Abstract
Meloidogyne polycephannulata n. sp. is described from specimens collected from an area cultivated with carrot cv. Brasilia, in the city of Rio Paranaíba, in the region of Alto Paranaíba, Minas Gerais State, Brazil. The perineal pattern of the female is circular to ovoid with a high dorsal arch that has widely spaced, coarse annulations. The lateral field may have a deep furrow separating the dorsal and ventral arches. The medial lips are short and wide, whereas the lateral lips are large and triangular. The female stylet is 15-16 μm long with wide knobs, distinctly divided by an indentation in the center. Its tip is slightly curved dorsally. The excretory pore opens 34-65 μm from the anterior end. Females retain eggs and second-stage juveniles in their body cavity, similar to that of the cyst-forming nematodes. Males are 1.3-1.7 mm long and have a high head cap that is rounded and slopes posteriorly. The labial disc is fused to the medial lips. The head region has several irregular annulations that are similar in appearance to the first or second body annules that are likewise irregular, making the head region appear to be extremely large. The stylet of the male is 21-24 μm long; it is slender, and has small, rounded knobs, that are distinctly indented medially and appear heart-shaped. The shaft has several tiny projections throughout its length. Mean second-stage juvenile length is 411.7 μm. The juvenile head cap is elevated, the medial lips are small, and the lateral lips are elongate to triangular-shaped. The head region has several short, incomplete and irregular transverse annulations. The juvenile stylet is 14-23 μm long with small, rounded, and sloping knobs. The thin tail ends with a short hyaline portion that is variable in size (16-26 μm) and with a small, rounded tip. Isozyme profiles of esterases from Meloidogyne javanica show 3 strong bands (SB) at Rm 46, 59, and 66; profiles of M. polycephannulata n. sp. show a SB at Rm 47 and a weak band (WB) at Rm 52; M. petuniae has two SB at Rm 44 and 53; M. phaseoli has a SB at 53, 58, and 64 Rm; M. brasilensis has three SB at Rm 40, 58, and 66 and a WB at Rm 71; M. pisi has a SB at Rm 40, 60, and 64 and two WB at 46 and 50 Rm. Data from sequencing the 18S rDNA region of M. polycephannulata n. sp. confirms that it is different from M. arabicida, M. arenaria, M. ethiopica, M. incognita, M. javanica, M. paranaensis, and M. thailandica. Sequence identity among these eight species ranged between 85 to 93.4%. Meloidogyne polycephannulata n. sp. reproduces very well on carrot and tomato; poorly on pepper; and not at all on cotton, peanut, tobacco, watermelon, and sweet corn.
Keywords: Daucus carotae, description, host range, Meloidogyne brasilensis, M. javanica, M. petuniae, M. phaseoli, M. pisi, morphology, morphometrics, scanning electron microscopy, taxonomy
Carrot plants (Daucus carotae L.) cv. Brasilia, collected from a commercial field in area #46, owned by Luiz Sasaki, city of Rio Paranaíba, in the region of Alto Paranaíba, Minas Gerais State, Brazil, were severely infected with root-knot nematodes. Symptoms included chlorosis, stunting, galled roots and the main taproot had numerous cracks, constrictions, and other deformities. The infected roots were examined in the EMBRAPA/CNPH (National Researcher Center for Vegetable Crops) Nematology Laboratory, Brasilia, Brazil.
Additional work on morphology and morphometrics was conducted at the Virginia Polytechnic Institute and State University and the host range was determined at the research center of EMBRAPA/CNPH in Brasilia, Brazil. Several unusual morphological characters and a unique host range were identified, which indicated that this root-knot nematode could be a new species. Although the perineal pattern of the female was somewhat similar to that of M. incognita (Kofoid & White, 1919) Chitwood, 1949, the head region of the female, male, and second-stage juvenile (J2) contained numerous short, irregular annulations that were different compared to any other described species. In addition, the unique biology in which the eggs and second-stage juveniles were retained inside the cuticle of the dead female has not been reported for any other known species of the genus Meloidogyne. Meloidogyne polycephannulata n. sp. is described herein, and the common name ‘carrot root-knot nematode’ is proposed.
This is the first report of a new species of root-knot nematode parasitizing carrot in Brazil. The four most common root-knot nematodes species, M. arenaria (Neal, 1889) Chitwood, 1949; M. hapla Chitwood, 1949; M. incognita; and M. javanica (Treub, 1885) Chitwood, 1949, are widely distributed in Brazil. Meloidogyne incognita and M. javanica are the more important species because they damage several species of vegetables and other cash crops. The Meloidogyne species previously described from Brazil are M. exigua Göldi, 1887; M. coffeicola Lordello & Zamith, 1960; and M. paranaensis Carneiro, et al., 1995 infecting coffee, Coffea arabica L. (Göldi, 1887; Lordello & Zamith, 1960; Carneiro, et al., 1995).
In the past several years, four new species of root-knot nematodes have been described from Brazil including M. petuniae Charchar et al., 1999 on Petunia hibrida L. (Charchar et al., 1999); M. brasilensis Charchar & Eisenback, 2002 on root-knot resistant tomato cv. Rossol (Solanum lycopersicum L.) (Charchar & Eisenback, 2002); M. pisi Charchar et al., 2008 on pea (Pisum sativum L.) cv. Mikado (Charchar, et al., 2008), and M. phaseoli Charchar et al., 2008 on bean (Phaseolus vulgaris L.) cv. Carioca (Charchar et al., 2008).
Materials and Methods
A stock culture of Meloidogyne polycephannulata n. sp. was established from a single egg mass from carrot cv. Brasilia collected from the type locality and propagated on tomato cv. Rutgers. The culture was kept in a greenhouse between 22-28°C. All nematodes used in morphology and morphometric studies were obtained from these cultures.
Morphological studies: Males and J2 were extracted from washed, galled roots and placed in a moist chamber. Light microscopy (LM) observations were made from specimens stored in a refrigerator for at least 48 hours and killed by gentle heat. Fixed specimens were always compared to live specimens mounted in 0.9% saline solution. Females, males, and J2 were prepared for scanning electron microscopy (SEM) according to Eisenback (1985). Perineal patterns were prepared for SEM according to Charchar & Eisenback (2001). Eggs were measured in fresh tap water. All LM observations were made with a bright field microscopy and at least 100 specimens were observed. Measurements were made of females in 2% glutaraldehyde in 0.1 M cacodylic acid buffer, pH 7.2, perineal patterns mounted in glycerin, and males and J2 were mounted in fresh tap water and killed with gentle heat from an alcohol lamp. Thirty specimens from each life-stage were randomly selected for measurements.
Host range test: Seedlings of carrot (cvs. Alvorada, Brasilia, Carandai, Nantes, and Kuronan), tomato (cv. Rutgers), tobacco (Nicotiana tabacum L. cv. NC 95), cotton (Gossypium hirsutum L. cv. Deltapine 61), pepper (Capsicum annuum L. cv. California Wonder), peanut (Arachis hypogea L. cv. Florunner), watermelon (Citrullus vulgaris Schard. cv. Charleston Gray), and sweet corn (Zea mays L. cv. Golden Cross Bantam) were transplanted as single plants to 11 cm diameter clay pots containing 500 cm3 of sterilized sandy loam soil. Transplants were inoculated with a suspension of 300 freshly hatched juveniles in 5 ml of water placed in shallow depressions around the root system. Each treatment was replicated three times and the plants were maintained at 22-28°C in a greenhouse. Roots were washed and stained with phloxine B (Dickson & Struble, 1965) to visualize egg masses for counting.
Esterase phenotypes: Phenotypes of esterases were prepared according to Esbenshade and Triantaphyllou (1985). Females of previously characterized populations of M. javanica were used for comparison.
