Abstract
As carbon and energy flow through the soil food web they are depleted by the metabolic and production functions of organisms. To be sustained, a “long” food web, with a large biomass at higher trophic levels, must receive a high rate of rhizodeposition or detrital subsidy, or be top-populated by organisms of slow growth and long life cycle. Disturbed soil food webs tend to be bottom heavy and recalcitrant to restoration due to the slow growth of upper predator populations, physical and chemical constraints of the soil matrix, biological imbalances, and the relatively low mobility and invasion potential of soil organisms. The functional roles of nematodes, determined by their metabolic and behavioral activities, may be categorized as ecosystem services, disservices or effect-neutral. Among the disservices attributable to nematodes are overgrazing, which diminishes services of prey organisms, and plant-damaging herbivory, which reduces carbon fixation and availability to other organisms in the food web. Unfortunately, management to ameliorate potential disservices of certain nematodes results in unintended but long-lasting diminution of the services of others. Beneficial roles of nematodes may be enhanced by environmental stewardship that fosters greater biodiversity and, consequently, complementarity and continuity of their services.
Keywords: Soil food webs, spatial patterns, functions, services, functional complementarity, functional continuity, feedback transitions
The organisms of the soil food web derive carbon and energy through plant- bacterial- or fungal-feeding and through successive levels of parasitism or predation. Mainly, the resources originate through photosynthetic fixation of C by autotrophs. Carbon is immobilized in the body structure of organisms or mineralized and respired during liberation of the energy bound in complex carbohydrates. That energy drives metabolic and physiological processes (Atkinson, 1980; Ingham et al., 1985). Consequently, C and energy dissipate during the course of successive exchanges and their availability affects the structure, dynamics and activities of the food web. Food webs driven by sparse resources, as in desert systems, or periodic pulses of resources, as in some agricultural systems, are likely to be short and dominated by opportunists at the entry level. Those with sustained energy supply may be longer, with greater abundance and biomass at higher trophic levels, provided the higher trophic organisms can thrive in the prevailing environmental conditions (Ferris et al., 2004; Ferris and Bongers, 2006).
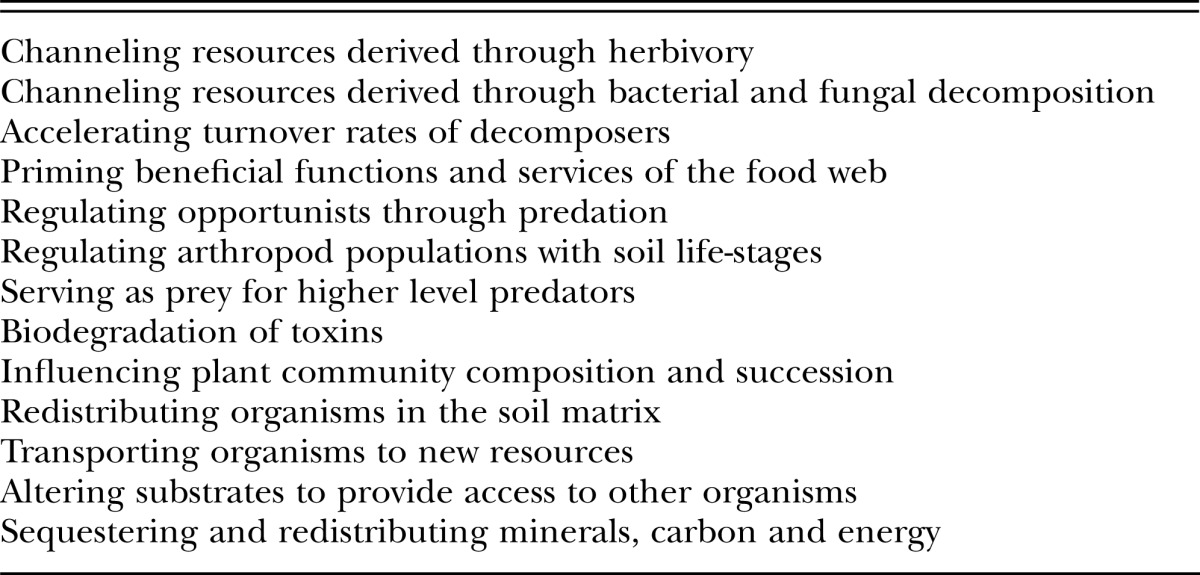
Functions and Services: Ecosystem functions are the activities and processes of the component organisms of the food web that impact on other organisms in the system or on the outputs and inputs of the system as a whole. Functions with positive impact are considered services; those with negative impact are disservices (Table 1). Most of the functions are services, some are disservices, and some may transition between the two categories due to positive or negative feedback among the parties, often mediated by resource availability. Some functions realized at a local level have effects that permeate to the ecosystem level, others have effects that remain local.
