Abstract
The antibiotic 2,4-diacetylphloroglucinol (DAPG), produced by some strains of Pseudomonas spp., is involved in suppression of several fungal root pathogens as well as plant-parasitic nematodes. The primary objective of this study was to determine whether Wood1R, a D-genotype strain of DAPG-producing P. fluorescens, suppresses numbers of both sedentary and migratory plant-parasitic nematodes. An experiment was conducted in steam-heated soil and included two seed treatments (with Wood1R and a control without the bacterium) and six plant-nematode combinations which were Meloidogyne incognita on cotton, corn, and soybean; M. arenaria on peanut; Heterodera glycines on soybean; and Paratrichodorus minor on corn. Wood 1R had no effect on final numbers of M. arenaria, P. minor, or H. glycines; however, final numbers of M. incognita were lower when seeds were treated with Wood1R than left untreated, and this reduction was consistent among host plants. Population densities of Wood1R were greater on the roots of corn than on the other crops, and the bacterium was most effective in suppressing M. incognita on corn, with an average reduction of 41%. Despite high population densities of Wood1R on corn, the bacterium was not able to suppress numbers of P. minor. When comparing the suppression of M. incognita on corn in natural and steam-heated soil, egg production by the nematode was suppressed in natural compared to steamed soil, but the presence of Wood1R did not result in additional suppression of the nematodes in the natural soil. These data indicate that P. fluorescens strain Wood1R has the capacity to inhibit some populations of plant-parasitic nematodes. However, consistent suppression of nematodes in natural soils seems unlikely.
Keywords: antibiotic, biological control, corn, DAPG, Heterodera glycines, Meloidogyne arenaria, Meloidogyne incognita, Paratrichodorus minor, Pseudomonas fluorescens, root-knot nematode, stubby-root nematode, Zea mays
The antibiotic 2,4-diacetylphloroglucinol (DAPG), produced by some strains of Pseudomonas spp., can inhibit the growth and/or activities of several fungal and oomycete root pathogens (Raaijmakers et al., 2002), as well as some plant-parasitic nematodes (Cronin et al., 1997; Siddiqui and Shaukat, 2003a). Motility of Globodera rostochiensis juveniles was reduced in vitro and in soil microcosms in the presence of P. fluorescens strain F113 compared to controls without the bacterium (Cronin et al., 1997). A DAPG-negative mutant of the bacterium had no effect on nematode motility, but complementation of the mutant with a plasmid containing a 6-kb fragment coding for DAPG synthesis restored nematostatic activity. Motility of G. rostochiensis juveniles was also reduced in the presence of synthetic DAPG. The antibiotic was also implicated in suppression of Meloidogyne javanica on tomato by P. fluorescens strain CHA0 (Siddiqui and Shaukat, 2003a). Root galling caused by the nematode was lowest in the presence of a strain genetically modified to overproduce DAPG, intermediate in the wild-type strain, and greatest in a DAPG-negative strain. In addition to a direct effect on the nematode, the antibiotic may also have an indirect effect by inducing plant defenses (Iavicoli et al., 2003). In a split-root experiment, Siddiqui and Shaukat (2003a) showed that strains that produced DAPG suppressed numbers of M. javanica even when physically separated from the nematode, whereas the DAPG-negative strain did not.
The ability of pseudomonads to colonize the rhizosphere is critical to biological control of root pathogens (Bull et al., 1991; Chin-A-Woeng et al., 2000). Among DAPG-producing fluorescent pseudomonads, there are over a dozen distinct genotypes (McSpadden Gardener et al., 2000; Mavrodi et al., 2001) displaying different levels of rhizosphere competence on various crops (McSpadden Gardener et al., 2005; Landa et al., 2002; Landa et al., 2006; Raaijmakers et al., 1998; Sharifi-Tehrani et al., 1998). Under controlled conditions, the D genotype has shown itself to be particularly competitive and can readily colonize roots to levels in excess of 105 cells per g fresh weight of root in both healthy and diseased plants (Raaijmakers and Weller, 2001; McSpadden Gardener and Weller, 2001). In the field, members of the D genotype were found to be the dominant genotype in the rhizosphere of corn (McSpadden Gardener et al., 2005) and their abundance has been correlated with crop stand and yield in that crop (Rotenberg et al., 2007).
The two strains of DAPG-producing P. fluorescens, F113 and CHA0, previously shown to have activity against plant-parasitic nematodes are members of the K and A genotypes, respectively (McSpadden Gardener et al., 2000). It is unknown whether the D genotype also has activity against nematodes or whether DAPG-producing pseudomonads can suppress migratory plant-parasitic nematodes. The primary objective of this study was to determine whether a D genotype of P. fluorescens (strain Wood1R) suppresses numbers of both sedentary and migratory plant-parasitic nematodes (M. incognita, M. arenaria, Heterodera glycines, and Paratrichodorus minor). In a preliminary study evaluating six DAPG-producing strains, Wood1R was the most effective in reducing penetration of tomato roots by M. incognita (Kiewnick and McSpadden Gardener, unpubl. data). Initially, we tested several genera of plant parasites on four different crops: corn (Zea mays), cotton (Gossypium hirsutum), peanut (Arachis hypogaea), and soybean (Glycine max). In later experiments we focused on M. incognita on corn.
