Abstract
Population dynamics of Aphelenchoides fragariae were assessed over three growing seasons and during overwintering for naturally-infected, container-grown lantana (Latana camara) plants in a North Carolina nursery. During the growing season, the foliar nematode population in symptomatic leaves peaked in July each year then remained above 100 nematodes/g fresh weight into late summer. Foliar nematodes were also detected in asymptomatic and abscised leaves. Results suggest that leaves infected with foliar nematodes first develop symptoms at populations of about 10 nematodes/g. Foliar nematodes were detected in symptomatic and asymptomatic plant leaves and in abscised leaves during overwintering in a polyhouse, but the number of infected plants was low. A steep disease gradient was found for infection of lantana plants by A. fragariae on a nursery pad with sprinkler irrigation. When the canopies of initially healthy plants were touching the canopies of an infected plants, 100% of the plants became infected within 11 wk, but only 5 to 10% became infected at a canopy distance of 30 cm. Overwintering of A. fragariae in infected plants and a steep disease gradient during the growing season suggests strict sanitation and an increase in plant spacing are needed to mitigate losses from this nematode pest.
Key words: Aphelenchoides fragariae, detection, dispersal, ecology, foliar nematode, Lantana camara, management, nursery, ornamental crop, population dynamics, Salvia farinaeae
Foliar nematodes, including Aphelenchoides fragariae, are frequently encountered and economically important pests in the ornamental industry causing damage on a broad range of landscape and nursery-grown herbaceous and woody perennials (LaMondia, 1999; Jagdale and Grewal, 2006; McCuiston et al., 2007). Foliar nematodes feed endoparasitically on the mesophyll and parenchyma tissues of leaves, resulting in chlorotic blotches or vein-delimited lesions that turn necrotic over time. Premature abscission of infected leaves has been observed for several woody perennials including Lantana camara and can render a plant unmarketable. Understanding the population dynamics and dispersal of foliar nematodes in ornamental crops in greenhouses and nurseries is important for developing integrated management practices because chemical controls are limited in availability and efficacy (LaMondia, 1999; Jagdale and Grewal, 2002; Warfield and Para, 2003; Warfield et al., 2004). Current cultural management recommendations include avoiding sprinkler irrigation, destroying infected plant material and sanitation (Daughtrey et al., 1995).
There are a limited number of studies on the effect of environmental variables on temporal population changes of A. fragariae. In Poland, populations of A. fragariae in strawberries increased in the late fall and early spring, during months when the air temperature was low and the relative humidity was high (Szczygiel and Hasior, 1971, 1972). In Japan, populations of A. fragariae in lilies increased during the rainy season (Yamada and Takakura, 1987). Jagdale and Grewal (2006) determined that foliar nematodes migrating on the outside of hosta leaves had a high survival rate at 100% relative humidity with less survival at lower relative humidity.
Limited research has been done on the spatial movement of Aphelenchoides in plant nurseries. Foliar nematodes spread to new leaves on a single plant, and from plant to plant, primarily by migrating through films of water over plant surfaces (Wallace, 1959). A. fragariae can spread to new plants when moisture is present and infected plant tissue, including leaves, plantlets, and the surfaces of seeds, come in contact with healthy tissue. In greenhouses and nurseries sprinkler irrigation and rainfall allow foliar nematodes to move by splash dispersal from plant to plant (Marlatt, 1970; Lehman, 1996). Hesling and Wallace (1961) observed that uninfected chrysanthemum plants growing in the same block as infected plants under sprinkler irrigation became infected with A. ritzemabosi after two months. Symptomatic leaves were observed in plants growing as far away as 76 cm from the original infection source; however, whether individual plant canopies were allowed to touch over the growing season was unclear (Hesling and Wallace, 1961). The lateral disease gradient for foliar nematode infection between plants in nursery crop production under sprinkler irrigation has not been determined.
The objectives of this research were 1) to determine the population dynamics of A. fragariae in foliage of lantana during the growing season in a commercial nursery and during overwintering in a polyhouse, and 2) to determine the disease gradient for healthy plants growing near an A. fragariae-infected plant in a nursery with sprinkler irrigation.
Materials and Methods
Foliar nematode population dynamics: Changes in the population of A. fragariae in the foliage of naturally-infected, container-grown plants of L. camara ‘Miss Huff’ (a woody perennial hardy in USDA zones 8-11) was followed at a commercial ornamental nursery in Pender County, North Carolina, over three growing seasons. In each growing season, a new set of 30 naturally-infected lantana plants were sampled from May through October. Sampled plants had been naturally infected with A. fragariae during the previous growing season and overwintered in a Quonset-type polyhouse. In April of each year, plants from the polyhouse were transplanted into larger 13.2 liter containers of pine bark and placed 60-cm apart on a nursery pad. The plants were sprinkler irrigated during the growing season for 5-10 min three times each day. Plants were pruned to half their size once per month after sampling to shape the plants and mimic typical nursery practices. Air temperature, relative humidity, and rainfall data were recorded at the nursery (GroWeather, Davis Instruments, Hayward, California) so that correlations between environmental variables and nematode populations could be made. Environmental variables were measured every 2 hr for a total of twelve readings per day. When occasional data skips occurred, missing data was supplemented with weather data recorded at the North Carolina State University, Horticultural Crops Research Station located 50 km away in Castle Hayne, NC. Daily high temperatures ranged from 26.9°C to 32.6°C in 2006, 24.5°C to 36.5°C in 2007, and 20.4°C to 32.4°C in 2008. Daily low temperatures ranged from 17.2°C to 23.1°C in 2006, 13.4°C to 26.7°C in 2007, and 14.6°C to 23.3°C in 2008. Relative humidity levels ranged from 71-81% in 2007 to 69-84% in 2008.
