Abstract
The activity of sensory circuits is shaped by neuromodulators, which can have downstream consequences for both sensorimotor integration and behavioral output. Recent evidence indicates that brain-derived estrogens (“neuroestrogens”) can act as local circuit modulators in the songbird auditory forebrain. Specifically, neuroestrogens fluctuate in the auditory caudomedial nidopallium (NCM) during social interactions and in response to song stimuli. Within minutes of elevation, neuroestrogens also enhance auditory response properties of NCM neurons, and acute blockade of estrogen production in NCM disrupts behavioral song preferences. Here, we test the hypothesis that fluctuating neuroestrogens within NCM influence stimulus selectivity in a downstream sensorimotor nucleus (HVC, used as a proper name) that receives indirect auditory input from NCM. Dual extracellular recordings coupled with retrodialysis delivery show that song selectivity in HVC is rapidly enhanced by increasing neuroestrogens in NCM in adult males. Conversely, inhibiting neuroestrogen production in NCM causes a rapid decline in song selectivity in HVC, demonstrating the endogenous nature of this modulatory network. In contrast, HVC selectivity is unaffected by neuroestrogen delivery to either nearby caudomedial mesopallium or into HVC itself, indicating that neuroestrogen actions are restricted to NCM. In juvenile males, identical neuroestrogen treatment in NCM also does not alter HVC selectivity, consistent with a developmental maturation of the auditory network. Lastly, the rapid actions of estrogens leading to enhanced HVC selectivity appear to be mediated by membrane-bound receptors in NCM. These findings indicate that steroid-dependent modulation of sensory processing is not locally restricted and can be transmitted transynaptically to influence downstream sensorimotor and premotor targets.
Introduction
Brain circuits are highly sensitive to steroid hormones derived both from the periphery and generated within the CNS itself. Steroids can sculpt the brain over long-term seasonal and developmental timescales, yet they can also modulate the excitability of neurons within seconds to minutes. These rapid, nonclassical actions on neuronal excitability have been described for all major steroids, including progestins (Joëls, 1997; Shen et al., 2007; Smith et al., 2009), glucocorticoids (Dallman, 2005; Coddington et al., 2007; Pasricha et al., 2011), androgens (Bass and Remage-Healey, 2008; Foradori et al., 2008), and estrogens (Dufy et al., 1979; Wong and Moss, 1992; Mermelstein et al., 1996; Remage-Healey and Bass, 2007; Woolley, 2007; Kuo et al., 2010). The nonclassical actions of estrogens have drawn particular attention because the activity of the estrogen-synthesis enzyme aromatase is rapidly regulated within discrete brain regions (Cornil et al., 2005; Balthazart et al., 2006; Charlier et al., 2011) and because aromatase is expressed in presynaptic boutons in the forebrain of rodents, primates, and songbirds (Naftolin et al., 1996; Hojo et al., 2004; Peterson et al., 2005; Srivastava et al., 2010). Estrogens therefore appear to exert rapid, neuromodulatory actions with high spatial and temporal resolution (Balthazart and Ball, 2006; Remage-Healey et al., 2011). However, despite current understanding of their capacity for neuromodulation, whether neurosteroids, such as estrogens, can rapidly alter the flow of information between identified brain circuits is unclear.
Here, we test this hypothesis using the zebra finch, a songbird with pronounced brain steroid production (London et al., 2006; Schlinger and Brenowitz, 2008; Remage-Healey et al., 2010a). Adult males have a specialized network of forebrain nuclei devoted to auditory processing and learned vocalizations (Mooney, 2009). One auditory region, the caudomedial nidopallium (NCM), is aromatase rich (Saldanha et al., 2000; Saldanha and Coomaralingam, 2005; Jeong et al., 2011) and is important for auditory discrimination and song memories (Gentner et al., 2004; Bolhuis and Gahr, 2006; Phan et al., 2006). Auditory signals processed by NCM ultimately reach nucleus HVC (via indirect projections; see Fig. 1A), a key sensorimotor structure that integrates auditory input with vocal motor commands and is critical for song production and perception (Brenowitz, 1991; Gentner et al., 2000; Long and Fee, 2008; Prather et al., 2008).
Figure 1.
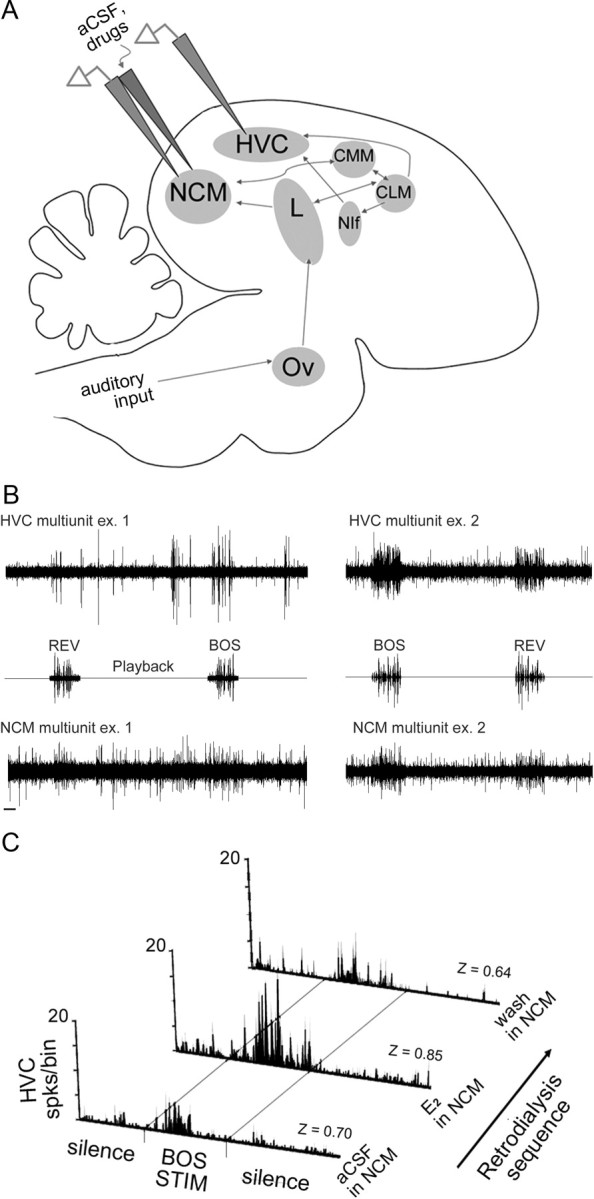
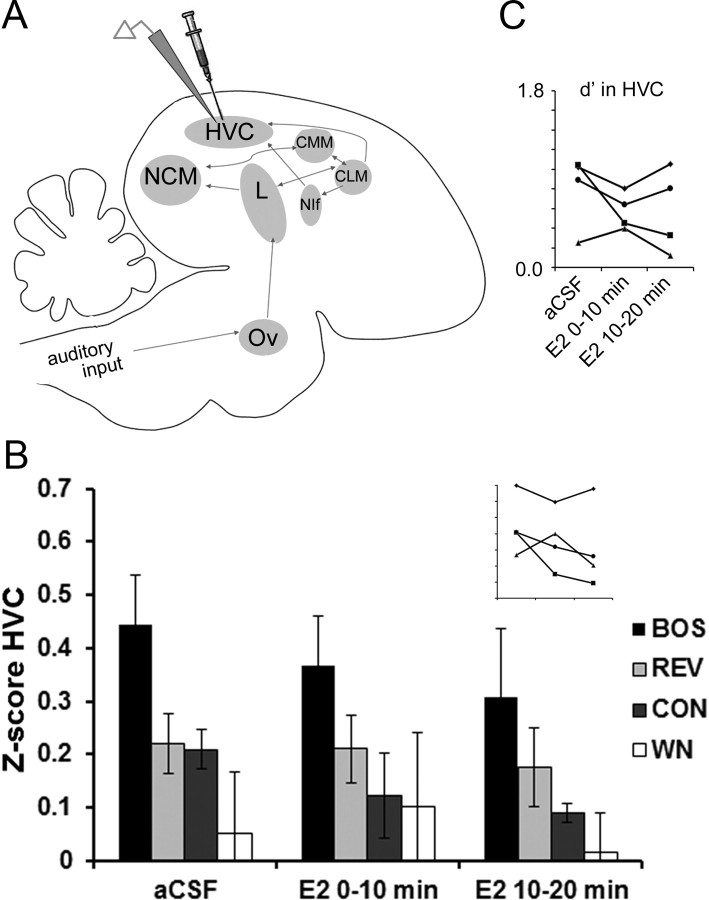
Estrogens acting locally within NCM rapidly enhance the neural representation of song in HVC. A, Sagittal schematic of the male zebra finch auditory circuit, showing dual extracellular electrodes in NCM and sensorimotor HVC. A retrodialysis probe is pictured coupled to the electrode in NCM, which allows acute manipulation of local concentrations of estrogenic drugs and inhibitors within discrete nuclei, along with washout with aCSF. The auditory pathway ascends from the cochlear nucleus (not pictured), via thalamic nucleus ovoidalis (Ov), primary thalamorecipient Field L (L), secondary auditory cortical nuclei NCM, CMM, CLM, and NIf, into the sensorimotor integration nucleus HVC. B, Two representative examples of dual multiunit recordings from two separate animals taken at baseline. Multiunit traces from HVC (top) and NCM (bottom) are shown for a 20 s sequence of presentation of both the BOS and REV (playback waveforms shown in middle). NCM exhibits characteristic auditory-evoked increased firing rates to most sounds, including REV and BOS, whereas HVC exhibits characteristic BOS selectivity (i.e., limited response to REV). Calibration bar, 1 s. C, PSTHs from multiunit activity in HVC during auditory playback and neuroestrogen manipulations in NCM. All three PSTHs are from the same experimental animal over the course of a typical experiment of serial 30 min treatments with aCSF, E2, and aCSF washout in NCM. Each PSTH depicts the summed HVC multiunit activity over 20 iterations of 6 s of 2 s silence, followed by 2 s of BOS playback (BOS STIM), followed by 2 s of silence. The corresponding Z-scores are presented above the x-axis for each recording.