DNA extraction: Genomic DNA was extracted from eight females using a modified 2x CTAB protocol with organic solvents.
PCR amplification of Mitochondrial DNA: The Primer pairs ‘C2F3’ (5’-GGTCAATGTTCAGAAATTTGTGG-3’) and ‘1108’ (5’-TACCTTTGACCAATCACGCT-3’) described by Powers & Harris (1993) were used to amplify the mitochondrial region located between the 3’ end of cytochrome oxidase subunit II (COII gene) and the 5’ end of the16S rRNA gene. ‘High fidelity’ PCR was performed in a 50 μL volume containing 50 mM Tris (pH 9.2), 16 mM ammonium sulphate, 1.75 mM MgCl2, 350 μM dNTPs, 400 pM of primers, 3 μL of DNA, 0.2 U of Platinum Taq DNA polymerase (Invitrogen, USA). Amplification was carried out using the following three steps: (1) one denaturation cycle at 94°C for 2 min; (2) 10 cycles of: denaturation at 94°C for 10 s, annealing at 48°C for 30 s and extension at 68°C for 2 min and (3) 25 cycles of: denaturation at 94°C for 10 s, annealing at 48°C for 30 s and extension at 68°C for 2 min plus 20 s added to every consecutive cycle. Electrophoresis was performed on a 1% TAE agarose gels.
DNA sequencing: Anti-sense and sense strands of the PCR products were directly sequenced using BigDye version 3.1 ABI PRISM™ (Perkin-Elmer Corp., Foster City, CA, USA) and the same primers that were used for amplification. DNA was analyzed in an automatic ABI Prism sequencer model 3100 (Applied Biosystems of Brazil, São Paulo-SP) at the Genomic Analysis Laboratory of the CNPH.
Sequence analysis: Sequences were assembled using SeqMAN 4.0 (Lasergene, Madison, WI, USA). Alignments were performed using Clustal W available on the Megalign package (Lasergene, Madison, WI, USA). The sequences used for comparison with M. polycephannulata n.sp. were obtained from GenBank (http://www.ncbi.nlm.nih.gov/Genbank/).
Results
Meloidogyne polycephannulata n. sp. (Figs. 1-8) (Table 1)
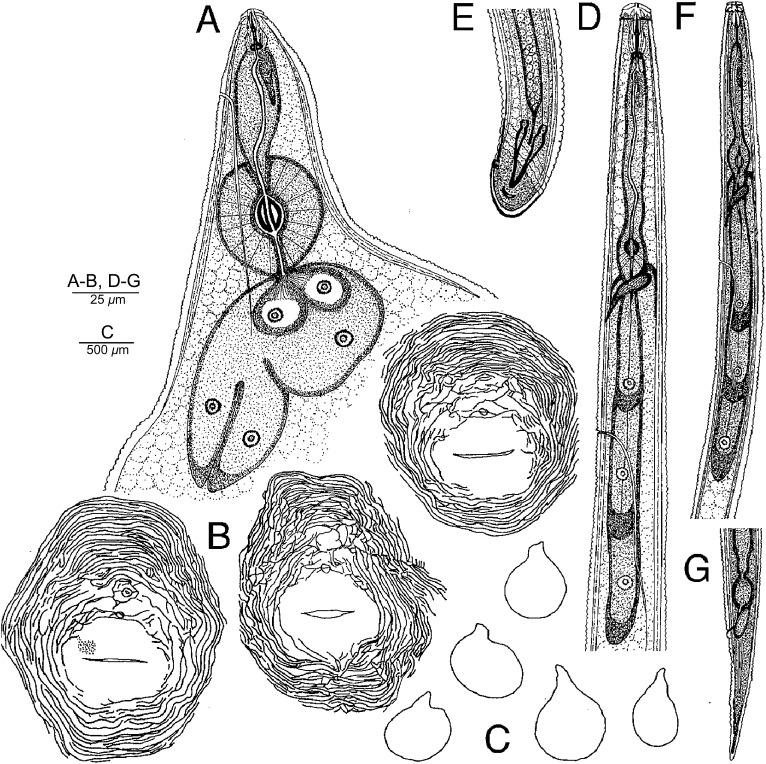
Fig. 1.
Drawings of female, male, and second-stage juvenile of Meloidogyne polycephannulata n. sp. A) Anterior end, lateral view. B) Perineal patterns. C) Outlines of whole female specimens, lateral view. D) Anterior end of male, lateral view. E) Male tail, ventral view. F) Anterior end of second-stage juvenile, lateral view. G) Tail of second-stage juvenile, lateral view.
Fig. 8.
Clustal alignment of the M. polycephannulata n. sp. amplicon sequence with available GenBank acessions: 1 = M. arabicida (AY942852), 2 = M. arenaria (EU364879), 3 = M. ethiopica (AY942848), 4 = M. incognita (AY635611), 5 = M. javanica (AY635612), 6 = M. paranaensis (AY942851), 7 = M. thailandica (EU364883), 8 = M. polycephannulata n. sp. (Bankit 1157541), and 9 = Meloidogyne sp. (AY942858).
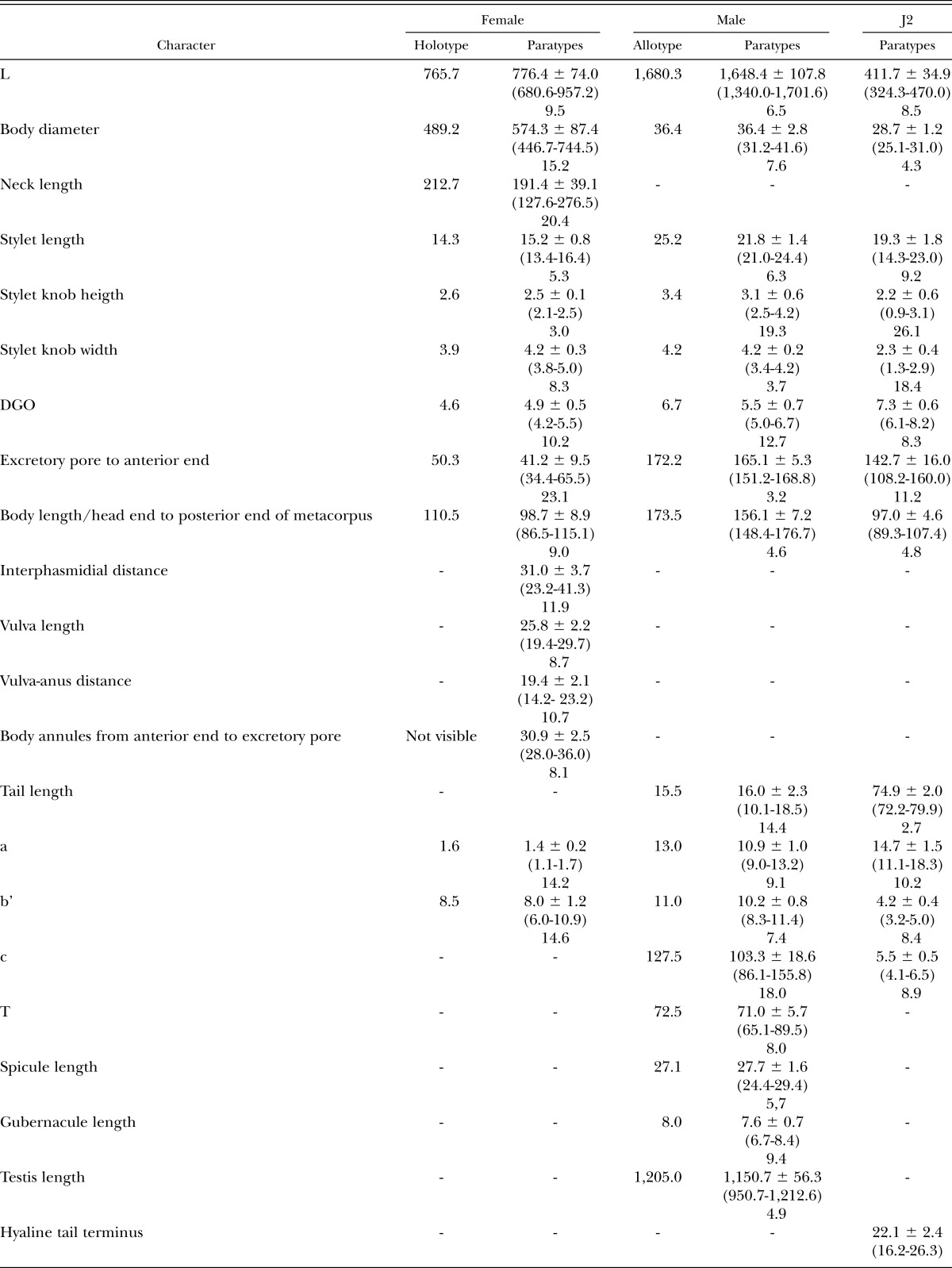
Table 1.