Materials and Methods
Bacterial seed treatment: The Wood1R strain of P. fluorescens was isolated from the rhizosphere of corn in Wood Co., Ohio and selected for rifampicin resistance (McSpadden Gardener et al., 2005). The bacterium was grown for 24 hr at 28 °C on 1/10th tryptic soy agar (TSA) containing 50 μg/ml rifampicin before the cells were scraped from the plate and suspended in sterile distilled water. The suspension was diluted to ca. 5 x 107 cells/ml and 2 ml was added to seed: 75 seeds of corn cv. Pioneer 3223 and soybean cv. Hutcheson and 30 seeds for cotton cv. DP 555 RR and peanut cv. Georgia Green. The seeds were mixed for 2 min to evenly coat the seed. An equal number of control seeds were treated with 2 ml of sterile distilled water.
To determine the density of Wood1R adhering to the seeds of each crop species, five treated and control seeds were placed individually in a 50-ml plastic tube containing 10 ml of sterile distilled water. The tubes were vortexed for 1 min and sonicated for 1 min in an ultrasonic cleaning bath (3510R-MT, Branson Ultrasonics Corp., Danbury, CT) to dislodge bacteria from seed surfaces. Serial dilutions of seed washings were plated on 1/10th TSA amended with rifampicin (50 μg/ml) and cycloheximide (100 μg/ml). After incubation at 28°C for 48 hr, colonies on the plates were counted and the mean population size was determined from the five individual seeds. Average bacterial populations were 3.1 x 104, 2.8 x 104, 3.8 x 104, and 2.2 x 104 CFU/seed on treated corn, soybean, peanut, and cotton seeds, respectively.
Root colonization by bacterium: The density of Wood1R in the rhizosphere of each crop species was assessed at both the time of nematode inoculation (3 wk after planting) and at the end of the experiment when nematodes were extracted from roots or soil (11 wk after planting). Plants set up to determine bacterial colonization were treated the same as other experimental plants except that they were not inoculated with nematodes. At each sampling time, five treated and control plants of each crop species were removed from the soil and the root systems cut from the plant. Soil loosely adhering to the roots was removed and the roots were cut into 1-cm pieces. Root samples (about 1 g) from each individual plant were placed in a 50-ml plastic tube containing 10 ml of sterile distilled water and bacterial populations on roots were recovered and enumerated as described above for seed.
Nematode inoculum: Meloidogyne incognita race 3 and M. arenaria race 1 were cultured on eggplant (Solanum melongena). To obtain second-stage juveniles (J2) for inoculum, roots with egg masses were placed on Baermann pans in a mist chamber (Barker, 1985). After 3 d, the J2 were collected from the pans onto a 25-μm-pore sieve and rinsed with tap water. Heterodera glycines were cultured on soybean (Glycine max). To obtain J2 for inoculum, eggs were freed from cysts using a tissue grinder and placed in a Baermann pan with 16 mMZnSO4 · 7H2O to induce hatching (Timper et al., 1999). After 3 d, the J2 were collected from the pan onto a 25-μm-pore sieve and rinsed with tap water. Paratrichodorus minor was cultured on tall fescue (Lolium arundinaceum) and mixed vermiform stages used for inoculum were extracted from soil by centrifugal flotation (Jenkins, 1964).
Nematode suppression by Wood1R in heated soil: The experiment included two seed treatments (with Wood1R and a control without the bacterium) and six plant-nematode combinations which were M. incognita on cotton, corn, and soybean; M. arenaria on peanut; H. glycines on soybean; and P. minor on corn. Each treatment combination was replicated eight times and the experiment was conducted twice. The soil used in the experiment was a sandy loam (82% sand, 7% silt, 11% clay, 1.5% organic matter; pH 5.4) that had been steam heated at 100 °C for 6 hr to kill potential plant pathogens. The soil was added to 10-cm square pots (700 cm3 of soil) and one seed planted ca. 15 mm deep in each pot. The control seed was planted first to avoid contamination with the bacterium. The pots were inoculated with nematodes 3 wk after planting by distributing 300 mixed stages of P. minor or 2,000 J2 of the other nematodes between two holes (3-cm-deep) at the base of the plant and covering with soil. The treatments were completely randomized on a single bench in a greenhouse where soil temperatures varied between 20 and 35 °C.
Two months after inoculation with nematodes, the tops of the plants were cut and the roots were removed from soil and rinsed with water; the roots were weighed after blotting dry. Eggs of M. incognita and M. arenaria were extracted from the entire root system by cutting the roots into ca. 5-cm pieces, placing them in a 1-liter flask, and agitating for 4 min in a 1% NaOCl solution (Hussey and Barker, 1973). Cysts of H. glycines were extracted by hand-rubbing roots in water, collecting them on a 250-μm-pore sieve nested under an 850-μm-pore sieve, and then removing root and soil debris using centrifugal flotation (Dunn, 1969). Cysts were crushed with a tissue grinder to release the eggs, which were then collected on a 25-μm-pore sieve. Vermiform stages of P. minor were extracted from 150 cm3 of soil from each pot by centrifugal flotation (Jenkins, 1964).