A recently-optimized in vivo microdialysis approach in behaving zebra finches reveals that estrogens in NCM are elevated during auditory stimulation and singing (Remage-Healey et al., 2008, 2012). In vivo recordings indicate that elevated NCM estrogens rapidly boost auditory response properties of NCM neurons (Tremere et al., 2009; Remage-Healey et al., 2010b). Furthermore, blocking production of estrogens in NCM disrupts auditory processing (Remage-Healey et al., 2010b) and song preferences in behaving individuals (Remage-Healey et al., 2010b; Tremere and Pinaud, 2011). Here, we demonstrate that local and acute estrogen signaling in NCM transforms the auditory response properties of HVC neurons and that these actions appear to be mediated by a membrane-bound estrogen receptor. These results therefore reveal that rapid steroid signaling within a sensory region has direct implications for downstream sensorimotor integration.
Materials and Methods
We recorded from 34 male zebra finches (Taeniopygia guttata; adults >120 d and juveniles <90 d; see below) obtained from the laboratory breeding colony (14/10 h light/dark cycle, group housed in mixed-sex aviaries). All experiments were approved by the Institutional Care and Use Committee at the University of Massachusetts. Males were housed individually inside sound-attenuation chambers (Eckel Industries) with a companion female to elicit song before surgery. Spontaneous song bouts were continuously recorded and sorted using Sound Analysis Pro (http://soundanalysispro.com/; version 2A.04). Electrophysiology procedures coupled to neuroestrogen microdialysis were performed according to previously established methods (Remage-Healey et al., 2010a, 2012) with some modifications. Dual multiunit extracellular recordings from ipsilateral NCM and HVC were obtained simultaneously and combined with reverse microdialysis (retrodialysis) of estrogens/inhibitors within ipsilateral NCM (Fig. 1A).
For craniotomy surgery to expose the caudal forebrain, birds were anesthetized with 20% urethane via intramuscular injections (3 × 30 μl injections over a 2 h period). After injection with lidocaine (2% in ethanol), a midline incision and craniotomy exposed the reference point (bifurcation of the midsagittal sinus), and the locations of NCM and HVC were stereotaxically marked on the skull (from the bifurcation of the midsagittal sinus NCM: 1.2 mm rostral and 0.7 mm lateral; HVC: 2.4 mm lateral). A stainless steel head post (Herb Adams Engineering) was attached to the rostral skull via dental acrylic/cyanoacrylate, and a recording chamber surrounding the entire caudal forebrain region was sealed with dental acrylic.
After craniotomy, the dura mater was carefully resected over the region overlying the HVC and NCM boundaries, within the recording chamber. The bird was stabilized on a head-post anchor stage (Herb Adams Engineering) inside a sound-attenuation chamber (Industrial Acoustics) and kept warm with a direct current heating pad (FHC Neurocraft). A CMA-7 microdialysis probe prefilled with aCSF (199 mm NaCl, 26.2 mm NaHCO3, 2.5 mm KCl, 1.0 mm MgS04, 2.5 mm CaCl, 11.0 mm glucose, and 1% bovine serum albumin, pH 7.4) was advanced into ventral NCM with a motorized micromanipulator (Warner Instruments). Experiments did not begin until the end of a 60 min stabilization period of steady dialysate flow (2.0 μl/min) to allow implantation-induced phenomena to subside (Remage-Healey et al., 2010, 2012). A motorized micromanipulator (Warner Instruments) advanced a carbon-fiber electrode (0.5–1.2 MΩ; Carbostar-1; Kation Scientific) into NCM immediately adjacent to the retrodialysis probe, and search stimuli included white noise (WN) and tones. Auditory sites were established in NCM by a combination of electrode depth (∼1.5 mm ventral from brain surface) and characteristic auditory-evoked activity. After stable auditory activity in NCM was established, a hydraulic micromanipulator (Narishige) advanced a second carbon-fiber electrode into HVC (beginning ∼0.3 mm ventral from brain surface), for which search stimuli included bird's own song (BOS) and BOS played in reverse (REV). All histologically confirmed HVC sites exhibited the characteristic enhanced neuronal selectivity to BOS relative to all other playback sounds (see Results below and Fig. 1A). Simultaneous, stable, auditory-evoked activity was obtained from both NCM and HVC typically within minutes of the initial encounter of auditory sites in NCM.
Sound stimuli for all animals included BOS, REV, a conspecific song (CON), and white noise (WN). All stimuli (∼2 s in duration) were bandpass filtered using Adobe Audition (0.5–10 kHz; Adobe Systems) and were presented in randomized order at an interstimulus interval of 10 ± 2 s at ∼70 dB SPL. Experimental treatments were divided into three successive 30 min playback periods. Each playback block (20× per stimulus) was 15 min in duration after an initial 15 min silent period to allow drug wash-in or washout, for a total treatment period of 30 min. The first 30 min recording/playback period consisted of aCSF retrodialysis to determine baseline responsiveness, followed by 30 min retrodialysis of estrogens/inhibitors, followed by a 30 min washout period of aCSF alone. Each experimental subject was treated with one compound exclusively, and all recordings were analyzed offline blind to treatment condition. At the conclusion of each experimental session, the NCM and HVC recording sites were each lesioned (+10 μA for 8 s) for histological verification.
The retrodialysis delivery method necessarily infuses estrogenic drugs and inhibitors into a ∼1.0 mm dorsoventral extent of tissue (CMA-7 probe membrane length of 1.0 mm; CMA/Microdialysis). This method enables a larger-scale modulation of the population of NCM neurons (within the NCM region, which is ∼1.4 × 1.2 mm) than is possible with pressure or micropipette injections from a discrete tip (Remage-Healey et al., 2010b, 2012). In adult males (n = 19), retrodialysis solutions in NCM were the predominant neuroestrogen 17-β-estradiol [E2; 30 μg/ml (110 μm); n = 9], the estrogen synthesis inhibitor fadrozole (FAD; 100 μm; n = 5), or the biotin-conjugated estradiol (E6biotin; 30 μg/ml; n = 5). The doses for each compound were identical to previous studies (Remage-Healey et al., 2010b, 2012) and were selected to target the approximate range of local neuroestrogen concentrations in NCM (Remage-Healey et al., 2008).
Dual recordings from NCM and HVC were amplified, bandpass filtered (0.3–5 kHz; A-M Systems 1700), digitized at 20 kHz (Micro 1401; Cambridge Electronic Design), and stored on a computer using Spike 2 software (Cambridge Electronic Design). Multiunit recordings were analyzed offline using thresholding methods (excluding low-amplitude spikes with less than ∼3:1 peak amplitude/background noise ratio) following established protocols (Coleman et al., 2007; Remage-Healey et al., 2010b, 2012). For each recording session, the threshold level was maintained for the entire set of recordings, so that all sampling periods per experiment (e.g., aCSF, E2, washout) were analyzed with the same voltage threshold (for representative multiunit traces and stimulus histograms, see Fig. 1B,C, respectively). Suprathreshold multiunit activity was then used to obtain average response strength (RS) for each 30 min recording. RS was computed by subtracting the mean firing rate for 2 s immediately before playback stimulation from the mean firing rate for 2 s during auditory stimulation. RS typically varies several-fold as a function of the “preferred” stimulus for any one auditory region (e.g., HVC exhibits a twofold to fivefold larger RS to BOS than any other stimulus; Coleman and Mooney, 2004; Nick and Konishi, 2005a). To standardize comparisons among playback treatments, auditory responses were z-transformed (expressed as Z-scores) for both HVC and NCM data. The Z-score is the difference between the mean response during the stimulus and during the baseline period (RS) divided by the SD of the difference between the stimulus and baseline periods (Coleman and Mooney, 2004; Bauer et al., 2008). We observed that results with manipulations of neuroestrogens in NCM were similar for both RS and Z-score measures; presented below are Z-scores for HVC and NCM to simplify stimulus and region response comparisons.