Measurements of 30 females, males and second-stage juveniles (J2) of Meloidogyne polycephanulata n. sp. [all measurements in μm and in the form: mean ± SD (range) coefficient of variation].
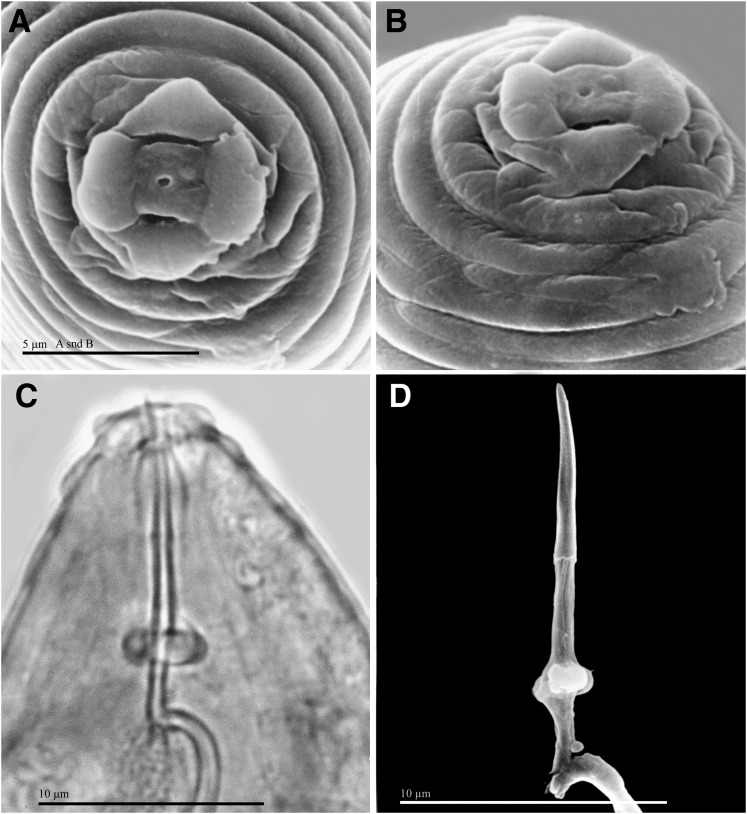
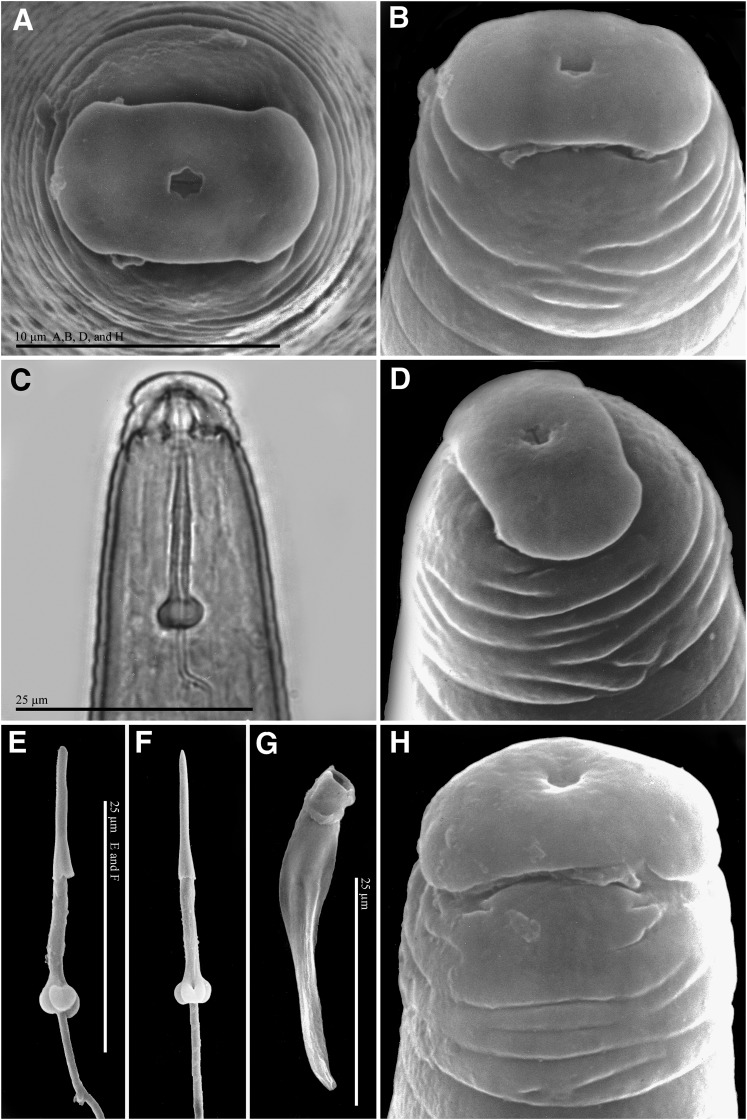
Female: Body translucent white, variable in size, pear-shaped to ovoid with short, thick neck, posteriorly rounded without tail protuberance (Fig. 1 A, C). In SEM, stoma slit-like, located in ovoid prestoma, surrounded by pit-like openings of six inner labial sensilla (Fig. 2 A, B). Labial disc squarish, slightly indented on one or both sides, and fused with the medial lips. Medial lips kidney-shaped short and wide. Lateral lips large, triangular-shaped, separated from medial lips and head region. Head region distinctly set off from regular body annules, often marked with several distinct, incomplete annulations, interrupted by transversal and longitudinal striae. In LM, cephalic framework strong, hexaradiate, lateral sectors slightly enlarged (Fig. 1 A). Vestibule and extension prominent. Cephalids and hemizonids not observed. Excretory pore located posterior to stylet base, variable in distance from the anterior end (34.4-65.5 μm); excretory duct very long. Stylet robust; cone base wider than shaft (Fig. 2 C, D). Shaft cylindrical, same width throughout, smaller than cone. Cone narrowing gradually posteriorly, tip slightly curved dorsally; stylet knobs wide; distinctly divided by an indentation in the center. Lumen lining with large, rounded thickenings between stylet base and median bulb. Distance of dorsal pharyngeal gland orifice (DGO) to the anterior end variable 4.2-5.0 μm; orifice branched into three channels; dorsal gland ampulla large. Subventral gland orifices branched, located immediately posterior to enlarged triradiate lumen lining of metacorpus. Metacorpus rounded to ovoid. Pharyngeal glands large, trilobed, with one large uninucleate dorsal lobe; two smaller nucleated subventral lobes, usually posterior to dorsal lobe; two pharyngo-intestinal cells, rounded, nucleated, located between metacorpus and intestine. Two ovaries and six rectal glands present, as characteristic of the genus.
