dressing in a granular clay formulation on cotton plants. Bars represent the sample standard deviation of six replications per treatment.
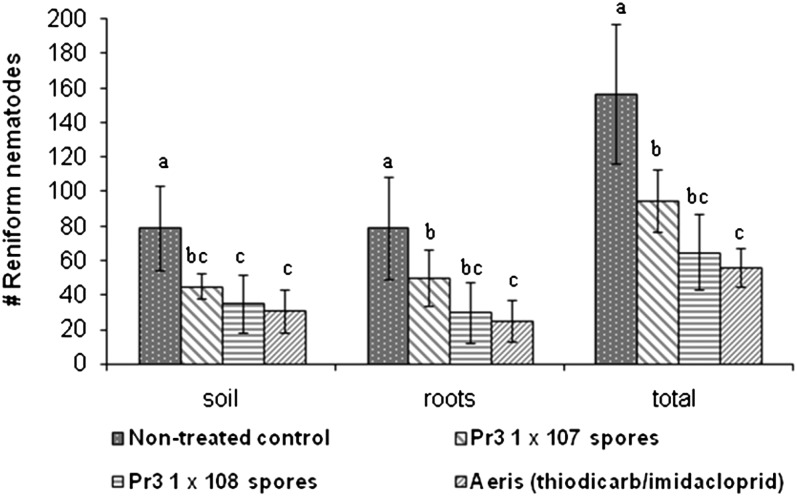
The results of the seed treatment trial with 1.0 x 107 and 1.0 x 108 Pasteuria Pr3 endospores/seed, or thiodicarb/imidacloprid showed a 39.5%, 58.6% and 64.3% decrease in total reniform nematodes, respectively, as compared to the untreated control (Figs. 9). Equivalent reductions were observed in both the soil-borne and root-borne reniform nematode populations.
Fig. 9.
Mean number of Rotylenchulus reniformis on cotton at 4 weeks post treatment following seed-coat application with Pasteuria Pr3 endospores or the nematocide/insecticide; thiodicarb/imidacloprid. Bars represent the sample standard deviation of seven replications per treatment. Letters indicate statistical significance at P ≤ 0.05.
Discussion
Rotylenchulus reniformis Pasteuria spp. endospores were shown to display a “universal” epitope recognized by the anti-Pasteuria monoclonal antibody MAb2A41D10 as previously observed for all Pasteuria spp. challenged with this MAb (Preston et al., 2003), including P. ramosa, the cladoceran parasite (Schmidt et al., 2008). This epitope has been shown to be expressed in the early stages of sporulation associated with parasporial fiber development (Stage III sporogenesis) and presents as a supramolecular component of mature endospores (Brito et al., 2003). The properties of this antigen are consistent with a putative adhesion conferring virulence to the nematode host. Detection of this epitope by immunofluorescent assay in this study provided initial confirmation that the spores observed attached to the cuticle of R. reniformis nematodes and also proliferating within the pseudocoelom were Pasteuria spp. prior to conducting genomic analysis. As in previous studies, MAb2A41D10 is demonstrated as a valuable tool in indentifying Pasteuria infection in phytopathogenic nematodes as a requisite to further analysis (Schmidt et al., 2003).
Morphologically, Pr3 and Pr4 endospores are distinctive from most described Pasteuria spp. in being highly vertically elongated and having an uncharacteristically large and broadly exposed spore cortex (central body). They most closely resemble the description given of the Mesocriconema ornatum (ring nematode) Pasteuria spp. (RNP) (Orajay, 2009). Typologically, the RNP and reniform nematode Pasteuria spp. are very similar. The endospore height:width for RNP reported by Orajay (2009) of 0.59, is intermediate to that observed here for Pr3 (0.63) and Pr4 (0.57). The central body presents as a horizonally-oriented ellipse similar to P. penetrans and P. nishizawae and contrasts with the rounded-ellipse of Candidatus Pasteuria aldrichi (Giblin-Davis et al., 2010) which is similar in shape to P. ramosa the Cladoceran parasite (Sayre et al., 1979). The inner and outer spore coat are of similar thickness dorsally and laterally, with the outer spore coat becoming thinner ventrally upon approach to the germinal pore. There is no evidence of a basal ring surrounding the germinal pore as evidenced in P. penetrans (Chen et al., 1997). There is no evidence of lateral microprojections in the outer spore coat as evidenced in P. penetrans, Candidatus Pasteuria usage and P. nishizawae.