Effects of Wood1R on nematode penetration of corn roots: To determine whether Wood1R reduces root penetration and development of M. incognita in corn, seeds treated with the bacterial strain and control seeds were planted in 10-cm square pots containing 700 cm3 of steam-heated soil as described earlier. The pots were inoculated with 2,000 J2 of M. incognita 3 wk after planting and 2 wk later the plants were removed from the soil. The roots were weighed, cut into ca. 3-cm-long pieces, stained with an acid fuchsin, lactoglycerol solution (Bridge et al., 1982), and homogenized in a blender for 15 sec. Stained nematodes were separated from root debris with nested 850-, 150-, and 25-μm sieves (Timper, 2000). Using an inverted microscope (10x and 40x), the stained nematodes were counted and classified as “developing” if they were larger than the infective J2. The seed treatment and control were replicated seven times each, and the experiment was conducted twice.
Comparison of M. incognita suppression by Wood1R in steamed and unsteamed soil: This 2 x 2 factorial experiment was designed to determine whether Wood1R could suppress M. incognita populations on corn in natural soil. There were two seed treatments, with and without Wood1R, and two soil treatments, steam-heated and natural soil. Each treatment combination was replicated eight times and the experiment was conducted twice. The natural and steamed soil (described in the previous section) was obtained from the Jones Farm, Tifton, GA. The field site had been planted with peanut and was infested with M. arenaria, but not M. incognita. The soil treatments were added to 10-cm square pots (700 cm3 of soil) and one seed planted ca. 15 mm deep in each pot. The pots were inoculated with 2,000 J2 of M. incognita 3 wk after planting and 8 wk later the plants were removed from the soil. The roots were rinsed with water and weighed after blotting dry. Nematode eggs were extracted as described previously.
Statistical analysis: Numbers of nematodes per pot and per gram root were square root-transformed prior to analysis. Analysis of variance was used to determine whether the seed treatment, crop species, soil treatment, trial, or the interaction of these factors influenced nematode numbers, root weight, or root colonization by Wood1R (PROC ANOVA, v. 9; SAS Institute, Cary, NC). If there was a trial x treatment interaction (P ≤ 0.05), the results for each trial are presented separately, otherwise, they are combined. In the first experiment, each nematode species was treated as a different experiment and analyzed separately. Fisher's LSD test was used to determine differences among means (P ≤ 0.05). Means in tables and figures are presented untransformed.
Results
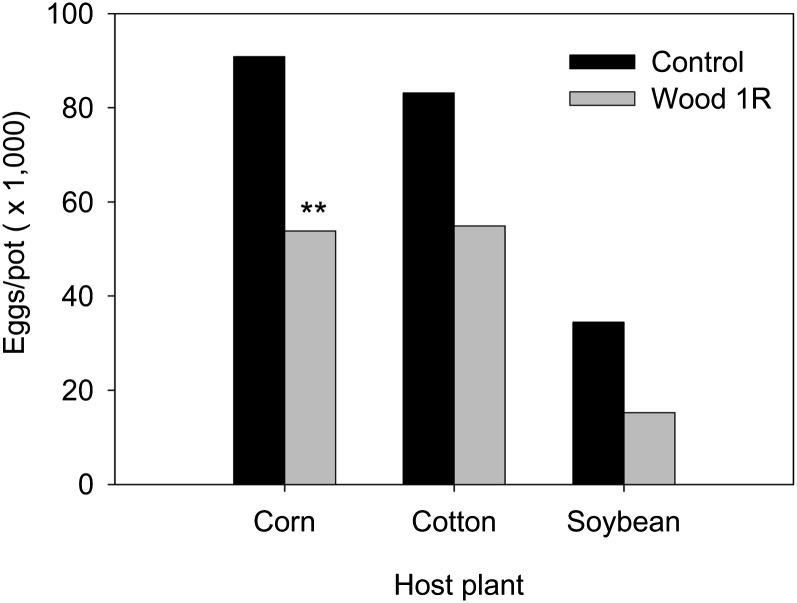
Nematode suppression by Wood1R in heated soil: In the first trial of the experiment, Wood1R did not affect final nematode numbers on any of the host plants (data not shown). When the experiment was repeated, the effect of Wood1R varied among nematode species (Wood 1R x nematode interaction, P = 0.048). As with the first trial, the bacterial strain again had no effect on final numbers of M. arenaria, P. minor, or H. glycines; however, final numbers of M. incognita were lower (P = 0.003) when seeds were treated with Wood1R than left untreated , and this reduction was consistent among host plants (Fig. 1). In pairwise comparisons within plant species, significant differences (P = 0.001) between the Wood1R and control seed treatments were only observed on corn. The experiment was repeated a third time with corn and the results were consistent with the second trial. The suppression on corn was observed to be 0, 42, and 38% reduction in the number of M. incognita eggs for the three experiments, respectively.
Fig. 1.
Final populations of Meloidogyne incognita on three host plants with and without a seed treatment of Pseudomonas fluorescens strain Wood1R. The data are the mean of two trials (n = 16) for corn and one trial for cotton and soybean (n = 8). In the first trial of the experiment, Wood1R did not affect egg production on any of the host plants (data not included in figure). ** indicates a significant difference (P = 0.001) between the control and Wood1R treatments.