Fig. 2.
Females of Meloidogyne polycephannulata n. sp. A, B) Scanning electron micrographs (SEM) of anterior end, face and lateral views, respectively. C) Light micrograph of anterior end, lateral view. D) SEM of excised stylet.
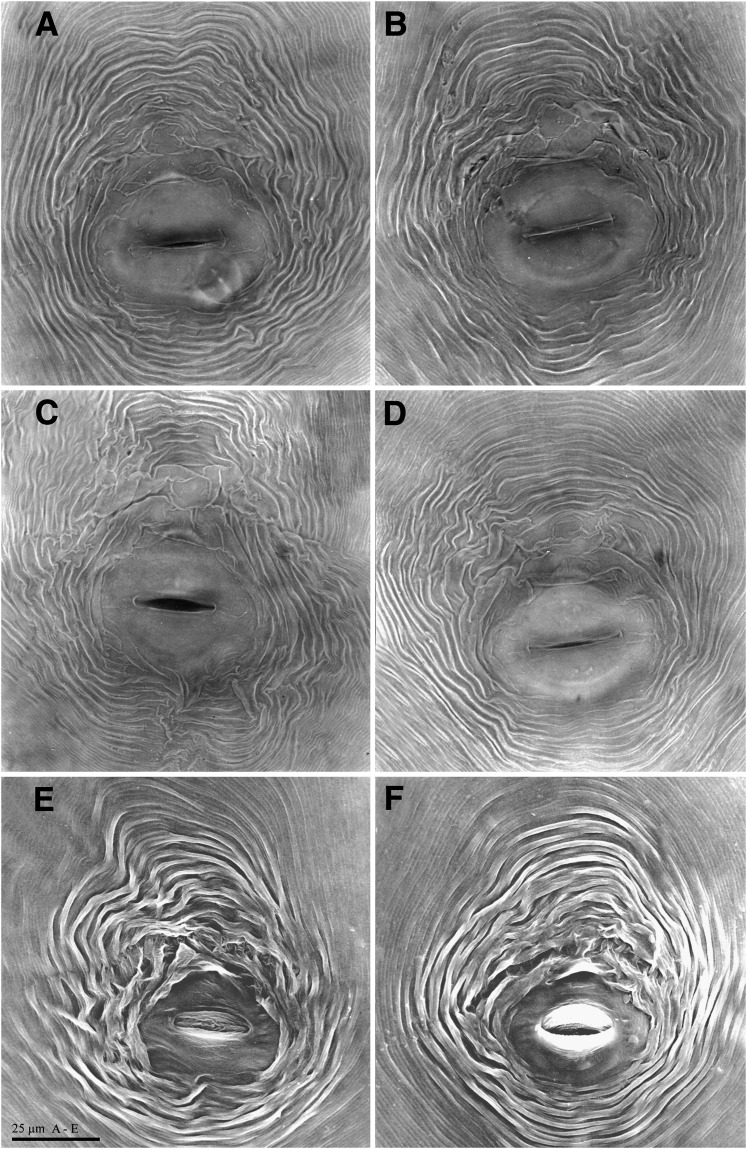
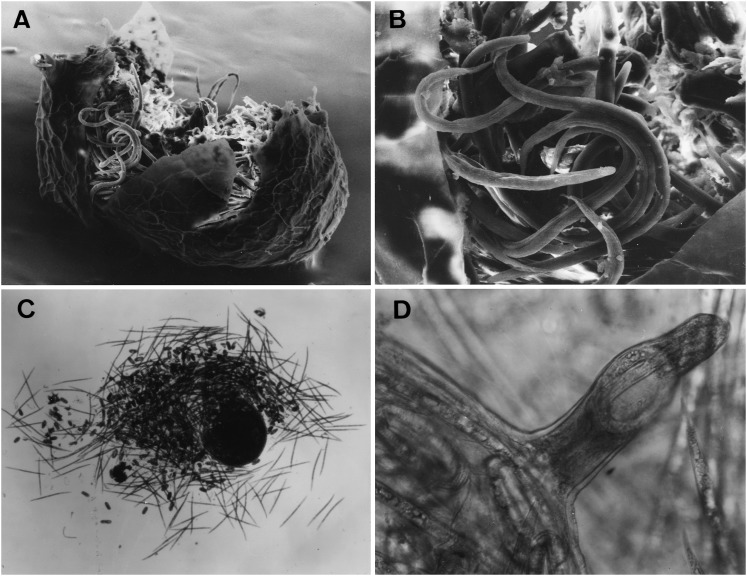
Perineal patterns of M. polycephannulata n. sp. vary from circular to ovoid. (Fig. 3) Dorsal arch flattened to high, rounded and widely spaced with coarse striations. Lateral fields may have wing-like striae on one side. Ventral striae varying from wavy to coarse striations. The lateral fields may be marked by discrete transverse striae. Tail tip area well-defined with discrete striations in most specimens. Perivulval region discretely striated, with punctations on the left side anterior to the vulva in some specimens. Phasmid small, surface structure not apparent in SEM. Anus often covered by a thick cuticular fold. The female of M. polycephannulata n. sp. induces galls in host roots and produces eggs externally in a gelatinous matrix; in addition she can retain the eggs and second-stage juveniles in the body shell that persists from the dead female (Fig. 4).
Fig. 3.
Perineal patterns of females of Meloidogyne polycephannulata n. sp. A-D) Light micrographs. E-F) Scanning electron micrographs.
Fig. 4.
Females of Meloidogyne polycephannulata n. sp. A) Scanning electron micrographs (SEM) of female body cuticle retaining eggs and second-stage juveniles. B) SEM of second-stage juveniles retained in the body of the female. C) Light micrograph (LM) of a mashed female body showing the eggs and second-stage juveniles that were retained. D) LM of the neck region of a female showing retained eggs and second-stage juveniles.
Male: Body translucent white, vermiform, tapering anteriorly, bluntly rounded posteriorly; tail twisting through 90° in heat-killed specimens (Figs, 1, 5). Head cap high, in face and lateral views, tapering posteriorly; head region elevated, distinctly set off from the body (Figs, 5 A-D, H). Hexaradiate cephalic framework well-developed; vestibule and extension distinct. Stoma slit-like, located in large hexagonal prestoma, surrounded by pore-like openings of six inner labial sensilla. In SEM (face view) labial disc rounded and large, fused with the medial lips. Medial lips large, crescent-shaped, sloping posteriorly. Four large cephalic sensilla are evident as depressions on the boundary of the medial lips with the labial disc. Amphidial aperture elongated, slit-like, between labial disc and lateral sectors of head region. Lateral lips fused with head region. Head region very high, with several interrupted annulations, varying from 5 to 8 incomplete annules. Body annules distinct. Four incisures in areolated lateral field. Stylet long, delicate, straight, pointed, gradually decreasing in diameter posteriorly; opening located slightly posterior to the tip; cone base wider than shaft; junction of cone and shaft uneven (Fig, 5 E, F). Shaft cylindrical, marked by numerous very tiny projections throughout visible in live specimens and extracted stylets viewed with SEM. Stylet knobs rounded, large, distinctly divided by an indentation in the center and set off from shaft. Dorsal pharyngeal gland orifice to stylet base of variable length (5.0-6.7 μm), dorsal gland duct branched into three channels, gland ampulla distinct. Procorpus distinctly outlined; metacorpus elongated, oval-shaped with enlarged, tri-radiate cuticular lumen lining; subventral pharyngeal gland orifices branched, located posteriorly in the metacorpus. Pharyngo-intestinal junction weakly sclerotized, at level of nerve ring. Gland lobe variable in length, with two or three nuclei visible. Excretory pore distinct, variable in position (151.2-168.8 μm), terminal duct long. Hemizonid not observed. Intestinal caecum extending anteriorly on dorsal side at level of metacorpus. Usually one testis, rarely two; outstretched, or reflexed anteriorly. Spicules long with thick blade, slightly arcuate very long, slender with narrow head, long and narrow shaft, blade without vellum, single tip (Fig. 5 G). Gubernaculum distinct, crescent-shaped. Tail short and rounded. Phasmids each with slit-like opening in lateral field at level of cloaca.