Inspection of the HVC data revealed a substantial shift in BOS selectivity relative to other stimuli during NCM E2 treatment (i.e., enhanced responses to BOS, unchanged responses to other stimuli; see Fig. 2A). To assess the neuroestrogen-dependent changes in HVC auditory selectivity, the selectivity index d′ (a psychophysical measure of discriminability between two stimuli; Green and Swets, 1966) was computed for all song stimuli compared with WN, according to the following equation (Coleman and Mooney, 2004; Bauer et al., 2008):
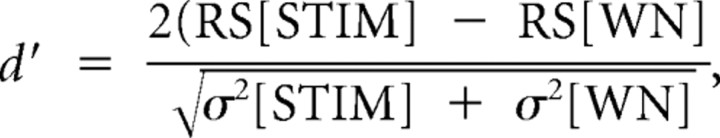 |
where RS[STIM] is the average RS for any stimulus (BOS, REV, CON), RS[WN] is the comparison average RS for WN, and σ2 is the variance for each corresponding parameter. The d′ selectivity index helps to account for distribution variance and is therefore used as a measure of discrimination between two environmental stimuli.
Figure 2.
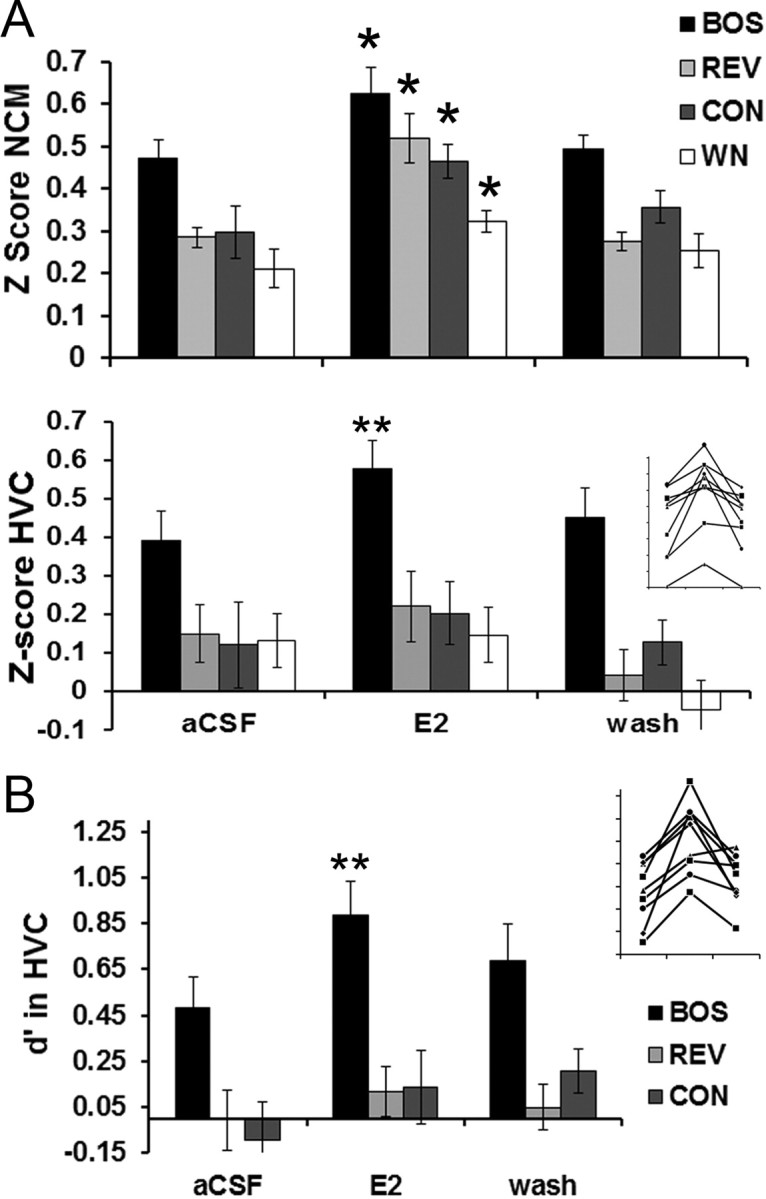
E2 delivered to NCM rapidly enhances neuronal responses to all auditory stimuli in NCM, but E2 selectively enhances only neuronal responses to BOS in HVC. A, The Z-score (neuronal response strength) in NCM is rapidly enhanced by E2 treatment within NCM for all four auditory stimuli (top; n = 9). In contrast, simultaneous recordings in HVC show that the Z-score in HVC is rapidly enhanced by E2 treatment within NCM for the BOS stimulus only. Inset, Individual data for all nine birds showing rapid changes in Z-score values in HVC for the neuronal responses to BOS during aCSF (left), E2 (middle), and wash (right) treatment periods (axes are unlabeled but are identical to bar graph). B, In this same experiment, the stimulus selectivity index d′ in HVC is rapidly enhanced for the BOS stimulus only, indicating that HVC exhibits enhanced neuronal selectivity for the BOS stimulus when E2 levels are acutely elevated in NCM. Inset, Individual data for all nine birds showing rapid changes in d′ values in HVC for the neuronal responses to BOS during aCSF (left), E2 (middle), and wash (right) treatment periods (axes are unlabeled but are identical to bar graph). Each time period is 30 min in duration. *p < 0.05; **p < 0.01 for Wilcoxon's signed-rank post hoc tests comparing E2 versus aCSF conditions on a within-stimulus basis.
HVC single-unit analyses.
To investigate whether E2-dependent changes in HVC auditory responses occurred at the level of single neurons, HVC recordings were sorted for single-unit analysis (procedures as performed by Remage-Healey et al., 2010b). Large-amplitude single units (signal/noise ratio >3:1; one to two units per bird) were identified via waveform spike sorting (Spike 2; Cambridge Electronic Design). Single units identified by the offline spike sorting algorithm were segregated by waveform characteristics and by principle component analysis (see Fig. 3A). In all cases, single units were verified to be auditory responsive by inspecting playback peristimulus time histograms (PSTHs; see Fig. 3B), and only units meeting refractory period criteria were included in the analyses [i.e., the number of interspike intervals (ISIs) within 1 ms ≤1% of the total number of ISIs; average ± SEM, 0.69 ± 0.33%]. This conservative analysis identified eight single units from the nine males for which spike-sorted waveform templates could be reliably maintained across treatment periods (aCSF, E2, wash). Z-scores and d′ values were generated for single units and analyzed as above.
Figure 3.
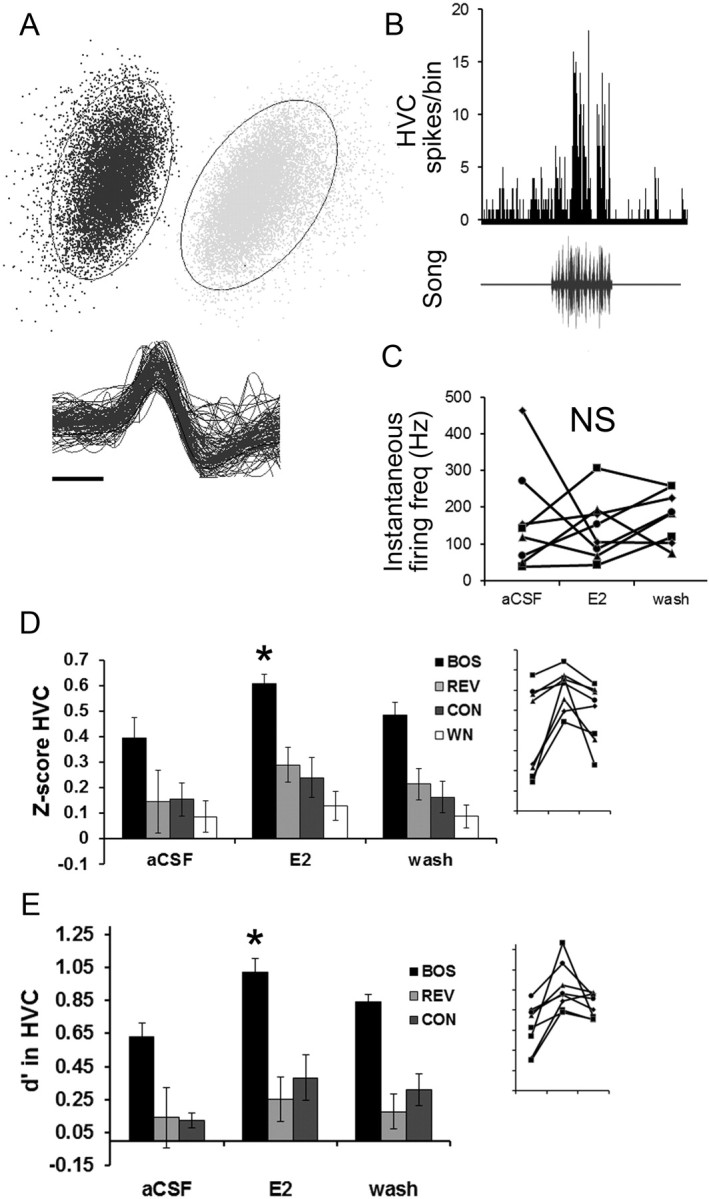
Estradiol in NCM enhances BOS selectivity of single neurons in HVC. A, Top, Principle component analysis plot for a typical single auditory unit in HVC (black dots, lines indicate SD) showing clear separation from a second HVC unit (gray dots). Bottom, Overlay of 100 random spike waveforms from the same single unit shows the corresponding unique distribution of spikes for the same unit (calibration bar, 0.2 ms). B, All identified HVC single units showed significant changes in auditory-evoked activity; shown here is a PSTH of a typical unit (top) along with the corresponding song stimulus (bottom). C, Estradiol treatment in NCM did not produce changes in spontaneous activity in HVC single units. The average instantaneous firing frequency is plotted here for all individual data for eight single units in this study, showing no significant change with E2 treatment. D, The Z-scores for all identified single units were rapidly enhanced for BOS in HVC after E2 treatment in NCM. Inset, Individual data for all eight units for BOS showing rapid changes in Z-scores during aCSF (left), E2 (middle), and wash (right) treatment periods (axes are unlabeled but are identical to bar graph). E, In this same experiment, the stimulus selectivity index d′ in HVC is rapidly enhanced for the BOS stimulus when E2 levels are acutely elevated in NCM. Inset, Individual data for all identified single units for BOS showing rapid changes during aCSF (left), E2 (middle), and wash (right) treatment periods (axes are unlabeled but are identical to bar graph). Each time period is 30 min in duration. *p < 0.05 for Wilcoxon's signed-rank post hoc tests comparing E2 versus aCSF conditions on a within-stimulus basis. NS, Nonsignificant.