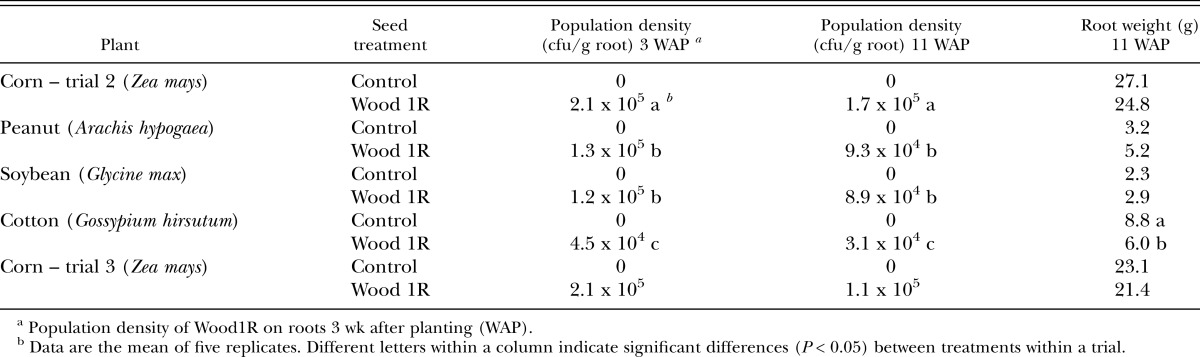
Treatment of crop seeds with Wood1R did not affect root weight except in two cases. In the first trial, root weights of corn were greater for Wood1R than for the control (P = 0.03; 67 vs. 45 g). In the second trial, root weights of cotton were less for Wood1R than for the control (Table 1). When the M. incognita egg counts from corn in trials 2 and 3 were analyzed on a per gram root basis, there were no significant differences between the Wood1R and control treatments. The discrepancy between the eggs per gram root and eggs per pot analyses was likely due to the additional variation from the root weights.
Table 1.
Population densities of Pseudomonas fluorescens strain Wood1R on plant roots at time of nematode inoculation and plant harvest, and effects of the bacterial strain on root weights in the second trial (all plants) and third trial (corn only).
At the time of nematode inoculation, the density of Wood1R in the root system was greatest for corn, intermediate for peanut and soybean, and lowest for cotton (Table 1). Although the density of Wood1R declined from 3 wk to 11 wk after planting, the relative abundance on the different crops remained the same. Bacterial densities also did not vary significantly between trials either at 3 wk (trial: P = 0.79; trial x plant: P = 0.84) or at 11 wk (trial: P = 0.30; trial x plant: P = 0.94) after planting.
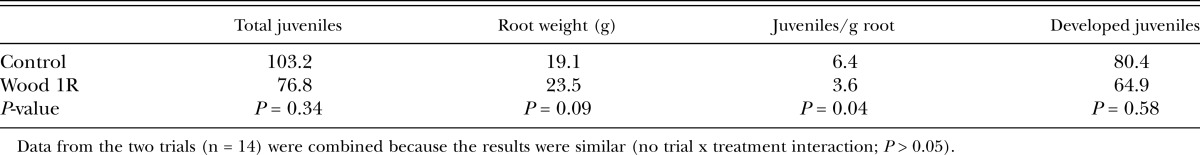
Effects of Wood1R on nematode penetration of corn roots: In both trials, there was no apparent effect of Wood1R inoculation on either the number of J2 that had penetrated the roots or the number that had begun to develop past the J2 stage (Table 2). However, there were fewer (P = 0.04) J2 penetrating per gram of root in the Wood1R-treated plants than in the control. This difference was due to a combination of lower J2 penetration and larger root systems in the Wood1R treatment compared to the control, even though these two parameters were not significantly different by themselves (Table 2).
Table 2.
Penetration and development of Meloidogyne incognita juveniles in corn plants treated with Pseudomonas fluorescens strain Wood1R and control plants.