Fig. 5.
Males of Meloidogyne polycephannulata n. sp. A, B, D & H) Scanning electron micrographs (SEM) of anterior end, face (A) and lateral views (B, D, & H). C) Light micrographs of anterior end, lateral view. E, F,) SEM of excised stylet. G) SEM of excised spicule.
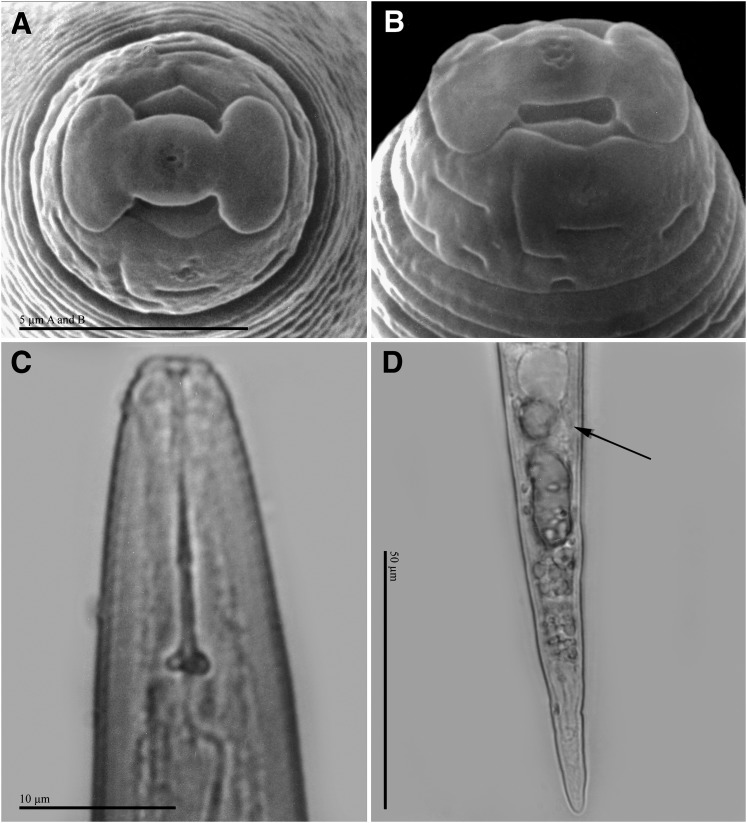
Second-stage juvenile: Body translucent white, vermiform, slender, tapering posteriorly (Fig. 1 F, G). Stoma slit-like, located in oval-shaped prestoma, surrounded by pore-like openings of six inner labial sensilla (Fig. 6 A, B). In SEM (face view) labial disc rounded, slightly raised above medial lips by a rounded groove, adjacent submedial lips fused with one another and with labial disc to form dumbbell-shaped lip structure; medial lips reniform. Lateral lips narrow, crescent-shaped, distinctly separated from labial disc and medial lips. Amphidial apertures wide, elongate, located between labial disc and lateral lips. Head region elevated, distinctly set off from body, and marked with numerous incomplete annulations Fig. 6 C-D). Body annulation distinct. Lateral field marked by four incisures and areolations. Cephalic framework weak; vestibule and extension distinct. Stylet moderately long, but delicate, cone sharply pointed, shaft cylindrical, may widen slightly posteriorly; knobs elongated, set off from shaft; knobs slope slightly (Fig. 6 C). Distance of dorsal pharyngeal gland orifice to stylet base moderately long, variable in position (6.1-8.2 μm); orifice branched into three channels; ampulla poorly defined. Procorpus distinct, metacorpus ovoid with distinct lumen lining; subventral pharyngo-intestinal junction weakly scerotized, at the level of nerve ring. Pharyngeal gland lobe variable in length with three small nuclei. Excretory pore distinct, variable in position (108.2-160.0μm), terminal duct long. Hemizonid not observed. Tail slender, ending in slightly rounded tip (Fig. 6 D). Hyaline tail terminus small, length variable (16.2-26.3 μm). Phasmid openings small, distinct, located posterior to level of anus; rectum dilated.
Fig. 6.
Second-stage juveniles of Meloidogyne polycephannulata n. sp. A, B) Scanning electron micrographs of head, face and lateral views. C) Light micrographs (LM) of head, lateral view. D) LM of tail, lateral view (arrow indicates rectum).
Eggs (n=30): Length 70.2-101.4 μm (879.6±6.6) coef. var. = 7.4; width 36.4-49.4 (40.5 μm ± 3.0) coef. v. 7.4; and length/width ratio of 1.9-2.5 (2.2 ± 0.2), coef. v. = 9.1.
Type host and locality: Roots of carrot plants (Daucus carotae L. cv. Brasilia), found on a commercial field located in the farm no. 46, owned by Makoto Edson Sekita, in the town of Rio Paranaíba, Alto Paranaíba region, Minas Gerais State, Brazil.
Type specimens: Holotype (female): Isolated from a culture that originated from a single egg mass and maintained in a greenhouse on tomato cv. Rutgers deposited in the USDA Nematode Collection (USDA-NC), Beltsville, Maryland (slide T-620t). Original population derived from type locality and host. Male: Same data as holotype, USDA-NC, Beltsville, Maryland. USDA Nematode Collection (USDA-NC), Beltsville, Maryland (slide T-621t). Paratypes (4 females, 4 males, and 4 J2): Same data as holotype (slide T-5671p). Paratypes (12 females, 18 males, and 24 J2) were deposited in the Virginia Tech Nematode Collection, Blacksburg, Virginia. (Slides T-1014, T-1015, and T-1016).
Diagnosis and Relationships
Meloidogyne polycephannulata n. sp. can be distinguished from the most common species of root-knot nematodes (M. arenaria, M. hapla, M. incognita and M. javanica) and other species in the genus (Eisenback et al., 1981) by the shape of the perineal pattern and the shape and morphology of the head and stylet in the female; the shape and morphology of the head and stylet in the male, and the morphology of the head in the second-stage juvenile. The morphology of the stylet of the male is very similar to that of M. arenaria, whereas, the perineal pattern morphology is similar to that of M. incognita. The lateral lips of the female are elongated, and crescentic to triangular shaped, thus differing from all other species. The head region of the female has several transversal and longitudinal annulations, that have not been observed in other species of root-knot nematodes. The stylet knobs of the female and male of M. polycephannulata n. sp. are slightly bigger than those of M. brasilensis (Charchar & Eisenback, 2002) and smaller than those observed in the four most common species. The length of the stylet of females of M. polycephannulata n. sp. is similar to that of a number of species (i.e. M. africana Whitehead, 1960, M. arenaria, M. kikuyensis de Grisse, 1961, M. mali Ohshima & Ichinohe, 1969, M. megadora Whitehead, 1968, M. oryzae Maas et al., 1978, M. enterolobii Yang & Eisenback, 1983, M. citri Zhang et al., 1991, M. pisi Charchar et al., 2008, M. incognita, and M. izalcoensus Carneiro et al., 2005); however, it differs from all of these, except for M. arenaria, in the distance of the DGO to the base of the stylet. Meloidogyne polycephannulata n. sp. differs from M. arenaria in the distance of the excretory pore to the anterior end of the female and by the distance between the phasmids in the perineal pattern. Although the distance from the excretory pore to the anterior end of the female is variable in most root-knot nematode species, the distance in M. polycephannulata n. sp. is long (34.4-65.5 μm), the pore opening between the stylet base and median bulb, is located 28-33 annules from the head region. The female of M. polycephannulata n. sp. is associated with galls in the host roots and it produces eggs externally in a gelatinous matrix as is the case for other species of root-knot nematode. However some individuals of M. polycephannulata n. sp. retain the eggs and J2s inside the dead cuticle of female similar to that of cyst nematodes.