Dual CMM–HVC recordings and HVC microinjections.
We performed two additional experiments to determine the region specificity of the rapid actions of E2 in NCM, in particular because there are no direct anatomical connections between NCM and HVC (Fig. 1A). First, dual extracellular recordings were obtained from HVC and caudomedial mesopallium (CMM), a secondary auditory region that, like NCM, provides auditory input into HVC via two intervening pathways (Fig. 1A). In a separate set of adult males (n = 5), dual multiunit recordings from ipsilateral CMM and HVC were obtained simultaneously and combined with reverse microdialysis (retrodialysis) of E2 (30 μg/ml) within ipsilateral CMM (see Fig. 5). Surgical and recording procedures were as presented above, with modifications to expose CMM in addition to HVC.
Figure 5.
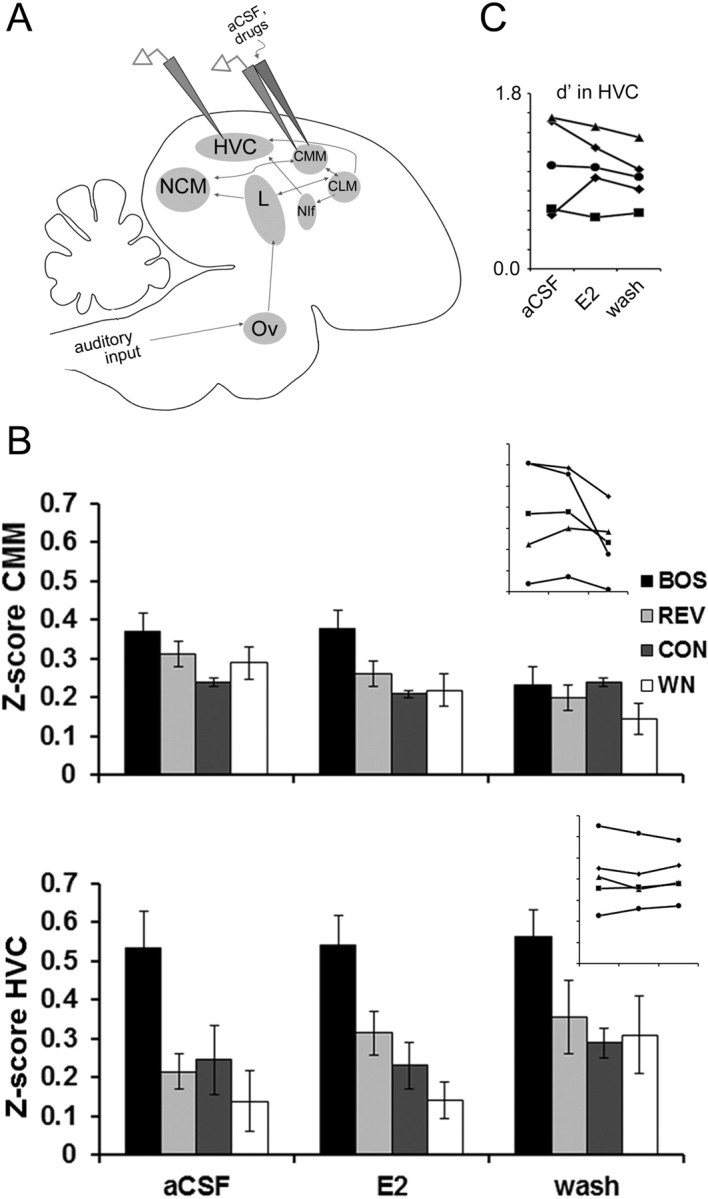
Estradiol in CMM does not alter HVC auditory responses in adult males. A, A sagittal schematic similar to Figure 1A showing the retrodialysis probe coupled to the extracellular electrode in CMM and a second extracellular electrode in HVC. B, Although CMM exhibits strong auditory-evoked activity (average Z-scores are positive for all stimuli during all treatment conditions), E2 treatment within CMM has no significant effect on auditory responses in either CMM (top) or HVC (bottom), demonstrating the region specificity of rapid E2 actions within nearby NCM. Insets, Individual data for all five birds for BOS showing no changes in Z-score values for CMM (top) and HVC (bottom; axes are unlabeled but are identical to bar graphs). C, Individual data for all five birds showing no change in BOS selectivity (index d′) in HVC during E2 manipulations in CMM. Other abbreviations are as defined in the legend to Figure 1.
Second, in a separate set of adult males (n = 4), we delivered E2 directly into HVC via microinjection (the dorsoventral extent of HVC is too narrow to permit targeted retrodialysis with current methods) using a modified picospritzer while recording within HVC (World Precision Instruments). After establishing stable multiunit activity in HVC, ∼100 nl of aCSF was delivered to HVC following established protocols for small-volume pressure delivery (Remage-Healey and Bass, 2010; Tremere et al., 2010). HVC multiunit activity was recorded for the 10 min after aCSF delivery to establish baseline responses, after which ∼100 nl of E2 (30 μg/ml) was delivered to HVC and two subsequent HVC recordings were collected (0–10 min after E2; 10–20 min after E2).
Dual NCM–HVC recordings in juvenile males.
BOS-selective responses emerge in the HVC of juvenile males by 70–90 d post-hatching (dph; Nick and Konishi, 2005b) and in the downstream anterior forebrain pathway by as early as 60 dph (Solis and Doupe, 1997; Sizemore and Perkel, 2011). During this period, juvenile males gradually refine their highly variable song, and full “crystallized ” adult song (i.e., highly stereotyped across renditions) only occurs after 90 dph (Korsia and Bottjer, 1991; Brainard and Doupe, 2002; Mooney, 2009), when gonadal androgens are elevated (Schlinger and Brenowitz, 2008). Because of the above results with adult males, we tested whether the E2-dependent modulation within NCM leads to downstream enhanced HVC BOS selectivity in juvenile males, before the full maturation of the auditory forebrain network. A set of six males was used to explore this hypothesis, with ages over the range of the sensorimotor phase of song development (ages: 60, 63, 67, 72, 76, and 81 dph). Dual extracellular recordings were obtained from HVC and NCM, after anesthesia and surgery as outlined above, in combination with retrodialysis of E2 (30 μg/ml) within ipsilateral NCM.
Statistical analyses.
Statistical analyses were performed using Statview 4.5 (Abacus) and R (GNU project). Z-score and d′ values were analyzed for each brain region using nonparametric repeated-measures Friedman's ANOVA tests (Shapiro-Wilk's W tests for parametric distributions were significant at p < 0.05; no multi-way repeated-measures ANOVA tests exist for nonparametric data). One-way repeated-measures Friedman's tests were computed for each auditory playback stimulus on a within-subject basis over time for a given treatment sequence (e.g., aCSF, E2, and wash). When initial Friedman's tests were found to be significant for an auditory playback stimulus (e.g., BOS), subsequent Wilcoxon's signed-rank post hoc tests (Siegel and Castellan, 1988; Sokal and Rohlf, 1995) were used to test for paired within-subject changes in Z-scores or d′ values from baseline (e.g., aCSF vs E2 treatment periods).