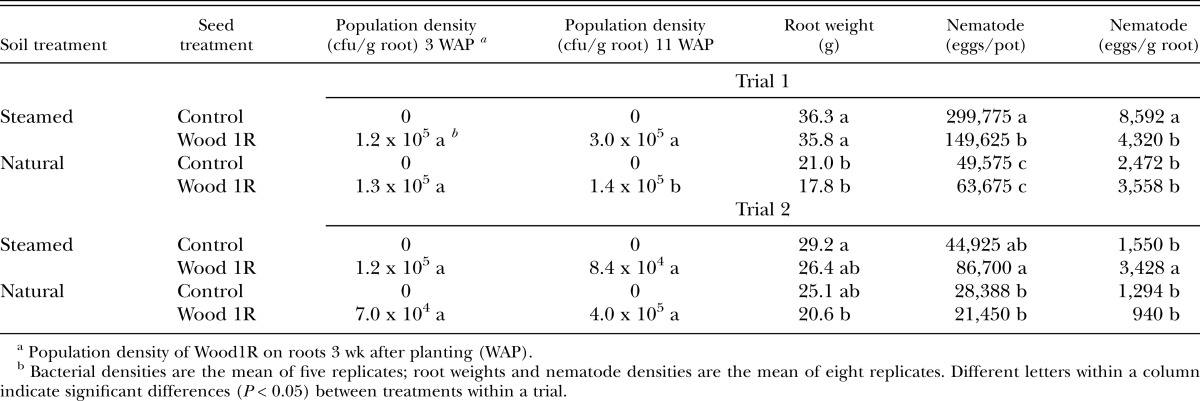
Comparison of M. incognita suppression by Wood1R in steamed and unsteamed soil: In the first trial, root weights were lower (P < 0.0001) in natural than in steam-heated soil, but they were not significantly affected by Wood1R (Table 3). The bacterial seed treatment reduced by 50% populations of M. incognita, both on an eggs per pot and eggs per gram root basis, in steamed soil only. Egg production was suppressed in natural soil compared to steamed soil, but the presence of Wood1R did not result in additional suppression. In the second trial, root weights were generally similar in natural and in steamed soil except that roots treated with Wood1R in natural soil were smaller than control roots in steamed soil (Table 3). The bacterial seed treatment did not reduce the number of nematode eggs per pot in either steamed or natural soil, but appeared to increase the density of eggs per gram root in steamed soil. Egg production by M. incognita was either numerically or statistically lower in the natural compared to the steamed soil.
Table 3.
Efficacy of Pseudomonas fluorescens strain Wood1R in suppressing egg production by Meloidogyne incognita on corn in steam-heated and natural soil.
In the first trial, the population densities of Wood1R was similar on corn roots in steam-heated and natural soil 3 wk after planting; however, at 11 wk, root densities were lower in the natural soil (Table 3). In the second trial, mean densities of Wood 1R on corn roots were similar in both soil treatments at 3 wk and 11 wk. However, the population dynamics of the bacterium differed in the two soil treatments. Somewhat surprisingly, the densities of Wood1R increased over time in the natural soil and decreased over time in the steamed soil.
Discussion
The results of this study indicate that a D genotype of P. fluorescens, represented by strain Wood1R, has the capacity to suppress populations of at least one plant-parasitic nematode. Wood 1R suppressed population densities of M. incognita on cotton, soybean, and corn, but was not able to suppress M. arenaria on peanut, H. glycines on soybean, or P. minor on corn. It is well established that the host plant can influence both the population density of pseudomonads in the root system and the production of DAPG (Bergsma-Vlami et al., 2005; De La Fuente et al., 2006; Landa et al., 2006; Notz et al., 2001). Moreover, continuous planting of a crop species has been shown to enrich populations of specific DAPG-producing genotypes from a mixture of genotypes in the soil, suggesting population selection for crop-adapted genotypes (Landa et al., 2006; Landa et al., 2002). Wood1R was originally isolated from corn in a corn-soybean rotation (McSpadden Gardener et al., 2005) and may be adapted to the corn rhizosphere. Although Wood1R reduced numbers of M. incognita on all crops, it was most effective in reducing nematode numbers on corn. And, population densities of the bacterium were significantly lower on peanut, soybean, and cotton than on corn. Thus, it may be that the biological control activities of this strain may be most pronounced on corn, where it is commonly found in the rhizosphere (McSpadden Gardener et al., 2005).
We suspect that the antibiotic DAPG was involved in nematode suppression; however, other secondary metabolites of the bacterium, such as hydrogen cyanide, may have also been involved (Siddiqui et al., 2006). The host plant can have a direct effect on DAPG production irrespective of bacterial densities. For example, Notz et al., (2001) showed that the expression of a gene involved in DAPG production was two- to four-fold greater in two monocots (corn and wheat) than in two dicots (bean and cucumber). Perhaps population densities of the bacterium or the production of DAPG or other inhibitory compounds secreted by Wood1R in the rhizosphere of peanut, soybean, and cotton were too low to affect the nematodes. In corn, population densities of Wood1R were sufficiently high to reduced numbers of M. incognita; however, P. minor was not affected. The latter species may be less sensitive to secretions of Wood1R than M. incognita. Additionally, because it is a migratory ectoparasite, P. minor may not be affected by the plant resistance induced by DAPG (Iavicoli et al., 2003; Siddiqui and Shaukat, 2003a). To date, induced plant resistance has only been shown to reduce populations of sedentary and migratory endoparasites (Collins et al., 2006; Dababat and Sikora, 2007; Hasky-Gunther et al., 1998; Vu et al., 2006).
Because DAPG can reduce the mobility and survival of infective stages of some plant-parasitic nematodes (Cronin et al., 1997; Siddiqui and Shaukat, 2003a), production of this antibiotic in the rhizosphere of plants should suppress nematode penetration of roots. As expected, the greatest reduction in root penetration by M. javanica occurred when P. fluorescens strain CHA0 was in the same root zone; though, the bacterium also reduced penetration when physically separated from the nematode (Siddiqui and Shaukat, 2003a). However, it is also known that DAPG can affect root morphology (Brazelton et al., 2008). Such changes in root architecture might alter the number of available infection sites and, therefore, lead to a complex response with regards to nematode suppression. In our study, the effect of P. fluorescens strain Wood1R on root infection by M. incognita was inconclusive: nematode infection was reduced per gram root, but the total number infecting roots was similar in Wood1R-treated and control plants.