Plant sampling: Only symptomatic leaves were collected during the 2006 growing season, from 6 June to 25 September. Each of the 30 plants was sampled every 14 d by removing a total of five symptomatic leaves from the bottom and middle tier of each plant. During the 2007 and 2008 growing seasons, from 16 May to 17 October, and 28 May to 27 August, respectively, symptomatic, asymptomatic, and abscised leaves were collected from each of the 30 plants. Three symptomatic and three adjacent asymptomatic leaves were removed from both the middle and bottom tiers of each plant canopy. If symptomatic leaves were not present, asymptomatic leaves were arbitrarily collected from at least two branches of each plant in accordance with the sampling pattern. Two to four abscised leaves with apparent symptoms were collected at each sampling date from the surface of the container substrate. The substrate surface of each container was cleared of fallen leaf debris after sampling so that all abscised leaf samples collected at the next sample date would have a maximum time of 14 d on the substrate surface. A total of 150 symptomatic leaves were collected at each sampling date in 2006. In 2007 and 2008, 180 symptomatic, 180 asymptomatic and 60-120 abscised leaves were collected at each sampling date. Leaf samples from each plant were placed by sample type into individual 50-ml centrifuge tubes and capped prior to transport inside an insulated cooler to the lab where samples were refrigerated at 4°C prior to assay within 24 to 48 hr.
Beginning in 2007, each plant was visually assessed twice per month for characteristic symptoms of foliar nematode damage. An overall disease severity rating, based on the percentage of symptomatic leaves, was assigned to each plant at each assessment date using the Horsfall-Barratt Scale (Horsfall and Barratt, 1945). Symptomatic leaf samples collected in 2007 and 2008 were photographed, and the percent leaf area of diseased tissue was calculated with ASSESS Image Analysis Software (APS Press, St. Paul, Minnesota). For those leaf samples in which the presence of foliar nematodes was confirmed, the percent area of diseased tissue was averaged across all infected leaves to obtain an average percentage of symptomatic leaf area at each sampling date. Correlation analysis of the percentage of symptomatic leaf area with the nematode population at each sampling date was performed using the Spearman rank correlation test (SAS, SAS Institute, Cary, NC).
Nematode assay: All collected leaves of one sample type (symptomatic, asymptomatic or abscised) from each plant were pooled, weighed, cut into small pieces and incubated in 10-15 ml of deionized water in a 50-ml centrifuge tube at room temperature to stimulate foliar nematode emergence (Esser and Riherd, 1981). After 48 hr, the leaf pieces and extraction water were passed through a set of nested sieves with a large mesh sieve to first remove leaf debris, followed by a 500-mesh (25-μm openings) sieve to collect any nematodes that had emerged. Nematodes were washed from the 500-mesh sieve in 5-10 ml of deionized water. The nematode samples then were refrigerated at 4°C until counted and quantified in a 3- × 7.5-cm counting dish under an inverted microscope at x40. The total number of A. fragariae in the dish was corrected for the total fresh weight of leaf tissue in each respective sample from each plant to obtain a nematode count per gram fresh weight per plant at each sampling date. Since leaf samples were always less than 1 g, counts were extrapolated. For comparison purposes, one gram of chopped, fresh, leaf tissue was equivalent to approximately 10 fully expanded, intact leaves. While both saprophytic and parasitic nematodes were recovered in leaf samples, the only pathogenic nematode species found during each growing season and overwintering period was A. fragariae based on morphological features and morphometrics (Sanwal, 1961; Siddiqi, 1975; and Hunt 1993).
Correlation analysis: The average nematode count per gram fresh weight at each sampling date, for each leaf sample type, was tested for correlation with the environmental data using the Spearman rank correlation test. This correlation test was used because it is a robust, non-parametric statistical method of analysis that makes no prior assumptions about the data. Environmental data including daily high air temperature, daily low air temperature, daily average air temperature, relative humidity, and rainfall collected 3, 7, 14 and 20 d prior to each sampling date was used for analysis.
Overwintering study: Each growing season, a companion group of 30 newly-rooted lantana ‘Miss Huff’ plants were grown in trade gallon (2.8 liter) containers of pine bark. These plants were placed on the nursery pad around the perimeter of the block of larger, infected plants in 13.2 liter containers in the current season's population trial. The smaller plants became naturally infected with A. fragariae by splash dispersal from the larger plants. In October of 2006 and 2007 the smaller plants were moved into the polyhouse at the Pender County nursery site for overwintering. Plants were tightly spaced, allowing adjacent plant canopies to touch per standard industry practice. The polyhouse was equipped with supplemental heat to maintain minimum temperatures just above freezing to prevent plant damage but daily fluctuations on sunny days could be quite large, requiring venting. Plants were sprinkler irrigated as needed. The top of each plant canopy was pruned once during winter 2006-07. Plants were not pruned in 2007-08 to prevent removal of potentially nematode-infected plant tissue. During 2007-08, leaf debris was removed from the surface of the container substrate after each sampling.
A maximum of six attached, symptomatic leaves (three from the lower canopy and three from the middle plant canopy) on each plant and a maximum of four abscised leaves from the substrate surface of each container were sampled at 6-wk intervals during overwintering. When plants were first moved to the polyhouse, infected leaves became senescent and defoliated, so six symptomatic leaves were not always available per plant at the first sampling dates in the polyhouse. Later as plant growth was stimulated during warm winter periods more leaves became symptomatic. Attached, asymptomatic leaves directly adjacent to symptomatic leaves also were collected during winter 2007-08. Foliar nematodes were detected by water extraction of collected leaf tissue and counts extrapolated to nematodes per gram as previously described. Environmental variables including daily minimum, maximum and average air temperature, and relative humidity collected 3, 7, 14 and 20 d prior to each sampling date were used in Spearman correlation tests with nematode counts for each leaf sample type. Daily high temperatures in the polyhouse ranged from 5.5°C in mid February 2006 to 29.6°C in late March 2007 and 26.2°C in mid December to 2.1°C in early January in 2007-08. Daily low temperatures ranged from -4.7°C in mid February to 17.6°C in late March during the 2006-07 period and 16.9°C in late December to -9.7°C in early January in 2007-08. The average daily temperatures ranged from 0.9°C to 23.0°C in 2006-07 and –1.9°C to 20.7°C in 2007-08. Relative humidity ranged from 42% to 98% in 2006-07 and from 43% to 98% in 2007-08.
