Abstract
A new Parasitorhabditis species with males and females was discovered from the southern pine beetle Dendroctonus frontalis and its galleries in loblolly pine, Pinus taeda, growing in Mississippi. Females of the new species have a cupola-shaped tail with a small spike; males possess a 2 + (3+2) + 3 ray pattern on the tail fan with ray 10 reaching the margin, and a distinctive stomatal tooth. Parasitorhabditis frontali n. sp. has some similarities to P. hylurgi Massey, 1974 from Hylurgops pinifex in New York, USA, P. terebranus Massey, 1974 from D. terebrans (Olivier, 1795) in Texas USA, P. ligniperdae Fuchs, 1915 from Hylergops ligniperda (Fabricius, 1787) and P. dendroctoni Rühm, 1956 from D. micans (Kugelann, 1794) in Europe, P. ateri Fuchs, 1915 isolated from the beetle Hylastes ater (Paykull, 1800) in Germany, and P. malii Devdariani and Kakulia,1970 from Scolytus mali (Bechstein, 1805) within the republic of Georgia. Morphometrics for 44 species of Parasitorhabditis are provided to update older keys. Parasitorhabditis frontali n. sp. was initially grown on Malt Extract (ME) agar with its own microbial contaminants that included a bacterium and fungus. The nematode also grew and reproduced after slices of ME agar with nematodes and microbial contaminants were transferred to water agar. It was killed by E. coli on NGM agar plates commonly used to raise other Rhabditida. Drawings of diagnostic anatomy and low-temperature SEM images of bodies, heads, and tails are provided for cultured specimens from pine beetle frass.
Keywords: morphology, nematode, Parasitorhabditis frontali n. sp., SEM, southern pine beetle, taxonomy, trophic interaction
A rhabditid nematode with males and females was discovered from the southern pine beetle Dendroctonus frontalis and its galleries in loblolly pine Pinua taeda growing in Mississippi. It was determined to be a new species of Parasitorhabditis Fuchs, 1937, a genus of parasitic or phoretic nematodes associated with bark beetles. These nematodes are generally benign to their hosts but at least two instances of damage to cerambycid beetles (Chapman, 1964) and scolytid beetles (Tomalak et al., 1989) were reported. Beetles of the genera Ips De Geer, 1775, Dendroctonus Erichson, 1836, Dryocoetes Eichhoff, 1864, Hylurgus Latreille, 1807, Hylurgops LeConte, 1876, Hylastes Erichson, 1836, Pityogenes Bedel, 1888, Cryphalus Erichson, 1836, Crypturgus Erichson, 1836, some Cerambycidae (Sudhaus, 1974) Phloeosinus Chapuis, 1869, Polygraphus Erichson, 1836 and Scolytus Geoffroy, 1762 (Massey, 1974) have had Parasitorhabditis associates. The U. S. Forest Service publication of Calvin Massey (Massey, 1974) listed 6 U.S. species of Parasitorhabditis sp. known at the time from various bark beetles, including one Parasitorhabditis sp. among the 18 nematode species listed from D. frontalis. Within other beetle species there were 19 unspeciated Parasitorhabditis isolates listed in the index. Much of the literature has been within Russian or German journals that may be difficult to access. Since the compendia of Blinova (1982) and Andrássy (1984), only one other new species, P. platidontus Reboredo and Camino, 2000, has been described. Approximately 44 species are currently known in this genus (Blinova, 1982; Andrássy, 1984, Reboredo and Camino, 2000). Some of these are species inquirenda due to inadequate descriptions. Nematodes identified in the U. S. and Canada are also designated (Table 1).
Table 1.
Measurements of Female Parasitorhabditis Fuchs, 1937. Range lengths in μm, a - V ratios.
Species within Parasitorhabditis have one of the most variable male tail ray patterns among genera of Rhabditida (Sudhaus and Fitch, 2001). GenBank accessions for large and small subunit ribosomal RNA sequence exist for only one morphologically characterized species, Parasitorhabditis obtusa (Fuchs, 1915) Chitwood and Chitwood, 1950, the type species. Phylogenetically this species branches between Mesorhabditis (Osche, 1952) Dougherty, 1953 and Teratorhabditis (Osche, 1952) Dougherty, 1955 in a well-resolved tree of ribosomal DNA and large subunit RNA polymerase II (Kiontke, et al., 2007). This position differed from expectations based on stomatal similarities presumed to be ancestral that grouped Parasitorhabditis with Protorhabditis (Osche, 1952) Dougherty, 1953 and Prodontorhabditis Timm, 1961 within Protorhabditinae Dougherty, 1955 (Sudhaus, 1976; Andrássy, 1984). However, current rhabditid molecular phylogeny shows these last two genera branch immediately outside Caenorhabditis (Osche, 1952) Dougherty, 1953, three clades removed from Parasitorhabditis (Kiontke et al., 2007). The close genetic relationship of Parasitorhabditis with Mesorhabditis is interesting since these and only two other genera of Rhabditidae, Cephaloboides (Rahm, 1928) Massey, 1974 and Bunonema Jägerskiöld, 1905, were identified as bark beetle associates in the U.S. (Massey, 1974).