While data on some D genotype strains indicate a degree of field efficacy with respect to improving crop health (e.g., Cook et al., 2002; McSpadden Gardener et al., 2006; Raudales et al., 2009), Wood1R did not appear to provide consistent nematode suppression. Even on corn, the strain did not consistently reduce populations of M. incognita. In some cases, this may have been due to low bacterial densities in the root system. It seemed that Wood1R was most effective in reducing nematode populations when densities were above 2.0 x 105 cfu/g root either at 3 or 11 weeks after planting (Table 1 and Table 3). However, low bacterial densities cannot explain the lack of nematode suppression in all cases. For example, in trial 1 of the first experiment, densities of Wood1R were nearly identical to those observed in trial 2, yet the bacterium did not suppress M. incognita on corn as it did in trial 2. Several abiotic and biotic factors, in addition to the host plant, can alter DAPG production (de Werra et al., 2008; Duffy and Defago, 1999; Shanahan et al., 1992). Metabolites from several common soil fungi have been shown to repress biosynthesis of DAPG (Notz et al., 2002; Siddiqui and Shaukat, 2003b; Siddiqui and Shaukat, 2005; Siddiqui et al., 2004). Moreover, the presence of some of these fungi (Fusarium solani, Rhizoctonia solani, and Aspergillus quadrilineatus) in soil reduced the ability of the bacterium to suppress populations of Meloidogyne spp. on tomato. The failure of Wood1R to suppress M. incognita in natural soil may be due to other microorganisms inhibiting root colonization and/or DAPG production by the bacterium. Such microbes might also re-colonize sterilized soil and interfere with antibiotic production.
At best, Wood1R provided only a moderate level of nematode suppression, 38% to 50%, compared with the untreated control. Such levels do not clearly indicate the use of this strain for nematode control. Similar levels of suppression of M. javanica on tomato were observed with P. fluorescens strain CHA0 (Siddiqui and Shaukat, 2003a). Strains that overproduce DAPG or produce additional nematicidal compounds may show greater levels of nematode suppression (Siddiqui and Shaukat, 2003a).
Footnotes
The authors thank David Clements, William Wilson, and Michael Purvis for technical assistance.
This manuscript was edited by Inga Zasada.
Literature Cited
- Barker KR. Nematode extraction and bioassays. Pp. 19–35. In: Barker KR, Carter CC, Sasser JN, editors. An Advanced Treatisse on Meloidogyne. Raleigh, NC: North Carolina State University Graphics; 1985. [Google Scholar]
- Bergsma-Vlami M, Prins ME, Raaijmakers JM. Influence of plant species on population dynamics, genotypic diversity and antibiotic production in the rhizosphere by indigenous Pseudomonas spp. FEMS Microbiology Ecology. 2005;52:59–69. doi: 10.1016/j.femsec.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Brazelton J, Pfeuffer E, Sweat T, McSpadden Gardener B, Coenen C. 2,4- diacetylphloroglucinol alters plant root development. Molecular Plant-Microbe Interactions. 2008;21:1349–1358. doi: 10.1094/MPMI-21-10-1349. [DOI] [PubMed] [Google Scholar]
- Bridge J, Page S, Jordon S. An improved method for staining nematodes in roots. Report of the Rothamsted Experiment Station for 1981 Part. 1982;1:171. [Google Scholar]
- Bull CT, Weller DM, Thomashow LS. Relationship between root colonization and suppression of Gaeumannomyces graminis var tritici by Pseudomonas fluorescens atrain 2-79. Phytopathology. 1991;81:954–959. [Google Scholar]
- Chin-A-Woeng TFC, Bloemberg GV, Mulders IHM, Dekkers LC, Lugtenberg BJJ. Root colonization by phenazine-1-carboxamide-producing bacterium Pseudomonas chlororaphis PCL1391 is essential for biocontrol of tomato foot and root rot. Molecular Plant-Microbe Interactions. 2000;13:1340–1345. doi: 10.1094/MPMI.2000.13.12.1340. [DOI] [PubMed] [Google Scholar]
- Collins HP, Navare DA, Riga E, Pierce FJ. Effect of foliar applied plant elicitors on microbial and nematode populations in the root zone of potato. Communications in Soil Science and Plant Analysis. 2006;37:1747–1759. doi: 10.1080/00103620600710538. [DOI] [Google Scholar]
- Cook RJ, Weller DM, Youssef El-Banna A, Vakoch D, Zhang H. Yield responses of direct-seeded wheat to rhizobacteria and fungicide seed treatments. Plant Disease. 2002;86:780–784. doi: 10.1094/PDIS.2002.86.7.780. [DOI] [PubMed] [Google Scholar]
