Abstract
Background
CC Chemokine receptor 4 (CCR4) is preferentially expressed on Th2 lymphocytes. CCR4-mediated inflammation may be important in the pathology of allergic rhinitis. Disruption of CCR4 – ligand interaction may abrogate allergen-induced inflammation.
Methods
Sixteen allergic rhinitics and six nonatopic individuals underwent both allergen and control (diluent) nasal challenges. Symptom scores and peak nasal inspiratory flow were recorded. Nasal biopsies were taken at 8 h post challenge. Sections were immunostained and examined by light or dual immunofluorescence microscopy for eosinophils, T-lymphocytes, CCR4+CD3+ and CXCR3+CD3+ cells and examined by in situ hybridization for CCR4, IL-4 and IFN-γ mRNA+ cells. Peripheral blood mononuclear cells were obtained from peripheral blood of nine normal donors and the CCR4+CD4+ cells assessed for actin polymerization in response to the CCR4 ligand macrophage-derived chemokine (MDC/CCL22) and the influence of a CCR4 antagonist tested.
Results
Allergic rhinitics had increased early and late phase symptoms after allergen challenge compared to diluent; nonatopics did not respond to either challenge. Eosinophils, but not total numbers of CD3+ T cells, were increased in rhinitics following allergen challenge. In rhinitics, there was an increase in CCR4+CD3+ protein-positive cells relative to CXCR3+CD3+ cells; CCR4 mRNA+ cells were increased and IL-4 increased to a greater extent than IFN-γ. CCR4+CD4+ T cells responded to MDC in vitro, and this response was inhibited by the selective CCR4 antagonist.
Conclusion
Lymphocyte CCR4 expression is closely associated with induction of human allergen-induced late nasal responses. Blocking CCR4-ligand interaction may provide a novel therapeutic approach in allergic disease.
Keywords: allergic rhinitis, CCR4, nasal mucosa, Th2-mediated inflammation
T lymphocyte trafficking to target organs is a critical component of host defence. Chemokines are low molecular weight soluble proteins produced by cells including epithelial cells and leucocytes. They are involved in inflammatory processes via leucocyte chemotaxis, upregulation of integrin-mediated adhesion and cytoskeletal rearrangement (1). They bind to corresponding families of chemokine receptors on leucocyte cell surfaces. Differential expression of various chemokine receptors and responses to chemokines has been demonstrated on Th1 and Th2 cells in vitro (2, 3). Allergen-induced pulmonary Th2 cell recruitment in mice is dependent on interactions between CCR3 and its ligand eotaxin, as well as between CC Chemokine receptor 4 (CCR4) and macrophage-derived chemokine (MDC) (4). Both these chemokines are members of the CC chemokine receptor family. Murine studies have also suggested the feasibility of blocking CCR4 - ligand interactions as a means of preventing Th2-mediated inflammatory responses (5). In man, the expression of CCR4 and its ligands (TARC & MDC) has been reported to be upregulated in the bronchial mucosa and in the skin of atopic subjects during late phase responses following bronchial/cutaneous allergen challenge (6–8).
Allergic rhinitis is common (9) and significantly impacts on quality of life and performance (10). Allergic rhinitis is an IgE-mediated disorder with increasing prevalence worldwide (9). Inflammatory responses in the nasal mucosa of allergic rhinitics comprise activated mast cells, eosinophils and CD4+Th2-type T helper lymphocytes that preferentially generate the cytokines IL-4 and IL-13 which promote ongoing IgE synthesis and IL-5 which is in turn essential for the selective recruitment and prolonged survival of eosinophils in tissues (11).
Increased expression of the CCR4 ligand TARC (CCL17) has been demonstrated in human nasal epithelium in response to allergen provocation (12). CCR4 is thought to be the most discriminating chemokine receptor marker of human Th2 cells (3, 5, 13, 14), yet little is known about its role in allergic rhinitis. Thus far, CCR3 and eotaxin have been more extensively studied in allergic rhinitis, with increased eotaxin expression in the nasal mucosa post allergen challenge (15) and increased CCR3 expression on circulating lymphocytes of individuals with allergic rhinitis compared to nonatopics (16).
This study examined T lymphocyte expression of CCR4 within the nasal mucosa during allergen-induced inflammation and the response of CCR4+ T cells to MDC in vitro. CXCR3 was used for comparison as a chemokine receptor preferentially associated with Th1 lymphocytes (1). We hypothesized that (i) allergen-induced inflammation in the nasal mucosa is associated with the recruitment of CCR4+ T-cells and (ii) the in vitro response of CCR4+ T-cells to MDC could be inhibited by a CCR4 antagonist.