Males of M. polycephannulata n. sp. have numerous short, irregular annules on the head region. Most males of root-knot nematodes have one large head annule, that in some species may be divided by a few irregular or incomplete annulations as described by Eisenback & Hirschmann, 1980. In SEM, males of M. polycephannulata n. sp. appear to have annulations on the head region that may vary from 5 to 8 incomplete annules. The head region is very high and distinctly set-off from body. The stylet of M. polycephannulata n. sp. is delicate with small knobs, very tiny projections occur throughout the shaft that are much smaller than those observed in M. konaensis and M. brasilensis (Eisenback et al., 1994; Charchar & Eisenback, 2002). The stylet length of the males of M. polycephannulata n. sp. is similar to that of M. litoralis Elmiligy, 1968, M. mersa Siddiqi & Booth, 1991, M. jianyangensis Yang, et al., 1990, M. ardenensis Santos, 1968, M. arenaria, M. californiensis Abdel-Rahman & Maggenti, 1987, M. cruciani Garcia-Martinez, et al., 1982, M. kirjanovae Terenteva, 1965, M. oteifae Elmiligy, 1968, and M. platani Hirschmann, 1982; however it differs from all of these by the distance of the DGO to the base of the stylet.
In SEM, the head region of the second-stage juveniles is very high, set off from the body, with several incomplete annules. The body length of M. polycephannulata n. sp. is similar to that of M. morrocciensis Rammah & Hirschmann, 1990, M. turkestanica Shagalina, et al., 1984, M. baetica Castillo, et al., 2003, M. fallax Karssen, 1996, M. mingnanica Zhang, 1993, M. ethiopica Whitehead, 1968, M. ulmi Palmisano & Ambrogioni, 2000, M. indica Whitehead, 1968, M. kongi Yang, et al., 1988, and M. fugianensis Pan, 1985; however, it differs from all of these by the very long stylet , very long distance from the DGO to the base of the stylet, and by the very long tail.
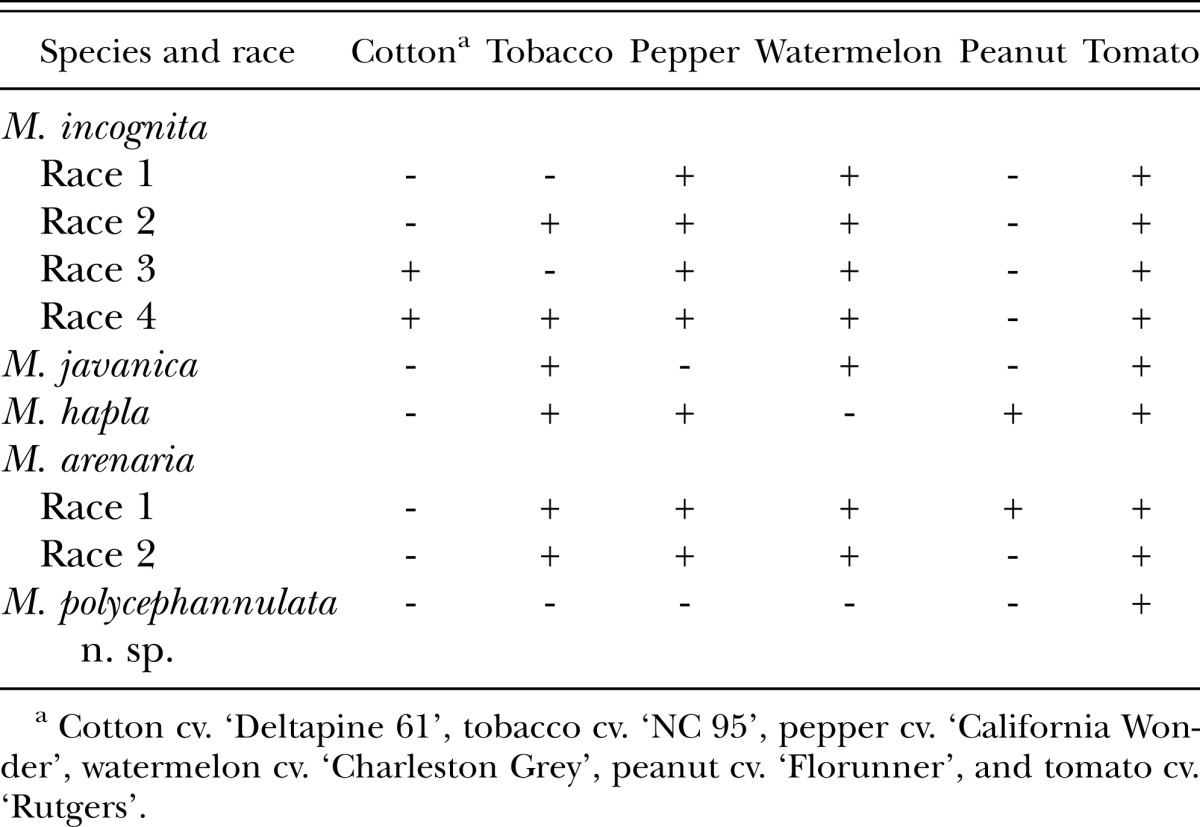
The differential host test showed that M. polycephannulata n. sp. reproduces very well on carrot (cvs. Alvorada, Brasilia, Carandai, Nantes, and Kuronan) and tomato cv. Rutgers, but poorly on pepper, and not at all on tobacco, peanut, watermelon, cotton, and sweet corn. Therefore, M. polycephannulata n. sp. can be easily differentiated from the four most common root-knot nematodes by host specificity, since pepper and watermelon are good hosts for all races of M. incognita; watermelon and tobacco are good hosts for M. javanica; tobacco, pepper, and peanut are good hosts for M. hapla; and tobacco, pepper and watermelon are good hosts for both races of M. arenaria (Table 2). The only good host for M. polycephannulata n. sp. in the North Carolina differential host test (Hartmann & Sasser, 1984) is tomato.
Table 2.
Comparison of response of M. polycephannulata n. sp. to the four most common species of root-knot nematodes and their host races based on the North Carolina differential host test (Hartmann & Sasser, 1984).
Etymology: The specific epithet is derived from the presence of numerous annules in the head region of the male.