Results
NCM E2 treatment enhances auditory encoding in NCM
Acute, 30 min retrodialysis of E2 (30 μg/ml; n = 9) into NCM caused a local upregulation of NCM auditory responses to all stimuli (Fig. 2A, top), consistent with previously published work in adult males and females of this species (Tremere et al., 2009; Remage-Healey et al., 2010b, 2012; Tremere and Pinaud, 2011). For response strength (Z-score values) in NCM, the nonparametric repeated-measures Friedman's tests revealed overall significant effects of E2 treatment for BOS (X2 = 7.60; p = 0.022), REV (X2 = 10.00; p = 0.007), CON (X2 = 6.40; p = 0.041), and WN (X2 = 7.60; p = 0.022). The source of this variation was determined by Wilcoxon's signed-rank post hoc tests, which revealed that response strength in NCM was significantly elevated over baseline (aCSF vs E2) for BOS, REV, CON, and WN (all p < 0.04).
NCM E2 treatment enhances BOS responses in HVC
The same acute, 30 min retrodialysis of E2 (30 μg/ml; n = 9) into NCM caused an upregulation of HVC auditory responses only to the playback of the BOS (Fig. 2A, bottom). For response strength (Z-score values) in HVC, the nonparametric repeated-measures Friedman's test revealed an overall significant effect of E2 treatment on the response to BOS (X2 = 13.50; p = 0.001). In contrast, Friedman's tests showed no effects of E2 treatment on the HVC response to REV (X2 = 0.06; p = 0.972), CON (X2 = 2.39; p = 0.303), or WN (X2 = 3.72; p = 0.147). The source of this variation was determined by Wilcoxon's signed-rank post hoc tests, which revealed that response strength in HVC was significantly elevated over baseline (aCSF vs E2) for BOS (p = 0.007) but not significantly different for REV, CON, and WN (all p > 0.37).
Similarly, for the selectivity index d′ (Fig. 2B), the nonparametric repeated-measures Friedman's test revealed an overall significant effect of E2 treatment on the HVC selectivity for BOS (X2 = 12.06; p = 0.002). In contrast, Friedman's tests showed no effects of E2 treatment on the HVC selectivity for REV (X2 = 1.17; p = 0.549) or CON (X2 = 2.11; p = 0.347). The source of this variation was determined by Wilcoxon's signed-rank post hoc tests, which revealed that d′ values in HVC were significantly elevated over baseline (aCSF vs E2) for BOS (p = 0.007) but not significantly different for REV and CON (all p > 0.37). Therefore, although E2 caused a rapid and generalized upregulation of auditory response strength in NCM, the same modulatory event was transformed by the time it reached HVC to become specific for a selective enhancement of the BOS.
HVC single-unit response properties enhanced by NCM E2 treatment
To investigate whether E2-dependent changes in HVC auditory responses occurred at the level of single neurons, HVC recordings were sorted for single-unit analysis (see Materials and Methods and Fig. 3A,B). Patterns of response for the n = 8 isolated single units were very similar to results with HVC multiunit data. E2 retrodialysis in NCM caused a local upregulation of HVC single-unit auditory responses to the BOS only (Fig. 3). For response strength (Z-score values) in HVC, the nonparametric repeated-measures Friedman's test revealed an overall significant effect of E2 treatment on the response to BOS (X2 = 10.75; p = 0.004). In contrast, Friedman's tests showed no effects of E2 treatment on the HVC response to REV (X2 = 1.75; p = 0.417), CON (X2 = 0.75; p = 0.687), or WN (X2 = 0.25; p = 0.882). The source of this variation was determined by Wilcoxon's signed-rank post hoc tests, which revealed that response strength in HVC was significantly elevated over baseline (aCSF vs E2) for BOS (p = 0.01) but not significantly different for REV, CON, and WN (all p > 0.21).
Similarly, for the selectivity index d′ (Fig. 3E), the nonparametric repeated-measures Friedman's test revealed an overall significant effect of E2 treatment on the HVC selectivity for BOS (X2 = 13.07; p = 0.001). In contrast, Friedman's tests showed no effects of E2 treatment on the HVC selectivity for REV (X2 = 0.27; p = 0.875) or CON (X2 = 3.47; p = 0.177). The source of this variation was determined by Wilcoxon's signed-rank post hoc tests which revealed that d' values in HVC were significantly elevated over baseline (aCSF vs E2) for BOS (p = 0.01) but not significantly different for REV and CON (all p > 0.21). Therefore, unsurprisingly, the enhanced representation of BOS in HVC was attributable to specific modulation of the activity of single HVC neurons.
There was no significant change in baseline firing frequency (i.e., in the absence of auditory stimulation) in HVC between aCSF versus E2 NCM retrodialysis conditions. The mean ± SEM instantaneous firing frequency in HVC for aCSF versus E2 conditions was unchanged at the level of multiunit spontaneous activity (309.5 ± 61 vs 338.9 ± 58 Hz, respectively; p = 0.83 for paired t test), as well as at the level of single-unit spontaneous activity (163.3 ± 50 vs 141.9 ± 30 Hz, respectively; p = 0.74 for paired t test; Fig. 3C). This suggests that E2-dependent modulation transformed the auditory input(s) to HVC and did not directly alter the intrinsic membrane properties of the HVC neurons we sampled (as reported previously, spontaneous activity is also unchanged in NCM during E2 treatment; Remage-Healey et al., 2010b). However, this hypothesis remains to be tested with intracellular recordings before conclusions can be made about the nature of the modulation at the level of HVC.
No enhancement of HVC BOS representation in juvenile males
Because of the above results collected in adult males, we tested whether the E2-dependent modulation within NCM led to downstream enhanced BOS selectivity in juvenile males (before song crystallization and maturation of the HVC–RA circuit, 60–81 dph). In contrast to adults, however, the same dose of E2 (30 μg/ml; n = 6) produced no significant changes in auditory-evoked activity in juveline HVC (Fig. 4). For response strength (Z-score values) in HVC, the nonparametric repeated-measures Friedman's tests showed no significant effect of E2 treatment on the responses to BOS (X2 = 4.33; p = 0.115), REV (X2 = 0.33; p = 0.846), CON (X2 = 0.33; p = 0.846), or WN (X2 = 0.33; p = 0.846). Therefore, the rapid modulation of HVC auditory processing by E2 in NCM very likely depends on a developmental maturation of the auditory pathway.
Figure 4.
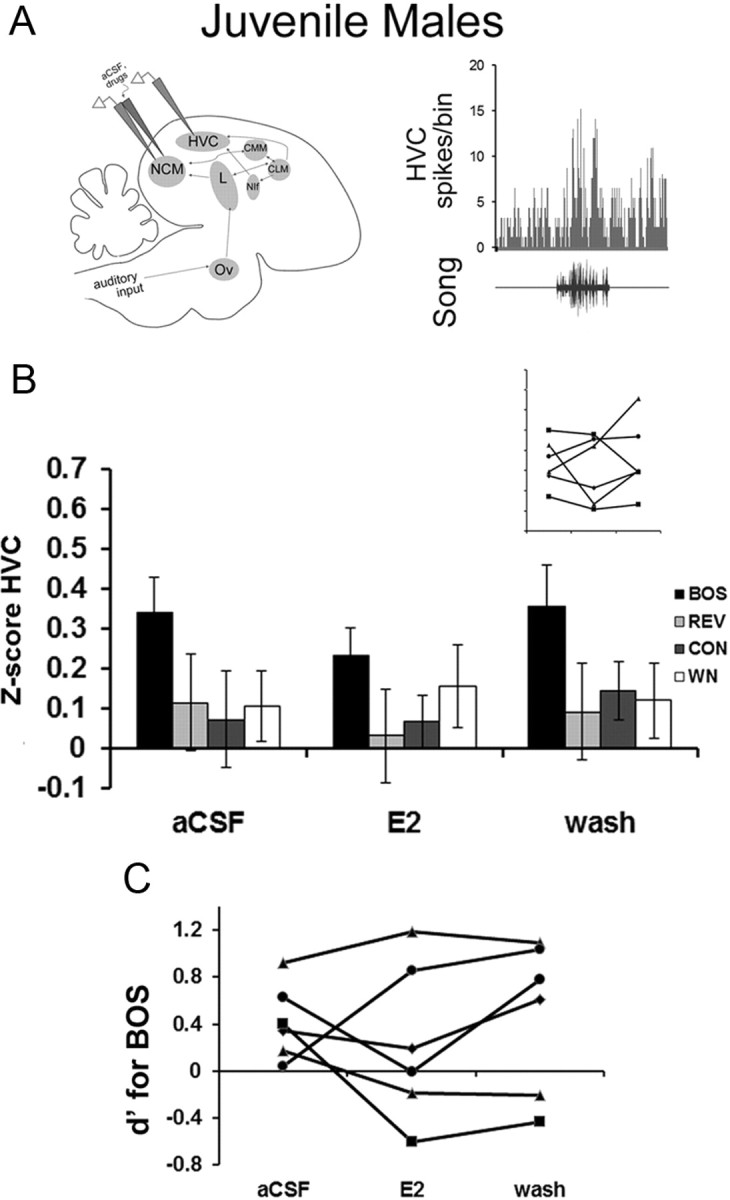
Estradiol in NCM does not alter HVC auditory responses in juvenile male zebra finches. A, At left is a sagittal schematic identical to Figure 1A showing the retrodialysis probe coupled to the extracellular electrode in NCM and a second extracellular electrode in HVC. At right is an example of HVC multiunit activity showing clear auditory-evoked activity in a juvenile male; shown here is a PSTH of a typical response (top) along with the corresponding song stimulus (bottom). Unlike adult males, E2 treatment in NCM did not cause rapid changes in Z-score values (B) or d′ values for BOS (C) in the HVC of juvenile males (60–81 dph). Individual data are presented for all six birds showing no consistent change in BOS response strength (B; Z-score values; axes are unlabeled but are identical to bar graph) and BOS selectivity (C; d′ values). Other abbreviations are as defined in the legend to Figure 1.
