Abstract
Aim
Carbamylation of proteins through reactive cyanate has been demonstrated to predict an increased cardiovascular risk. Cyanate is formed in vivo by break-down of urea and at sites of inflammation by the phagocyte protein myeloperoxidase. Since myeloperoxidase (MPO) associates with high-density lipoprotein (HDL) in human atherosclerotic intima, we examined in the present study whether cyanate specifically targets HDL.
Results
Mass spectrometry analysis revealed that protein carbamylation is a major post-translational modification of HDL. The carbamyllysine content of lesion derived HDL was more than 20-fold higher in comparison to 3-chlorotyrosine levels, a specific oxidation product of MPO. Notable, the carbamyllysine content of lesion-derived HDL was 5 to 8-fold higher when compared to lesion derived low-density lipoprotein (LDL) or total lesion protein and increased with lesion severity. Importantly, the carbamyllysine content of HDL, but not of LDL, correlated with levels of 3-chlorotyrosine, suggesting MPO mediated carbamylation in the vessel wall. Remarkably, one carbamyllysine residue per HDL associated apolipoprotein A-I was sufficient to induce cholesterol accumulation and lipid droplet formation in macrophages through a pathway requiring the HDL receptor scavenger receptor class B, type I.
Conclusion
The present results raise the possibility that HDL carbamylation contributes to foam cell formation in atherosclerotic lesions.
Introduction
There is a consensus that atherosclerosis represents a state of heightened oxidative stress characterized by lipid and protein modifications in the vascular wall (31). LDL and HDL are prone to be modified in atherosclerotic lesions, but there is considerably less known about the role of HDL modifications in atherogenesis. Increasing evidence suggests that endogenously generated aldehydes are involved in pathophysiologies associated with cardiovascular diseases such as atherosclerosis (33;36). Recent observations demonstrated that HDL isolated from subjects with cardiovascular disease is dysfunctional and shows pro-inflammatory activities (3;26;39), indicating that dysfunctional HDL is involved in the pathogenesis of atherosclerosis.
Functional impairment of proteins through carbamylation is of particular relevance as clinical studies have shown that carbamylated proteins (measured as plasma levels of protein-bound carbamyllysine) are independent risk factors for development of coronary artery disease, future myocardial infarction and stroke (37). Proteins are carbamylated through cyanate (OCN−), a reactive nucleophile that irreversibly transforms lysine to ε-amino-carbamyllysine, also know as homocitrulline. One potential major enzymatic source for OCN− generation within human atheroma is the heme protein myeloperoxidase (MPO) (37). MPO utilizes hydrogen peroxide in the presence of the preferred substrate thiocyanate to generate OCN−. In line with this observation previous immunohistochemical data showed that polymorphonuclear neutrophils and monocytes, MPO-rich cells, are markedly enriched with carbamylated proteins (12).
OCN− is also a decomposition product of urea. This is particularly relevant in renal disease, where plasma concentrations of urea and carbamylated proteins increase (27). Notably, carbamylated LDL was shown to be the most abundant LDL modification found in human plasma (2). Moreover, in a mouse model of renal disease, the oral administration of urea increased carbamylated LDL significantly resulting in more severe atherosclerosis (1). However, there is very little information available about the vulnerability of HDL to carbamylation: with only one study reporting that carbamylation decreases anti-apoptotic activities of HDL (37).
We could previously demonstrate that proteins oxidized by hypochlorous acid (specific oxidation products of the MPO/H2O2/Cl− system) localize with apoA-I in the human atheroma (19). Subsequently, it was found that 3-chlorotyrosine (a specific fingerprint of the MPO/H2O2/Cl− system) is enriched on lesion-derived HDL indicating that MPO selectively targets HDL for oxidative modification in atherosclerotic lesions (4;11;18;19;39). We therefore hypothesized that MPO-H2O2-thiocyanate derived OCN− specifically targets HDL in atherosclerotic lesions, thereby modulating the functional integrity of HDL. This could be of particular importance, since thiocyanate, even at low physiologic concentrations, is reported to be the preferred substrate for MPO (7;34).
In this study, we conducted liquid chromatography tandem mass spectrometry (LC-MS/MS) to demonstrate that carbamyllysine levels were markedly elevated in HDL isolated from human atherosclerotic tissue of subjects free of renal disease. We observed that less than one carbamyllysine residue per HDL-associated apoA-I was sufficient to induce cholesterol accumulation in macrophages through a pathway involving scavenger receptor class B, type 1 (SR-BI). Overall, our observations raise the possibility that OCN− promotes human atherogenesis by promoting macrophage cholesterol accumulation.
Material and Methods
RPMI1640, DMEM, fetal bovine serum (FBS) and penicillin/streptomycin (PS) were obtained from PAA (Pasching, Austria). Radiochemicals were purchased from Hartman Analytic (Braunschweig, Germany). Phorbol 12-myristate 13-acetate (PMA) was purchased from Merck (Darmstadt, Germany), homocitrulline (carbamyllysine) from Bachem (Weil am Rhein, Germany), 13C3-homocitrulline from Ascent Scientific (Bristol, UK), 13C6-3-chlorotyrosine from Polypeptide Group (Strasbourg, France), and L-α-phosphatidylcholine (PC) from Avanti Polar Lipids (Alabama, US). All other reagents were obtained from Sigma (Vienna, Austria) unless otherwise specified.
Isolation of HDL and apolipoprotein A-I (apoA-I)
For HDL isolation, plasma density from normolipidemic blood donors was adjusted with KBr to 1.24 g/ml. Afterwards a two-step density gradient was generated in centrifuge tubes (16 × 76 mm, Beckman) by layering the density-adjusted plasma (1.24 g/ml) underneath a KBr-density solution (1.063 g/ml). Tubes were sealed and centrifuged at 90.000rpm for 4 hours in a 90TI fixed angle rotor (Beckman Instruments, Krefeld, Germany). After centrifugation, the clearly separated HDL-containing band was collected, desalted via a PD10 column (GE Healthcare, Vienna, Austria) and immediately used for experiments. ApoA-I was isolated as described previously (17).
Preparation of reconstituted HDL
Discoidal reconstituted HDL (rHDL) containing L-α-phosphatidylcholine (PC), free cholesterol (FC) and apoA-I were prepared using the cholate dialysis method (23). 3.29 μmol of PC was mixed with 13.9 nmol of free cholesterol (FC) and chloroform was evaporated under a stream of argon. Dried PC/FC was resuspendend during vortexing by drop wise addition of ~200 μl sodium-cholate (10% solution in 0.2 mol/L potassium phosphate buffer, pH 7.4) to achieve a clear solution. Afterwards, 559.2 μl apoA-I (3.29 nmol in 0.2 mol/L potassium phosphate buffer, pH 7.4) was drop wise added and the solution was vortexed twice for 30 seconds. The resulting solution was extensively dialyzed against PBS under argon at 4°C.