Parasitorhabditis species have been cultured from juveniles to adults on Malt agar (Massey, 1945) or on water agar or potato dextrose agar supporting fungi (Hunt and Poinar, 1971). The morphology and culture of the new P. frontali n. sp. are described below, including a diagnosis of related species with a mosaic distribution of similar characters.
Materials and Methods
Nematode isolation: Loblolly pine logs (Pinus taeda L.) infested with the southern pine beetle (Dendroctonus frontalis) were collected in the Homochitto National Forest near Meadville, MS, USA in June, 2008. One or two types of nematodes, including an aphelenchid, were active in the mycangial area at the prothorax and in mouth parts of beetle adults, and in the head area of larvae.
Nematode culture: Bark frass containing nematodes were applied to Malt Extract (ME) plates. After nematodes and their microbial associates began to reproduce, plates were maintained by cutting slices of the medium with nematodes using a flamed spatula to new plates. They were refrigerated for several months with good viability. However, nematodes died within a day when transferred to plates of Nematode Growth Medium (NGM) agar with E. coli used to support other soil and insect-associated nematodes (Stiernagle, 1999). Slices of ME agar were also applied to 4% water agar plates with some fungal mycelium carried from the ME frass culture that supported moderate, sustained growth and egg-laying of the nematodes. There were sufficient numbers of clean adult nematodes for low-temperature scanning electron microscopy (LT-SEM), however nematodes in the culture died before DNA could be extracted.
Nematode imaging, drawing and measuring: Most measurements and photomicrographs of live and fixed specimens were taken on a Zeiss Ultraphot with DIC (differential interference contrast) (Carl Zeiss, Inc., Jena, Germany/Baltimore Instrument Co., Baltimore MD, USA) and a Q-I Micropublisher 5 camera (Q-Imaging Inc., Austin, TX, USA). Some measurements of annules and phasmids were made from LT-SEM micrographs. Scale bars and increased contrast were applied in Adobe Photoshop 7.0 (Adobe Systems Inc., San Jose, CA, USA). Drawings were made from images and specimens at multiple planes. Morphological conventions were based on Sudhaus and Fitch, 2001. Stoma illustration style was based on Massey, 1974.
LT-SEM: Approximately 50 Parasitorhabditis frontali n. sp. adult specimens were gently lifted from 4% water agar with a pig's eyelash and placed in a BPI dish of M9 buffer. The nematodes were refrigerated overnight, after which, excess M9 buffer was removed and sterile deionized water was replaced three times. Nematodes were placed in 1.5 ml microcentrifuge tubes and concentrated at the base by gravity. In order to optimize the likelihood that nematodes would turn their heads upward before flash-freezing, specimens were mounted in water on leaves with a variety of elevated structures, as well as the standard filter paper. Rectangles 25 mm x 14 mm were cut from the dorsal and ventral surfaces of fresh azalea (Rhododendron sp.) leaves or filter paper and tacked onto rough-surfaced, rectangular, copper plates (1.5-cm-wide, 3.0-cm-long and 1.0-mm thick) with tissue mounting medium (Triangle Biomedical Sciences, Durham, NC). Nematodes were pipetted onto the leaf or filter paper surface and excess moisture was wicked away with absorbent paper under a dissecting microscope (Carta et al., 2003). A single fungus colony grown on water agar was sampled both at the edge and toward the center with a forceps holding sticky, round, ultra smooth carbon adhesive tabs (Electron Microscopy Sciences, Hatfield, PA) pressed onto the agar. The other sides of the sticky tabs were peeled away and pressed onto rough-surfaced, rectangular, copper plates (1.5-cm-wide, 3.0-cm-long and 1.0-mm thick). The mounted specimens were placed on a –196 °C liquid nitrogen-precooled 14-mm square brass tube within a 20.5 cm x 15 cm x 4 cm styrofoam box on a lab bench. This contact freeze immobilization method cryofixes the nematodes milliseconds after contact with the metal surface and preserves their natural biological positioning and actions (Wergin et al., 2000). Samples were mounted on a specimen holder and transferred into a Polaron CryoPrep PP2000T (Energy Beam Sciences, East Granby, CT) Cryotrans system. Samples were etched at –90 °C to remove surface water and coated with platinum. Samples were observed in a Hitachi S-4700 field emission SEM.