No enhancement of HVC BOS representation by E2 treatment in either CMM or HVC
One explanation for the rapid actions of E2 delivered to NCM on downstream response properties in HVC in adults could be rapid diffusion of E2 through the caudal forebrain to have a direct effect on HVC. We performed two separate experiments to test this possibility and thereby determine the region specificity of the rapid actions of E2 in NCM. First, dual extracellular recordings were obtained from HVC and CMM, an adjacent secondary auditory region that (like NCM) provides indirect input into HVC (Fig. 5A). Dual extracellular recordings revealed that the same dose of E2 (30 μg/ml; n = 5) delivered to CMM did not have significant effects on auditory processing within CMM or upstream in HVC, despite the similar HVC proximity of CMM as that of NCM. For response strength (Z-score values) in CMM (Fig. 5B, top), the nonparametric repeated-measures Friedman's tests showed no significant effect of E2 treatment on the responses to BOS (X2 = 2.80; p = 0.246), REV (X2 = 0.40; p = 0.819), CON (X2 = 3.60; p = 0.165), or WN (X2 = 3.60; p = 0.165). For corresponding response strength in HVC (Fig. 5B, bottom), the nonparametric repeated-measures Friedman's tests showed no significant effect of E2 treatment on the responses to BOS (X2 = 1.60; p = 0.449), REV (X2 = 1.20; p = 0.549), CON (X2 = 2.80; p = 0.246), or WN (X2 = 2.80; p = 0.246). Thus, elevated E2 levels in nearby CMM did not cause rapid changes in CMM activity nor did they mimic the rapid actions of NCM E2 on downstream HVC processing.
Second, E2 delivered directly into HVC produced no changes in the auditory response properties of HVC itself (Fig. 6). For response strength (Z-score values) in HVC (Fig. 6B), the nonparametric repeated-measures Friedman's tests showed no significant effect of local HVC E2 treatment on the responses to BOS (X2 = 4.50; p = 0.105), REV (X2 = 1.50; p = 0.472), CON (X2 = 2.00; p = 0.368), or WN (X2 = 1.50; p = 0.472). Similarly, the selectivity index d′ was unchanged for BOS after delivery of E2 (Fig. 6C; X2 = 1.500; p = 0.472). These results are in direct contrast with those presented above in which E2 is delivered into NCM (Fig. 2A,B). Together, experiments with E2 delivery to CMM and HVC indicate that the modulation of HVC response properties by the actions of E2 in the auditory forebrain appear to be specifically localized to NCM and not dependent on passive diffusion of E2 from NCM (or CMM) into HVC itself.
Figure 6.
Estradiol delivered directly to HVC does not alter HVC auditory responses in adult males. A, A sagittal schematic similar to Figure 1A showing the HVC microinjection site coupled to the extracellular electrode in HVC. B, Auditory response strength (Z-score values) in HVC are unchanged after E2 treatment within HVC, further demonstrating the region specificity of rapid E2 actions within nearby NCM. Inset, Individual data for all four birds for BOS showing no changes in Z-score values (axes are unlabeled but are identical to bar graph). C, Individual data for all four birds showing no change in BOS selectivity (index d′) in HVC during E2 manipulations in HVC. Other abbreviations are as defined in the legend to Figure 1.
Local inhibition of NCM aromatase diminishes neuronal selectivity in HVC
To test whether endogenous estrogen production within NCM has consequences for HVC auditory selectivity, we retrodialyzed the potent aromatase inhibitor FAD (100 μm; n = 5) into NCM while recording from HVC. The same dose of FAD has been shown previously via retrodialysis delivery to suppress local E2 levels, auditory processing, as well as behavioral song preferences in NCM (Remage-Healey et al., 2008, 2010b). For response strength (Z-score values) in HVC (Fig. 7C, top), the nonparametric repeated-measures Friedman's test revealed an overall significant effect of FAD treatment on the response to BOS (X2 = 8.40; p = 0.015). In contrast, Friedman's tests showed no effects of FAD treatment on the HVC response to REV (X2 = 1.60; p = 0.449), CON (X2 = 0.40; p = 0.819), or WN (X2 = 0.40; p = 0.819). However, the Wilcoxon's signed-rank post hoc test showed a trend but no significant change for Z-scores in response to BOS during FAD retrodialysis relative to the preceding baseline period (Z = 1.753; p = 0.051 for FAD vs aCSF).
Figure 7.
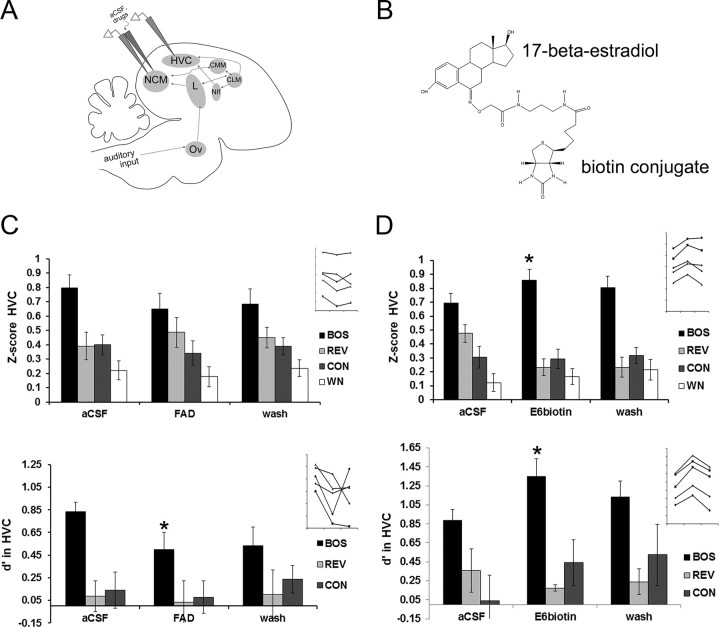
Blocking endogenous estrogen production in NCM suppresses neuronal selectivity for BOS in HVC, and the rapid actions of estrogens in NCM appear to depend on acute membrane-mediated actions. A, Schematic of dual NCM–HVC electrophysiology recordings (identical to Fig. 1A) used for retrodialysis delivery of the aromatase inhibitor FAD and the cell-membrane-impermeable biotin-conjugated estradiol (E6biotin). B, Chemical structure of E6biotin, showing the biotin conjugate in the carbon-6 position of E2, which retains receptor binding capacity of E2 but restricts diffusion through cell membranes. Adapted with permission from Steraloids. C, Acute suppression of local estrogen production within NCM (via FAD-mediated aromatase inhibition) does not alter Z-score values in HVC (top) but significantly suppresses the selectivity index d′ for BOS in HVC (bottom). In contrast, the d′ values for REV and CON remain unchanged. Insets show individual data for all five birds showing rapid changes in d′ values in HVC for the neuronal responses to BOS during aCSF (left), FAD (middle), and wash (right) treatment periods and no consistent changes for Z-score values (axes are unlabeled but are identical to bar graphs). Each time period is 30 min in duration. *p < 0.05 for the Wilcoxon's signed-rank post hoc test comparing FAD versus aCSF conditions on a within-stimulus basis. D, Retrodialysis of the cell-membrane-impermeable compound E6biotin into NCM fully mimics the acute actions of unconjugated E2 on Z-score values for BOS (top) and selectivity index d′ for BOS (bottom) in HVC. Insets show individual data, respectively, for all five birds showing rapid changes for BOS during aCSF (left), E6biotin (middle), and wash (right) treatment periods (axes are unlabeled but are identical to bar graphs). Each time period is 30 min in duration. *p < 0.05 for Wilcoxon's signed-rank post hoc tests comparing E6biotin versus aCSF conditions on a within-stimulus basis. Other abbreviations are as defined in the legend to Figure 1.