Carbamylation of HDL, rHDL and apoA-I
HDL, rHDL or lipid free apoA-I (1 mg protein/ml) were carbamylated with potassium cyanate (1 mmol/L up to 10 mmol/L) in phosphate buffered saline (pH 7.4) containing 100 μmol/L diethylenetriaminepentaacetic acid (DTPA) for 4 hours at 37 °C. Control HDL was incubated under same conditions in the absence of potassium cyanate. The carbamyllysine content was assessed by LC-MS/MS, which confirmed that a relevant degree of protein carbamylation was present.
(0.51 up to 5.27 HCit/apoA-I). All modified HDL preparations were passed through a PD10 column to remove unreacted reagents and used immediately for experiments.
Phospholipid analysis
Lipids were extracted with methyl-tert-butyl ether (22), resuspended in 100 μl CHCl3 / MeOH 1:1 and 100 pmol 12:0/13:0 PE was added as an internal standard. Chromatography was performed using Accela UHPLC (Thermo Scientific, Vienna, Austria) equipped with a Thermo Hypersil GOLD C18, 100 × 1 mm, 1.9 μm column. Solvent A was water with 1% ammonia acetate and 0.1% formic acid, and solvent B was acetonitrile/2-propanol 5:2 with 1% ammonia acetate and 0.1% formic acid. The gradient ran from 35% to 70% B in 4 min, then to 100% B in another 16 min where it was held for 10min. The flow rate was 250 μl/min. Phospholipids were determined by a TSQ Quantum Ultra (Thermo Scientific) in positive ESI mode. Total phosphatidylcholine was detected in a precursor ion scan on m/z 184 at 30 eV and phosphatidylethanolamine was detected with a neutral loss scan on mass 141 at 25 eV.
Measurement of lipoprotein hydroperoxide content
Conjugated dienes develop in lipoproteins through the oxidation of polyunsaturated fatty acids with isolated double bonds to polyunsaturated fatty acid hydroperoxides with conjugated double bonds (= dienes). The formation of conjugated dienes can be monitored by an increase in UV-absorption at 234 nm. Baseline absorption of native HDL was determined and compared to absorption of HDL after carbamylation.
Sources of human tissue
Aorta abdominalis of 15 subjects who died of cerebral haemorrhage were harvested during multi-organ procurement according to a protocol that had been approved by the Ethics Committee of the Medical University of Graz. The harvested arteries were snap-frozen and stored in liquid nitrogen for further analysis. The subjects were 61.4 +/− 15.2 years old with normal total cholesterol (< 180 mg/dl) and no increase in plasma urea (< 45 mg/dl). The morphology of the aortas investigated ranged from thickened intima up to pronounced atheroma containing calcium inclusions. The morphology of the aortas was classified according to previously described methodology (30).
Isolation of HDL-like particles from atherosclerotic lesions
HDL- or LDL-like particles were isolated from human arteries as previously described (4) with modifications. Briefly, the tissue was frozen, pulverized and suspended in 2 ml extraction buffer (0.15 mol/L NaCl, 100 μmol/L DTPA, 100 μmol/L butylated hydroxyl toluene, protease inhibitor cocktail (Sigma), 10 mmol/L Na3PO4, pH 7.4 and incubated overnight with gently shaking. Tissue was pelleted by centrifugation, the supernatant collected, and the pellet extracted a second time with 1 ml extraction buffer for 1 hour. The supernatants were pooled and used for isolation of lipoprotein-like particles (HDL, LDL) by sequential density gradient ultracentrifugation. Following centrifugation, HDL was purified with a polyclonal anti-apoA-I and LDL with a polyclonal anti-apoB antibody bound to magnetic bead reagent (Dynabeads, Invitrogen, Lofer, Austria) according to the manufacturers instructions. ApoA-I was identified as the main component of lesion-derived HDL by silver staining and by LC-MS/MS analysis and apoB-100 by silver staining and subsequent Western blot immunodetection with a monoclonal anti-apoB antibody.
Homocitrulline, 3-chlorotyrosine and amino acid quantification
Protein samples were hydrolyzed with a high-throughput low-volume hydrolysis method as described previously (6). Briefly, protein samples (3 to 20 μg) were placed into Qsert vials (Waters, Vienna) and 10 μl internal standard was added (containing 10 ng 13C6-HCit, 10 ng 13C6-3-CT, 0.3 μg 13C6-tyrosine and 1 μg 13C6-lysine). Hydrobromic acid with 0.25% phenol was added to a final concentration of 6 N, vials were flushed with argon, sealed and hydrolyzed at 160°C for 5 min. Afterwards, hydrobromic acid was evaporated in a speedvac. Protein hydrolysates were suspendend in 100 μl 0.2 mol/L Li-Citrat buffer (pH 2.8) and derivatized with the EZ:faast Kit (Phenomenex, Aschaffenburg, Germany) according to the manufacturers instructions.
Electrospray ionization tandem mass spectrometry (LC-MS/MS) with online HPLC was used for quantification of HCit and lysine. Calibrations curves were prepared by using varying amino acids and HCit levels with fixed amounts of internal standards. The calibration curves had a linearity range from 50 pg – 100 ng for HCit and 3-CT (R2: 0.998 and R2: 0.999) and from 100 ng – 3 μg for lysine and tyrosine (R2: 0.997 and R2: 0.998).
The HPLC column (250×4 mm, AAA-MS HPLC column, Phenomenex, Aschaffenburg, Germany) was equilibrated for 15 min with 100% solvent A at 35°C. Solvent A was 10 mmol/L ammonium formiate in water and solvent B was 10 mmol/L ammounium formiate in methanol. After equilibration, the sample (10 μl) was injected onto the HPLC column at a flow rate of 0.25 ml/min. Compounds were eluted with a discontinuous gradient starting with 83% solvent B for 13 min followed by 68% of solvent B for 4 min. The HPLC column effluent was introduced into an API 200 triple quadrupole mass spectrometer. Ions were generated by electrospray ionization in the positive-ion mode with multiple reactions monitoring of parent and characteristic daughter ions. Following transitions were monitored indicated by their mass-to-charge ratio (m/z): m/z 318→127 for HCit; m/z 324→132 for 13C6-HCit; m/z 430→170 for 3-CT; m/z 436→176 for 13C6-3-CT; m/z 361→170 for lysine; m/z 367→175 for 13C6-lysine; m/z 396→136 for tyrosine; m/z 402→142 for 13C6-tyrosine. Following mass spectrometry analysis, the generated calibration curves were used to quantify HCit, 3-CT, tyrosine and lysine.