Results and Discussion
Parasitorhabditis frontali n. sp.
Table 2.
Morphometrics of Parasitorhabditis frontali n. sp. (LKC43) Measures in μm unless measure is a ratio or %. Length = L, Gub = Gubernaculum
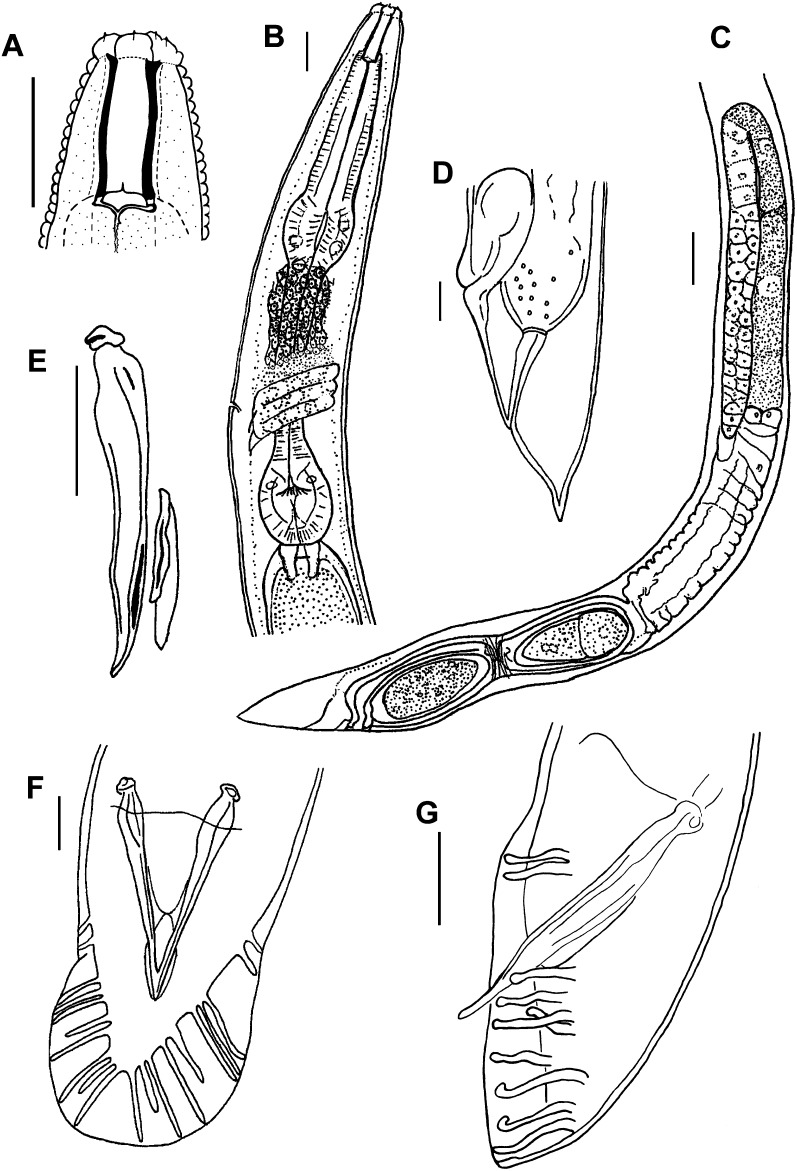
Fig. 1.
Parasitorhabditis frontali n. sp. Drawings with scale bars of 10 μm for A, B, D – F, and 20μm for C. A. Female stoma, lateral view, B. Female pharynx, lateral view, C. Female reproductive system, lateral view, D. Female tail, lateral view, E. Male spicule, slipper-shaped gubernaculum, lateral view, F. Male tail with spicules, ventral view, open bursal fan, fused spicule tips, 10 rays, 2 + (3 + 2) + 3 ray pattern. G. Male tail with spicules, lateral view, 10 rays, 2 + (4 + 1) + 3, with ray 6 trapped under ray 5 so that ray tip order is reversed from typical pattern.