Methods
Participants
Twenty-two volunteers were recruited for the study in response to an advertisement. The study was approved by the ethics committee of The Royal Brompton and Harefield Hospitals NHS Trust and was performed with the subjects’ written informed consent. Sixteen atopic rhinitic individuals and six normal nonatopic healthy controls were enrolled into the study. Inclusion criteria were a history of seasonal or perennial allergic rhinitis of at least 2 years duration and a positive skin test to mixed grass pollen or house dust mite. Inclusion criteria for nonatopic controls were an absence of nasal or allergic symptoms and negative skin testing to common aeroallergens. Exclusion criteria for both groups were a history of anaphylaxis, current systemic steroid or immuno-suppressive medication, other nasal or systemic disease, asthma requiring regular inhaled or oral corticosteroids (i.e. step 2 or above of Global Initiative for Asthma treatment guidelines (17)), or specific immunotherapy within the last 5 years. At screening, participants were examined, including a nasal examination to rule out the presence of nasal polyps or infective rhinosinusitis. Skin prick testing was performed with standardized reagents (ALK Abello, Horsholm, Denmark); a weal of >3 mm with adequate negative control was considered positive. Following screening, blood samples were taken for full blood count, total IgE and grass pollen and house dust mite specific IgE.
Study design
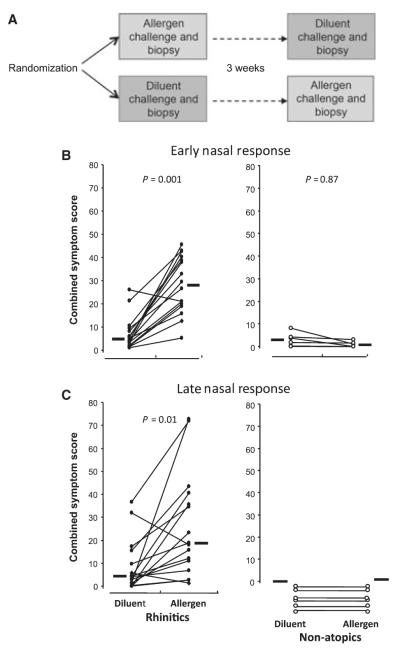
This was a prospective clinical experimental study. Participants underwent separate nasal challenges with diluent and allergen followed by nasal biopsies 8 h later. Challenges were separated by at least 3 weeks. The order of challenges (allergen or diluent) was randomized (Fig. 1A). Symptom scores and peak nasal inspiratory flow were measured before and at 15-min intervals for 1 h then hourly for 8 h after each challenge.
Figure 1.
(A) Participants were randomized to one of two nasal challenge protocols: either undergoing allergen challenge to one nostril selected at random plus biopsy, followed by diluent challenge to the other nostril 3 weeks later, or vice versa. (B) Early phase response to intranasal challenge with 2500 BU allergen as nasal spray or equal volume of diluent alone. Symptom scores for nasal itch/sneezing, running and blockage were recorded on a visual analogue scale at 15, 30 and 60 min and plotted on a graph. The area under the curve was then calculated and expressed as the early phase combined symptom score. (C) Late phase response to nasal allergen or diluent challenge. Symptom scores were recorded at hourly intervals; the combined symptom score was calculated as the area under the curve between 2 and 8 h. Circles represent individual participants; lines connect responses in the same individual. Horizontal bars represent median values. P-values represent comparison by Wilcoxon matched-pairs sign rank test.
Nasal challenge
Challenges were performed outside of the major UK pollen seasons. All challenges were performed at the Allergy unit of the Royal Brompton Hospital, London. Volunteers were asked not to take caffeine-containing drinks on the day of the challenge. Volunteers arrived at the unit and were allowed to acclimatize at room temperature (20 °C) for at least 30 min prior to the procedure; they were asked to refrain from eating or drinking during this period. Allergen (ALK Abello) was diluted in a nasal spray applicator at 5000 BU/ml. One spray was standardized to release 0.14 ml. The procedure was explained to the volunteer who sat facing the examiner. The volunteer’s nose was visualized using a nasal speculum and head mirror. Two sprays of allergen or diluent alone were administered to the lateral wall of one nasal cavity by the examiner under direct visualization. Administration of two further sprays was performed 15 min later. This provided a total dose of approximately 2500 BU, a dose previously shown to be sufficient to induce late phase nasal responses (18).
Symptom scores were measured by means of a visual analogue scale immediately before and at 15, 30 and 60 min post challenge and then hourly to 8 h. Three parameters were measured: nasal itch/sneeze, running and blockage, then combined (Fig. 1B).
Peak nasal inspiratory flow (VBM Medizintechnik GmbH, Baden-Württemberg, Germany) was recorded before and at 15, 30 min and 8 h after challenge. The best of three measurements was recorded at each stage.
Nasal biopsy
Biopsies were taken at 8 h post challenge. Specimens (2.5 mm) were taken from the under surface of the inferior turbinate using Gerritsma forceps with lidocaine 5%/phenylephrine 0.5% and 10% cocaine as local anaesthetic as described previously (19). Tissue specimens were divided into two, one specimen was embedded in OCT (Tissuetek; Raymond Lamb, Eastbourne, UK) and immediately snap frozen in liquid nitrogen, the other was preserved in 4% paraformaldehyde dehydrated in 15% sucrose for 1 h and then again overnight, before being embedded in OCT and snap frozen. Five-micron thick sections from snap frozen tissue were cut onto slides before fixing in acetone, drying and storage at −80 °C. Paraformaldehyde-fixed tissue was cut into six-micron sections onto slides, dried overnight at 37 °C and then stored at −80 °C.