- Cronin D, MoenneLoccoz Y, Fenton A, Dunne C, Dowling DN, Ogara F. Role of 2,4-diacetylphloroglucinol in the interactions of the biocontrol pseudomonad strain F113 with the potato cyst nematode Globodera rostochiensis. Applied and Environmental Microbiology. 1997;63:1357–1361. doi: 10.1128/aem.63.4.1357-1361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dababat AEFA, Sikora RA. Induced resistance by the mutualistic endophyte, Fusarium oxysporum strain 162, toward Meloidogyne incognita on tomato. Biocontrol Science and Technology. 2007;17:969–975. doi: 10.1080/09583150701582057. [DOI] [Google Scholar]
- De La Fuente L, Landa BB, Weller DM. Host crop affects rhizosphere colonization and competitiveness of 2,4-diacetylphloroglucinol-producing Pseudomonas fluorescens. Phytopathology. 2006;96:751–762. doi: 10.1094/PHYTO-96-0751. [DOI] [PubMed] [Google Scholar]
- de Werra P, Baehler E, Huser A, Keel C, Maurhofer M. Detection of plant-modulated alterations in antifungal gene expression in Pseudomonas fluorescens CHA0 on roots by flow cytometry. Applied and Environmental Microbiology. 2008;74:1339–1349. doi: 10.1128/AEM.02126-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy BK, Defago G. Environmental factors modulating antibiotic and siderophore biosynthesis by Pseudomonas fluorescens biocontrol strains. Applied and Environmental Microbiology. 1999;65:2429–2438. doi: 10.1128/aem.65.6.2429-2438.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn RA. Extraction of cysts of Heterodera species from soils by centrifugal flotation in high density solutions. Journal of Nematology. 1969;1:7. [Google Scholar]
- Hasky-Gunther K, Hoffmann-Hergarten S, Sikora RA. Resistance against the potato cyst nematode Globodera pallida systemically induced by the rhizobacteria Agrobacterium radiobacter (G12) and Bacillus sphaericus (B43) Fundamental and Applied Nematology. 1998;21:511–517. [Google Scholar]
- Hussey RS, Barker KR. A comparison of methods of collecting inocula for Meloidogyne spp., including a new technique. Plant Disease Reporter. 1973;57:1025–1028. [Google Scholar]
- Iavicoli A, Boutet E, Buchala A, Metraux JP. Induced systemic resistance in Arabidopsis thaliana in response to root inoculation with Pseudomonas fluorescens CHA0. Molecular Plant-Microbe Interactions. 2003;16:851–858. doi: 10.1094/MPMI.2003.16.10.851. [DOI] [PubMed] [Google Scholar]
- Jenkins WR. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Disease Reporter. 1964;48:692. [Google Scholar]
- Landa BB, Mavrodi OV, Raaijmakers JM, Gardener BBM, Thomashow LS, Weller DM. Differential ability of genotypes of 2,4-diacetylphloroglucinol-producing Pseudomonas fluorescens strains to colonize the roots of pea plants. Applied and Environmental Microbiology. 2002;68:3226–3237. doi: 10.1128/AEM.68.7.3226-3237.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa BB, Mavrodi OV, Schroeder KL, Allende-Molar R, Weller DM. Enrichment and genotypic diversity of phlD-containing fluorescent Pseudomonas spp. in two soils after a century of wheat and flax monoculture. FEMS Microbiology Ecology. 2006;55:351–368. doi: 10.1111/j.1574-6941.2005.00038.x. [DOI] [PubMed] [Google Scholar]
- Mavrodi OV, Gardener BBM, Mavrodi DV, Bonsall RF, Weller DM, Thomashow LS. Genetic diversity of phlD from 2,4-diacetylphloroglucinol-producing fluorescent Pseudomonas spp. Phytopathology. 2001;91:35–43. doi: 10.1094/PHYTO.2001.91.1.35. [DOI] [PubMed] [Google Scholar]
- McSpadden Gardener B, Benitez M-S, Camp A, Zumpetta C. Evaluation of a seed treatment containing a phlD+ strain of Pseudomonas fluorescens on organic soybeans, 2005. Biological and Cultural Tests Report 21:FC046. 2006 [Google Scholar]
- McSpadden Gardener BB, Gutierrez LJ, Joshi R, Edema R, Lutton E. Distribution and biocontrol potential of phlD(+) pseudomonads in corn and soybean fields. Phytopathology. 2005;95:715–724. doi: 10.1094/PHYTO-95-0715. [DOI] [PubMed] [Google Scholar]
- McSpadden Gardener B, Schroeder K, Kalloger S, Raaijmakers J, Thomashow LS, Weller DM. Genotypic and phenotypic diversity of phlD-containing Pseudomonas isolated from the rhizosphere of wheat. Applied and Environmental Microbiology. 2000;66:1939–1946. doi: 10.1128/aem.66.5.1939-1946.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSpadden Gardener BB, Weller DM. Changes in populations of rhizosphere bacteria associated with take-all disease of wheat. Applied and Environmental Microbiology. 2001;67:4414–4425. doi: 10.1128/AEM.67.10.4414-4425.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notz R, Maurhofer M, Dubach H, Haas D, Defago G. Fusaric acid-producing strains of Fusarium oxysporum alter 2,4-diacetylphloroglucinol biosynthetic gene expression in Pseudomonas fluorescens CHA0 in vitro and in the rhizosphere of wheat. Applied and Environmental Microbiology. 2002;68:2229–2235. doi: 10.1128/AEM.68.5.2229-2235.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notz R, Maurhofer M, Schnider-Keel U, Duffy B, Haas D, Defago G. Biotic factors affecting expression of the 2,4-diacetylphloroglucinol biosynthesis gene phlA in Pseudomonas fluorescens biocontrol strain CHA0 in the rhizosphere. Phytopathology. 2001;91:873–881. doi: 10.1094/PHYTO.2001.91.9.873. [DOI] [PubMed] [Google Scholar]