Cell culture
Human monocytic THP-1 cell line was maintained in RPMI 1640 medium while murine RAW264.7 macrophages were maintained in DMEM, both media being supplemented with 2 mmol/L glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin and 10% FBS. To induce differentiation, THP-1 cells were cultured for 48 hours in the presence of 100 nmol/L PMA.
Recombinant adenovirus preparation
Adenoviral vectors encoding human SR-BI (Ad/SR-BI) or LacZ cDNA (Ad/LacZ) were generated as described previously (17).
Induction of SR-BI expression in THP-1 macrophages
THP-1 macrophages (3×106 cells) were plated on 6-well plates and differentiated with PMA (100 nM) for 48 hours. In initial experiments, cells were infected with adenoviral vectors encoding human SR-BI or LacZ with a multiplicity of infection (moi) ranging from 50 - 500 for 1 hour, inducing moderate (50 moi), moderate to high (100 moi) and very high (500 moi) SR-BI expression. Infections were performed in the absence of serum followed by a medium change (RPMI 1640, 10% FBS) as described (17). After infection, THP-1[Ad/SR-BI] or THP-1[Ad/LacZ] cells were cultured for two days in RPMI 1640 containing 10% FBS to allow SR-BI expression (17). SR-BI expression was verified by Western blotting. Cell surface expression of SR-BI was assessed by immunofluorescence staining using a primary polyclonal SR-BI (Abcam, Cambridge, UK) antibody for 1 hour and secondary anti-rabbit AF488 antibody (Invitrogen) for 1 hour. Cells were imaged using the Olympus IX70 system. Cells infected with moi 100 were used for further experiments.
Association of lipoproteins to THP-1 cells
Binding studies of [125I]-labeled lipoproteins to THP-1 cells were performed as described (20). Total cholesterol accumulation in THP-1 macrophages was determined as described (21). Each condition was measured in triplicates.
To assess neutral lipid accumulation, THP-1 macrophages were differentiated for 72 hours in RPMI1640 containing 3% lipoprotein-deficient serum. Cells were incubated with 200 μg/ml lipoproteins for 48 hours and lipid droplets were stained in formaldehyde fixed cells with 1 μl/ml Bodipy.
Cholesterol efflux experiments
THP-1 macrophages were labeled with [3H] cholesterol (1 μCi/ml) for 48 hours. After cholesterol loading, the cells were rinsed twice with Tris-buffered saline (TBS) containing 5% (wt/vol) BSA and twice with TBS. Efflux experiments were initiated by the addition of HDL, or carbamylated HDL in DMEM without FBS. Two hours later, the medium was collected and radioactivity was counted. Cells were rinsed and lysed to estimate both, the cellular protein content and cell-associated radioactivity. Efflux of the radioactive label into the medium was calculated as percentage of radioactivity associated with cells before the addition of the indicated cholesterol acceptors.
SDS-Page and Western blotting
SDS-Page for protein separation and protein transfer to nitrocellulose membranes for Western blotting were performed as described previously (20)
Statistical analysis
Statistical analyses were performed using PASW Software V.18. Mean values of 2 independent groups were compared with the Mann-Whitney U-test (for non-parametric data) or 2-tailed student’s t-test (for parametric data). Comparisons of 2 dependent groups were performed using the Wilcoxon signed-rank test. Significances were accepted at * p<0.05, ** p<0.01 and *** p<0.001.
Results
HDL isolated from human atherosclerotic lesions is enriched with carbamyllysine
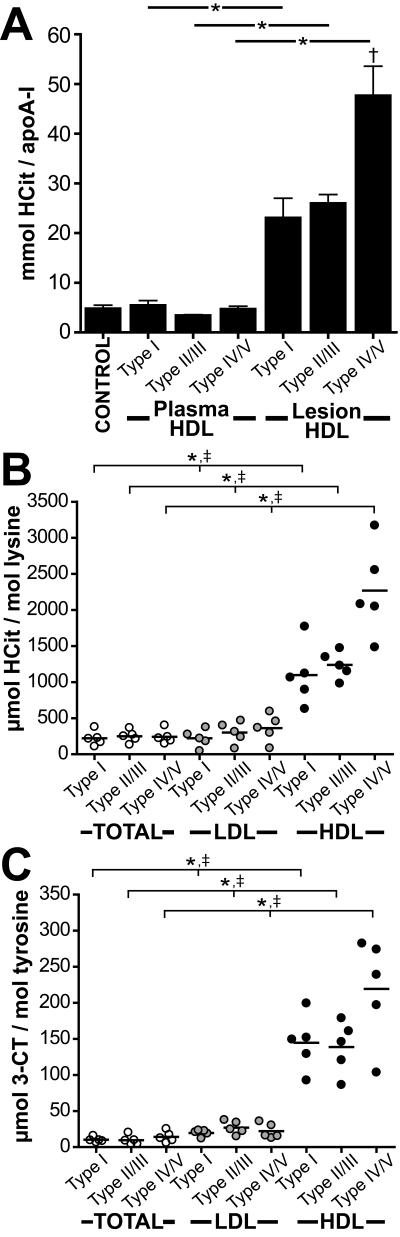
To address whether MPO-derived OCN− specifically targets HDL in the vessel wall, HDL was isolated by sequential density gradient ultracentrifugation from human plasma and atherosclerotic tissue. HDL recovered from atherosclerotic tissue was further purified by immunoprecipitation using a monoclonal apoA-I antibody bound to magnetic bead reagent. The morphology of the aortas investigated ranged from thickened intima to pronounced atheroma containing calcium inclusions. Both, control and atherosclerotic subjects had normal total cholesterol and no increase in plasma urea (Supplemental Table 1). In order to determine the purity and composition of lesion derived HDL, HDL was subjected to LC-MS/MS analysis or separated by SDS-PAGE and visualized by silver staining. We detected mainly apoA-I, lower amounts of apoA-II and albumin followed by low levels of apoE and antitrypsin (Supplemental Fig. 1). Next, mass spectrometry analysis was performed to quantitatively assess the carbamyllysine content of HDL isolated from plasma and atherosclerotic lesions. To test whether MPO contributes to HDL carbamylation in vivo, the specific MPO oxidation product 3-chlorotyrosine was determined in parallel (9). Measurement of 3-chlorotyrosine levels is currently the best method available for probing the MPO-mediated oxidation in the pathology of inflammatory diseases (38). Mass spectrometry analysis showed that both, control and atherosclerotic subjects had comparable carbamyllysine levels in plasma HDL (Fig. 1A). Remarkably, the carbamyllysine content of lesion-derived HDL was significantly higher in comparison with total lesion tissue or lesion-derived LDL (isolated from the same atherosclerotic tissue) (Fig. 1B). The carbamyllysine levels of lesion derived HDL were dependent on lesion severity and increased significantly from initial or moderate lesions (type I – type III) to advanced lesions (type IV-V) (Fig. 1B). The 3-chlorotyrosine content of lesion derived HDL followed the trend observed for carbamyllysine (Fig. 1C). However, 3-chlorotyrosine levels of lesion derived HDL were more than 20-fold lower when compared to the carbamyllysine content. This clearly indicates that protein carbamylation is a major post-translational modification of HDL in the vessel wall.