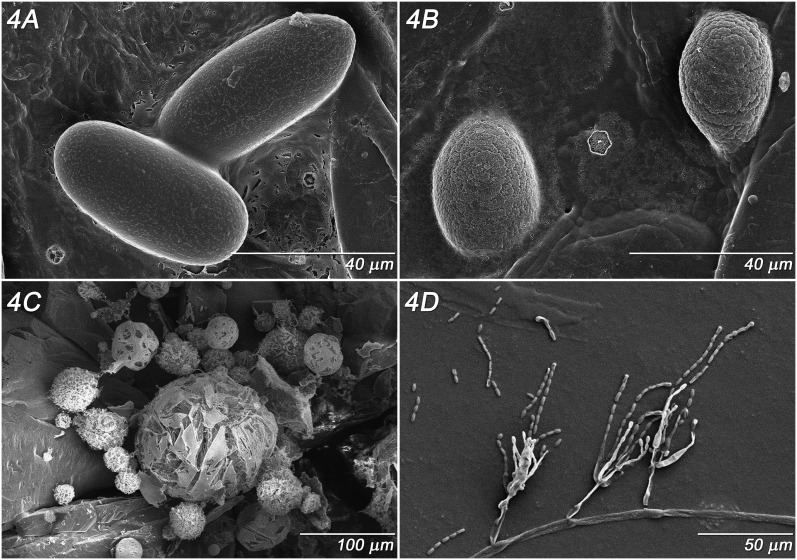
Fig. 4.
Parasitorhabditis frontali n. sp. LT-SEM images from culture A. Eggs, B. Microbial residue in water, C. Actinomycete-like structures from bacterial colonies on agar substrate, D. Gliocladium sp. hyphae and conidiospores on sticky tab surface.
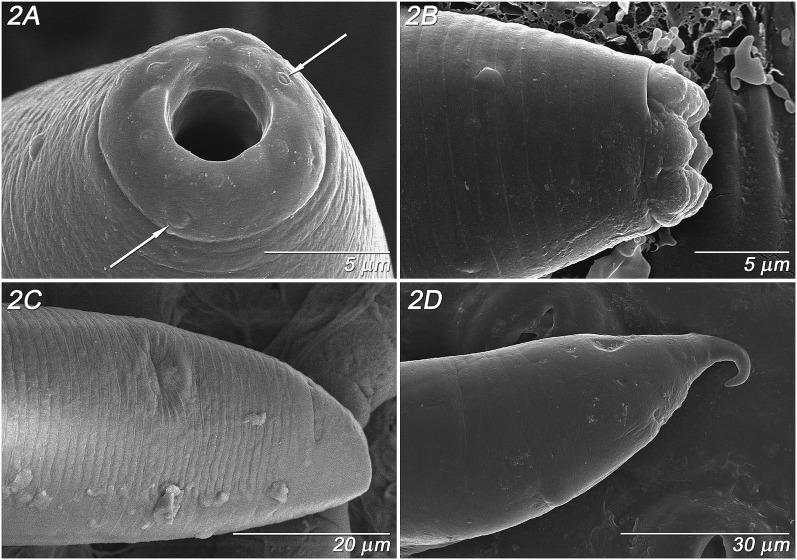
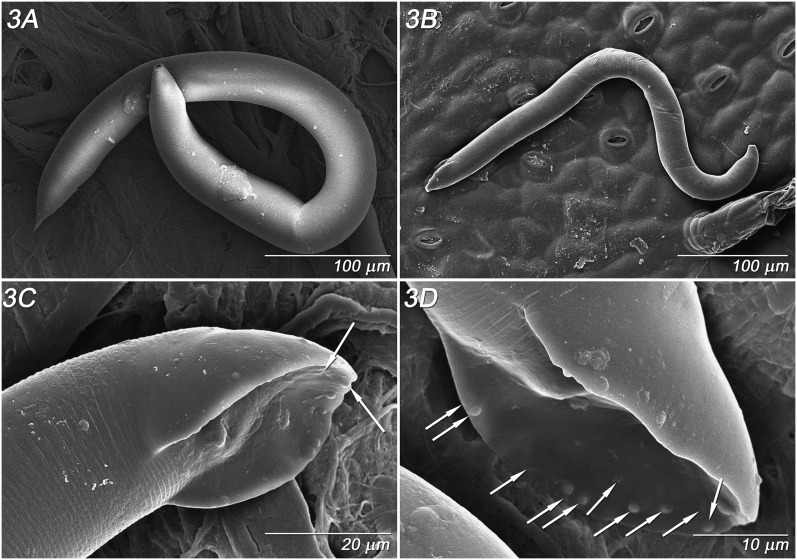
Adult Females (n = 10): (Fig. 1 A - D, Fig. 2 A - D; Fig. 3 A, Table 2). Stoma 3.2 ± 0.3 (2.9 – 3.5) μm wide, bearing a single small thin tooth visible in lateral view; convex walls slightly broader at base with a small bifurcation at the anterior end of stoma column and slight flare at basal walls (Fig. 1A), about 1 μm thick. No stomatal collar, glottoid apparatus nor punctations on cuticle of head region. Pseudolips of six sectors bearing three types of sensory structures: apical, labial papillae recessed within striated, open pustules; cephalic papillae at base of lips, and obscure amphid pore on base of lips (Fig. 2A). Lip height 2.5 μm (Fig. 2B). Prominent annulations with 1.3 – 1.4 μm interval between head annules (Fig. 2B). Trasverse ridging in lumen of procorpus, characteristic of genus. Pharynx procorpus with moderately swollen median bulb and isthmus about equal in length, each about twice the length of the basal bulb; proportion of procorpus length divided by length of long isthmus plus basal bulb, 69 ± 9% (60 – 80%) (n = 5) (Fig. 1B). Nerve ring 60 – 75 % of pharynx at mid- to posterior mid- isthmus. Excretory pore obscure (n = 5), 96.2 ± 10.7 (90 - 114) μm from lips, 74 ± 8 (68 - 87%) of pharynx length at posterior isthmus. Single anteriorly-directed gonad extends up to 2/3 body length, with prominent crustaformeria often containing 1 – 2 embryos (Fig. 1C). Vulva a transverse slit below anterior lip annule, above semicircular posterior lip region flanked laterally by oblique stress folds (Fig. 2C), variably protruding. Vagina short, oblique with muscular walls (Fig. 1C, D). Vulval-anal distance in flash-frozen specimens about 17 - 24 μm, with anus 10 - 19 annules below vulval opening, and phasmid 10 - 11 annules below anus, about 11.5 - 19 μm (Fig. 2C, D). Vulval-anal distance in fixed specimens slightly longer than tail length. Interval between major annules 1.7 μm on tail (Fig. 2C). Tail cupola-shaped, with short spike often curled in live specimens (Fig. 2D). Body plump with relatively steep angle from region of stoma to pharynx (Fig. 3A), with posture sinusoidal to straight when dead.
Fig. 2.
Parasitorhabditis frontali n. sp. LT-SEM images of female morphology. A. Pseudolips, face view, with cephalic papillae (left arrow) labial papillae (right arrow), B. Pseudolips, lateral view, C. Tail, ventro-lateral view with vulval slit (upper middle surface) and anal slit (upper right surface), D. Tail, lateral view with vulval slit (lower middle surface) and anal slit (upper right surface), curved tail spike.