Table 1.
Some functions of nematodes in the soil food web.
The relocation of organisms to new resources, facilitated by the size, body configuration and motility of nematodes, is an important service, particularly where the transported organisms provide other ecosystem services. Nematodes encumbered by propagules of fungal parasites may retain the ability to move in the soil matrix for some time, thus distributing their parasites to new locations (Jaffee and Muldoon, 1989). Similarly, bacteria are distributed to new resources, either by survival of passage through the intestine or by external adherence (Ingham et al., 1985). The bacteria transported to new resources generate additional food for their nematode transporters. Negative feedback causes the service to transition to a disservice when resources for the bacteria are depleted and the nematode predators consequently overgraze the limited prey (Fu et al., 2005).
Nematodes feeding on other organisms, including plants, fungi, bacteria, microarthropods and other nematodes, take in more N than they need for their body structure. Often there are differences in the C:N content of predator and prey and; additionally, 40-60% of the ingested C may be mineralized through respiration. The N associated with respired C is usually in excess of body needs (Ferris et al., 1995; 1997). Excess N is mineralized as ammonia, excreted and available for uptake by plants and bacteria (Ferris et al., 1998).
Soil mineral N levels are increased by 20% or more by the feeding of bacterial- and fungal-feeding nematodes in microcosm experiments (Ferris et al., 1998; Chen and Ferris, 1999). In field plots in which sufficient N was fixed by a winter cover crop, the subsequent agronomic crop often exhibited N deficiency because the mineral was immobilized in the flush of microbial biomass that followed cover crop incorporation. When plots received additional organic matter and were irrigated during the dry post-harvest period of the previous year, the abundance of fungal- and bacterial-feeding nematodes and, by inference, the abundance of protozoa, was increased at the time of cover crop incorporation. Available mineral N was at higher levels in plots with abundant bacteria-grazing organisms, thus alleviating the N-deficiency (Ferris et al., 2004).
Nematode feeding that affects growth rates and fitness of higher plants may confer relatively greater fitness on their competitors with consequent effects on ecosystem succession (Van der Putten, 2003). In natural systems, susceptible and less fit plant species are reduced in abundance or even eliminated from the plant community (Yeates et al., 2009). In agricultural systems, reduction of vigor by nematode parasitism of the agronomic crop may reduce its competitiveness with weeds, which amplifies yield losses (Alston et al., 1991; Schroeder et al., 2005).
Higher predator nematodes (specialist and generalist), and non-nematode components of that functional guild, feed on and regulate the abundance of opportunistic species. Absent these regulators, herbivory may increase in an uncontrolled manner, reducing autotrophic production and hence supply to the system – another example of negative feedback (Sánchez-Moreno et al., 2007, 2008). Interestingly, the predator nematodes provide an indicator service as their presence and abundance may be correlated with those of predaceous microarthropods (Sánchez-Moreno et al., 2009).