Immunohistochemistry
Eosinophil staining was carried out on tissue sections using an Avidin Biotin Alkaline Phosphatase Immune Complex technique (ABC-AP). Endogenous tissue Fc receptors were blocked with polyclonal horse serum. Human major basic protein (MBP) was stained using mouse anti-MBP monoclonal antibody BMK 13 (kindly donated by Professor AB Kay). Labelling was achieved by incubation with biotinylated anti-mouse antibody (Vector Labs, Burlingame, CA, USA), followed by Avidin Biotin Complex Alkaline Phosphatase. Fast Red (Fast Red TR/Naphthol, AS-Mx; Sigma, St. Louis, MO, USA) in alkaline phosphatase substrate buffer was then applied before counterstaining with haematoxylin (BDH Laboratory Supplies, Lutterworth, UK). Washing in tris-buffered saline was carried out between steps as appropriate. Slides were mounted in Ultramount (Dako, Ely, UK).
CCR4CD3 and CXCR3CD3 staining was carried out as follows. Endogenous biotin was blocked (Vectastain, avidin/ biotin blocking kit, Vector Laboratories). Endogenous Fc receptors were blocked with polyclonal donkey serum and the sections were incubated with primary antibodies: mouse anti-CD3 IgG1 monoclonal antibody (Dako, Cambridge, UK), goat polyclonal IgG anti-CCR4 or goat polyclonal IgG anti-CXCR3 (Santa Cruz, Santa Cruz, CA, USA). Following several washes in PBS, sections were incubated with biotinylated anti-goat antibody (Stratech Scientific, Cambridge, UK). Finally, streptavidin-conjugated Alexa-Fluor 594 (Cambridge Biosciences, Cambridge, UK) and fluorescein isothiocyanate (FITC)-labelled rabbit anti-mouse antibody (Dako) were applied. Slides were mounted using fluorescence mountant, Prolong Anti Fade Kit (Molecular Probes Limited, Eugene, OR, USA).
The concentration of each antibody was individually optimized. Isotype control antibodies were applied as appropriate: mouse IgG1 (Dako) for eosinophil and CD3 staining, and goat IgG (Autogen Bioclear) for CCR4- and CXCR3-expressing cells.
Positively stained eosinophils were counted using an Olympus BH2 microscope (Olympus Optical, Tokyo, Japan) at 200 × magnification. Cells to a depth of approximately one grid (0.45 mm) below the epithelium were counted along the length of two sections; results were expressed as mean number of cells/ mm2. CCR4CD3 and CXCR3CD3 stained sections were visualized with a Nikon Eclipse E400 microscope at 400 × magnification and alternate fields of one or two grid depths beneath the epidermal border along the length of the specimen captured electronically using a Lucia 4.8 software package (Prague, Czech Republic). Single CD3 positive and dual positive stained cells were counted and expressed as mean number of cells/mm2.
In situ hybridization
Anti-sense and sense riboprobes were prepared from cDNA encoding human CCR4, IL-4 and IFN-γ. cDNA inserted into different pGEM vectors was linearized with appropriate enzymes prior to transcription in the presence of 35S-UTP and either T7 or SP6 RNA polymerase. Prior to hybridization, cryostat sections were permeabilized (Triton X-100; Sigma, UK) and treated with proteinase K (Sigma), then treated with iodoacetamide and N-ethylmalemide followed by acetic anhydride/triethanolamine to prevent nonspecific binding. Sections were then hybridized with the 35S-labelled riboprobes, washed and dipped in photographic emulsion. Three weeks later, slides were developed and counterstained.
Slides were examined at 200 × magnification using a Zeiss microscope (Axiolab). Positively stained cells were counted and expressed as cells/mm2. Specimens for both immunochemistry and in situ hybridization were counted in a coded fashion with the examiner blind to the status of the donor.