Fig. 3.
Parasitorhabditis frontali n. sp. LT-SEM images of female and male morphology A. Female body, filter paper background, B. Male body, leaf background, C. Male tail, latero-ventral view, phasmid (upper arrow), and ray 10 tip (lower arrow), D. Male tail, ventro-lateral view, positions of rays 1 – 10 indicated by arrows from anterior to posterior.
Adult Males (n = 7): (Fig. 1 E-F, Fig. 3 B-D, Table 2). Stoma as in female. Pharynx proportion of procorpus length divided by length of long isthmus plus basal bulb 60 – 70% (n = 4). Nerve ring 65 – 75 % of pharynx, just posterior to mid-isthmus. Excretory opening as in female. Testis extends up to 2/3 of body length, with packed hexagonal sperm diameter of 7 μm. Lateral field extends down to about the third annule of fan. Spicule has nearly straight to minimally curved axis and very short distal fusion near acute, bluntly rounded tip, not curved (Fig. 1E). Gubernaculum slipper-shaped with posterior lateral thickening, 33 - 46% (n = 8) of spicule length (Fig. 1E). Open peloderan tail with bursal fan with 2 + (3+2) + 3 typical ray pattern (Fig. 1F), exceptionally 2 + 5 + (1 + 2) or 2 + (4+1) + 3 (Fig. 1G), with rays 1 and 2 preanal, rays 3, 6 and 9 retracted from edge of fan and opening dorsally, 6 generally thickened at base, and 10 extending to edge of fan (Figs. 3C, D). In one large male rays 6 and 9 extended close to the edges of the fan. A few punctuations visible by light microscopy on the fan, especially toward the posterior end. Body plump with relatively steep angle from region of stoma to pharynx, with posture sinusoidal to (Fig. 3B) straight when dead. Papillar phasmids at ventral junction of tail body and fan (Fig. 3C). Interval between major annules 1.5 μm above tail (Fig. 3C).
Eggs: (Fig. 4 A) 52 – 61 μm length x 23 – 29 μm width (n = 4). Egg shell with slightly elevated, interrupted network pattern.
Type host and locality: Southern pine beetle (Dendroctonus frontalis) frass from loblolly pine (Pinus taeda L.) trees located within the Homochitto National Forest near Meadville, MS, USA.
Type specimens (Adult Females and Males): Subcultured on agar culture after isolation from frass of Dendroctonus frontalis on type plant host and locality, male holotype: one of three specimens on T-5884p, female paratypes: T-5880p, T-5881p, T-5882p, and male paratypes: T-5883p, T-5884p, T-5885p, T-5886p, deposited in the U.S. Department of Agriculture Nematode Collection, Beltsville, Maryland. A female paratype slide deposited at the National Russian Collection of Nematodes, Moscow, Russia.