The pine bark substrate in each container was sampled for foliar nematodes in winter 2006-07. A 2-cm diameter soil sampling tube was used to remove a core of substrate from each container. The resulting hole was re-filled with fresh pine bark. Substrate samples were placed in Baermann funnels in a modified Seinhorst mist apparatus (Barker et al., 1986) for 24 hr. The resulting water solution from each sample was drained into a test tube then examined microscopically in a counting dish for the presence of Aphelenchoides. Foliar nematodes were not detected in any substrate sample in 2006-07; therefore, container substrate was not sampled in 2007-08.
Disease gradient study: Plant-to-plant dispersal of foliar nematodes was evaluated in a simulated nursery setting at the Horticultural Field Lab located at North Carolina State University in Raleigh, North Carolina from 3 August to 12 October 2007, and 20 July to 7 October 2008. Healthy, asymptomatic lantana ‘Miss Huff’ plants grown in trade gallon containers of pine bark were placed on a nursery pad into 15 blocks, each composed of four lantana plants spaced equidistantly around a Salvia farinaeae (2007) or a lantana (2008) plant naturally infected with A. fragariae. For each of the blocks, one of three container spacings was used to separate the canopy of the nematode-infected plant from the canopies of the four, healthy lantana plants: 0-cm (touching), 30-cm, or 100-cm apart. Spacing distance was replicated five times, for a total of 20 lantana plants placed at each spacing distance in each year. Blocks were separated by a minimum of 100 cm to avoid inter-plot interference. The average canopy size of the lantana plants at the start of each trial was 30 × 38 cm. As the plants grew, they were re-spaced to maintain the original distance between plant canopies. Plants were watered twice daily (2.8-cm water/day) by sprinkler irrigation except on days when at least 1.2 cm of rain occurred. Natural rainfall during the trial periods totaled 13.1 and 31.4 cm in 2007 and 2008, respectively. The ambient air temperature during the trial period in each year ranged from a minimum of 12°C to a maximum of 40°C.
The lantana plants were evaluated visually once per week for characteristic symptoms of foliar nematode infection. Symptomatic leaves, when observed, were sampled and processed by water extraction to confirm the presence of foliar nematodes. If foliar nematodes were detected in a leaf sample, the corresponding plant was recorded as positive. The data are presented as a series of plot maps representing the weekly sampling periods throughout the duration of each year's trial. At the end of each trial, a disease gradient was estimated by regression of the percentage of infected plants at each spacing distance against the spacing distance to determine the best fit equation.
Results
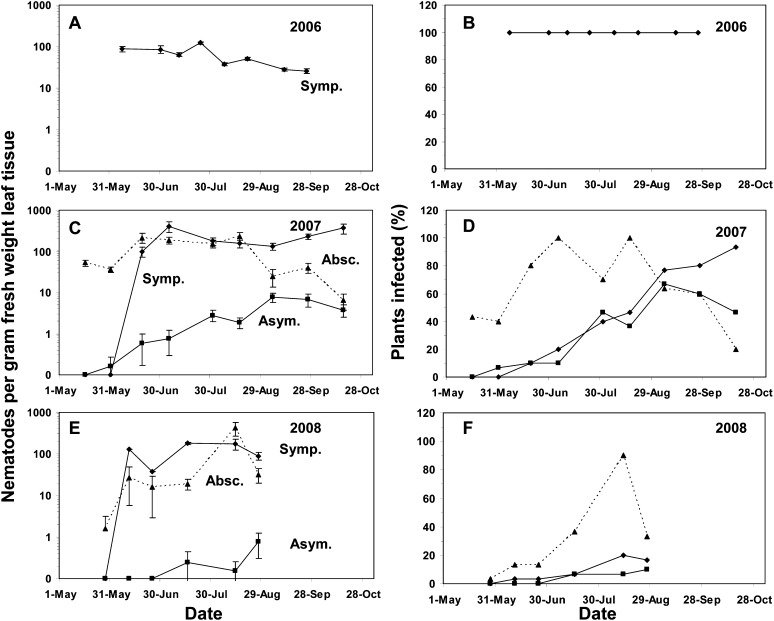
Population dynamics study: In 2006, the first samples were not collected until plants had symptomatic leaves. The nematode population in symptomatic leaves was already near 100 nematodes/g fresh weight of symptomatic leaf tissue on 7 June then declined slowly throughout the growing season with the exception of a small increase in July (Fig 1A). By 25 September, the nematode population detected in symptomatic leaves had fallen to near 25 nematodes/g fresh weight. The percentage of plants with symptomatic leaves remained at 100% throughout the 2006 season (Fig. 1B). In 2007 and 2008, no symptomatic leaves were observed at the first sampling dates in May but by mid-June symptomatic leaves were present and had near 100 nematodes/g fresh weight in both years (Fig 1C, E). In all three years, the highest nematode populations were detected in July of each growing season (Fig. 1A, C, D). Unlike the 2006 growing season, nematode populations in 2007 and 2008 remained at or above 100 nematodes/g fresh weight throughout the growing season (Fig. 1C, E). In 2007 the percentage of lantana plants with symptomatic leaves was low throughout the first weeks of the growing season with only about 40% plants infected by 31 July, but by the end of the growing season on 28 October 93% of the plants had symptomatic leaves (Fig. 1D). In 2008, the percentage of plants with symptomatic leaves was only 20% by the first of August (Fig. 1F).
Fig. 1.
Population of A. fragariae in (A, C, E) symptomatic leaf tissue (Symp., ♦), (C, E) asymptomatic leaf tissue (Asym., ▪) and (C, E) abscised leaves (Absc., ▴ with dashed line) of lantana, and percent infected plants by leaf class over three growing seasons (B, D, F). Each data point for the nematode populations represents the mean number of nematodes counted per gram of fresh weight leaf tissue per plant from 30 plants ± the standard error of the mean.