The process of infection and etiology of disease is universal in Pasteuria spp. (Tian et al., 2007), however, the rate of growth and differentiation varies between hosts and is influenced by ambient environmental temperature (Chen and Dickson, 1997; Serracin et al., 1997). The rate of growth of the Pasteuria spp. parasitizing R. reniformis is rapid compared to the growth rate of P. penetrans in Meloidogyne spp.; reaching sporulation in 208 degree days verses 500 degree days for P. penetrans at 28 oC (Serracin et al., 1997). Interestingly, bacterial development and differentiation was also observed in the absence of a host plant, demonstrating reniform Pasteuria can infect and multiply in non-feeding hosts, however, development was more rapid in the presence of the host plant. Pr3 endospores were also observed to infect and complete their life-cycle in juvenile, male and female reniform nematodes. Pasteuria spp. associated with principally ectoparasitic nematodes are observed to complete their life cycles in various life-stages and host sexes (Ciancio et al., 1992; Ciancio et al., 1998). Pasteuria penetrans has been observed only rarely to develop in Meloidogyne sp. males and juveniles (Chen and Dickson, 1997). Pr3 development was found to be asynchronous, wherein, multiple developmental growth and sporulation stages were observed within a single individual. Multiple infection events are likely to occur as spores were observed attached to the cuticle of advanced-stage infected vermiform nematodes.
Phylogenetic analysis using the partial cds of the 16 rDNA gene of Pasteuria spp. infecting two regionally distinct R. reniformis populations showed very little intra-“species” variation and supported placement of these Pasteuria congeneric within the phytopathogenic nematode Pasteuria. Two newly sequenced isolates, Bl1 from B. longicaudatus and Ph4 a “small-spore” Pasteuria spp., (potentially the same isolate first described by Giblin-Davis, 1990) from H. galeatus were closely related to Pr3 and Pr4 indicating similar evolutionary histories. A robustly supported monophyletic clade (bootstrap support 100%) separates the Tylenchid nematode Pasteuria spp. from the bacteriovorous nematode Pasteuria and the Cladoceran parasite P. ramosa. The observed lack of a strongly supported lineage definition within the Pasteuria infecting phytopathogenic nematodes (bootstrap support of 52%) is the result of overall strong 16s rDNA sequence conservation within the Pasteuria infecting different hosts. Pairwise identity compilation of the Pasteuria isolated from R. reniformis (Pr3 and Pr4), Meloidogyne arenaria Race 1 (Pp1), B. longicaudatus (Bl1), H. galeatus (Ph4), and Heterodera glycines (Pn1) demonstrated 97.0% or greater sequence similarity and identity based on 1170 BP of the 5'-biased end of the 16s rDNA molecule. Pairwise alignment reveals the first approximately 300-400 Bp of the 5'-end of 16s rDNA in Pasteuria harbors the greatest degree of sequence heterogeneity in isolates infecting the same host, with the remainder of the molecule showing very little relative sequence diversity. A surprising degree of 16s rDNA sequence diversity was found within what was considered a single strain of Pasteuria penetrans infecting M. arenaria (Duan et al., 2003). Clones containing variable coding sequences were observed in this first reported environmental screen for Pasteuria conducted by Duan et al., 2003 and their findings shed light, and likely represent only the “tip of the iceburg” with regard to the heterogeneity of 16s rDNA of Pasteuria occupying a host defined niche. Evidence suggests that construction of phylogeny and speciation using the 16s rDNA model standard is an insufficient and somewhat arbitrary measure in defining Pasteuria spp. ecological role and host association. Preston et at., 2003, importantly introduced the concept of Pasteuria species-“biotypes” based on presence of variable molecular weight glycopeptides forming a supramolecular structure recognized by a universal Pasteuria-specific monoclonal antibody (Preston et al., 2003; Schmidt et al., 2008). These carbohydrate ligands are formed in the process of sporulation (Brito et al., 2003) and are believed to perform a primary role in host recognition and attachment (Persidis, 1991; Davies and Danks, 1993; Davies and Redden, 1997; Charnecki, 1998). Clearly, the genes conferring the biochemistry that allows for host-specific virulence may more clearly distinguish the Pasteuria spp. and elucidate parity with regard to host-parasite interactions.