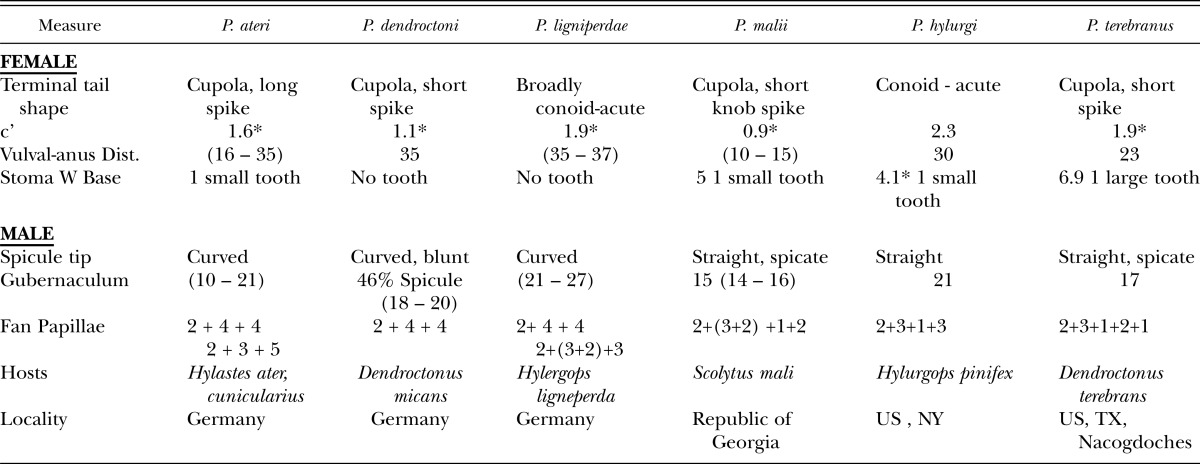
Diagnosis and Relationships: (Tables 2, 3.) Parasitorhabditis frontali n. sp. LKC43 female with broad, cupola-shaped tail with short spike, sometimes curved, and single thin tooth at stoma base. Diagnostic morphology for males was the 2 + (3+2) + 3 ray pattern on the tail fan with rays 3, 6 and 9 opening dorsally, with retraction from fan edge in rays 3, retracted, often thickened ray 6, and extension of ray 10 to edge of fan, and a straight, bluntly pointed spicule tip. In comparison with other similar species there was a shorter female body and stoma length, and the last three male rays generally formed an exclusive group (Blinova, 1982; Devdariani, 1974; Devdariani and Kakuliya, 1970; Fuchs, 1915, Massey, 1974). Compared to P. hylurgi, there were shorter female and male body lengths and stoma lengths, widths, lower ‘c’ ratio (0.5 – 1.7 vs 2.3), female procorpus divided by isthmus plus terminal bulb ratio of 0.69 vs 0.95, and lack of obvious head punctations. Compared to P. terebranus, presence of a single thin stomatal tooth vs 4 teeth, shorter female and male body lengths and stoma lengths and widths, a procorpus-median bulb divided by isthmus plus terminal bulb ratio of 0.69 vs 1, and separation of male ray pattern. Compared to the morphometrically similar P. ligniperdae having similar ‘a – c’ ratios and slightly overlapping female body lengths, the new species had a small tooth vs no tooth in the shorter stoma (13 – 17 vs 27 - 28 μm), a discretely shorter pharynx (113 – 136 vs 193 – 217 μm), shorter vulval-anal distance (23 – 27 vs 35 – 37 μm), shorter male body lengths (503 – 661 vs 885 – 1110 μm), shorter spicule (28 - 36 vs 41- 49 μm) and gubernaculum (11 – 13 vs 21 – 27 μm), relatively shorter gubernaculum relative to spicule (35 - 40 vs 55%), a retracted ray 3, and ray 10 reaching the edge of the fan, 2 + 4 + 4 ray pattern not seen, and larger eggs (52 – 61 x 23 – 29 vs 44 – 49 x 20 – 26 μm). Compared to P. dendroctoni the new species had a thin stomatal tooth vs no tooth, shorter female and male body lengths and stoma lengths and widths, a discretely shorter pharynx (113 – 136 vs 189 – 205 μm), vulval-anal distance (23 – 27 vs 35 μm), and 2 + 4 + 4 ray pattern not seen. Compared to P. ateri there were shorter female and male body lengths and stoma lengths, widths, and male rays had a 2 + (3+2) + 3 pattern vs 2 + 4 + 4 or 2 + 3 + 5. There was a longer vulval-anal distance (23 – 27 vs 10 – 15 μm), and a retracted ray 3 and separation of male ray 8 from 9-10 on the male tail compared to P. malii. The presence of a tooth on the stoma base, shorter female stoma length (13 – 17 vs 21 μm), shorter vulva position (up to 93.5 vs 97% of body), shorter spicule (32.5 vs 38 μm), and retracted ray 3 distinguished the new species from P. welchi.