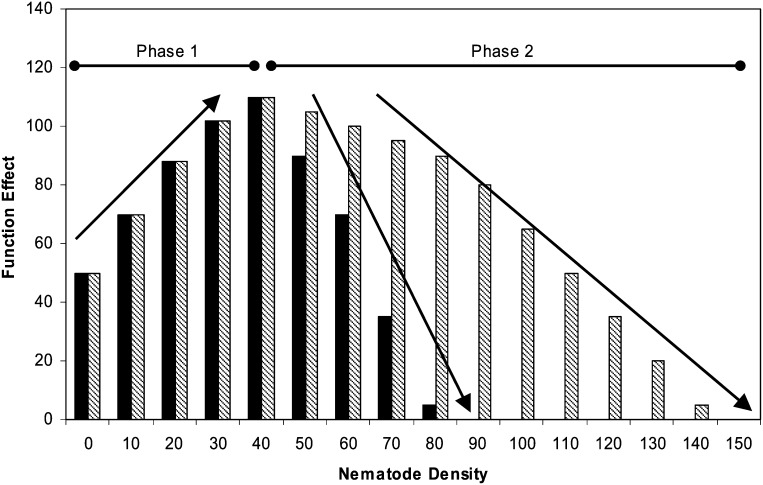
The transition of ecosystem functions between services and disservices can be explained by a generalized model of intensity of interactions among organisms or between organisms and their environment (Fig. 1). In phase 1, the service increases with the abundance of interacting organisms. In phase 2, the organisms are too abundant to be sustained by available resources and the function may become a disservice. The rate of transition and magnitude of the disservice depends on the intensity of interaction as indicated by the two scenarios in Fig. 1. When feeding on plants at low population densities without causing evident damage to their hosts (phase 1), nematodes provide a resource that primes the beneficial services of other organisms in the food web, either by enhancing leakage of exudates or by serving as prey for parasites and predators. At higher population densities (phase 2), plant physiological processes are compromised, C fixation is restricted and resources for the food web are diminished. The herbivory function has transitioned to a disservice. Some nematodes are less damaging to plants than others and the consequent effects are portrayed by the differing trajectories of the disservices (Fig. 1). At one extreme, many of the known species of plant associate nematodes in the family Tylenchidae do not seem to cause measurable plant damage. In that case, the function of supplying resources that prime other activities of the food web may only transition to a disservice under extreme circumstances.
Fig. 1.
Feedback effects from the relationships of population density and resource abundance, or predator and prey abundance, mediate transitions of ecosystem functions between services and disservices.
Phases 1 and 2 of the interaction may be sequential or they may be alternatives that depend on the state of the system and abundance of the interacting organisms (Fig. 1). The model can be applied to the transport of bacteria to new resources by bacterial-feeding nematodes. In patches of high nematode abundance, or at some point in time, the abundance of nematodes supersedes the supply of bacteria, particularly if resources become limiting for the bacteria (e.g., Fu et al., 2005). Similarly, the function of providing resources for higher trophic levels, either predator nematodes or nematophagous fungi, may transition from service to disservice depending on voracity of the predators and reproductive capabilities of the prey. The interaction of predators and prey is an interesting transition of a function because it supports inverse services. The overgrazing of prey by their predators may be a disservice in the function of providing resources to higher trophic levels but performs a biological control service if the prey are pest species. In that case, the subjective determination of service or disservice may depend on whether the individual with a stake in the interaction is a farmer or a predator nematode! Of course, it is important to avoid the simplifications of assuming directional linearity in such trophic cascades. A fecund prey with abundant resources may increase at a rate faster than the resource needs of its predators. Alternatively, generalist predators may have alternative available resources so that their abundance is not bottom-up controlled by a single taxon or guild.
Importance of Diversity: Organisms differ in their life history characteristics, life course duration, physiological and behavioral attributes, and their adaptation to prevailing conditions. Dispersal in the three-dimensional matrix of the soil, with its myriad combinations of moisture, aeration, texture and chemistry, determines that organisms of the same functional guild are seldom in competition for resources. They exploit different microsites and niches so that their services, rather than being offsetting or redundant, are complementary.
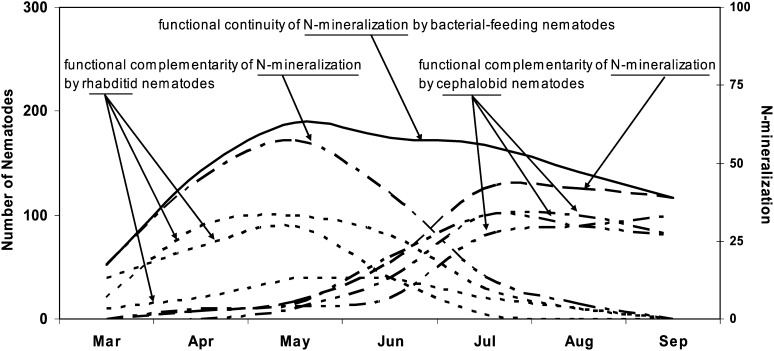
The multitude of organisms with apparently similar function, but differing in their adaptations, provides additive contributions to specific services, for example, mineralization or regulation. Further, a functional guild is not comprised of nematodes alone; organisms of other taxa perform similar functions and services. Those organisms expand the range of physiologies and behaviors within the guild and contribute to the service in a complementary manner. The diversity of organisms underpins functional resilience whereby the service continues even if conditions become unfavorable for some components of the guild. It is also an essential component of functional complementarily (Loreau, 2004) and functional continuity (Ferris et al., 1996; 2004). As an example (Fig. 2), a diverse assemblage of rhabditid nematodes, adapted to cooler temperature conditions, provides complementary input to the service of N-mineralization in the spring while, in the summer, the complementary service is provided by cephalobid nematodes adapted to warmer conditions. The very diversity of the organisms ensures functional continuity; the function or service continues although the players change in response to environmental conditions (Ferris et al., 1995; 1997; 1998; 2004).