Inhibiting estrogen production in NCM with FAD had clear effects on stimulus selectivity in HVC. For the selectivity index d′ (Fig. 7C, bottom), the nonparametric repeated-measures Friedman's tests revealed an overall significant effect of FAD treatment on the HVC selectivity for BOS (X2 = 6.40; p = 0.041) and no effects of FAD treatment on the HVC selectivity for REV (X2 = 0.40; p = 0.819) or CON (X2 = 5.20; p = 0.074). The source of this variation was tested by Wilcoxon's signed-rank post hoc tests, which revealed that d′ values in HVC were significantly reduced relative to baseline (aCSF vs FAD) for BOS (p = 0.04) but not significantly different for REV and CON (all p > 0.50). Therefore, the auditory selectivity for BOS in HVC was significantly and specifically suppressed when endogenous estrogen production was blocked within the NCM.
A membrane-impermeable estrogen mimics rapid effects on HVC selectivity
To test for possible rapid E2 actions at putative neuronal membrane receptors, the membrane-impermeable biotinylated E2 (E6biotin; Fig. 7B) was retrodialyzed into NCM in a separate set of adult males (30 μg/ml; n = 5) while recording from HVC. For response strength (Z-score values) in HVC (Fig. 7D, top), the nonparametric repeated-measures Friedman's test revealed an overall significant effect of E6biotin treatment on the response to BOS (X2 = 6.40; p = 0.041). In contrast, Friedman's tests showed no effects of E6biotin treatment on the HVC response to REV (X2 = 1.20; p = 0.549), CON (X2 = 0.40; p = 0.819), or WN (X2 = 1.60; p = 0.449). The source of this variation was tested by Wilcoxon's signed-rank post hoc tests, which revealed that response strength was significantly elevated above baseline (aCSF vs E6biotin) for BOS (p = 0.04) but not significantly different for REV (p = 0.07), CON (p = 0.99), and WN (p = 0.71).
Similarly, for the selectivity index d′ (Fig. 6D, bottom), the nonparametric repeated-measures Friedman's test revealed an overall significant effect of E6biotin treatment on the HVC selectivity for BOS (X2 = 8.40; p = 0.015). In contrast, Friedman's tests showed no effects of E6biotin treatment on the HVC selectivity for REV (X2 = 2.80; p = 0.246) or CON (X2 = 0.40; p = 0.819). The source of this variation was determined by Wilcoxon's signed-rank post hoc tests, which revealed that d′ values in HVC were significantly elevated above baseline (aCSF vs E6biotin) for BOS (p = 0.04) but not significantly different for REV and CON (all p > 0.27). Therefore, the rapid actions of E2 in NCM on downstream HVC BOS selectivity were fully mimicked by the cell-membrane-specific estrogen E6biotin.
Discussion
Here, we show that local and acute estrogen signaling in the auditory forebrain transforms downstream auditory response properties of neurons in a sensorimotor nucleus and that these actions appear to be mediated by a membrane-bound estrogen receptor. Although E2 caused a rapid and generalized upregulation of auditory responses to all stimuli in the auditory NCM, the same modulatory event was markedly transformed by the time it reached sensorimotor HVC, enhancing the representation of BOS exclusively. This transformation was also evident in the evoked activity of single HVC auditory neurons. Moreover, the auditory representation of BOS in HVC was specifically suppressed when endogenous estrogen production was blocked within NCM. Together, this evidence is consistent with the hypothesis that locally derived estrogens in NCM can rapidly modulate the stream of auditory information that is ultimately transmitted to HVC. These findings indicate that steroid-dependent modulation of activity in a sensory forebrain region is not locally restricted and can be transmitted to an interconnected network of sensorimotor and premotor targets.
Region and age specificity
One potential explanation for the rapid actions of NCM neuroestrogens on downstream HVC response properties is that retrodialyzed E2 diffused through the caudal forebrain into HVC itself for direct local actions. We feel confident in rejecting this possibility based on the following reasoning. First, in separate experiments, E2 was rapidly retrodialyzed into the nearby secondary auditory nucleus CMM, which receives reciprocal connections from NCM and projects to HVC [via caudolateral mesopallium (CLM) and nucleus interfacialis (NIf); Fig. 1A]. E2 had no local effects on CMM auditory responses, and similarly E2 did not have significant downstream effects on HVC response properties. Thus, identical neuroestrogen delivery to a nearby auditory nucleus (CMM) did not mimic the rapid actions of E2 in NCM. Second, there was no effect of NCM E2 manipulations on baseline (i.e., non-auditory evoked) firing properties of HVC neurons, suggesting instead that the afferent auditory inputs of HVC were modulated. Third, E2 delivered directly into HVC itself produced no changes in HVC response properties to song stimuli. Together, these findings indicate that E2 acts within NCM to indirectly modulate HVC response properties rather than diffusing and acting directly on the HVC microenvironment. The nature of this indirect, transynaptic, and rapid modulation by E2 has now become an active area of interest.
The region specificity of rapid E2 actions in NCM is consistent with the distribution of the estrogen-synthesis enzyme aromatase in the caudal forebrain of zebra finches. The NCM is highly enriched with aromatase mRNA and protein expression, whereas other nuclei in the ascending auditory pathway (such as Field L, CMM, and NIf) are essentially devoid of aromatase (Saldanha et al., 2000; Saldanha and Coomaralingam, 2005; Jeong et al., 2011). NCM also exhibits predominant expression of two estrogen receptors, ERβ (Saldanha et al., 2000; Saldanha and Coomaralingam, 2005; Jeong et al., 2011) and GPR30 (Acharya and Veney, 2012), that could mediate rapid and local neuroestrogen signaling. NCM therefore occupies a critical node that enables estrogen-dependent modulation of the network of ascending auditory signals into HVC. It is noteworthy that HVC itself contains no aromatase cell bodies but does contain terminal boutons that are aromatase positive (Saldanha et al., 2000; Peterson et al., 2005), and it remains to be determined whether this population is independently controlled (with respect to rapid estrogen signaling and auditory modulation) from the abundant terminal aromatase in NCM (Remage-Healey et al., 2011).
The HVC of juvenile male songbirds exhibits increasing BOS selectivity as the song learning period unfolds (Volman, 1993; Nick and Konishi, 2005a), and the neuronal selectivity for tutor songs in the song system wanes by ∼70 dph in favor of BOS (Solis and Doupe, 1997; Yazaki-Sugiyama and Mooney, 2004; Nick and Konishi, 2005b). Our data show that the ability of NCM neuroestrogens to drive enhanced neural representations of BOS in HVC does not strictly parallel the developmental emergence of baseline HVC selectivity for the BOS. Rather, the E2-dependent modulation by NCM is only evident in adult males (>120 dph), after song crystallization has occurred, and this modulation is absent in juvenile males (<90 dph). These findings indicate that auditory inputs to the HVC undergo a maturation process in the late juvenile period that is necessary for modulatory signals from NCM to be transmitted to HVC. This maturation may be coincident with and/or dependent on elevated gonadal androgens that permanently alter the response properties of song system nuclei during the transition to adulthood (White et al., 1999).
Estrogens can transform information flow
Transynaptic communication of steroid actions from one brain region to another have been documented previously across long-term timescales (i.e., actions that occur within days to weeks of estrogen treatment). For example, estradiol can exert transynaptic effects on cortical BDNF release via actions on afferent inhibitory neurons (Blurton-Jones and Tuszynski, 2006), on cholecystokinin expression in the medial amygdala via actions in the bed nucleus of the stria terminalis (Micevych et al., 1996), and on seasonal firing patterns in premotor robust nucleus of the arcopallium (RA) via actions in sensorimotor HVC (Meitzen et al., 2007).
This study now demonstrates that estrogens can exert transynaptic actions on a rapid, modulatory timescale (i.e., within minutes) by altering the neural representation of song stimuli between auditory and sensorimotor nuclei in the songbird brain. This mode of action is reminiscent of how classical neuromodulators can influence information flow between brain circuits. Dopamine fluctuations in ventral forebrain can initiate locomotor sequences via modulating the input to spinal pattern generators (Grillner, 2003), and similarly, in prairie voles the coupling of forebrain dopamine and neuropeptide signaling is considered crucial for maintaining conditioned partner preferences via downstream motor nuclei (Young and Wang, 2004). In the songbird forebrain, dopamine signaling in the striatum can alter auditory responses in a cortical nucleus via intervening thalamic nuclei (Leblois et al., 2010), and this transynaptic modulation is thought to occur via changing ISIs of inhibitory input onto the thalamus. To the extent that rapid neuroestrogen signaling identified in the current study shares similarities with transynaptic modulation by catecholamines, it is noteworthy that rapid estrogen modulation within NCM causes a shift in ISIs and burst firing of NCM neurons (Remage-Healey et al., 2010b). Based on this model, intervening neural substrates between NCM and HVC should therefore exhibit changes in spike timing and/or synaptic drive in response to neuroestrogen manipulations in NCM.