- Raaijmakers JM, Weller DM. Natural plant protection by 2,4-diacetylphloroglucinol - producing Pseudomonas spp. in take-all decline soils. Molecular Plant-Microbe Interactions. 1998;11:144–152. [Google Scholar]
- Raaijmakers JM, Weller DM. Exploiting genotypic diversity of 2,4-diacetylphloroglucinol- producing Pseudomonas spp.: Characterization of superior root- colonizing P. fluorescens strain Q8r1-96. Applied and Environmental Microbiology. 2001;67:2545–2554. doi: 10.1128/AEM.67.6.2545-2554.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaijmakers JM, Vlami M, de Souza JT. Antibiotic production by bacterial biocontrol agents. Antonie Van Leeuwenhoek International Journal of General and Molecular Microbiology. 2002;81:537–547. doi: 10.1023/a:1020501420831. [DOI] [PubMed] [Google Scholar]
- Raudales RE, Stone E, McSpadden Gardener B. DAPG-producing pseudomonads improve crop health in acidic soils by altering patterns of nutrient uptake. Phytopathology. 2009;99:506–511. doi: 10.1094/PHYTO-99-5-0506. [DOI] [PubMed] [Google Scholar]
- Rotenberg D, Joshi R, Benitez MS, Gutierrez Chapin L, Camp A, Zumpetta C, Osborne A, Dick WA, McSpadden Gardener B. Complex effects of farm management on rhizosphere colonization by native populations of phlD-containing Pseudomonas spp. and the relative contribution of those bacteria to crop stand and productivity. Phytopathology. 2007;97:756–766. doi: 10.1094/PHYTO-97-6-0756. [DOI] [PubMed] [Google Scholar]
- Shanahan P, Osullivan DJ, Simpson P, Glennon JD, Ogara F. Isolation of 2,4-diacetylphloroglucinol from a fluorescent pseudomonad and investigation of physiological parameters influencing its production. Applied and Environmental Microbiology. 1992;58:353–358. doi: 10.1128/aem.58.1.353-358.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi-Tehrani A, Zala M, Natsch A, Moenne-Loccoz Y, Defago G. Biocontrol of soil-borne fungal plant diseases by 2,4-diacetylphloroglucinol-producing fluorescent pseudomonads with different restriction profiles of amplified 16S rDNA. European Journal of Plant Pathology. 1998;104:631–643. [Google Scholar]
- Siddiqui IA, Shaukat SS. Suppression of root-knot disease by Pseudomonas fluorescens CHA0 in tomato: importance of bacterial secondary metabolite, 2,4-diacetylpholoroglucinol. Soil Biology & Biochemistry. 2003a;35:1615–1623. [Google Scholar]
- Siddiqui IA, Shaukat SS. Non-pathogenic Fusarium solani represses the biosynthesis of nematicidal compounds in vitro and reduces the biocontrol of Meloidogyne javanica by Pseudomonas fluorescens in tomato. Letters in Applied Microbiology. 2003b;37:109–114. doi: 10.1046/j.1472-765x.2003.01349.x. [DOI] [PubMed] [Google Scholar]
- Siddiqui IA, Shaukat SS. Phenylacetic acid-producing Rhizoctonia solani represses the biosynthesis of nematicidal compounds in vitro and influences biocontrol of Meloidogyne incognita in tomato by Pseudomonas fluorescens strain CHA0 and its GM derivatives. Journal of Applied Microbiology. 2005;98:43–55. doi: 10.1111/j.1365-2672.2004.02457.x. [DOI] [PubMed] [Google Scholar]
- Siddiqui IA, Shaukat SS, Khan A. Differential impact of some Aspergillus species on Meloidogyne javanica biocontrol by Pseudomonas fluorescens strain CHA0. Letters in Applied Microbiology. 2004;39:74–83. doi: 10.1111/j.1472-765X.2004.01540.x. [DOI] [PubMed] [Google Scholar]
- Siddiqui IA, Shaukat SS, Sheikh IH, Khan A. Role of cyanide production by Pseudomonas fluorescens CHA0 in the suppression of root-knot nematode, Meloidogyne javanica in tomato. World Journal of Microbiology & Biotechnology. 2006;22:641–650. [Google Scholar]
- Timper P, Holbrook CC, Xue HQ. Expression of nematode resistance in plant introductions of Arachis hypogaea. Peanut Science. 2000;27:78–82. [Google Scholar]
- Timper P, Riggs RD, Crippen DL. Parasitism of sedentary stages of Heterodera glycines by isolates of a sterile nematophagous fungus. Phytopathology. 1999;89:1193–1199. doi: 10.1094/PHYTO.1999.89.12.1193. [DOI] [PubMed] [Google Scholar]
- Vu T, Hauschild R, Sikora RA. Fusarium oxysporum endophytes induced systemic resistance against Radopholus similis on banana. Nematology. 2006;8:847–852. [Google Scholar]