Fig. 2.
Functional complementarity and functional continuity of N-mineralization (based on lifetime μg N per individual) for diverse assemblages of rhabditid nematodes, adapted to cooler temperature conditions and more abundant in the spring, and cephalobid nematodes, adapted to warmer conditions and more abundant in the summer. (From studies reported in Ferris et al., 1995; 1997; 1998; 2004).
Diversity Indices and Application: There are many useful indices of ecosystem diversity; they measure species richness and relative abundance of component taxa (e.g., Shannon and Weaver, 1949; Simpson, 1951; Pielou, 1966; Hill, 1973). Some, like the Maturity Index (MI) family are developed specifically for nematode assemblages (Bongers, 1990; Bongers et al., 1991; Ferris and Bongers, 2009). There are fewer indicators of ecosystem function that are conveniently applicable across functional guilds of organisms. The research and model validation associated with the Maturity Index family led to a functional guild classification of nematodes as a basis for studying and comparing ecosystem processes (Bongers and Bongers, 1998; Bongers and Ferris, 1999; Ferris et al., 2001).
While diversity indices are useful descriptive tools for assessment of food web and ecosystem condition, they do not provide information on the magnitude or nature of ecosystem functions. For example, within the nematode assemblage, it is possible to have the same diversity index value, the same MI value or the same structure and enrichment indices with an abundance of nematodes or with a few nematodes (Ferris and Bongers, 2009). Documentation of metabolic activity levels of different indicator guilds of nematodes would convey more information on the importance of the food web or ecosystem attributes suggested by the indices.
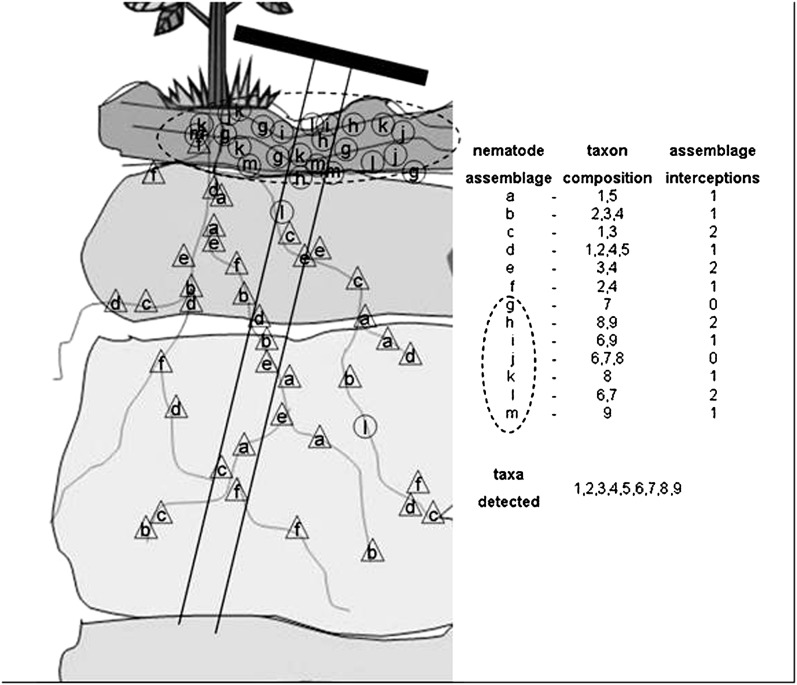
Spatial Pattern of the Soil Food Web: Nematode assemblages and associated communities of other organisms are distributed in patches centered on resources available from plant sources, herbivores and primary decomposers. Some patches, with unique assemblages of taxa, are associated with organic layers near the soil surface (Fig 3, upper oval). while other assemblages are associated with plant roots. What is the size of such patches? How much interaction is there between patches? Some interaction potentially results from soil tillage and from the phoretic associations, mutualistic or inadvertent, referred to earlier. However, the metadynamics within the soil food web are difficult and labor-intensive to ascertain.
Fig. 3.