In vitro actin polymerization
Blood was taken from normal volunteers who had taken no medication within the previous 10 days and mixed with 1/9 of the volume of 3.8% tri-sodium citrate solution. Chemokine-induced increases in the F-actin content of CD4+ CCR4+ T cells were measured by a modification of the method of Pilette et al. (8). Peripheral blood mononuclear cells (PBMC) were isolated by dextran sedimentation followed by Percoll density gradient centrifugation. The PBMCs were incubated with FITC-conjugated anti-human CD4 and PE-conjugated anti-CCR4 antibodies or appropriate isotype controls (all from BD Biosciences) for 15 min. The cell suspensions were then centrifuged at 400 g for 10 min and the pellets resuspended in assay buffer (phenol red-free RPMI 1640 medium containing 10 mM HEPES and 1% bovine serum albumin) at 107/ml. The resulting cell suspension was incubated with antagonist (10 nM) or vehicle (0.1% DMSO) for 30 min at 37 °C before stimulation with the MDC for 15 s. The assay was terminated by addition of 3% formaldehyde. The fixed cells were washed twice with PBS (centrifuging at 1000 g for 5 min to recover the cells) and incubated at room temperature with Alexa fluor-647 phalloidin (0.075 U/ml) in the presence of lysophosphatidylcholine (93.75 μg/ml) for 20 min to stain F-actin. The cells were washed once more with PBS, and the relative F-actin content of the cells was measured flow cytometrically. The mean fluorescence intensity of 1000 CD4+ CCR4+ cells per sample was determined and expressed as a fraction of the mean intensity of the CD4+ CCR4− cells in that sample.
Acquisition of the blood samples was approved by the Hertfordshire Research Ethics Committee, and all donors gave informed consent prior to donation.
Statistical analysis
A commercial software package (minitab release 9.2, Minitab Inc., State College, PA, USA) was used. Nonparametric statistical methods were applied. Results are expressed as median and interquartile range. Between-group comparisons were made by Mann–Whitney U-test, within-group comparisons by Wilcoxon matched-pairs test. Statistical significance was considered at P < 0.05.
Results
Clinical parameters
Table 1 summarizes the demographic characteristics of the participants. Ten of the atopic patients were polysensitized, six were monosensitized to grass pollen. Nasal challenge was performed with mixed grass pollen in 14 and with house dust mite extract in two allergic rhinitic (‘atopic’) individuals. The most relevant allergen for challenge was determined on the basis of the clinical history and the results of serum allergen-specific IgE concentrations and skin testing. All six nonatopic individuals were challenged with mixed grass pollen extract (Allergen extracts, ALK Abello).
Table 1.
Summary of characteristics of participants recruited into the study
| Atopic rhinitis (n = 16) |
Normal controls (n = 6) |
|
|---|---|---|
| Age (years) | 30.3 | 30 |
| Gender (M:F) | 11 : 5 | 5 : 1 |
| Total IgE (IU/ml) | 407 (115) | 16.4 (3.1) |
| Allergen specific IgE (IU/ml) | 42.5 (9.3) | <0.34 |
Data are expressed as mean (standard error) where appropriate. Allergen-specific IgE refers to mixed grass pollen in 14 and house dust mite in two atopic rhinitics, and mixed grass pollen in all six normal controls.
Atopic participants demonstrated both early and late phase symptomatic responses to allergen challenge but not to diluent challenge (P = 0.001 and P = 0.01, respectively, Fig. 1B, C). Nonatopics showed no differences in response to allergen or diluent. Allergen challenge resulted in a fall in peak nasal inspiratory flow in atopics at 15 min (median 150 l/min at baseline, 86 l/min at 15 min, P = 0.001). No such fall occurred following diluent challenge. No changes in peak nasal inspiratory flow were detected at 8 h following allergen or diluent challenge in atopics. No falls were detected in nonatopics at any time point.
Immunofluorescence and in situ hybridization studies
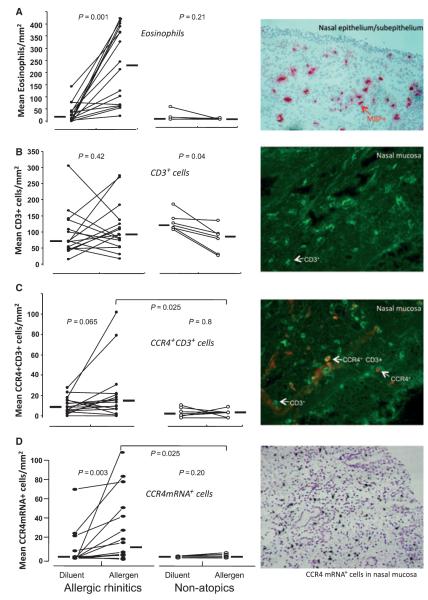
Atopics had significantly more eosinophils in the nasal mucosa at 8 h following allergen challenge compared to the diluent challenge (P = 0.001, Fig. 2A). Nonatopics had low numbers of eosinophils following both allergen and diluent challenge with no difference between them (P = 0.21).
Figure 2.
(A) left; mean number of eosinophils per square millimeter counted by light microscopy in nasal mucosal biopsy specimens taken from atopic and nonatopic participants at 8 h after intranasal challenge with diluent or allergen, right; example of eosinophils as identified by positive immunostaining for major basic protein (MBP) in nasal mucosa (× 200 magnification). (B) left; mean number of CD3+ cells per square millimeter counted by fluorescent microscopy following immunostaining in nasal biopsy specimens, right; example of staining. (C) left; mean number of CC Chemokine receptor 4 (CCR4)+CD3+ cells per square millimeter in the nasal mucosa, right; example of individual and dual CCR4+CD3+ cells following dual immunohistochemistry identified by fluorescence microscopy (× 400 magnification). (D) left; mean number of CCR4 mRNA+ cells per square millimeter following in situ hybridization in nasal mucosa specimens, right; example of CCR4 mRNA positive cells identified by dense accumulation of silver grains overlying individual cells (× 200 magnification). Circles represent individual participants; lines connect measurements in the same individual. Horizontal bars represent median values. P-values represent within-group comparison by Wilcoxon matched-pairs sign rank test or between-group comparison by Mann–Whitney U-test.