Figure 1. Homocitrulline content is increased in lesion-derived HDL.
(A) LC-MS/MS quantification of homocitrulline (HCit) in plasma HDL and lesion-derived HDL from subjects with atherosclerotic lesions, classified as type I (initial lesion, n=5), type II-III (intermediate, n=5) and type IV-V (complicated lesion, n=5). Quantification of HCit (B) and 3-chlorotyrosine (3-CT) (C) levels in total atherosclerotic tissue protein (TOTAL), lesion-derived LDL and lesion-derived HDL isolated from type I (n=5), type II-III (n=5) and type V (n=5) lesions.
* p<0.05; total tissue protein vs. lesion-derived HDL.
† p<0.05; lesion-derived HDL (type I) vs. lesion-derived HDL (type IV/V).
‡ p<0.05; lesion-derived LDL vs. lesion-derived HDL.
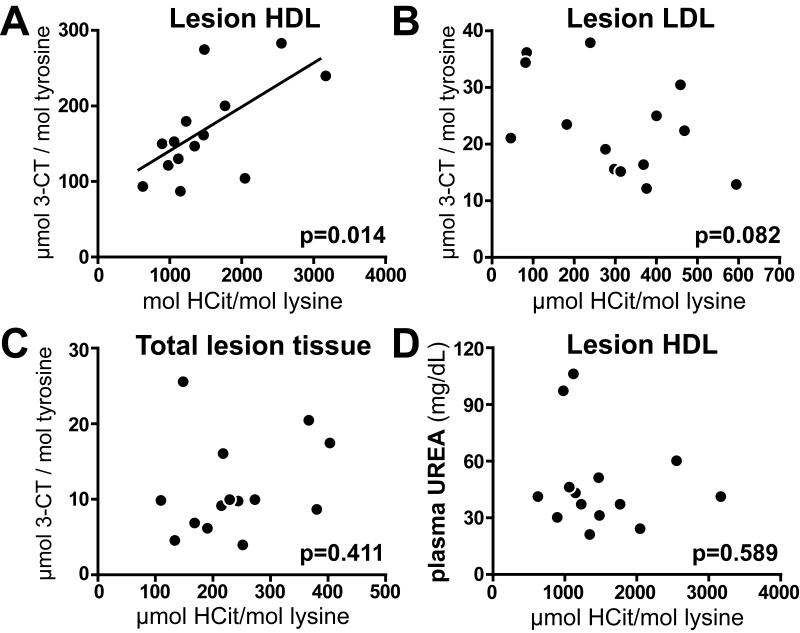
Correlation between carbamyllysine content of lesion-derived HDL and 3-chlorotyrosine levels
Both, carbamyllysine and 3-chlorotyrosine levels were increased in lesion derived HDL. Further data analysis revealed a positive correlation between carbamyllysine and 3-chlorotyrosine levels in lesion-derived HDL (Fig. 2A), whereas the carbamyllysine content of LDL or total lesion tissue did not correlate with 3-chlorotyrosine levels (Fig. 2B, C). Importantly, neither plasma urea concentration (Fig. 2D) nor plasma lipid levels correlated with the carbamyllysine content of lesion derived HDL (Supplemental Table 2). These results implicate that MPO significantly contributes to HDL carbamylation in atherosclerotic lesions.
Figure 2. Carbamylation of lesion-derived HDL correlates with the MPO marker 3-CT.
(A) The content of HCit and 3-CT present in lesion-derived HDL correlate significantly (n=15). (B) HCit and 3-CT content do not correlate in lesion-derived LDL. (C) HCit and 3-CT content do not correlate in total atherosclerotic tissue protein. (D) Plasma urea levels do not correlate with the HCit content in lesion-derived HDL. The significance level of Pearson’s correlation is noted for each plot.
Increased binding affinity of carbamylated HDL to the HDL receptor SR-BI
A previous study had demonstrated that carbamylated epitopes co-localize with MPO in human atherosclerotic lesions (37). These findings suggest that macrophage associated MPO generates OCN−, which subsequently reacts with HDL-associated apolipoproteins to generate carbamyllysine. Given that macrophages express high levels of the HDL receptor SR-BI in human atherosclerotic lesions (5;10;32), we hypothesized that carbamylation of apoA-I in the artery wall might modulate binding of HDL to SR-BI in macrophages.
To test this hypothesis, HDL was carbamylated with OCN− and the carbamyllysine content was assessed by LC-MS/MS, which confirmed that a relevant degree of protein carbamylation was present (0.57 and 5.25 HCit/apoA-I). Carbamylated HDL was separated by SDS-PAGE and visualized by silver staining, revealing that OCN− treatment did not induce crosslinking or degradation of apoA-I (Fig. 3A). We also tested whether HDL associated phospholipids were modified upon OCN− treatment, since the primary amino group of phosphatidylethanolamine (PE), a minor fraction of HDL associated phospholipids (about 3% of total HDL phospholipids, 1-2 molecules PE per HDL) (8), is prone to be modified by reactive aldehydes (28). As seen in Figure 3B, total phospholipid content was not significantly altered in carbamylated HDL, whereas the PE content was significantly decreased. Importantly, OCN− treatment did not induce hydroperoxide formation, since no increase in UV-absorption was observed (Fig. 3C).
Figure 3. Carbamylation of HDL does not alter HDL integrity.
HDL and carbamylated HDL (cHDL, 5.25 HCit/apoA-I) was analyzed for (A) protein integrity by silver staining; (B) for changes in phospholipid (PL) and phosphatidylethanolamine (PE) composition and (C) for changes in the absorption at 234 nm indicating hydroperoxide generation (formation of dienes). Results shown in B and C represent the mean of triplicate determinations ± SD of a representative experiment performed at least two times. **p<0.01
In contrast to macrophages in atherosclerotic lesions, cultured macrophages generally express low levels of SR-BI (5;25). Therefore, we used a human monocytic leukemia macrophage cell line (THP-1), that can be infected by adenoviral vectors to induce SR-BI expression (Supplemental Fig. 2).