In both 2007 and 2008, any symptomatic leaves present had abscised as plants were moved from the polyhouse, re-potted to larger containers, and placed under sprinkler irrigation on the container growing area such that no plants had symptomatic leaves at the first sampling dates, as mentioned above. However at the beginning of the 2007 growing season, these abscised leaves collected from the surface of the container had nematodes populations near 50 nematodes/g fresh weight, and the population remained near 100 nematodes/g fresh weight throughout the growing season (Fig. 1C). In 2008, nematode populations in abscised leaves were lower at the beginning of the growing season. Populations above 100 nematodes/g fresh weight were not detected in abscised leaves until August (Fig. 1 E). The shedding of symptomatic leaves was also apparent from the marked increase in the percentage of plants with abscised leaves in the first months (June and July) of the 2007 growing season (Fig. 1D). Foliar nematode populations in abscised leaves were comparable to populations in symptomatic leaves from June to mid-August 2007 but mean populations were less than populations in symptomatic leaves in September and October (Fig. 1C). In 2008, mean populations in abscised leaves were always less than in symptomatic leaves except at the 15 August sampling date (Fig. 1E).
In 2007 and 2008, asymptomatic leaves were collected next to symptomatic leaves in the plant canopies to determine if nematodes could be detected in leaves before symptoms appeared. In both years, A. fragariae was detected in asymptomatic leaves but mean populations were always less than 10 nematodes/g fresh weight (Fig. 1 C, E). In 2008, the percentage of lantana plants with infected, asymptomatic leaves was less than 20% but in 2007, over 65% of the plants had infected, asymptomatic leaves by September (Fig. 1D).
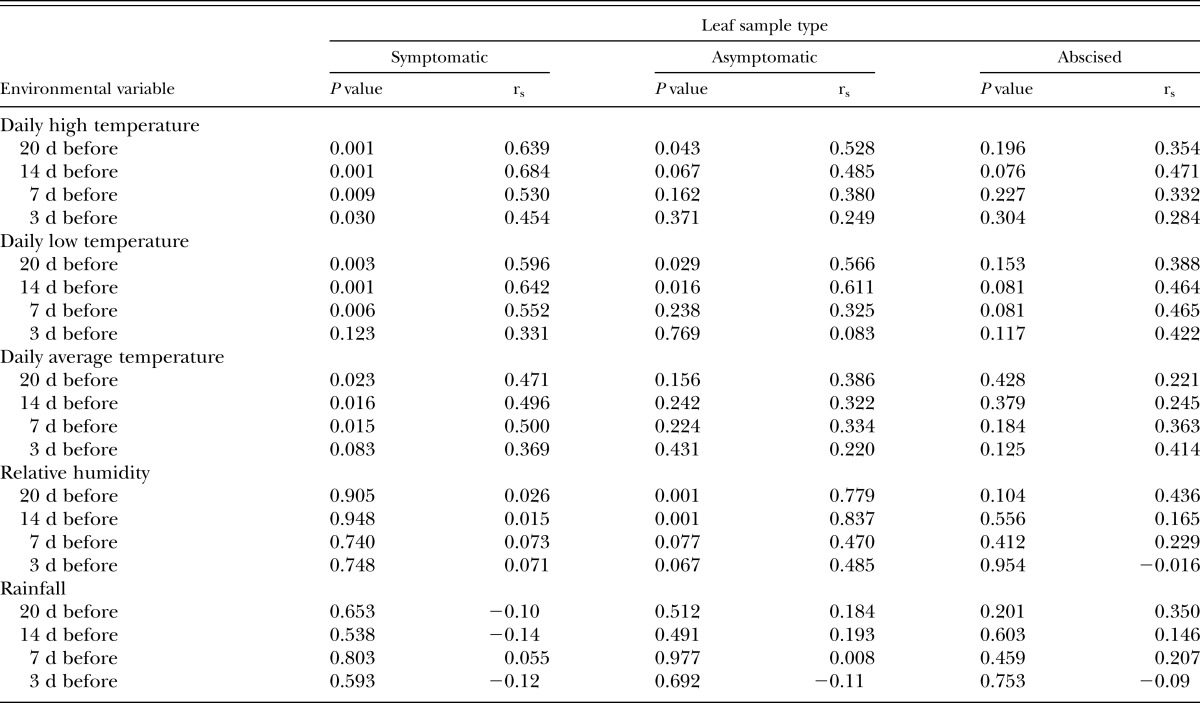
Nematode populations and correlation with environment: Nematode populations in symptomatic leaf tissue were positively correlated to daily high temperatures at 3, 7, 14 and 20 days before sampling and daily low temperatures 7, 14, and 20 days before sampling (Table 1). Daily average temperature was correlated to nematode populations at 7, 14, and 20 days before to sampling. There were no significant correlations between foliar nematode populations in symptomatic leaves and relative humidity or rainfall (Table 1). Nematode population in asymptomatic leaves was positively correlated with daily high temperature 20 days before sampling; with daily low temperature 14 and 20 days before sampling; and with relative humidity at both 14 and 20 days before sampling (Table 1). Populations of A. fragariae in abscised leaves were never correlated with any measured environmental variables (Table 1).
Table 1.
Spearman rank correlation values (rs) and level of significance for environmental variables collected at four intervals before sampling and nematode populations in leaves of lantana collected from container plants with symptomatic, asymptomatic and abscised leaves during the growing season.
Disease severity based on individual plant assessments across the 30 naturally-infected lantana plants increased over the 2007 and 2008 growing seasons. In 2007, the mean plant disease severity reached 50% by the end of the season in mid-October. In 2008, a similar rate of increase was observed through August when disease severity assessments ended. On a leaf area basis, symptomatic area only reached 13% and 6.5% in 2007 and 2008, respectively. There was no correlation between the percent area of symptomatic leaf tissue and the population of nematodes per gram of leaf tissue in 2007 (P = 0.0899) or 2008 (P = 0.0724).