The precedent for Pasteuria as an agent for biocontrol of a number of important phytopathogenic nematodes pathogens is well established (Davies and Danks, 1992; Chen and Dickson, 1998; Atibalentja et al., 1998). Belonoliamus longicaudatus populations where reported to decline linearly upon application of in vitro produced endospores (PBI-Bl1) at a soil spore loading level ≥ 2.8 x 104 endospores/ cm3 soil, and Pasteuria proliferation within nematode cadavers was also observed (Luc et al., “in press”). In a related study, Pasteuria Bl1 in vitro produced endospores where shown to suppress B. longicaudatus populations from 20% to 63% when compared to the non-treated control depending upon the type of formulation applied (Luc et al., “in press”). Pasteuria penetrans present at a level of 1.0 x 105 endospores/g of soil provide immediate control of Meloidogyne arenaria (Chen and Dickson, 1998). This is the first reported study of a Pasteuria spp. to control reniform nematode. The efficacy of R. reniformis in vitro Pasteuria Pr3 endospores to reduce reniform nematode populations on cotton was established using differing methods of endospore application. A seed-coating density of approximately 1 x 108 endospores/seed provides control equivalent to the commercial seed treatment Aeris™. The results of these tests indicate that Pr3 Pasteuria endospores demonstrate the ability to suppress reniform nematodes and may effectively control reniform populations on cotton in a commercial application.
Discovery, characterization and commercial development/application of a Pasteuria biotype to control reniform nematode constitutes significant progress in expanding the role of Pasteuria spp. in addressing the serious damage and economic losses associated with nematode predation on cotton. Future development is expected to address another important cotton pest, Meloidogyne incognita. The potential for Pasteuria as a natural and robust biocontrol agent of phytopathogenic nematodes has been espoused over several decades by many nematologists. As investigators continue to characterize new isolates, and perform research to better understand the mechanisms of differentiation and virulence, and full-scale commercial application is realized, we may then begin to witness fruition of this most anticipated reality.
Literature Cited
- Atibalentja N, Noel GR, Liao TF, Gertner GZ. Population changes in Heterodera glycines and its bacterial parasite Pasteuria sp. in naturally infested soil. Journal of Nematology. 1998;30(1):81–92. [PMC free article] [PubMed] [Google Scholar]
- Atibalentja N, Noel GR, Domier LL. Phylogenetic position of the North American isolate of Pasteuria that parasitizes the soybean cyst nematode, Heterodera glycines, as inferred from 16s rDNA sequence analysis. International Journal of Systematic and Evolutionary Microbiology. 2000;50:605–613. doi: 10.1099/00207713-50-2-605. [DOI] [PubMed] [Google Scholar]
- Bird AF, Brisbane PG. The influence of Pasteuria penetrans in field soils on the reproduction of root-knot nematodes. Revue de Ne´matologie. 1988;11:75–81. [Google Scholar]
- Blasingame D. Memphis, TN: National Cotton Council of America; 2006. Cotton disease loss estimate. Pp. 155–157 in Proceedings of the Beltwide Cotton Conferences, January 3-5, 2006. [Google Scholar]
- Brito JA, Preston JF, Dickson DW, Giblin-Davis RM, Williams DS, Aldrich HC, Rice JD. Temporal Formation and immunolocalization of an endospore surface epitope during Pasteuria penetrans sporogenesis. Journal of Nematology. 2003;35(3):278–288. [PMC free article] [PubMed] [Google Scholar]
- Charnecki JH, Rice JD, Dickson DW, Preston JF. Determinants for the attachment of endospores of Pasteuria penetrans to phytopathogenic nematodes. Annual Meeting of the American Society for Microbiology; Atlanta, GA. 1998. (Abstr.). [Google Scholar]
- Chen ZX, Dickson DW. Minimal growth temperature of Pasteuria penetrans. Journal of Nematology. 1997;29(4S):635–639. [PMC free article] [PubMed] [Google Scholar]
- Chen ZX, Dickson DW, McSorley R, Mitchell DJ, Hewlett TE. Suppression of Meloidogyne arenaria race 1 by soil application of endospores of Pasteuria penetrans. Journal of Nematology. 1996;28:159–168. [PMC free article] [PubMed] [Google Scholar]