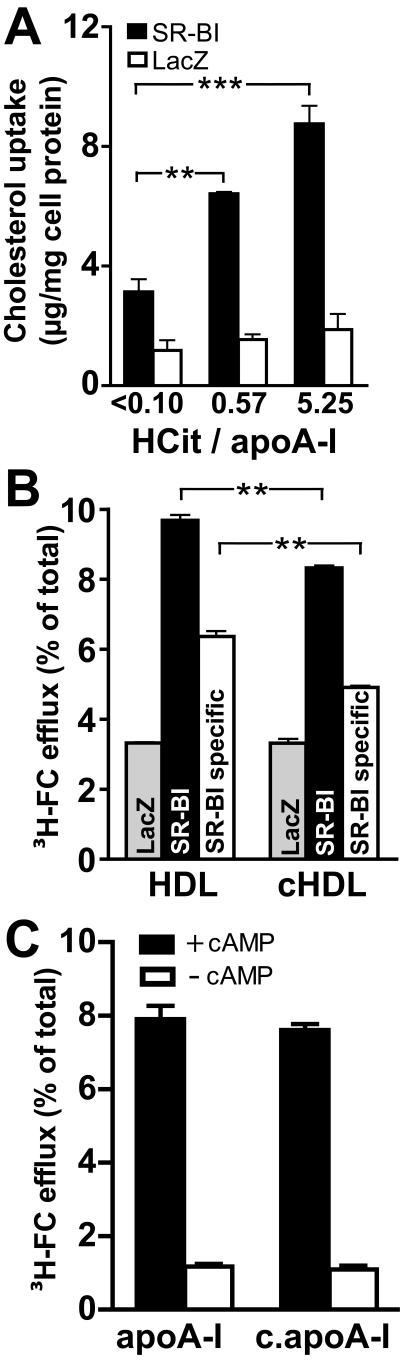
We first investigated whether OCN− induced HDL carbamylation could affect the binding properties of HDL to SR-BI expressing THP-1 cells. As seen in Figure 4A, SR-BI specifically mediated the binding of 125I-labeled carbamylated HDL and 125I-labeled native HDL. Carbamylation of HDL increased the binding affinity and capacity to SR-BI (Table 1), whereas binding of HDL to control macrophages was unaltered (Fig. 4A).
Figure 4. Carbamylation of HDL increases binding affinity to SR-BI.
THP1 macrophages infected with adenoviral vectors encoding SR-BI or LacZ (control) were incubated for 2 h at 4 °C in the presence of increasing concentrations of (A)125I-labeled native HDL or 125I-labeled carbamylated HDL (cHDL) or (B) 125I-labeled reconstituted HDL (rHDL) or 125I-labeled carbamylated rHDL. Values obtained with LacZ expressing cells were subtracted from SR-BI expressing cells to calculate SR-BI specific binding. Results represent the mean of triplicate determinations ± SD of a representative experiment performed at least two times.
Table 1.
Calculated Kd and Bmax values for binding of 125 I-labeled HDL, reconstituted HDL (rHDL) and lipid-free apoA-I to SR-BI. Calculations were performed by nonlinear regression analysis.
| Hcit / apoA-l | Kd [μg/ml] |
Bmax [ng/mg] |
|
|---|---|---|---|
| HDL | <0.01 | 32 ± 7.9 | 173 ± 15 |
| cHDL | 0.57 | 23 ±4.9 | 175 ± 12 |
| cHDL | 5.25 | 15 ±3.9 | 251 ± 19 |
|
| |||
| rHDL | <0.01 | 2.2 ±0.2 | 248 ± 13 |
| c.rHDL | 0.51 | 2.0 ± 0.2 | 299 ± 10 |
| c.rHDL | 1.87 | 1.7 ±0.2 | 289 ±11 |
|
| |||
| apoA-l | <0.01 | 293 ± 53 | 205 ± 28 |
| c.apoA-l | 0.58 | 138 ±27 | 171 ±21 |
| c.apoA-l | 2.04 | 57 ±35 | 93 ± 14 |
To directly demonstrate that carbamyllysine formation in HDL associated apoA-I increases binding affinity to SR-BI, we prepared reconstituted HDL (rHDL) using human apoA-I and PC, an inert phospholipid. As seen in Figure 4B, binding affinity and capacity of rHDL to SR-BI increased after OCN− treatment (Table 1). Moreover, already 2 carbamyllysine residues present on lipid-free apoA-I increased binding affinity to SR-BI more than 5-fold (Table 1).
Carbamylated HDL-induced cholesterol accumulation in macrophages is SR-BI dependent
SR-BI mediates selective HDL cholesteryl-ester uptake by formation of a productive lipoprotein/receptor complex, which requires specific structural domains and conformation states of apoA-I (15). We hypothesized that increased binding of HDL through carbamylation might confer pro-atherosclerotic properties by inducing SR-BI dependent cholesterol accumulation in macrophages. Remarkably, HDL exposed to OCN− induced marked total cholesterol accumulation in SR-BI expressing THP-1 cells, but not in control cells (Fig. 5 A). A recent study demonstrated that cholesterol efflux of human macrophages is dependent on SR-BI and ATP-binding cassette transporter A1 (ABCA1), but independent of ABCG1 (14). Therefore, we tested whether OCN− modulates the ability of HDL to induce SR-BI and ABCA1 mediated cholesterol efflux from macrophages. Interestingly, carbamylation moderately, but significantly reduced the ability of HDL to promote SR-BI dependent cholesterol efflux (Fig. 5 B), whereas cholesterol efflux of control cells was not altered. Lipid-poor apoA-I removes cellular cholesterol from macrophages exclusively by an active transport process mediated by ABCA1. The process appears to involve the amphipathic α-helical domains of apoA-I. Modification of lysine residues may alter the ability of lipid poor apoA-I to remove ABCA1 dependent cholesterol from lipid-laden macrophages (29). However, carbamylation of apoA-I did not decrease ABCA1 mediated cholesterol efflux (Fig. 5 C).
Figure 5. Carbamylated HDL induces cholesterol accumulation in THP-1 macrophages via SR-BI.
(A) THP1 macrophages infected with adenoviral vectors encoding SR-BI or LacZ (control) were incubated with 100 μg/ml native HDL or carbamylated HDL for 24 h at 37°C. Subsequently, cells were rinsed and cell associated total cholesterol (TC) (sum of free cholesterol and cholesterolester) was estimated. HDL-induced cholesterol-uptake was calculated by subtracting cholesterol content of cells grown in the absence of HDL (16.1 ± 2 μg/mg cell protein). (B) To measure SR-BI dependent cellular cholesterol efflux, THP-1 cells expressing SR-BI or LacZ were labeled with [3H]-cholesterol. Subsequently, cells were incubated with 100 μg/ml native HDL or carbamylated HDL (0.57 HCit/apoA-I) as cholesterol acceptors for 2 h at 37°C. At the end of the experiment media and cells were separately collected, and efflux was determined as described in “Methods”. To calculate SR-BI specific [3H]-cholesterol (FC)-efflux, values obtained with LacZ expressing cells were subtracted from SR-BI expressing cells. (C) RAW264.7 cells were [3H]-cholesterol-labeled and incubated in the presence or absence of cAMP to induce ABCA1 expression. Cells were then incubated for 2 h at 37°C with 10 μg/ml native or carbamylated apoA-I (5.62 HCit / apoA-I). Media and cells were collected separately to determine cholesterol efflux. Specific cholesterol efflux was calculated by subtracting efflux in the absence of acceptors from efflux in the presence of acceptors. Results represent the mean of triplicate determinations ± SD of a representative experiment performed at least three times. **p<0.01; ***p<0.001
Carbamylated HDL induces SR-BI dependent lipid droplet formation in macrophages
The cholesterol uptake and efflux experiments indicate that carbamylation of HDL destabilizes the HDL/SR-BI mediated balance between cholesterol-uptake versus efflux, indicating that net cholesterol content of macrophages increases. Therefore, further assessment whether carbamylated HDL could induce lipid-droplet formation in macrophages was performed. A significant SR-BI-dependent lipid droplet formation was induced upon exposure of macrophages to carbamylated HDL for 48 hours (Fig. 6).