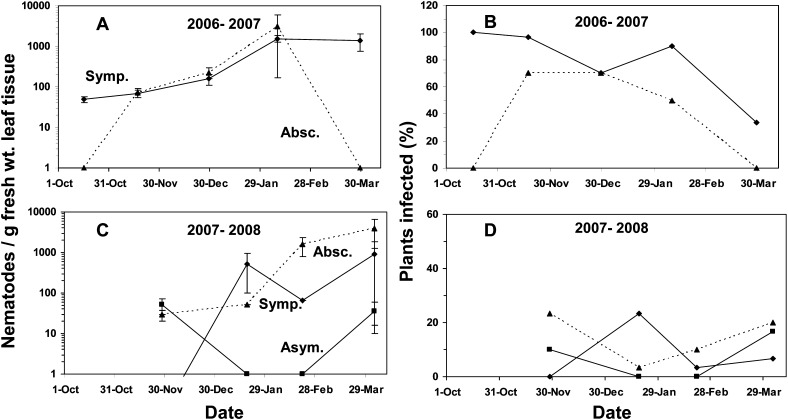
Overwintering study: At the time the smaller plants (2.8 liter containers) were moved into the overwintering polyhouse in the fall of 2006, the nematode population in symptomatic leaves was about 50 nematodes/g fresh weight, and 100% of the plants were infected (Fig. 2A, B). Interestingly, the nematode population increased in symptomatic leaves reaching a peak of over 1,000 nematodes per gram by early February 2007; a population that persisted into late March (Fig. 2A). However, the number of plants with infected, symptomatic leaves decreased from 100% to 33% by late March as symptomatic leaves abscised (Fig. 2B). No infected, abscised leaves were present on the container surface when the smaller plants (2.8 liter containers) were moved into the polyhouse. However, the symptomatic leaves present began to abscise such that by the middle of November, the nematode population in abscised leaves was the same as that in symptomatic attached leaves for the next three sampling dates (Fig. 2A).
Fig. 2.
Population of A. fragariae in (A, C) symptomatic leaves (Symp., ♦), (C) asymptomatic leaves (Asym., ▪) and (A, C) abscised leaves (Absc., ▴ with dashed line) of lantana and (B, D) percent infected plants by leaf class over two overwintering periods in a polyhouse. Nematode population values represent the mean number of nematodes counted per gram of fresh weight leaf tissue per plant from 30 plants ± the standard error of the mean.
In 2007-2008, none of the plants moved into the polyhouse had symptomatic leaves at the first sampling date in late November (Fig. 2C, D). By mid-January, symptomatic leaves were present with nematode population near 500 nematodes/g and then ranging from about 60-900 nematodes/g through April (Fig. 2C). The number of plants with infected, symptomatic leaves remained below 25% throughout the overwintering period (Fig. 2D). However, foliar nematodes were detected in asymptomatic leaf tissue in November, ranging from less than 1-50 nematodes/g across the various sampling dates (Fig. 2C). The number of plants with asymptomatic infected leaves ranged from 0-17% (Fig. 2D). Nematodes were also detected in abscised leaves ranging from about 30-4,000 nematodes/g during the overwintering period (Fig. 2C). However, the number of plants with abscised leaves only ranged between 3 and 23% throughout the overwintering period (Fig. 2D). The number of nematodes detected per gram of symptomatic leaf was not correlated (P > 0.05) to daily high temperature, daily low temperature, average daily temperature, or to the relative humidity in the polyhouse in either overwintering period.
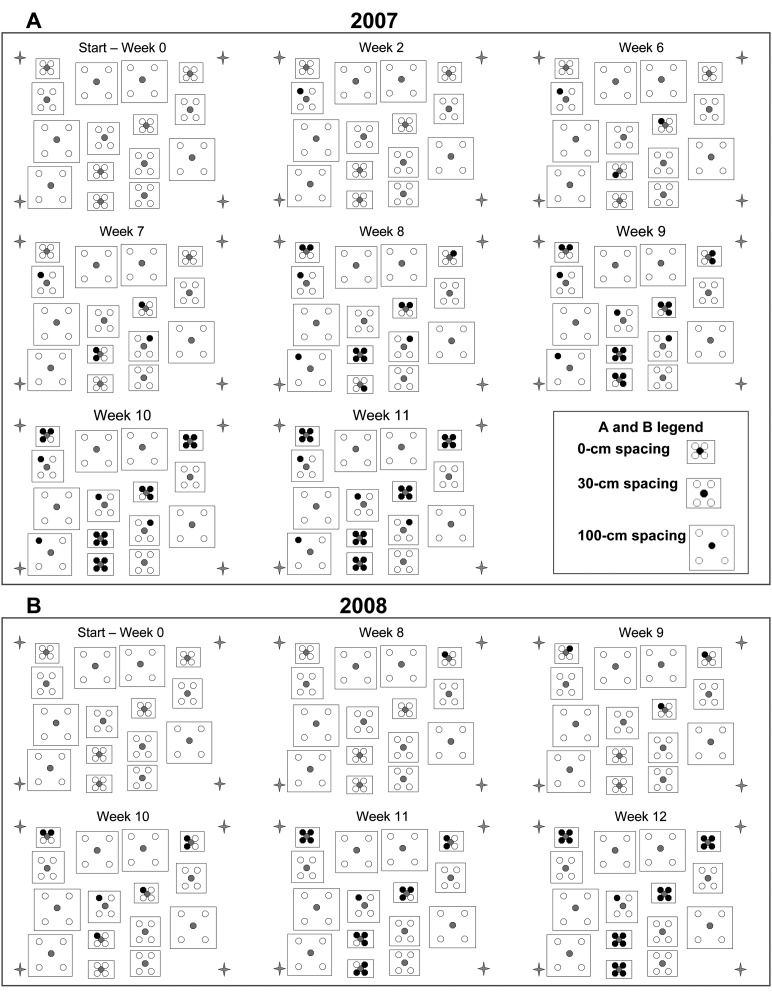
Disease gradient study: Symptoms of foliar nematode infection were first observed on the healthy lantana plants placed at the 0-cm spacing at six (2007) and eight (2008) weeks after trial initiation (Fig. 3A, B). The number of lantana plants with foliar nematode symptoms at the 0-cm spacing continued to increase each week until 100% of the plants were symptomatic by week 11 in 2007 and week 12 in 2008. At the 30-cm spacing, two initially healthy lantana plants (10%) developed symptoms in 2007 and only one lantana plant (5%) in 2008 (Fig. 3A, B). Foliar nematode symptoms were observed on a third lantana plant at the 30-cm spacing by week 2 in 2007. However, this plant was presumed to have been infected prior to the start of the trial since no other plant at this distance became infected until at least 7 weeks. One lantana plant at the 100-cm spacing became symptomatic in 2007 during week 8, but no plants were infected at the 100-cm spacing in 2008.