There was no difference in the total number of CD3+ cells following allergen or diluent challenge in atopic individuals (P = 0.42, Fig. 2B). There were fewer CD3+ cells following allergen challenge than diluent challenge in nonatopics (P = 0.04).
In atopic individuals, there was a trend towards greater numbers of dual CCR4+CD3+ following allergen than diluent challenge (P = 0.065, Fig. 2C), whereas no changes were seen in nonatopics. Greater numbers of CCR4+CD3+ cells were present following allergen challenge in atopics than nonatopics (P = 0.025). Amongst the nine atopic participants randomized to receive diluent challenge prior to allergen challenge, there was a significant increase in dual CCR4+CD3+ cells after allergen compared to diluent (P = 0.04); whereas this was not observed in the seven atopics who underwent allergen challenge first (data not shown). In contrast, there were no differences in the number of dual CXCR3+CD3+ cells in response to allergen for either atopic or nonatopic participants (data not shown).
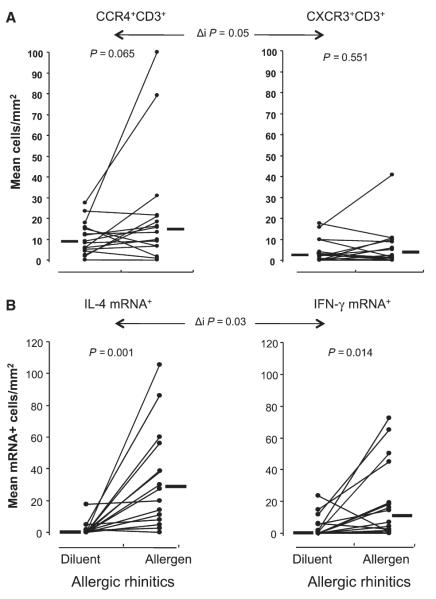
In atopics, greater numbers of CCR4 mRNA+ cells were seen after allergen compared with diluent (P = 0.003), and these differences were significant compared to the corresponding values for nonatopic subjects (P = 0.025, Fig. 2 D). In atopics, the magnitude of increase in CCR4+CD3+ cells after allergen compared with diluent was significantly greater than that seen for CXCR3+CD3+ cells (P = 0.05, Fig. 3A).
Figure 3.
(A) mean numbers of CC Chemokine receptor 4+CD3+ (left) and CXCR3+CD3+ cells (right) as identified by dual immunofluorescence in the nasal mucosa at 8 h after diluent or allergen challenge in atopic individuals only. (B) mean number of IL-4 (left) and IFN-γ (right) mRNA+ cells per square millimeter following in situ hybridization of nasal mucosa specimens from atopic participants after diluent or allergen challenge. Circles represent individual participants; lines connect measurements in the same individual. Horizontal bars represent median values. P-values represent comparison by Wilcoxon matched-pairs sign rank test, Δi P-values represent comparison of degree of change in median number of positive cells between diluent and allergen challenge between groups by Mann–Whitney U-test.
The median number of IL-4 mRNA+ cells in atopics was approximately 30 times higher, and IFN-γ mRNA+ cells approximately 15 times higher, following allergen compared with diluent (P = 0.001 and P = 0.014, respectively, Fig. 3 B) and the magnitude of increase was significantly greater for IL-4 than for IFN-γ (P = 0.03).
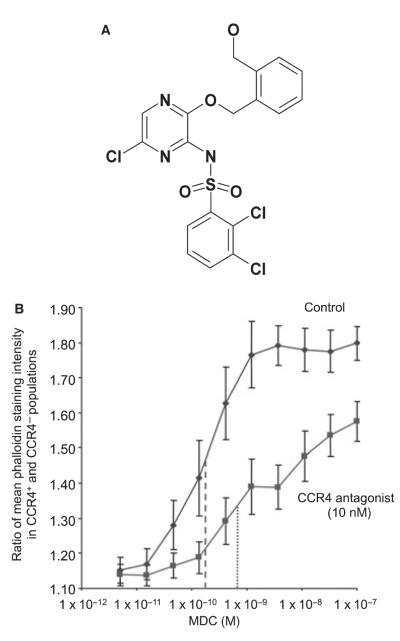
Actin polymerization
The chemical structure of the AstraZeneca CCR4 antagonist used in these experiments is shown in Fig. 4A. Macrophage-Derived Chemokine induced concentration-dependent increases in the F-actin content of CCR4+ CD4+ cells relative to CCR4− CD4+ cells in PBMCs with a pEC50 (negative log of the dose of MDC providing half-maximal relative increase in F-actin) of 9.88 ± 0.18 (Fig. 4B). After pre-incubation with CCR4 antagonist (10 nM), the pEC50 of MDC was 9.13 ± 0.16, representing an approximate fivefold increase in the concentration of MDC required to give a half-maximal relative increase in F-actin in the presence of antagonist. This increase was highly statistically significant (P ≤ 0.0002, paired t-test). There was no effect of MDC on CD4+ CCR4− cells in these experiments (data not shown).