Table 3.
Supplemental morphometrics of Parasitorhabditis spp. for diagnosis. * denotes derivation from published drawing. Vulval-anal distances from Blinova, 1982.
Microbes in Water Agar Culture: (Fig. 4 B - D) The microbial residue from rinsing nematodes from plates (Fig. 4B) is present as small to large, oval globules that may be produced from a biofilm-forming Rahnella Izard et al. 1981 sp. bacteria in the bark. The microbial flora will be further characterized in another publication. It is possible that the fine markings on the eggs (Fig. 4A) may be a result of this microbial sludge, since at least one other species lacks egg markings (Reboredo and Camino, 2000). What appeared to be typical bacterial colonies on agar were relatively dense but fragmentable actinomycete-like organisms (Fig. 4C). An unidentified species of Gliocladium Corda, 1840 also grew and produced spores within both ME and water agar (Fig. 4D).
Microbial Ecology: Nematodes in bark beetles often have complex trophic associations with fungi and other organisms (Klepzig et al., 2001; Cardoza et al., 2006; Scott et al., 2008; Klepzig et al., 2009). Inability of Parasitorhabditis LKC43 to grow on bacteria yet survive with a fungus was also seen in another Parasitorhabditis sp. that fed on the bluestain fungus Ceratocystis minor Sydow and P. Sydow, 1919 (Hunt and Poinar, 1971), now known as a junior synonym of Ophiostoma minus Sydow and P. Sydow (Hedgcock). Ophiostoma minus was also present in the bark associated with this nematode in the wild (Scott et al., 2008). The Gliocladium Corda, 1840 fungus with asexual spores (Fig. 4D) found in these culture plates is unrelated to those ascomycetous wood fungi.
Remarks: Some of the measurements of fixed specimens, particularly pharyngeal length and body width differ somewhat from those of live material (Table 2) which may be due in part to sampling variation, but in the case of the pharynx may be due to shrinkage from fixation. Since relatively few measurements of fixed material are given in the literature, with almost no mention of any differences from live material, it is difficult to assess the true range of measurements for comparison. Parasitorhabditis frontali n. sp. is part of a species complex with P. hylurgi, P. terebranus (U. S. species from NY and TX), P. ligniperdae, and P. dendroctoni (species found in Dendroctonus), since they share a mosaic distribution of similar features. Some transitional morphometrics and qualitative morphological characters may yet be demonstrated to allow synonymy of these currently distinct morphotaxa.
An early coevolutionary phylogenetic scheme for four major groups of species within genus Parasitorhabditis (Rühm, 1956) included the Obtusa, Chalcographi, Autographi and Ateri groups of nematode species. While loosely associated with beetle genera, these nematode assemblages lacked unifying characters to distinguish them (Sudhaus and Fitch, 2001). The species compared in the diagnosis fall near the Ateri group, the left pole of the transitional Parasitorhabditis group spectrum. These nematodes are associated with Hylastes and Hylurgops bark beetles, with a subgroup containing P. ligniperdae and P. dendroctoni associated with Hylurgus and Dendroctonus beetles (Sudhaus and Fitch, 2001).
Footnotes
The authors are grateful for the assistance of Jenny Kramer, Lori Adams and Jonelle Agurs of the Nematology Laboratory, Beltsville, MD, Chris Pooley of the Electron and Confocal Microscopy Unit, Beltsville, MD and Erich Vallery of USDA Forest Service, Pineville, LA. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
This paper was edited by Kris Lambert.