Figure 6. Carbamylated HDL induces SR-BI dependent lipid droplet formation.
(A) SR-BI or LacZ (control) expression was induced in THP-1 macrophages by infection with adenoviral vectors encoding SR-BI or LacZ. Cell surface expression of SR-BI was visualized by immunofluorescence staining with an anti-SR-BI antibody of non-permeabilized cells. (B) SR-BI and LacZ expressing macrophages were incubated with 200 μg/ml native HDL (control) or carbamylated HDL (0.57 or 5.25 HCit/apoA-I) for 48 h at 37°C. The intracellular uptake of neutral lipid was visualized by Bodipy staining.
Discussion
In the present study, we have provided evidence that HDL is a major target for carbamylation in human atherosclerotic lesions. First, HDL recovered from human atherosclerotic tissue showed a remarkable enrichment in apoA-I carbamyllysine content that increased from initial to advanced lesions. Second, the carbamyllysine content of lesion derived HDL was more than 20-fold higher when compared with 3-chlorotyrosine levels, a specific oxidation product of MPO. Third, the analyses of the HDL-, LDL- and total lesion tissue indicate that HDL is a selective target for carbamylation. Moreover, we observed that already less than one carbamyllysine per HDL associated apoA-I induced lipid droplet formation in macrophages by a pathway requiring SR-BI.
The marked enrichment in the carbamyllysine content of apoA-I in lesions that showed a correlation with the MPO product 3-chlorotyrosine strongly supports the notion that macrophage associated MPO generates OCN−, which subsequently reacts with HDL-associated apolipoproteins to generate carbamyllysine. In line with our observation that MPO mediates carbamylation of HDL in the vessel wall, a previous study had demonstrated that carbamylated epitopes were found to extensively co-localize with MPO and macrophages in human atherosclerotic lesions (37).
Another remarkable finding is that increasing levels of carbamyllysine present on HDL trigger lipid droplet formation in macrophages in an SR-BI dependent manner. Macrophages in human atherosclerotic lesions express high levels of SR-BI (5;10;32). The multiligand receptor SR-BI has been described to mediate (i) selective HDL cholesteryl-ester uptake, (ii) uptake of oxidized lipoproteins as well as (iii) secretion of cholesterol to high-density lipoproteins (13). Interestingly, macrophage SR-BI in early stage lesions was shown to induce cholesterol accumulation, thereby triggering fatty streak development in mice (35). In advanced lesions, macrophage SR-BI seems to be atheroprotective, at least in mice (35). In the present study, we made a novel observation that even a minimal carbamylation of HDL (about one lysine residues per apoA-I modified) modulated the interaction of HDL with macrophage SR-BI in a fashion that carbamylated HDL induced lipid droplet formation in macrophages. Our studies demonstrate for the first time that modification of HDL by OCN− destabilizes the HDL/SR-BI mediated balance between cholesterol-uptake versus efflux, resulting in net cholesterol uptake.
Several modified proteins that are being recognized by the multiligand receptor SR-BI hold in common a negative charge, suggesting that SR-BI recognizes the negative charge on proteins. Our findings indicate that modification of HDL-lysine residues by OCN− (leading to a decrease of positive charge) affects the interaction of HDL with SR-BI, consistent with the observation that oxidation of (lipo)proteins with hypochlorous acid (resulting in the oxidation of lysine residues), showed an increased binding affinity to SR-BI (16;19;21).
Besides the important role of SR-BI in HDL-cholesterol transport, the HDL-SR-BI tandem confers additional actions. For instance, HDL binding to SR-BI protects endothelial cells from apoptosis (24). Interestingly, exposure of bovine aortic endothelial cells to carbamylated HDL was recently shown to induce apoptosis (37), thus it is intriguing to speculate whether interaction of carbamylated HDL with endothelial SR-BI would induce apoptosis.
High concentrations of carbamylated LDL have been reported to accumulate in plasma of patients with chronic renal failure, indicating that carbamylated LDL is by far the most abundant modified LDL found in human plasma (2). In line with that important study, we observed that the carbamyllysine content of plasma proteins of non-renal subjects correlated significantly with plasma urea concentrations (Supplemental Table 2). Thus it can be concluded, that plasma proteins are mainly carbamylated by urea derived cyanate. This may be of particular importance in end stage renal disease, where urea concentrations are dramatically high.
In summary, the results of this study raise the possibility that post-translational modification of HDL through carbamylation may promote atherogenesis by counteracting the established antiatherogenic effects of HDL. Carbamylation might critically impair anti-atherogenic properties of HDL in the atherosclerotic intima, thereby destabilizing the cellular balance between macrophage mediated cholesterol-uptake versus efflux, a critical step in the development of atherosclerosis.
Supplementary Material
Acknowledgments
Sources of Funding
M. H. and T. P. were funded by the PhD Program Molecular Medicine of the Medical University of Graz. This work was supported by the Jubiläumsfonds of the Austrian National Bank (Grants 13487 and 13533), the Austrian Science Fund FWF (Grants P21004-B02, P22521-B18 and P22976-B18), and the Franz Lanyar Foundation (Grant 329).
Non-standard Abbreviations
- 3-CT
3-chlorotyrosine
- ABCA1
ATP-binding cassette transporter A 1
- ABCG1
ATP-binding cassette transporter G 1
- apoA-I
apolipoprotein A-I
- cAMP
cyclic adenosine monophosphate
- CE
cholesterolester
- DTPA
diethylenetriaminepentaacetic acid
- FC
free cholesterol
- HCit
homocitrulline, carbamyllysine
- HDL
high-density lipoprotein
- HOCl
hypochlourus acid
- KOCN
pottasium cyanate
- LC-MS/MS
liquid chromatography tandem mass spectrometry
- LDL
low-density lipoprotein
- Lys
lysine
- MPO
myeloperoxidase
- OCN−
cyanate
- PC
L-α-phosphatidylcholine
- PE
phosphatidyl ethanolamine
- rHDL
reconstituted HDL
- SCN−
thiocyanate
- SR-BI
scavenger receptor class B, type I
Footnotes
Author Disclosure Statement
No competing financial interests exist
Contributor Information
Michael Holzer, Institute of Experimental and Clinical Pharmacology, Medical University of Graz, Austria.