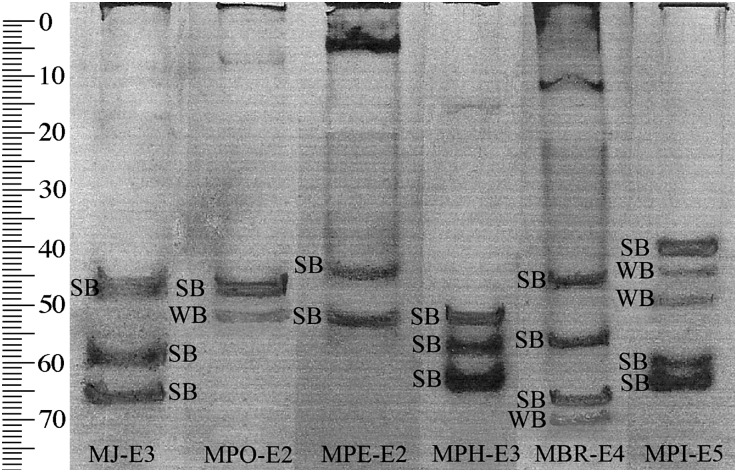
Isozyme phenotype: Isozyme profiles of esterases are illustrated in Fig. 7 for M. javanica, M. polycephannulata n. sp., M. petuniae, M. phaesoli, M. brasilensis, and M. pisi. Meloidogyne M. javanica has 3 strong bands (SB) at Rm 46, 59, and 66; M. polycephannulata n. sp. has a SB at Rm 47 and a weak band (WB) at Rm 52; M. petuniae has two SB at Rm 44 and 53; M. phaseoli has SB at 53, 58, and 64 Rm; M. brasilensis has three SB at Rm 40, 58, and 66 and a WB at Rm 71; M. pisi has SB at Rm 40, 60, and 64 and two WB at 46 and 50 Rm. The phenotype of M. polycephannulata n. sp. is similar to that of M. incognita, except for the occurrence of a second weak band at Rm 52. These two species may have similar esterase isozyme patterns; however, M. exigua and M. chitwoodi also share an identical esterase phenotype (Esbenshade and Triantaphyllou, 1990).
Fig. 7.
Esterase phenotypes of Meloidogyne javanica (MJ-E3), M. polycephannulata n. sp. (MPO-E2), M. petuniae (MPE-E2), M. phaseolus (MPH-E3), M. brasilensis (MBR-E4), and M. pisi (MPI-E5). Meloidogyne javanica is type E3 with three strong bands (SB), M. polycephannulata n. sp. is type E2 with two bands, one SB and one weak (WB) band, SB1 and WB1 have the medium migration speed (M), M. petuniae is type E2 with two SB, M. phaseolus is type E3 with three SB, M. brasilensis is type E4 with four bands, SB1, SB2, SB3 and WB1. SB1 has a slow migration speed (S), SB2 is medium (M), SB3 and WB1 are very fast (VF), and M. pisi is type E5 with three SB and two WB.
DNA amplification and sequence analysis: A single amplicon of 750 bp was obtained with the PCR primers ‘C2F3’ and ‘1180’ (Powers & Harris, 1993) using genomic DNA of M. polycephannulata n. sp. as template, indicating the primer pair specificity. The direct sequence obtained from this single DNA band was compared with eight other nematode COII genes available at GenBank (Fig. 8). The percent identity of M. polycephannulata n. sp. with other species of root-knot was 85% (M. paranaensis); 91% (M. arabicida Lopez & Salazar, 1989); and 93% (M. arenaria, M. ethiopica, M. javanica, M. incognita, and M. thailandica Handoo et al., 2005). Large deletions were observed in M. incognita (31 nucleotides) and M. paranaensis (46 nucleotides) compared with M. polycephannulata n. sp.
Remarks
The carrot root-knot nematode, M. polycephannulata n. sp., is the first species to be described from carrot in Brazil. This new species may become a very serious agricultural problem in the future because of its ability to parasitize the two most important vegetables crops (i.e. carrot and tomato) that are extensively cultivated throughout Brazil. M. polycephannulata n. sp. is able to parasitize several carrot cultivars (Alvorada, Brasilia, Carandai, Nantes, and Kuronan) and tomato cultivars (Rutgers, Santa Clara, and Santa Cruz); however, the cultivars tested of pepper, watermelon, peanut, tobacco, cotton, and corn are not hosts for M. polycephannulata n. sp. These plants may be useful in crop rotation programs in Brazil to control this nematode in regions where it is a problem.
Additional research is needed to identify resistant cultivars of carrot and tomato for use in crop rotations. Field experiments will be also necessary to evaluate the role of M. polycephannulata n. sp. as a pathogenic agent alone or in combination with other soil organisms as bacteria and fungi. Surveys in other carrot and tomato fields are needed to determine the distribution of M. polycephannulata n. sp. beyond its type locality, and to estimate the economic loss caused by this new species on other vegetable and field crops in Brazil.
Footnotes
This research was funded by CNPq – Conselho Nacional de Desenvolvimento Científico e Tecnológico – Subprograma Biotecnologia/RHAE, and EMBRAPA-Empresa Brasileira de Pesquisa Agropecuária by Acordo de Empréstimo EMBRAPA/BIRD/PRODETAB. Thanks to Dr. Ruy Rezende Fontes, the EMBRAPA/CNPH Head for obtaining the funding to allow the conclusion of this research at the Virginia Polytechnic Institute and State University.
This paper was edited by Luma Albanna.
Literature Cited
- Abdel-Rahman F, Maggenti AR. Meloidogyne californiensis n. sp. (Nemata:Meloidogyninae), parasitic on bulrush, Scirpus robustus Pursh. Journal of Nematology. 1987;19:207–217. [PMC free article] [PubMed] [Google Scholar]
- Carneiro RMG, Carneiro RG, Abrantes I, Castagnone P, Almeida M. Meloidogyne paranaensis n. sp. (Nematoda: Meloidogynidae) a root-knot nematode parasitizing coffee tree from Brazil. Journal of Nematology. 1995;28:177–189. [PMC free article] [PubMed] [Google Scholar]
- Carneiro RMG, Almeida M, Gomes ACM, Hernandez A. Meloidogyne izalcoensis n. sp. (Nematoda: Meloidogynidae), a root-knot nematode parasitising coffee in El Salvador. Nematology. 2005;7:819–832. [Google Scholar]
- Castillo P, Vovlas N, Subbotin S, Troccoli A. A new root-knot nematode, Meloidogyne baetica n. sp. (Nematoda: Heteroderidae), parasitizing wild olive in southern Spain. Phytopathology. 2003;93:1093–1102. doi: 10.1094/PHYTO.2003.93.9.1093. [DOI] [PubMed] [Google Scholar]
- Charchar JM, Eisenback JD. Improved preparation of perineal patterns of root-knot nematodes for SEM. Nematology. 2001;3:717–719. [Google Scholar]
- Charchar JM, Eisenback JD. Meloidogyne brasilensis n. sp. (Nematoda: Meloidogynidae), a root-knot nematode parasitizing tomato cv. Rossol in Brazil. Nematology. 2002;4:629–643. [Google Scholar]
- Charchar JM, Eisenback JD, Charchar MJ, Boiteux ME. Meloidogyne pisi n. sp. (Nematoda: Meloidogynidae), a root-knot nematode parasitizing pea in Brazil. Nematology. 2008;10:479–493. [Google Scholar]
- Charchar JM, Eisenback JD, Charchar MJ, Boiteux ME. Meloidogyne phaseoli n. sp. (Nematoda: Meloidogynidae), a root-knot nematode parasitizing bean in Brazil. Nematology. 2008;10:525–538. [Google Scholar]
- Charchar JM, Eisenback JD, Hirschmann H. Meloidogyne petuniae n. sp. (Nemata: Meloidogynidae), a root-knot nematode parasitizing petunia in Brazil. Journal of Nematology. 1999;31:81–91. [PMC free article] [PubMed] [Google Scholar]
- Chitwood BJ. Root-knot nematodes"–Part I. A revision of the genus Meloidogyne Goeldi, 1887. Proceedings of the Helminthological Society of Washington. 1949;16:90–104. [Google Scholar]
- DeGrisse A. Meloidogyne kikuyensis n. sp., a parasite of Kikuyu grass (Pennisetum clandestinum) in Kenya. Nematologica. 1960;5:303–308. [Google Scholar]
- Dickson DW, Struble FB. A sieving-staining technique for extraction of egg mass of Meloidogyne incognita from soil. Phytopathology. 1965;55:497. [Google Scholar]