Figure 4.
(A) Structure of the selective CC Chemokine receptor 4 (CCR4) antagonist used in this study. (B) Ratio of mean phalloidin-staining intensity between CD4+CCR4+ and CD4+CCR4− cells measured following stimulation with increasing concentrations of the CCR4 agonist macrophage-derived chemokine (MDC) after prior incubation with CCR4 antagonist or buffer alone. Fluorescence intensity of 1000 CD4+CCR4+ cells following cell lysis and staining with FITC-phalloidin was normalized to mean intensity of CD4+CCR4− cells in the same sample. Individual data points represent the mean of nine experiments, bars represent standard error. Approximate EC50 values are plotted without (- - - -) and with (· · · ·) the addition of the CCR4 antagonist.
Discussion
In atopic subjects following nasal allergen challenge, we observed significant increases in eosinophils, IL-4, IFN-γ (with a greater ratio of IL-4 : IFN-γ) and CCR4 mRNA-expressing cells in the nasal mucosa, consistent with a local Th2-type allergic response. These changes occurred in parallel with subjective and objective clinical responses to nasal provocation. We also observed increases in the recruitment of CCR4+CD3+ compared to CXCR3+CD3+ cells. These events are consistent with the differentiation of antigen-specific T-cell responses and emphasize the potential importance of the CCR4 receptor – ligand interaction in this clinical setting. In vitro, CCR4+CD4+ cells undergo cytoskeletal change, as evidenced by actin polymerization, in response to MDC. This process was inhibited by a selective CCR4 antagonist indicating that the response to MDC in this cell population is indeed CCR4-dependent. CCR4 likely plays an important role in vivo in the inflammatory response to allergen in the nasal mucosa and may provide a target for future pharmacological manipulation in allergic rhinitis and other allergic diseases.
The study used well-matched allergic rhinitis sufferers (‘atopics’) and nonatopic controls. Moreover, the use of diluent control challenges allowed each individual to act as their own negative control. Biopsies were undertaken outside of both grass and tree pollen seasons to reduce baseline levels of inflammation of the nasal mucosa. The lack of responses in nonatopic individuals throughout the study emphasizes the necessity for IgE sensitization to the relevant allergen and argues against the possibility that the response in atopics could have been because of a component of the allergen extract other than allergen, such as preservative or contaminating endotoxin. All atopic participants were sensitized to grass pollen, but two were challenged with house dust on the basis of greater reported symptoms on exposure to dust and greater radioallergosorbent test (RAST) and skin test results to house dust mite. Clinical scores and histological findings did not differ in these two patients from the rest of the atopic group. A standard allergen dose that was sufficient to induce late phase responses without untoward immediate side-effects was used for challenge based on our previous experience (18), rather than performing a more labour intensive up-dosing of allergen in each individual. The order of challenge affected the results in that atopics who received allergen challenge first demonstrated mildly elevated counts after diluent challenge 3 weeks later, compared to counts after diluent in those who received diluent first. This implies persisting inflammation after allergen that may last for 3 weeks or longer and highlights the need to perform diluent first in all subjects, or leave longer than 3 weeks after allergen challenge, to avoid this priming effect.
These findings add to the appreciation of the role of CCR4 in allergic inflammation within the nasal mucosa. In vitro, CCR4 is predominantly expressed on Th2 cells. These cells migrate in response to MDC (2), upregulate CCR4 in response to T-cell receptor stimulation and demonstrate intracellular calcium mobilization in response to CCR4-ligands (3). In mice, anti-MDC antibodies may inhibit recruitment of antigen-specific T cells to the lung following serial allergen challenge, accompanied by reduced eosinophils, IL-4 and bronchial hyperreactivity (4). However, CCR4 knockout mice still develop significant allergic inflammation in the lung (20), reflecting redundancy in the chemokine network. Furthermore, other strategies to block CCR4-mediated recruitment of inflammatory cells in mice have had mixed results (5). Recent work by Perros and colleagues using a humanized murine model of allergic asthma has successfully demonstrated that pretreatment with a CCR4-blocking antibody can abolish the effects of bronchial allergen challenge (21).