Martin Gauster, Institute of Cell Biology, Histology and Embryology, Medical University of Graz, Austria.
Thomas Pfeifer, Institute of Molecular Biology and Biochemistry, Medical University of Graz, Austria.
Christian Wadsack, Department of Obstetrics and Gynecology, Medical University of Graz, Austria.
Guenter Fauler, Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Austria.
Philipp Stiegler, Division of Transplantation Surgery, Medical University of Graz, Austria.
Harald Koefeler, Center for Medical Research, Medical University of Graz, Austria.
Eckhard Beubler, Institute of Experimental and Clinical Pharmacology, Medical University of Graz, Austria.
Rufina Schuligoi, Institute of Experimental and Clinical Pharmacology, Medical University of Graz, Austria.
Akos Heinemann, Institute of Experimental and Clinical Pharmacology, Medical University of Graz, Austria.
Gunther Marsche, Institute of Experimental and Clinical Pharmacology, Medical University of Graz, Austria, Universitätsplatz 4, 8010 Graz, Tel.: +43 316 380 4513, Fax: +43 316 380 9645, gunther.marsche@medunigraz.at.
References
- 1.Apostolov EO, Ray D, Savenka AV, Shah SV, Basnakian AG. Chronic uremia stimulates LDL carbamylation and atherosclerosis. J. Am. Soc. Nephrol. 2010;21:1852–1857. doi: 10.1681/ASN.2010040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apostolov EO, Shah SV, Ok E, Basnakian AG. Quantification of carbamylated LDL in human sera by a new sandwich ELISA. Clin. Chem. 2005;51:719–728. doi: 10.1373/clinchem.2004.044032. [DOI] [PubMed] [Google Scholar]
- 3.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ. Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 4.Bergt C, Pennathur S, Fu X, Byun J, O’Brien K, McDonald TO, Singh P, Anantharamaiah GM, Chait A, Brunzell J, Geary RL, Oram JF, Heinecke JW. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc. Natl. Acad. Sci. U. S. A. 2004;101:13032–13037. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinetti G, Gbaguidi FG, Griglio S, Mallat Z, Antonucci M, Poulain P, Chapman J, Fruchart JC, Tedgui A, Najib-Fruchart J, Staels B. CLA-1/SR-BI is expressed in atherosclerotic lesion macrophages and regulated by activators of peroxisome proliferator-activated receptors. Circulation. 2000;101:2411–2417. doi: 10.1161/01.cir.101.20.2411. [DOI] [PubMed] [Google Scholar]
- 6.Damm M, Holzer M, Radspieler G, Marsche G, Kappe CO. Microwave-assisted high-throughput acid hydrolysis in silicon carbide microtiter platforms-A rapid and low volume sample preparation technique for total amino acid analysis in proteins and peptides. J. Chromatogr. A. 2010 doi: 10.1016/j.chroma.2010.10.062. [DOI] [PubMed] [Google Scholar]
- 7.Dua S, Maclean MJ, Fitzgerald M, McAnoy AM, Bowie JH. Is the hypothiocyanite anion (OSCN)- the major product in the peroxidase catalyzed oxidation of the thiocyanate anion (SCN)-? A joint experimental and theoretical study. J. Phys. Chem. A. 2006;110:4930–4936. doi: 10.1021/jp058144t. [DOI] [PubMed] [Google Scholar]
- 8.Fournier N, Paul JL, Atger V, Cogny A, Soni T, Llera-Moya M, Rothblat G, Moatti N. HDL phospholipid content and composition as a major factor determining cholesterol efflux capacity from Fu5AH cells to human serum. Arterioscler. Thromb. Vasc. Biol. 1997;17:2685–2691. doi: 10.1161/01.atv.17.11.2685. [DOI] [PubMed] [Google Scholar]
- 9.Hazen SL, Crowley JR, Mueller DM, Heinecke JW. Mass spectrometric quantification of 3-chlorotyrosine in human tissues with attomole sensitivity: a sensitive and specific marker for myeloperoxidase-catalyzed chlorination at sites of inflammation. Free Radic. Biol. Med. 1997;23:909–916. doi: 10.1016/s0891-5849(97)00084-1. [DOI] [PubMed] [Google Scholar]
- 10.Hirano K, Yamashita S, Nakagawa Y, Ohya T, Matsuura F, Tsukamoto K, Okamoto Y, Matsuyama A, Matsumoto K, Miyagawa J, Matsuzawa Y. Expression of human scavenger receptor class B type I in cultured human monocyte-derived macrophages and atherosclerotic lesions. Circ. Res. 1999;85:108–116. doi: 10.1161/01.res.85.1.108. [DOI] [PubMed] [Google Scholar]
- 11.Klebanoff SJ. Myeloperoxidase: friend and foe. J. Leukoc. Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 12.Kraus LM, Elberger AJ, Handorf CR, Pabst MJ, Kraus AP., Jr. Urea-derived cyanate forms epsilon-amino-carbamoyl-lysine (homocitrulline) in leukocyte proteins in patients with end-stage renal disease on peritoneal dialysis. J. Lab Clin. Med. 1994;123:882–891. [PubMed] [Google Scholar]
- 13.Krieger M. Scavenger receptor class B type I is a multiligand HDL receptor that influences diverse physiologic systems. J. Clin. Invest. 2001;108:793–797. doi: 10.1172/JCI14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larrede S, Quinn CM, Jessup W, Frisdal E, Olivier M, Hsieh V, Kim MJ, Van EM, Couvert P, Carrie A, Giral P, Chapman MJ, Guerin M, Le GW. Stimulation of cholesterol efflux by LXR agonists in cholesterol-loaded human macrophages is ABCA1-dependent but ABCG1-independent. Arterioscler. Thromb. Vasc. Biol. 2009;29:1930–1936. doi: 10.1161/ATVBAHA.109.194548. [DOI] [PubMed] [Google Scholar]
- 15.Liu T, Krieger M, Kan HY, Zannis VI. The effects of mutations in helices 4 and 6 of ApoA-I on scavenger receptor class B type I (SR-BI)-mediated cholesterol efflux suggest that formation of a productive complex between reconstituted high density lipoprotein and SR-BI is required for efficient lipid transport. J. Biol. Chem. 2002;277:21576–21584. doi: 10.1074/jbc.M112103200. [DOI] [PubMed] [Google Scholar]