Fig. 3.
Pattern of initially healthy lantana plants infected with A. fragariae from a central infected plant under daily sprinkler irrigation during August to October 2007 (A) and July to October 2008 (B). White circles indicate healthy lantana plants, center circles indicate initially-infected plants placed as sources of inoculum, and black circles are lantana plants that became infected during the growing season. Plant canopy spacing tested was 0- (touching), 30- or 100-cm distant from the infected central plant as represented by the three different sized rectangles in the map. There were five replicates for each distance. Stars represent the position of the four irrigation sprinklers.
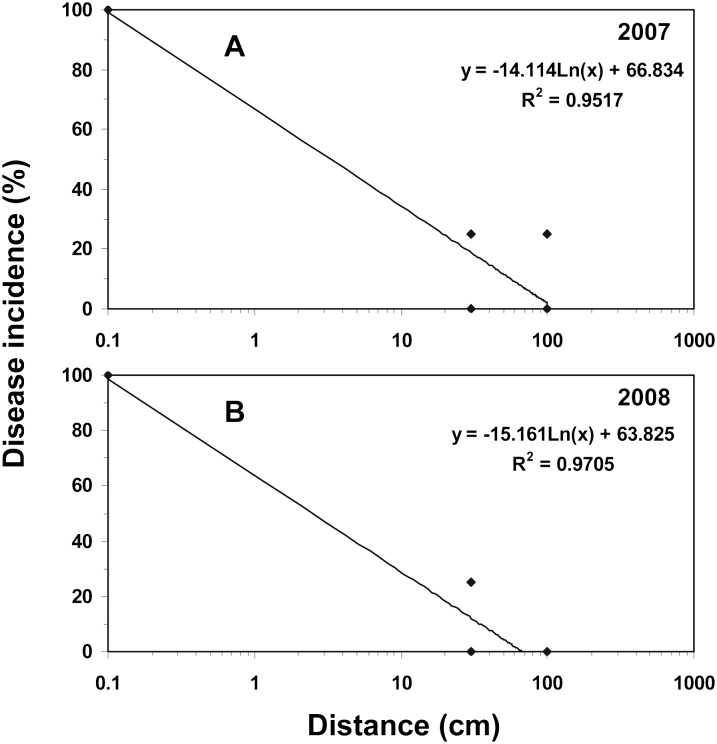
A steep disease gradient was found for dispersal of foliar nematodes from the central infected plant and infection of nearby healthy plants in both years. As expected for disease gradients, a logarithmic regression gave the best r-square values as dispersal efficiency falls off with the logarithm of the distance healthy plants were placed from an infected plant. In 2007, the regression equation was y = -14.1Ln(x) + 66.8 (R2 = 0.95) where Ln(x) is the natural logarithm of the distance from the source plant (Fig. 4A). In 2008, the regression equation was y = -15.2Ln(x) + 63.8 (R2 = 0.97) (Fig. 4B).
Fig. 4.
Logarithmic regression plots for the dispersal of A. fragariae from an infected central plant to healthy plants at 0-, 30-, or 100-cm away under sprinkler irrigation on a nursery pad during the (A) 2007 and (B) 2008 growing seasons.
Discussion
During each growing season nematode populations of A. fragariae in symptomatic leaves of lantana followed a similar trend with the highest nematode populations occurring in July in all three years. We confirmed the population trend observed by Warfield et al. (2004) in a 2003 trial at the same commercial nursery, in which the foliar nematode population in symptomatic lantana leaves began to decline in late August, continued through September and increased again by October. The nematode populations in our study were about two-fold lower than the populations recorded in the 2003 trial suggesting that nematode populations can be quite variable depending on the environment as well as initial inoculum source. Although nematode populations for symptomatic leaves were about 100 nematodes/g or more beginning in July and August, overall disease severity of plants did not increase until the beginning of September, at least in 2007. This suggests that foliar nematodes did not begin to migrate from initially infected leaves to asymptomatic leaves within the canopy until later in the growing season as seasonal air temperatures decreased. Alternatively, the nematode reproductive rate may have been slower during the heat of the summer resulting in the slower development of visible leaf damage. Conversely, our sampling method required removal of infected plant tissue at each sampling date, hence the number of remaining symptomatic leaves per plant was reduced, and thus the potential for production of secondary inoculum slowed the epidemic. Removing symptomatic leaves also may have affected plant to plant dispersal and nematode infection resulting in a lower percentage of infected plants particularly in 2007 and 2008 when the initial number of infected plants was low at the beginning of the growing season.
Fluctuations of nematode populations of A. fragariae in symptomatic leaf tissue during the growing season may have been due to plant growth stage and/or air temperatures. Since we found positive correlations for daily high, low, and average air temperatures 7, 14 and 20 days prior to sampling and nematode population, fecundity may have been affected as well as development of other stages in the nematode life cycle. Szczygiel and Hasior (1972) determined for strawberry, a cool season crop that higher populations of A. fragariae were detected during periods of cool weather and high humidity in Poland, but our peak nematode populations in lantana, a warm season plant, occurred in July, when temperatures and humidity were high.