Spatial pattern of nematode assemblages associated with organic layers near the soil surface and with roots deeper in the soil. Some nematode assemblages and taxa are associated only with the surface organic zone as indicated by the oval marker; others are associated mainly with plant roots. The soil core depicted encounters 15 patches of nematode which represent 11 of the 13 unique assemblages occurring in the soil profile; all 9 taxa present in the profile are detected in the core.
Often, the device for determining the presence or abundance of soil organisms is a sampling tube that removes a core of soil (Fig. 3). That core may represent several soil horizons and a vast range of biota and abiotic conditions. The soil core depicted in Fig. 3 encounters 15 patches of nematodes, which represent 11 of the 13 unique assemblages occurring in the model soil profile. Although the assemblages are comprised of many different combinations of taxa, all nine taxa present in the profile are detected in the soil core. The inference drawn from assessment of such a core, or of a composite sample of such cores, is that the organisms detected are interacting with each other.
The myriad food web and community interactions inferred from soil core samples may be gross inflations of reality. Nematode assemblages and associated communities near the soil surface may be exploiting organic resources in that area. Communities associated with tips of feeder roots exploiting the nutrients in the upper soil may be in frequent interaction with predators associated with the decomposer food chains. Those associated with root tips deeper in the soil may be quite isolated from such interactions (Fig. 3).
Managing Structure and Function of the Soil Food Web: The general goal of management is to enhance the services and reduce the disservices of the soil food web or ecosystem. Usually that requires preserving and sustaining the diversity and activity of the system by providing an adequate and continuous resource supply, and preserving favorable conditions for component organisms. Where food web services involve interaction of diverse organisms, a challenge in management is to engineer or facilitate the co-location of those organisms. Often, management to ameliorate potential disservices results in unintended but long-lasting diminution of services, as exemplified by the difficulties often experienced in transitioning from conventional to organic management systems. Management activities and inputs should enhance, not constrain, the presence, abundance and activities of taxa important to services of interest.
Footnotes
This paper was edited by Haddiish Melakeberhan.
Literature Cited
- Alston FG, Bradley JR, Coble HD, Schmitt DP. Impact of population density of Heterodera glycines on soybean canopy growth and weed competition. Plant Disease. 1991;75:1016–1018. [Google Scholar]
- Atkinson HJ. Respiration in nematodes. In: Zuckerman BM, editor. Nematodes as Biological Models. Vol. 2. New York: Academic Press; 1980. pp. 116–142. [Google Scholar]
- Bongers T. The maturity index: an ecological measure of environmental disturbance based on nematode species composition. Oecologia. 1990;83:14–19. doi: 10.1007/BF00324627. [DOI] [PubMed] [Google Scholar]
- Bongers T, Alkemade R, Yeates GW. Interpretation of disturbance-induced maturity decrease in marine nematode assemblages by means of the Maturity Index. Marine Ecology Progress Series. 1991;76:135–142. [Google Scholar]
- Bongers T, Bongers M. Functional diversity of nematodes. Applied Soil Ecology. 1998;10:239–251. [Google Scholar]
- Bongers T, Ferris H. Nematode community structure as a biomonitor in environmental monitoring. Trends in Ecology and Evolution. 1999;14:224–228. doi: 10.1016/s0169-5347(98)01583-3. [DOI] [PubMed] [Google Scholar]
- Chen J, Ferris H. The effects of nematode grazing on nitrogen mineralization during fungal decomposition of organic matter. Soil Biology and Biochemistry. 1999;31:1265–1279. [Google Scholar]
- Ferris H, Bongers T. Nematode indicators of organic enrichment. Journal of Nematology. 2006;38:3–12. [PMC free article] [PubMed] [Google Scholar]
- Ferris H, Bongers T. Indices for analysis of nematode assemblages. In: Wilson M, Kakouli-Duarte T, editors. Nematodes as Environmental Biondicators. Wallingford, U.K: CABI; 2009. pp. 124–145. [Google Scholar]