- Chen ZX, Dickson DW, Mitchell DJ, McSorley R, Hewlett TE. Suppression mechanisms of Meloidogyne arenaria by Pasteuria penetrans. Journal of Nematology. 1997;29:1–8. [PMC free article] [PubMed] [Google Scholar]
- Chen ZX, Dickson DW. Review of Pasteuria penetrans: Biology, ecology, and biological control potential. Journal of Nematology. 1998;30(3):313–340. [PMC free article] [PubMed] [Google Scholar]
- Ciancio A. Density-dependent parasitism of Xiphinema diversicaudatum by Pasteuria penetrans in a naturally infested field. Phytopathology. 1995;85:144–149. [Google Scholar]
- Ciancio A, Farfan VV, Torres EC, Grasso G. Observations on a Pasteuria isolate parasitic on Hoplolaimus galeatus in Peru. Journal of Nematology. 1998;30(2):206–210. [PMC free article] [PubMed] [Google Scholar]
- Ciancio A, Mankau R, Mundo-Ocampo M. Parasitism of Helicotylenchus lobus by Pasteuria penetrans in naturally infested soil. Journal of Nematology. 1992;24(1):29–35. [PMC free article] [PubMed] [Google Scholar]
- Davies KG, Danks C. Interspecific differences in the nematode surface coat between Meloidogyne incognita and M. arenaria related to the adhesion of the bacterium Pasteuria penetrans. Parasitology. 1992;105:475–480. [Google Scholar]
- Davies KG, Danks C. Carbohydrate/protein interactions between the cuticle of infective juveniles of Meloidogyne incognita and spores of the obligate hyperparasite Pasteuria penetrans. Nematologica. 1993;39:53–64. [Google Scholar]
- Davies KG, Redden M. Diversity and partial characterization of putative virulence determinants in Pasteuria penetrans, the hyperparasite bacterium of root-knot nematodes (Meloidogyne spp.) Journal of Applied Microbiology. 1997;83:227–235. doi: 10.1046/j.1365-2672.1997.00223.x. [DOI] [PubMed] [Google Scholar]
- Doi RH. Sporulation and germination. In: Harwood CR, editor. Bacillus. London, UK: Pleum; 1989. pp. 169–215. [Google Scholar]
- Duan YP, Castro HF, Hewlett TE, White JH, Ogram AV. Detection and characterization of Pasteuria 16S rRNA gene sequences from nematodes and soils. International Journal of Systematic and Evolutionary Microbiology. 2003;53:105–112. doi: 10.1099/ijs.0.02303-0. [DOI] [PubMed] [Google Scholar]
- Ebert D, Rainey P, Embley TM, Scholz D. Development, life cycle, ultrastructure and phylogenetic position of Pasteuria ramosa Metchnikoff 1888: rediscovery of an obligate endoparasite of Daphnia magna Straus. Philosophical Transactions of the Royal Society. London B. 1996;351:1689–1701. [Google Scholar]
- Ellis EA. Solutions to the Problem of Substitution of ERL 4221 for Vinyl Cyclohexene Dioxide in Spurr Low Viscosity Embedding Formulations. Microscopy Today (July) 2006:32–33. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Giblin-Davis RM, McDaniel LL, Bilz FG. Isolates of the Pasteuria penetrans group from phytopathogenic nematodes in bermudagrass turf. Journal of Nematology. 1990;22(4S):750–762. [PMC free article] [PubMed] [Google Scholar]
- Giblin-Davis, R.M., Nong, G., Preston III, J.F., Williams, D.S., Center, B.J., Brito, J.A., and Dickson, D. W. 2010. ‘Candidatus Pasteuria aldrichii' sp. nov., an obligate endoparasite of the bacterivorous nematode, Bursilla sp. International Journal of Systematic and Evolutionary Microbiology, first published on Sep 24, 2010 as doi: doi:10.1099/ijs.0.021287-0. [DOI] [PubMed] [Google Scholar]
- Giblin-Davis RM, Williams DS, Bekal S, Dickson DW, Brito JA, Becker JO, Preston JF. ‘Candidatus Pasteuria usgae' sp. nov., an obligate endoparasite of the phytoparasitic nematode Belonolaimus longicaudatus. International Journal of Systematic and Evolutionary Microbiology. 2003;53:197–200. doi: 10.1099/ijs.0.02292-0. [DOI] [PubMed] [Google Scholar]
- Heald CM, Robinson AF. Survey of the current distribution of Rotylenchulus reniformis in the United States. Supplement to the Journal of Nematology. 1990;22(4S):695–699. [PMC free article] [PubMed] [Google Scholar]
- Hewlett TE, Dickson DW. A centrifuge method for attaching endospores of Pasteuria spp. to nematodes. Supplement to the Journal of Nematology. 1993;25:785–788. [PMC free article] [PubMed] [Google Scholar]