In man, bronchial allergen challenge results in upregulation of both CCR4 and its ligands, MDC and TARC (6–8). MDC in bronchoalveolar lavage (BAL) fluid in asthmatics has also been found to correlate with bronchial hyperresponsiveness (22). Intradermal allergen challenge is associated with increased CCR4 mRNA+ cells in atopics, and increased CLA+CCR4+ lymphocytes are found in the skin of patients with atopic dermatitis (14). Increased CCR4 expression has been demonstrated in adenoidal tissue of atopic children (13). There is limited data in the literature on the role of chemokines in allergic rhinitis. Francis et al. (16) found greater numbers of CCR3+CD4+ cells in peripheral blood of allergic rhinitics than nonatopics or individuals treated with grass pollen-specific immunotherapy; yet they found similar levels of CCR4 expression.
This study demonstrates the potential in vivo importance of CCR4 in the nasal mucosa in allergic rhinitis. The data presented here suggest a role for CCR4 in the recruitment of Th2 cells and the development of inflammation in the nasal mucosa in response to allergen. Confirmation of a response of CCR4+ CD4+ cells to MDC in vitro, coupled with the inhibitory effect of a CCR4 antagonist, validates CCR4 as a potential drug target for allergic rhinitis and possibly allergic asthma. However, we acknowledge that the considerable redundancy in the chemokine network may mean that blockade of CCR4 alone does not guarantee efficacy. Moreover, we are making the additional assumption that in vitro f-actin polymerization is an accurate surrogate for T cell chemotaxis in vivo. Use of CCR4 antagonists as adjuvants during immunization, as a means of boosting Th1 immunity, is under investigation and raises the question as to whether a related approach could in future be applied to specific allergen immunotherapy (23). A caveat to this approach is that CD4+CD25+ regulatory T cells have also been shown to express CCR4 (23, 24), hence blocking of this ligand-receptor interaction may have additional unwanted counter-regulatory effects.
Further research is needed in allergic rhinitis, asthma and atopic dermatitis as to the relative importance of CCR4 and other chemokine receptors, such as CCR3, CCR8 and CRTH2. Redundancy in the chemokine network may mean that interfering with a single chemokine or its receptor has only limited efficacy. Nonetheless, our controlled data in the target organ in man, together with supporting in vitro human data using a CCR4 antagonist, provide proof of concept for clinical testing of CCR4 antagonists. Further appreciation of the role of CCR4 and other chemokine receptors on regulatory T cells will also be required.
In summary, CCR4 mRNA expression and CCR4+ CD3+ cells relative to CXCR3+CD3+ cells are increased in the nasal mucosa during allergen-induced late nasal responses. These findings are accompanied by predominant Th2 cytokine expression and eosinophilic inflammation, supporting a role for this chemokine receptor in mediating allergic inflammation in the nose. Our demonstration of the ability of a CCR4 antagonist to block MDC/CCR4 ligand interaction and associated cytoskeletal changes in human T cells suggests CCR4 or its ligands may be targets for the development of drugs to control allergic disease.
Acknowledgments
The study was funded by the Academic Drug Discovery Initiative between Imperial College Trust and GlaxoSmithK-line. MRJ received funding from the Medical Research Council and SRD from a grant from Asthma UK. We are grateful to Ms Sandra Martins and to Ms Rebecca Roberts for technical support.
Footnotes
Conflicts of interest None.
References
- 1.Cosmi L, Annunziato F, Maggi E, Romagnani S, Manetti R. Chemoattractant receptors expressed on type 2 T cells and their role in disease. Int Arch Allergy Immunol. 2001;125:273–279. doi: 10.1159/000053827. [DOI] [PubMed] [Google Scholar]
- 2.Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Ambrosio D, Iellem A, Bonecchi R, Mazzeo D, Sozzani S, Mantovani A, et al. Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J Immunol. 1998;161:5111–5115. [PubMed] [Google Scholar]