- Ferris H, Bongers T, de Goede RGM. A framework for soil food web diagnostics: extension of the nematode faunal analysis concept. Applied Soil Ecology. 2001;18:13–29. [Google Scholar]
- Ferris H, Bongers T, de Goede R. Nematode faunal analyses to assess food web enrichment and connectance. In: Cook RC, Hunt DJ, editors. Proceedings of the Fourth International Congress of Nematology. 2004. pp. 503–510. Nematology Monographs and Perspectives 2. Leiden; Brill. [Google Scholar]
- Ferris H, Eyre M, Venette RC, Lau SS. Population energetics of bacterial-feeding nematodes: Stage-specific development and fecundity rates. Soil Biology and Biochemistry. 1996;28:271–280. [Google Scholar]
- Ferris H, Lau S, Venette R. Population energetics of bacterial-feeding nematodes: respiration and metabolic rates based on carbon dioxide production. Soil Biology and Biochemistry. 1995;27:319–330. [Google Scholar]
- Ferris H, Venette RC, Lau SS. Population energetics of bacterial-feeding nematodes: Carbon and Nitrogen budgets. Soil Biology and Biochemistry. 1997;29:1183–1194. [Google Scholar]
- Ferris H, Venette RC, Lau SS. Dynamics of nematode communities in tomatoes grown in conventional and organic farming systems, and their impact on soil fertility. Applied Soil Ecology. 1996;3:161–175. [Google Scholar]
- Ferris H, Venette RC, van der Meulen HR, Lau SS. Nitrogen mineralization by bacterial-feeding nematodes: verification and measurement. Plant and Soil. 1998;203:159–171. [Google Scholar]
- Ferris H, Venette RC, Scow KM. Soil management to enhance bacterivore and fungivore nematode populations and their nitrogen mineralization function. Applied Soil Ecology. 2004;24:19–35. [Google Scholar]
- Fu S, Ferris H, Brown D, Plant R. Does the positive feedback effect of nematodes on the biomass and activity of their bacteria prey vary with nematode species and population size? Soil Biology and Biochemistry. 2005;37:1979–1987. [Google Scholar]
- Hill MO. Diversity and evenness: A unifying notation and its consequences. Ecology. 1973;54:427–432. [Google Scholar]
- Ingham RE, Trofymow JA, Ingham ER, Coleman DC. Interactions of bacteria, fungi, and their nematode grazers: Effects on nutrient cycling and plant growth. Ecological Monographs. 1985;55:119–140. [Google Scholar]
- Jaffee BA, Muldoon AE. Suppression of cyst nematode by natural infestation of nematophagous fungus. Journal of Nematology. 1989;21:505–510. [PMC free article] [PubMed] [Google Scholar]
- Loreau M. Does functional redundancy exist? Oikos. 2004;104:606–611. [Google Scholar]
- Pielou EC. The measurement of diversity in different types of biological collections. Journal Theoretical Biology. 1966;13:131–144. [Google Scholar]
- Sánchez-Moreno S, Ferris H. Suppressive service of the soil food web: Effects of environmental management. Agriculture, Ecosystem and Environment. 2007;119:75–87. [Google Scholar]
- Sánchez-Moreno S, Ferris H, Nicola NL, Zalom FG. Effects of agricultural management on nematode – mite assemblages: soil food web indices as predictors of mite community composition. Applied Soil Ecology. 2009;41:107–117. [Google Scholar]
- Sánchez-Moreno S, Ferris H, Guil N. Role of tardigrades in the suppressive service of a soil food web. Agriculture Ecosystems and Environment. 2008;124:187–192. [Google Scholar]
- Sánchez-Moreno S, Smukler S, Ferris H, O'Geen AT, Jackson LE. Nematode diversity, food web condition, and chemical and physical properties in different soil habitats of an organic farm. Biology and Fertility of Soils. 2008;44:727–744. [Google Scholar]
- Schroeder J, Thomas SH, Murray LW. Impacts of crop pests on weeds and weed-crop interactions. Weed Science. 2005;53:918–922. [Google Scholar]
- Shannon CE, Weaver W. Urbana: University of Illinois Press; 1949. The Mathematical Theory of Communication. [Google Scholar]
- Simpson EH. The interpretation of interaction in contingency tables. Journal of the Royal Statistical Society, Ser. B. 1951;13:238–241. [Google Scholar]
- Van der Putten WH. Plant defense belowground and spatio-temporal processes in natural vegetation. Ecology. 2003;84:2269–2280. [Google Scholar]
- Yeates GW. Nematodes as soil indicators: functional and biodiversity aspects. Biology and Fertility of Soils. 2003;37:199–210. [Google Scholar]
- Yeates GW, Ferris H, Moens T, van der Putten WH. The role of nematodes in ecosystems. In: Wilson M, Kakouli-Duarte T, editors. Nematodes as Environmental Biondicators. Wallingford, U.K: CABI; 2009. pp. 1–44. [Google Scholar]