- Jenkins WR. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Disease Reporter. 1964;48:692. [Google Scholar]
- Kariuki GM, Dickson DW. Transfer and Development of Pasteuria penetrans. Journal of Nematology. 2007;39(1):55–61. 2007. [PMC free article] [PubMed] [Google Scholar]
- Koenning SR, Kirkpatrick TL, Starr JL, Wrather JA, Walker NR, Mueller JD. Plant-parasitic nematodes attacking cotton in the United States: Old and emerging production challenges. Plant Disease. 2004;88:100–113. doi: 10.1094/PDIS.2004.88.2.100. [DOI] [PubMed] [Google Scholar]
- Linford MB, Olivera JM. Rotylenchulus reniformis, nov. gen., n. sp., a nematode parasite of roots. Proceedings of the Helminthological Society of Washington. 1940;7:35–42. [Google Scholar]
- Luc JE, Pang W, Crow WT, Giblin-Davis RM. Effects of formulation and nematode population and the ability of in vitroproduced Pasteuria endospores to control its host Belonolaimus longicaudatus. “In press” Journal of Nematology. [PMC free article] [PubMed] [Google Scholar]
- Luc JE, Pang W, Crow WT, McSorely R, Giblin-Davis RM. Suppression of Belonolaimus longicaudatus with in vitroproduced Pasteuria sp. endospores. “In press” Nematropica. [Google Scholar]
- Mankau R. Biological control of Meloidogyne populations by Bacillus penetrans in West Africa. Journal of Nematology. 1980;12:230. (Abstr.). [Google Scholar]
- McSorley R. Nematodes associated with sweetpotato and edible aroids in southern Florida. Proceedings of Florida State Horticultural Society. 1980;93:283–285. [Google Scholar]
- McSorley R, Campbell W, Parrado JL. Nematodes associated with tropical and subtropical fruit trees in south Florida. Proceedings of Florida State Horticultural Society. 1982;95:132–135. [Google Scholar]
- McSorley R, Parrado JL, Stall WM. Aspects of nematode control on snapbean with emphasis on the relationship between nematode density and damage. Proceedings of Florida State Horticulture Society. 1981;94:134–136. [Google Scholar]
- Metchnikoff E. Pasteuria ramosa unrepresentant des bacteries a division longitudinale. Annals of the Institute Pasteur. (1888);2:165–170. [Google Scholar]
- Needleman SB, Wunsch CD. A general method applicable to the search for similarities in the amino acid sequence of two proteins. Journal of Molecular Biology. 1970;48:443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Nong G, Chow V, Schmidt LM, Dickson DW, Preston JF. Multiple-strand displacement and identification of single nucleotide polymorphisms as markers of genotypic variation of Pasteuria penetrans biotypes infecting root-knot nematodes. FEMS Microbiology Ecology. 2007;61:327–336. doi: 10.1111/j.1574-6941.2007.00340.x. [DOI] [PubMed] [Google Scholar]
- Orajay JI. Population dynamics of Pasteuria penetrans in a peanut field and description of a Pasteuria isolate infecting Mesocriconema ornatum. Dissertation presented to the University of Florida Graduate School; Gainesville, Florida. 2009. [Google Scholar]
- Persidis A, Lay JG, Manousis T, Bishop AH, Ellar DJ. Characterization of potential adhesins of the bacterium Pasteuria penetrans, and of putative receptors on the cuticle of Meloidogyne incognita, a nematode host. Journal of Cell Science. 1991;100:613–622. doi: 10.1242/jcs.100.3.613. [DOI] [PubMed] [Google Scholar]
- Preston JF, Dickson DW, Maruniak JE, Nong G, Brito JA, Schmidt LM, Giblin-Davis RM. Pasteuria spp.: Systematics and Phylogeny of These Bacterial Parasites of Phytopathogenic Nematodes. Journal of Nematology. 2003;35(2):198–207. [PMC free article] [PubMed] [Google Scholar]
- Regoes RR, Hottinger JW, Sygnarski L, Ebert D. The infection rate of Daphnia magna by Pasteuria ramosa conforms with the mass action principal. Epidemiology and Infection. 2003;131:957–966. doi: 10.1017/s0950268803008793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, P., Longden, I., and Bleasby, A. 2000. EMBOSS: The European Molecular Biology Open Software Suite Trends in Genetics 16(6):276–277. [DOI] [PubMed] [Google Scholar]