The life cycle of A. fragariae can be completed in 14 days (Strumpel, 1967; Siddiqi, 1975), with daily temperatures no doubt affecting egg-laying of females within leaf tissue. Past studies have demonstrated that A. fragariae females lay eggs at 18°C (Strumpel, 1967), and females of the related species A. ritzemabosi lay eggs at temperatures from 13-18°C (French and Barraclough, 1961), but the optimum temperature for foliar nematode reproduction and maturation is unknown. Since daily temperatures for July-August at the nursery averaged 27°C in 2006, 28°C in 2007, and 26°C in 2008 for the 14 days before each sampling date, A. fragariae can reproduce at much higher temperatures than observed in previous fecundity studies (Strumpel, 1967). Although no attempt was made to develop a model that describes the relationship of environmental variable to populations of A. fragariae in lantana leaves, our data set may prove useful in this regard in the future.
In 2007 and 2008, nematode populations in asymptomatic leaves on plants transferred from the polyhouse to the nursery pad were one source of inoculum that resulted in infected, symptomatic leaves at the mid-June sampling date. Over the two growing seasons, however, the mean nematode population in asymptomatic leaves was 10 or less nematodes/g fresh weight. As the nematode population increases in an asymptomatic leaf, the leaf eventually becomes symptomatic as the nematode damage becomes apparent. However, there was no correlation between the area of symptomatic leaf tissue and the number of nematodes per gram of leaf tissue. Our results suggest that the change from asymptomatic to symptomatic leaf status occurs between 10-100 nematodes/g as populations in asymptomatic leaves never exceeded 10 nematodes/g, but symptomatic leaves always had 100 nematodes/g or more. As the nematodes emerge from infected leaves and migrate, or are moved or splashed, to adjacent, healthy leaves new infections result in asymptomatic leaves with initially low nematode populations.
Another source of inoculum for foliar nematode infections at the start of the growing season as well as throughout the season is nematodes present in abscised leaves on the surface of the container substrate. In both 2007 and 2008, nematodes were present in abscised leaf samples at the beginning of the growing season from symptomatic leaves that dropped off after plants were transferred from the polyhouse to the nursery pad. Leaf drop was attributed to senescence due to nematode infection, the stress of repotting to larger containers, as well as changes in relative humidity from the humid polyhouse to the relatively dry environment of the outdoor nursery pad. As symptomatic leaves continued to abscise over the growing season, nematode populations in abscised leaves were similar to those in symptomatic leaves. Since abscised leaves can harbor viable foliar nematodes, growers should remove these leaves from the container surface or nursery pad at transplanting, shearing or whenever plants are handled to reduce inoculum levels and subsequent new infections.
In both 2006-2007 and 2007-2008, foliar nematodes overwintered in infected leaves of lantana plants maintained in the polyhouse under minimal heating. Nematodes were detected in both symptomatic and asymptomatic leaves as well as abscised leaves during overwintering but not in the container substrate. Presumably, nematodes could move into the substrate as infected, abscised leaves decomposed on the container surface. However, removal of all abscised leaves at each sampling date possibly reduced the opportunity for foliar nematodes to migrate into the container substrate. In contrast, Jagdale and Grewal (2006) recovered viable juveniles, females, and males of A. fragariae in frozen soil samples cut from containers of hosta plants whose foliage had died back to the soil line during overwintering in an unheated polyhouse in Ohio. In addition to soil, Jagdale and Grewal (2006) also found A. fragariae in the outer layers of bud scales present just below the soil line on hosta plants in the same polyhouse. We did not investigate if A. fragariae could also survive in the bud scales of lantana plants, since infected leaves were always present on some plants throughout the overwintering period.
Although high nematode populations were detected in symptomatic and abscised leaves at certain sampling dates of overwintered plants, the number of infected plants declined markedly from January to March 2007. In 2008, only about 10% of the plants moved into the polyhouse had become infected on the nursery pad. So even though high populations were detected in leaves of infected plants, the percentage of infected plants remained low. As a result, in the 2008 growing season the number of plants already infected, or became infected after being moved back to the nursery pad remained low as well. Thus, incidence of foliar nematode-infected plants during overwintering has a direct relationship to the number of plants in the population (block of plants on the nursery pad) that will become infected during the following growing season. Therefore sanitation practices, such as removing both symptomatic and abscised leaves, that reduce the number of infected plants placed in the overwintering structure will affect the number that subsequently becomes infected during the next growing season. However, the ability of the nematodes to overwinter on containerized plants in the polyhouses clearly demonstrates the risk of holding nematode-infected plant material over to the next season.
Nematodes were found in asymptomatic leaves during both the growing season and during overwintering. In a commercial nursery, asymptomatic plants are easy to overlook as a source of inoculum, but our results clearly show that asymptomatic leaves can harbor foliar nematodes. This is a concern for nursery growers and landscapers who want to avoid shipping and buying infected plant material.
Plant to plant dispersal of foliar nematodes was observed during the growing season when initially healthy plants at the research nursery were placed at different distances from an infected plant. Dispersal was most efficient between plants only when canopies were touching (0-cm distance) and resulted in 100% infection of initially healthy plants within 11-12 wk in both years. In a previous trial with lantana at the same nursery, 100% of the healthy plants whose canopies were allowed to touch infected plants became infected within 9 wk whether irrigated by overhead sprinklers or low-volume spray stakes (Warfield et al., 2004).
At a distance of 30 cm between canopies, only 5 and 10% of the initially healthy plants became infected in 2008 and 2007, respectively, suggesting that plant spacing can have a dramatic effect on nematode dispersal. The steep disease gradient observed in our study suggests that a canopy spacing of 10 cm would result in about 45-50% fewer infected plants while a larger spacing would have an even greater effect on limiting disease incidence. Although labor intensive, the simple process of re-spacing containers in a nursery block as plant canopies grow during the growing season could have a dramatic impact on management of this pest. Since plant infection at 100 cm was negligible, leaving an aisle of 100 cm between blocks of plants on a nursery pad could also help to prevent splash dispersal of foliar nematodes in the nursery.