Literature Cited
- Andrássy I. Berlin: Gustav Fischer Verlag; 1984. Klasse Nematoda. 509 pages. [In German.] [Google Scholar]
- Blinova SA. Moscow: Science Publishing House; 1982. Entomopathogenic nematodes – parasites of forest pests. 133 pages. [In Russian.] [Google Scholar]
- Cardoza YS, Paskewitz S, Raffa KF. Traveling through time and space on wings of beetles: A tripartite insect-fungi nematode association. Symbiosis. 2006;41:71–79. [Google Scholar]
- Carta LK, Wergin WP, Erbe EF, Murphy CA. A comparison of low temperature and ambient temperature SEM for viewing nematode faces. Journal of Nematology. 2003;35:78–81. [PMC free article] [PubMed] [Google Scholar]
- Chapman JA. Nematode infestation and sex difference in response to log odours, in the cerambycid beetle, Leptura obliterata (Haldeman) Bi-Monthly Progress Report Forestry, Canada. 1964;20:3. [Google Scholar]
- Devdariani TG, Kakuliya GA. A new species Parasitorhabditis malii sp. nov. (Nematoda: Rhabditidae) Soobshcheniya Akademii Nauk Gruzinskoi SSR. 1970;59:201–203. [In Russian.] [Google Scholar]
- Devdariani TG. New nematode species of a small, black spruce Capricorn beetle (Monochamus sutor L.) Soobshcheniya Akademii Nauk Gruzinskoi SSR. 1974;76:709–712. [In Russian.] [Google Scholar]
- Fuchs G. Die Naturgeschichte der Nematoden und einiger anderer Parasiten: I. Des Ips typographus L. des Hylobios abietis L. Zoologische Jahrbücher Abteilung für Systematik. 1915;38:109–222. [Google Scholar]
- Hunt RS, Poinar GO. Culture of a Parasitorhabditis sp. (Rhabditida: Protorhabditinae) on a fungus. Nematologica. 1971;17:321–322. [Google Scholar]
- Kiontke K, Barrière A, Kolotuev I, Podbilewicz B, Sommer R, Fitch DHA, Félix MA. Trends, stasis, and drift in the evolution of nematode vulva development. Current Biology. 2007;17:1925–1937. doi: 10.1016/j.cub.2007.10.061. [DOI] [PubMed] [Google Scholar]
- Klepzig KD, Moser JC, Lombardero MJ, Hofstetter RW, Ayres MP. Symbiosis and competition: Complex interactions among beetles, fungi, and mites. Symbiosis. 2001;30:83–96. [Google Scholar]
- Klepzig KD, Adams AS, Handelsman J, Raffa KF. Symbioses: A key driver of insect physiological processes, ecological interactions, evolutionary diversification, and impacts on human. Environmental Entomology. 2009;38:67–77. doi: 10.1603/022.038.0109. [DOI] [PubMed] [Google Scholar]
- Massey CL. Nematode parasites and associates of the Engelmann spruce beetle (Dendroctonus engelmanni Hopk.) Proceedings of the Helminthological Society of Washington. 1956;23:14–24. [Google Scholar]
- Massey CL. Biology and taxonomy of nematode parasites and associates of bark beetles in the United States. Washington D.C.: USDA Forest Service Agriculture Handbook No. 1974 446, 233 pp. [Google Scholar]
- Reboredo GR, Camino NB. Two new Rhabditida species (Nematoda: Rhabditidae) parasites of Cyclocephala signaticollis (Coleoptera: Scarabaeidae) in Argentina. Journal of Parasitology. 2000;86:819–821. doi: 10.1645/0022-3395(2000)086[0819:TNRSNR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Rühm W. Die Nematoden der Ipiden. Parasitologische Schriftenreihe. 1956;6:1–435. [Google Scholar]
- Scott JJ, Oh D-C, Yuceer MC, Klepzig KD, Clardy J, Currie CR. Bacterial protection of beetle-fungus mutualism. Science. 2008;322:63. doi: 10.1126/science.1160423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernagle T. Maintenance of C. elegans. Chapter 4. In: Hope IA, editor. C. elegans: A practical approach. New York: Oxford University Press; 1999. pp. 51–68. [Google Scholar]
- Sudhaus W. Zur Systematik, Verbreitung, Ökologie und Biologie neuer und wenig bekannter Rhabditiden (Nematoda) 2. Teil. Zoologische Jahrbucher. Abteilung fur Systematik, Okologie und Geographie der Tiere. 1974;101:417–465. [In German.] [Google Scholar]
- Sudhaus W, Fitch D. Comparative studies on the phylogeny and systematics of the Rhabditidae (Nematoda) Journal of Nematology. 2001;33:1–70. [PMC free article] [PubMed] [Google Scholar]
- Tomalak M, Welch HE, Galloway TD. Parasitism of Parasitorhabditis obtusa and P. autographi (Nematoda: Rhabditidae) in the digestive tract of their bark beetle (Coleoptera: Scolytidae) hosts. Journal of Invertebrate Pathology. 1989;53:140–141. [Google Scholar]
- Wergin WP, Yaklich RW, Carta LK, Erbe EF, Murphy CA. Effect of an ice nucleating activity (INA) agent on subzero survival of nematode juveniles. Journal of Nematology. 2000;32:198–204. [PMC free article] [PubMed] [Google Scholar]