- Eisenback JD. Techniques for preparing nematodes for scanning electron microscopy. In: Barker KR, Carter CC, Sasser JN, editors. An advanced treatise on Meloidogyne, vol. 2. Methodology. Raleigh, NC, USA: North Carolina State University Graphics; 1985. pp. 79–105. [Google Scholar]
- Eisenback JD, Bernard EC, Schmidt DP. Description of the Kona root-knot nematode, Meloidogyne konaensis n. sp. Journal of Nematology. 1994;26:363–374. [PMC free article] [PubMed] [Google Scholar]
- Eisenback JD, Hirschmann H. Morphological comparison of Meloidogyne males by scanning electron microscopy. Journal of Nematology. 1980;12:23–32. [PMC free article] [PubMed] [Google Scholar]
- Eisenback JD, Hirschmann H, Sasser JN, Triantaphyllou AC. International Meloidogyne Project. Raleigh, NC, USA: A Cooperative Publication of the Department of Plant Pathology and Genetics, North Carolina State University and the United States Agency for International Development; 1981. A guide to the four most common species of root-knot nematodes (Meloidogyne species) p. 48. [Google Scholar]
- Elmiligy E. Three new species of the genus Meloidogyne Goledi, 1887 (Nematoda: Heteroderidae) Nematologica. 1968;14:577–590. [Google Scholar]
- Esbenshade PR, Triantaphyllou AC. Use of enzyme phenotypes for identification of Meloidogyne spp. Journal of Nematology. 1985;17:6–20. [PMC free article] [PubMed] [Google Scholar]
- Garcia-Martinez R, Taylor AL, Smart GC., Jr Meloidogyne cruciani n. sp., a root-knot nematode from St. Croix (U.S. Virgin Islands) with observations on morphology of this and two other species of the genus. Journal of Nematology. 1982;14:292–303. [PMC free article] [PubMed] [Google Scholar]
- Göldi EA. Relatório sôbre a moléstia do cafeeiro na província do Rio de Janeiro. Arquivos do Museu Nacional. Rio de Janeiro. 1887;8:1–121. [Google Scholar]
- Handoo ZA, Skantar AM, Carta LK, Erbe EF. Morphological and molecular characterization of a new root-knot nematode, Meloidogyne thailandica n. sp. (Nematoda: Meloidogynidae), parasitizing ginger (Zingiber sp.) Journal of Nematology. 2005;37:343–353. [PMC free article] [PubMed] [Google Scholar]
- Hartmann KM, Sasser JN. Identification of Meloidogyne species on the basis of differential host test and perineal pattern morphology. In: Barker KR, Carter CC, Sasser JN, editors. An advanced treatise on Meloidogyne, vol. 2. Methodology. Raleigh, NC, USA: North Carolina State University Graphics; 1984. pp. 69–77. [Google Scholar]
- Hirschmann H. Meloidogyne platani n. sp. (Meloidgynidae), a root-knot nematode parasitizing American sycamore. Journal of Nematology. 1982;14:84–95. [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Ohshima Y, Ichinoe M. A root-knot nematode, Meloidogyne mali n. sp. on apple tree from Japan. Applied Entomology and Zoology. 1969;4:194–202. [Google Scholar]
- Karssen G. Description of Meloidogyne fallax n. sp. (Nematoda: Heteroderidae), a root-knot nematode from The Netherlands. Fundamental and Applied Nematology. 1996;19:593–599. [Google Scholar]
- Lopez R, Salazar L. [Meloidogyne arabicida sp.n, (Nemata:Heteroderidae) from Costa Rica: a new and severe pathogen of coffee.] Turrialba. 1989;39:313–323. [Google Scholar]
- Lordello LGE, Zamath PL. Meloidogyne coffeicola sp. n., a pest of coffee trees in the state of Paraná. Brail. Revista Brasileira de Biologia. 1960;20:375–379. [Google Scholar]
- Maas PWT, Sanders H, Dede J. Meloidogyne oryzae n. sp. (Nematoda: Meloidogynidae) infesting irrigated rice in Surinam (South America) Nematologica. 1978;24:305–361. [Google Scholar]
- Palmisano AM, Ambrogioni L. Meloidogyne ulmi sp. n., a root-knot nematode from elm. Nematologia Mediterranea. 2000;28:279–293. [Google Scholar]
- Pan C. [Studies on plant-parasitic nematodes on economically important crops in Fujian. III. Description of Meloidogyne fujianensis n. sp. (Nematoda: Meloidogynidae) infesting citrus in Nanjing County.] Acta Zooligica Sinica. 1985;32:263–268. [Google Scholar]
- Powers TO, Harris TS. A polymerase chain reaction method for identification of five major Meloidogyne species. Journal of Nematology. 1993;25:1–6. [PMC free article] [PubMed] [Google Scholar]
- Rammah A, Hirschmann H. Meloidogyne morocciensis n. sp. (Meloidogyninae), a root-knot nematode from Morocco. Journal of Nematology. 1990;22:279–291. [PMC free article] [PubMed] [Google Scholar]
- Santos SMA. Meloidogyne ardenensis n. sp. (Nematoda: Heteroderidae), a new British species of root-knot nematode. Nematologica. 1967;13:593–598. [Google Scholar]
- Shalinga L, Ivanova T, Krall E. Two new root-knot nematodes species of the genus Meloidogyne (Nematoda: Meloidogynidae) –Parasites of shrubs and trees. Proceedings of the Academy of Sciences of the Estonian SSR. Biology. 1984;34:279–286. [Google Scholar]
- Siddiqi MR, Booth W. Meloidogyne (Hypsoperine) mersa n. sp. (Nematoda: Tylenchida) attacking Sonnneratia alba trees in mangrove forest in Brunei Darussalam. Afro-Asian Journal of Nematology. 1991;1:212–220. [Google Scholar]
- Terentyeva TG. [A new species of the root-knot nematode, Meloidogyne kirjanovae n. sp. (Nematoda: Heteroderidae)] Mater. Nauch. Konf. Vses Obshch. Gel'mint. 1965;4:277–281. [Google Scholar]
- Whitehead AG. The root-knot nematodes of east Africa. I. Meloidogyne africana n. sp., a parasite of aribica coffee (Coffea arabica L.) Nematologica. 1959;4:272–278. [Google Scholar]
- Whitehead AG. Taxonomy of Meloidogyne (Nematodea: Heteroderidae) with descriptions of four new species. Transactions of the Zoological Society, London. 1968;31:263–401. [Google Scholar]
- Yang B, Hu K, Chen H, Zhu W. [A new species of root-knot nematodes Meloidogyne jianyangensis n. sp parasitizing mandarin orange.] Acta Phytopathologica Sinicia. 1990;20(4):259–264. [Google Scholar]
- Yang B, Eisenback JD. Meloidogyne enterolobii n. sp. (Meloidogynidae), a root-knot nematode parasitizing pacara ear pod tree in China. Journal of Nematology. 1983;15:381–391. [PMC free article] [PubMed] [Google Scholar]
- Yang BJ, Wang QL, Feng RZ. [Meloidogyne kongi n. sp. (Nematoda: Meloidogynidae), a root-knot nematode parasitizing Citrus sp. in Guangxi, China.] Journal of Guanxi Agricultural College. 1989;7(3):1–9. [Google Scholar]
- Zhang Shaosheng. Meloidogyne mingnanica n. sp. (Meloidogynidae) parasitizing Citrus in China. Journal of Fujian Agricultural University (Natural Sciences Edition) 1993;22:69–76. [Google Scholar]
- Zhang SS, Gao RX, Weng ZM. [Meloidogyne citri n. sp. (Meloidogynidae), a new root-knot nematode parasitizing citris in China.] Journal of Fujian Agricultural College. 1991;19:305–311. [Google Scholar]