Based on the results of our dispersal trials, the time between nematode infection and expression of leaf symptoms is at least 6-8 wk. In the trial mentioned above, half of the healthy plants had developed symptoms within 4 wk (Warfield et al., 2004). This relatively long incubation period for foliar nematode symptom expression on lantana complicates management strategies since infected, asymptomatic plants are hard to detect and remove from the plant population.
Overall management of foliar nematodes in lantana and other herbaceous and perennial ornamentals in nurseries is difficult since most nematicides are ineffective, especially when populations are high (Lamondia, 1999; Warfield and Para, 2003; Warfield, et al., 2003). In addition, foliar nematodes can overwinter in both symptomatic and asymptomatic leaves on lantana plants as well as in abscised leaves in polyhouses with these populations providing initial inoculum for epidemics during the subsequent growing season. Since most nurseries allow plant canopies to grow together due to container spacing requirements, sprinkler irrigation and rain can promote plant to plant dispersal of foliar nematodes. Our results suggest that both sanitation and plant spacing to avoid canopy to canopy contact can have dramatic impact on the management of foliar nematodes.
Literature Cited
- Barker KR, Townshend JL, Bird GW, Thomason IJ, Dickson DW. Determining nematode population responses to control agents. In: Hickey KD, editor. Methods for evaluating pesticides for control of plant pathogens. St. Paul, MN: American Phytopathological Society (APS) Press; 1986. pp. 283–296. [Google Scholar]
- Daughtrey ML, Wick RL, Peterson JL. St. Paul, MN: American Phytopathological Society (APS) Press; 1995. Compendium of flowering potted plant diseases. [Google Scholar]
- Esser RP, Riherd CC. Distribution of Aphelenchoides fragariae in leaves of Ficus elastica and Asplenium nidus. Plant Disease. 1981;65:425–426. [Google Scholar]
- French N, Barraclough RM. Observations on the reproduction of Aphelenchoides ritzemabosi (Schwartz) Nematologica. 1961;6:89–94. [Google Scholar]
- Hesling JJ, Wallace HR. Observations on the biology of chrysanthemum eelworm Aphelenchoides ritzema-bosi (Schwartz) Steiner in florists' chrysanthemum. I. Spread of eelworm infestation. Annuals of Applied Biology. 1961;49:195–203. [Google Scholar]
- Horsfall JG, Barratt RW. An improved grading system for measuring plant diseases. Phytopathology. 1945;35:655–655. [Google Scholar]
- Hunt DJ. Wallingford: CAB International; 1993. Aphelenchida, Longidoridae and Trichodoridae: Their systematics and bionomics. [Google Scholar]
- Jagdale GB, Grewal PS. Identification of alternatives for the management of foliar nematodes in floriculture. Pest Management Science. 2002;58:451–458. doi: 10.1002/ps.472. [DOI] [PubMed] [Google Scholar]
- Jagdale GB, Grewal PS. Infection behavior and overwintering survival of foliar nematodes, Aphelenchoides fragariae, on hosta. Journal of Nematology. 2006;38:130–136. [PMC free article] [PubMed] [Google Scholar]
- LaMondia JA. Efficacy of insecticides for control of Aphelenchoides fragariae and Ditylenchus dipsaci in flowering perennial ornamentals. Supplement to the Journal of Nematology. 1999;31:644–649. [PMC free article] [PubMed] [Google Scholar]
- Lehman PS. Dispersal modes for foliar nematodes. Nematology Circular No. 216:July/August. Florida Department of Agriculture & Consumer Services. 1996 Division of Plant Industry. 2 pages. [Google Scholar]
- Marlatt RB. Transmission of Aphelenchoides besseyi to Fiscus elastica leaves via Sporobolus poiretti inflorescences. Phytopathology. 1970;60:543–544. [Google Scholar]
- McCuiston JL, Hudson LC, Subbotin SA, Davis EL, Warfield CY. Conventional and PCR detection of Aphelenchoides fragariae in diverse ornamental host plant species. Journal of Nematology. 2007;39:343–355. [PMC free article] [PubMed] [Google Scholar]
- Sanwal KC. A key to the species of the nematode genus Aphelenchoides Fischer, 1894. Canadian Journal of Zoology. 1961;39:143–148. [Google Scholar]
- Siddiqi MR. Commonwealth Institute of Helminthology Descriptions of Plant-Parasitic Nematodes, no. 74. St. Albans, England: Commonwealth Agricultural Bureaux; 1975. Aphelenchoides fragariae. 4 pages. [Google Scholar]
- Strumpel H. Beobachtungen zur lebensweise von Aphelenchoides fragariae in Lorraine-begonien. Nematologica. 1967;13:67–72. [Google Scholar]
- Szczygiel A, Hasior H. Possibility of persistence of leaf and bud nematodes (Aphelenchoides fragariae) on strawberry plants and in the soil. Zeszyty problemowe postepow nauk rolniczych. 1971;121:101–106. [Google Scholar]
- Szczygiel A, Hasior H. Seasonal variations in population of plant parasitic nematodes in strawberry plantations. Ekologia Polska. 1972;20:507–522. [Google Scholar]
- Wallace HR. Movement of eelworms. V. Observations on Aphelenchoides ritzema-bosi (Schwartz 1912) Steiner 1932 on florists' chrysanthemums. Annals of Applied Biology. 1959;47:350–360. [Google Scholar]
- Warfield CY, Para GR. Evaluation of pesticides for control of foliar nematodes on lantana, 2002. Fungicide and Nematicide Tests 58:OT021. 2003 [Google Scholar]
- Warfield CY, Dudley JB, Hight PA. Evaluation of chemical and cultural methods for managing foliar nematodes on woody ornamental crops in nurseries. Proceedings of Southern Nursery Association (SNA) Research Conference. 2004;49:290–293. [Google Scholar]
- Yamada E, Takakura S. Ecological investigations on the strawberry nematode, Aphelenchoides fragariae on lilies. Japanese Journal of Nematology. 1987;17:1–7. [Google Scholar]