- Robinson AF, Akridge R, Bradford JM, Cook CG, Gazaway WS, Kirkpatrick TL, Laurence GW, Lee G, McGawley EC, Overstreet C, Padgett B, Rodriquez-Kabana R, Westphal A, Young LD. Vertical distribution of Rotylechulus reniformis in cotton fields. Journal of Nematology. 2005;37(3):265–271. [PMC free article] [PubMed] [Google Scholar]
- Robinson AF, Inserra RN, Caswell-Chen EP, Vovlas N, Troccoli A. Rotylenchulus species: Identification, distribution, host ranges, and crop plant resistance. Nematropica. 1997;27:127–180. [Google Scholar]
- Robinson AF, Westphal A, Overstreet C, Padgett GB, Greenberg SM, Wheeler TA, Stetina SR. Detection of suppressiveness against Rotylenchulus reniformis in soil from cotton (Gossypium hirsutum) fields in Texas and Louisiana. Journal of Nematology. 2008;40(1):35–38. [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Kábana R, Pope MH. A simple incubation method for the extraction of nematodes from soil. Nematropica. 1981;11:175–186. [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sayre RM, Adams JR, Wergin WP. Bacterial Parasite of a Cladoceran: Morphology, Development. In Vivo, and Taxonomic Relationships with Pasteuria ramosa Metchnikoff 1888. International Journal of Systematic Bacteriology. 1979;29(3):252–262. [Google Scholar]
- Sayre RM, Starr MP. Pasteuria penetrans (ex Thorne, 1940) nom. rev., comb. n., sp. n., a mycelial and endospore-forming bacterium parasitic in plant-parasitic nematodes. Proceedings of the Helminthological Society of Washington. 1985;52:149–165. [Google Scholar]
- Sayre RM, Starr MP. Bacterial diseases and antagonisms of nematodes. In: Poinar GO Jr, Jansson H-B, editors. Diseases of nematodes. Boca Raton, FL: CRC Press; 1988. pp. 69–101. [Google Scholar]
- Sayre RM, Wergin WP. Bacterial parasite of a plant nematode: Morphology and ultrastructure. Journal of Bacteriology. 1977;129:1091–1101. doi: 10.1128/jb.129.2.1091-1101.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayre RM, Wergin WP, Schmidt JM, Starr MP. Pasteuria nishizawae sp. nov., a mycelia and endospore-forming bacterium parasitic on cyst nematodes of genera Heterodera and Globodera. Research in Microbiology. 1991;142:551–564. doi: 10.1016/0923-2508(91)90188-g. [DOI] [PubMed] [Google Scholar]
- Schmidt LM, Preston JF, Dickson DW, Rice JD, Hewlett TE. Environmental quantification of Pasteuria penetrans endospores using in situ antigen extraction and immunodetection with a monoclonal antibody. FEMS Microbiology Ecology. 2003;44:17–26. doi: 10.1111/j.1574-6941.2003.tb01086.x. [DOI] [PubMed] [Google Scholar]
- Schmidt LM, Mouton L, Nong G, Ebert D, Preston JF. Genetic and Immunological Comparison of the Cladoceran Parasite Pasteuria ramosa with the Nematode Parasite Pasteuria penetrans. Applied Environmental Microbiology. 2008;74(1):259–264. doi: 10.1128/AEM.01778-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serracin M, Schuerger AC, Dickson DW, Weingartner DP. Temperature dependent development of Pasteuria penetrans in Meloidogyne arenaria. Journal of Nematology. 1997;29(2):228–238. [PMC free article] [PubMed] [Google Scholar]
- Starr MP, Sayre RM. Pasteuria thornei sp. nov. and Pasteuria penetrans sensu strict emend., mycelial and endospore-forming bacteria parasitic respectively, on plant-parasitic nematodes of the genera Pratylenchus and Meloidogyne. Annuals of the Institute Pasteur Microbiology. 1988;139:11–31. doi: 10.1016/0769-2609(88)90094-4. [DOI] [PubMed] [Google Scholar]
- Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceedings of the National Academy of Sciences (USA) 2004;101:11030–11035. doi: 10.1073/pnas.0404206101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins GD, Gibson TJ. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties, and weight matrix choice. Nucleic Acids Research. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KH, Hooks CRR. Plant-parasitic nematodes and their associated natural enemies within banana (Musa spp.) plantings in Hawaii. Nematropica. 2009;39(1):57–73. [Google Scholar]
- Weibelzahl-Fulton E, Dickson DW, Whitty EB. Suppression of Meloidogyne incognita and M. javanica by Pasteuria penetrans in field soil. Journal of Nematology. 1996;28:43–49. [PMC free article] [PubMed] [Google Scholar]