- 4.Lloyd CM, Delaney T, Nguyen T, Tian J, Martinez-A C, Coyle AJ, et al. CC chemokine receptor (CCR3)/eotaxin is followed by CCR4/monocyte-derived chemokine in mediating pulmonary T helper lymphocyte type 2 recruitment after serial antigen challenge in vivo. J Exp Med. 2000;191:265–274. doi: 10.1084/jem.191.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lloyd CM, Rankin SM. Chemokines in allergic airway disease. Curr Opin Pharmacol. 2003;3:443–448. doi: 10.1016/s1471-4892(03)00069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panina-Bordignon P, Papi A, Mariani M, Di Lucia P, Casoni G, Bellettato C, et al. The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J Clin Invest. 2001;107:1357–1364. doi: 10.1172/JCI12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nouri-Aria KT, Wilson D, Francis JN, Jopling LA, Jacobson MR, Hodge MR, et al. CCR4 in human allergen-induced late responses in the skin and lung. Eur J Immunol. 2002;32:1933–1938. doi: 10.1002/1521-4141(200207)32:7<1933::AID-IMMU1933>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 8.Pilette C, Francis JN, Till SJ, Durham SR. CCR4 ligands are up-regulated in the airways of atopic asthmatics after segmental allergen challenge. Eur Respir J. 2004;23:876–884. doi: 10.1183/09031936.04.00102504. [DOI] [PubMed] [Google Scholar]
- 9.Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J. 2004;24:758–764. doi: 10.1183/09031936.04.00013904. [DOI] [PubMed] [Google Scholar]
- 10.Walker S, Khan-Wasti S, Fletcher M, Cullinan P, Harris J, Sheikh A. Seasonal allergic rhinitis is associated with a detrimental effect on examination performance in United Kingdom teenagers: case-control study. J Allergy Clin Immunol. 2007;120:381–387. doi: 10.1016/j.jaci.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 11.Durham SR. Mechanisms of mucosal inflammation in the nose and lungs. Clin Exp Allergy. 1998;28(Suppl 2):11–16. [PubMed] [Google Scholar]
- 12.Terada N, Nomura T, Kim WJ, Otsuka Y, Takahashi R, Kishi H, et al. Expression of C-C chemokine TARC in human nasal mucosa and its regulation by cytokines. Clin Exp Allergy. 2001;31:1923–1931. doi: 10.1046/j.1365-2222.2001.01152.x. [DOI] [PubMed] [Google Scholar]
- 13.Banwell ME, Robinson DS, Lloyd CM. Adenoid-derived TH2 cells reactive to allergen and recall antigen express CC chemokine receptor 4. J Allergy Clin Immunol. 2003;112:1155–1161. doi: 10.1016/j.jaci.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 14.Vestergaard C, Bang K, Gesser B, Yoneyama H, Matsushima K, Larsen CG. A Th2 chemokine, TARC, produced by keratinocytes may recruit CLA+CCR4+ lymphocytes into lesional atopic dermatitis skin. J Invest Dermatol. 2000;115:640–646. doi: 10.1046/j.1523-1747.2000.00115.x. [DOI] [PubMed] [Google Scholar]
- 15.Minshall EM, Cameron L, Lavigne F, Leung DY, Hamilos D, Garcia-Zepada EA. Eotaxin mRNA and protein expression in chronic sinusitis and allergen-induced nasal responses in seasonal allergic rhinitis. Am J Respir Cell Mol Biol. 1997;17:683–690. doi: 10.1165/ajrcmb.17.6.2865. [DOI] [PubMed] [Google Scholar]
- 16.Francis JN, Lloyd CM, Sabroe I, Durham SR, Till SJ. T lymphocytes expressing CCR3 are increased in allergic rhinitis compared with non-allergic controls and following allergen immunotherapy. Allergy. 2007;62:59–65. doi: 10.1111/j.1398-9995.2006.01253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 18.Varney VA, Jacobson MR, Sudderick RM, Robinson DS, Irani AM, Schwartz LB, et al. Immunohistology of the nasal mucosa following allergen-induced rhinitis. Identification of activated T lymphocytes, eosinophils, and neutrophils. Am Rev Respir Dis. 1992;146:170–176. doi: 10.1164/ajrccm/146.1.170. [DOI] [PubMed] [Google Scholar]
- 19.Nouri-Aria KT, Wachholz PA, Francis JN, Jacobson MR, Walker SM, Wilcock LK, et al. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J Immunol. 2004;172:3252–3259. doi: 10.4049/jimmunol.172.5.3252. [DOI] [PubMed] [Google Scholar]
- 20.Chvatchko Y, Hoogewerf AJ, Meyer A, Alouani S, Juillard P, Buser R, et al. A key role for CC chemokine receptor 4 in lipopolysaccharide-induced endotoxic shock. J Exp Med. 2000;191:1755–1764. doi: 10.1084/jem.191.10.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perros F, Hoogsteden HC, Coyle AJ, Lambrecht BN, Hammad H. Blockade of CCR4 in a humanised model of asthma reveals a critical role for DC-derived CCL17 and CCL22 in attracting Th2 cells and inducing airway inflammation. Allergy. 2009;64:995–1002. doi: 10.1111/j.1398-9995.2009.02095.x. [DOI] [PubMed] [Google Scholar]
- 22.Lezcano-Meza D, Negrete-Garcia MC, Dante-Escobedo M, Teran LM. The monocyte-derived chemokine is released in the bronchoalveolar lavage fluid of steady-state asthmatics. Allergy. 2003;58:1125–1130. doi: 10.1034/j.1398-9995.2003.00273.x. [DOI] [PubMed] [Google Scholar]
- 23.Bayry J, Tchilian EZ, Davies MN, Forbes EK, Draper SJ, Kaveri SV, et al. In silico identified CCR4 antagonists target regulatory T cells and exert adjuvant activity in vaccination. Proc Natl Acad Sci USA. 2008;105:10221–10226. doi: 10.1073/pnas.0803453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahern D, Lloyd CM, Robinson DS. Chemokine responsiveness of CD4+ CD25+ regulatory and CD4+ CD25- T cells from atopic and nonatopic donors. Allergy. 2009;64:1121–1129. doi: 10.1111/j.1398-9995.2008.01962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]