- 16.Marsche G, Frank S, Hrzenjak A, Holzer M, Dirnberger S, Wadsack C, Scharnagl H, Stojakovic T, Heinemann A, Oettl K. Plasma-advanced oxidation protein products are potent high-density lipoprotein receptor antagonists in vivo. Circ. Res. 2009;104:750–757. doi: 10.1161/CIRCRESAHA.108.193169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsche G, Frank S, Raynes JG, Kozarsky KF, Sattler W, Malle E. The lipidation status of acute-phase protein serum amyloid A determines cholesterol mobilization via scavenger receptor class B, type I. Biochem. J. 2007;402:117–124. doi: 10.1042/BJ20061406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marsche G, Furtmuller PG, Obinger C, Sattler W, Malle E. Hypochlorite-modified high-density lipoprotein acts as a sink for myeloperoxidase in vitro. Cardiovasc. Res. 2008;79:187–194. doi: 10.1093/cvr/cvn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsche G, Hammer A, Oskolkova O, Kozarsky KF, Sattler W, Malle E. Hypochlorite-modified high density lipoprotein, a high affinity ligand to scavenger receptor class B, type I, impairs high density lipoprotein-dependent selective lipid uptake and reverse cholesterol transport. J. Biol. Chem. 2002;277:32172–32179. doi: 10.1074/jbc.M200503200. [DOI] [PubMed] [Google Scholar]
- 20.Marsche G, Levak-Frank S, Quehenberger O, Heller R, Sattler W, Malle E. Identification of the human analog of SR-BI and LOX-1 as receptors for hypochlorite-modified high density lipoprotein on human umbilical venous endothelial cells. FASEB J. 2001;15:1095–1097. doi: 10.1096/fj.00-0532fje. [DOI] [PubMed] [Google Scholar]
- 21.Marsche G, Zimmermann R, Horiuchi S, Tandon NN, Sattler W, Malle E. Class B scavenger receptors CD36 and SR-BI are receptors for hypochlorite-modified low density lipoprotein. J. Biol. Chem. 2003;278:47562–47570. doi: 10.1074/jbc.M308428200. [DOI] [PubMed] [Google Scholar]
- 22.Matyash V, Liebisch G, Kurzchalia TV, Shevchenko A, Schwudke D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008;49:1137–1146. doi: 10.1194/jlr.D700041-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matz CE, Jonas A. Micellar complexes of human apolipoprotein A-I with phosphatidylcholines and cholesterol prepared from cholate-lipid dispersions. J. Biol. Chem. 1982;257:4535–4540. [PubMed] [Google Scholar]
- 24.Mineo C, Shaul PW. Role of high-density lipoprotein and scavenger receptor B type I in the promotion of endothelial repair. Trends Cardiovasc. Med. 2007;17:156–161. doi: 10.1016/j.tcm.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Murao K, Terpstra V, Green SR, Kondratenko N, Steinberg D, Quehenberger O. Characterization of CLA-1, a human homologue of rodent scavenger receptor BI, as a receptor for high density lipoprotein and apoptotic thymocytes. J. Biol. Chem. 1997;272:17551–17557. doi: 10.1074/jbc.272.28.17551. [DOI] [PubMed] [Google Scholar]
- 26.Navab M, Reddy ST, Van Lenten BJ, Anantharamaiah GM, Fogelman AM. The role of dysfunctional HDL in atherosclerosis. J. Lipid Res. 2009;50(Suppl):S145–S149. doi: 10.1194/jlr.R800036-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oimomi M, Nishimoto S, Matsumoto S, Hatanaka H, Ishikawa K, Kawasaki T, Yoshimura Y, Baba S. Carbamylated plasma protein in renal failure. Nippon Jinzo Gakkai Shi. 1986;28:269–271. [PubMed] [Google Scholar]
- 28.Papahadjopoulos D, Weiss L. Amino groups at the surfaces of phospholipid vesicles. Biochim. Biophys. Acta. 1969;183:417–426. doi: 10.1016/0005-2736(69)90156-4. [DOI] [PubMed] [Google Scholar]
- 29.Shao B, Pennathur S, Pagani I, Oda MN, Witztum JL, Oram JF, Heinecke JW. Modifying apolipoprotein A-I by malondialdehyde, but not by an array of other reactive carbonyls, blocks cholesterol efflux by the ABCA1 pathway. J. Biol. Chem. 2010;285:18473–18484. doi: 10.1074/jbc.M110.118182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr., Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW, A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 31.Stocker R, Keaney JF., Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 32.Svensson PA, Englund MC, Snackestrand MS, Hagg DA, Ohlsson BG, Stemme V, Mattsson-Hulten L, Thelle DS, Fagerberg B, Wiklund O, Carlsson LM, Carlsson B. Regulation and splicing of scavenger receptor class B type I in human macrophages and atherosclerotic plaques. BMC. Cardiovasc. Disord. 2005;5:25. doi: 10.1186/1471-2261-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uchida K. Role of reactive aldehyde in cardiovascular diseases. Free Radic. Biol. Med. 2000;28:1685–1696. doi: 10.1016/s0891-5849(00)00226-4. [DOI] [PubMed] [Google Scholar]
- 34.van Dalen CJ, Whitehouse MW, Winterbourn CC, Kettle AJ. Thiocyanate and chloride as competing substrates for myeloperoxidase. Biochem. J. 1997;327(Pt 2):487–492. doi: 10.1042/bj3270487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van EM, Bos IS, Hildebrand RB, Van Rij BT, Van Berkel TJ. Dual role for scavenger receptor class B, type I on bone marrow-derived cells in atherosclerotic lesion development. Am. J. Pathol. 2004;165:785–794. doi: 10.1016/S0002-9440(10)63341-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang GW, Guo Y, Vondriska TM, Zhang J, Zhang S, Tsai LL, Zong NC, Bolli R, Bhatnagar A, Prabhu SD. Acrolein consumption exacerbates myocardial ischemic injury and blocks nitric oxide-induced PKCepsilon signaling and cardioprotection. J. Mol. Cell Cardiol. 2008;44:1016–1022. doi: 10.1016/j.yjmcc.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Nicholls SJ, Rodriguez ER, Kummu O, Horkko S, Barnard J, Reynolds WF, Topol EJ, Didonato JA, Hazen SL. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat. Med. 2007;13:1176–1184. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 38.Winterbourn CC, Kettle AJ. Biomarkers of myeloperoxidase-derived hypochlorous acid. Free Radic. Biol. Med. 2000;29:403–409. doi: 10.1016/s0891-5849(00)00204-5. [DOI] [PubMed] [Google Scholar]
- 39.Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, Schmitt D, Fu X, Thomson L, Fox PL, Ischiropoulos H, Smith JD, Kinter M, Hazen SL. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J. Clin. Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.