An in-depth examination of the candidate transynaptic factors that mediate this effect now becomes necessary to fully understand the significance of the rapid actions of E2 on the ascending auditory forebrain pathway. Because NIf (Fig. 1A) provides the primary BOS-selective input into HVC (Janata and Margoliash, 1999; Coleman and Mooney, 2004), it is likely that the selective enhancement of BOS in HVC is dependent on the intermediary nucleus NIf. This prediction is also supported by anatomical evidence in which HVC receives substantial input from NIf and no reported direct input from NCM (Fortune and Margoliash, 1995). Altering activity within NIf also produces changes in HVC response properties; in particular, catecholamines delivered to NIf can shift HVC BOS selectivity (Cardin and Schmidt, 2004). Together, these observations provide testable predictions in which the rapid, transynaptic modulation of HVC firing properties via E2-dependent actions in NCM occur via modulation of NIf. Importantly, despite our observation that E2 does not act in CMM to modulate HVC response properties, the CMM/CLM complex likely also plays a vital role in the transformation of information between the broadly tuned NCM and the BOS-selective structures NIf and HVC (Bauer et al., 2008; Keller and Hahnloser, 2009; Jeanne et al., 2011).
The NCM E2-dependent transition into a more highly selective state in HVC resembles the changes in HVC and RA response properties that have been observed during transitions in behavioral arousal. A current model proposes that individual HVC neurons experience shifts in the balance of excitatory and inhibitory input, which leads to changes in BOS selectivity at the cellular level (Rauske et al., 2003). Intracellular recordings in HVC show that alterations in BOS selectivity arise from shifting network dynamics within the HVC “microcircuit” of projection neurons and interneurons (Mooney, 2000; Mooney and Prather, 2005). We therefore predict that the rapid E2-mediated transformation in NCM is likely to use similar mechanisms to shift BOS selectivity in HVC via indirect modulation of afferent neural substrates, such as NIf. In other systems, steroids have been shown to act locally in brain circuits to rapidly influence the release of classical neurotransmitters, such as catecholamines and acetylcholine (Zheng, 2009), which can in turn enhance signal/noise ratios in sensory cortex. The ultimate outcome of estrogen-enhanced stimulus selectivity in a sensorimotor structure, such as HVC, may be improved feedback sensitivity and/or perception. This hypothesis is consistent with the recent observations that local suppression of estrogen production in NCM causes a disruption of song preference behavior (Remage-Healey et al., 2010b; Tremere and Pinaud, 2011). This study therefore offers a promising new look at how estrogens may rapidly enhance information transfer between sensory and motor structures and provides experimental insight into the improvements in cognition associated with the actions of estrogens in the forebrain (Frick, 2012; McEwen et al., 2012).
Footnotes
This work was supported by National Institute of Neurological Disorders and Stroke Grant K99/R00 NS066179, the Andrew W. Mellon Foundation, and the University of Massachusetts. We thank Juli Wade and Kamal Sen for generous contribution of birds to found the University of Massachusetts breeding colony, Sarah M. N. Woolley and Melissa Coleman for technical advice, John Meitzen, Andrew Bass, Jonathan Prather, and two anonymous reviewers for comments on this manuscript, and Barney Schlinger for advice and encouragement to launch this study.
References
- Acharya KD, Veney SL. Characterization of the G-protein coupled membrane bound estrogen receptor GPR30 in the zebra finch brain reveals a sex difference in gene and protein expression. Dev Neurobiol. 2012 doi: 10.1002/dneu.22004. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology. 2006;147:359–366. doi: 10.1210/en.2005-0845. [DOI] [PubMed] [Google Scholar]
- Bass AH, Remage-Healey L. Central pattern generators for social vocalization: androgen-dependent neurophysiological mechanisms. Horm Behav. 2008;53:659–672. doi: 10.1016/j.yhbeh.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer EE, Coleman MJ, Roberts TF, Roy A, Prather JF, Mooney R. A synaptic basis for auditory-vocal integration in the songbird. J Neurosci. 2008;28:1509–1522. doi: 10.1523/JNEUROSCI.3838-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blurton-Jones M, Tuszynski MH. Estradiol-induced modulation of estrogen receptor-beta and GABA within the adult neocortex: a potential transsynaptic mechanism for estrogen modulation of BDNF. J Comp Neurol. 2006;499:603–612. doi: 10.1002/cne.21122. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nat Rev Neurosci. 2006;7:347–357. doi: 10.1038/nrn1904. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. What songbirds teach us about learning. Nature. 2002;417:351–358. doi: 10.1038/417351a. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA. Altered perception of species-specific song by female birds after lesions of a forebrain nucleus. Science. 1991;251:303–305. doi: 10.1126/science.1987645. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MF. Noradrenergic inputs mediate state dependence of auditory responses in the avian song system. J Neurosci. 2004;24:7745–7753. doi: 10.1523/JNEUROSCI.1951-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier TD, Newman AE, Heimovics SA, Po KW, Saldanha CJ, Soma KK. Rapid effects of aggressive interactions on aromatase activity and oestradiol in discrete brain regions of wild male white-crowned sparrows. J Neuroendocrinol. 2011;23:742–753. doi: 10.1111/j.1365-2826.2011.02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coddington E, Lewis C, Rose JD, Moore FL. Endocannabinoids mediate the effects of acute stress and corticosterone on sex behavior. Endocrinology. 2007;148:493–500. doi: 10.1210/en.2006-0740. [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Mooney R. Synaptic transformations underlying highly selective auditory representations of learned birdsong. J Neurosci. 2004;24:7251–7265. doi: 10.1523/JNEUROSCI.0947-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MJ, Roy A, Wild JM, Mooney R. Thalamic gating of auditory responses in telencephalic song control nuclei. J Neurosci. 2007;27:10024–10036. doi: 10.1523/JNEUROSCI.2215-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, Ball GF, Balthazart J. Rapid decreases in preoptic aromatase activity and brain monoamine concentrations after engaging in male sexual behavior. Endocrinology. 2005;146:3809–3820. doi: 10.1210/en.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF. Fast glucocorticoid actions on brain: back to the future. Front Neuroendocrinol. 2005;26:103–108. doi: 10.1016/j.yfrne.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Dufy B, Vincent JD, Fleury H, Du Pasquier P, Gourdji D, Tixier-Vidal A. Dopamine-inhibition of action potentials in a prolactin secreting cell-line is modulated by estrogen. Nature. 1979;282:855–857. doi: 10.1038/282855a0. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Front Neuroendocrinol. 2008;29:169–181. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune ES, Margoliash D. Parallel pathways and convergence onto HVc and adjacent neostriatum of adult zebra finches (Taeniopygia guttata) J Comp Neurol. 1995;360:413–441. doi: 10.1002/cne.903600305. [DOI] [PubMed] [Google Scholar]
- Frick KM. Building a better hormone therapy? How understanding the rapid effects of sex steroid hormones could lead to new therapeutics for age-related memory decline. Behav Neurosci. 2012;126:29–53. doi: 10.1037/a0026660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH, Bentley GE, Ball GF. Individual vocal recognition and the effect of partial lesions to HVc on discrimination, learning, and categorization of conspecific song in adult songbirds. J Neurobiol. 2000;42:117–133. doi: 10.1002/(sici)1097-4695(200001)42:1<117::aid-neu11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH, Ball GF. Functional differences in forebrain auditory regions during learned vocal recognition in songbirds. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004;190:1001–1010. doi: 10.1007/s00359-004-0556-x. [DOI] [PubMed] [Google Scholar]
- Green D, Swets J. Signal detection theory and psychophysics. New York: Wiley; 1966. [Google Scholar]
- Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat Rev Neurosci. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017 alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janata P, Margoliash D. Gradual emergence of song selectivity in sensorimotor structures of the male zebra finch song system. J Neurosci. 1999;19:5108–5118. doi: 10.1523/JNEUROSCI.19-12-05108.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanne JM, Thompson JV, Sharpee TO, Gentner TQ. Emergence of learned categorical representations within an auditory forebrain circuit. J Neurosci. 2011;31:2595–2606. doi: 10.1523/JNEUROSCI.3930-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JK, Burrows K, Tremere LA, Pinaud R. Neurochemical organization and experience-dependent activation of estrogen-associated circuits in the songbird auditory forebrain. Eur J Neurosci. 2011;34:283–291. doi: 10.1111/j.1460-9568.2011.07743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M. Steroid hormones and excitability in the mammalian brain. Front Neuroendocrinol. 1997;18:2–48. doi: 10.1006/frne.1996.0144. [DOI] [PubMed] [Google Scholar]
- Keller GB, Hahnloser RH. Neural processing of auditory feedback during vocal practice in a songbird. Nature. 2009;457:187–190. doi: 10.1038/nature07467. [DOI] [PubMed] [Google Scholar]
- Korsia S, Bottjer SW. Chronic testosterone treatment impairs vocal learning in male zebra finches during a restricted period of development. J Neurosci. 1991;11:2362–2371. doi: 10.1523/JNEUROSCI.11-08-02362.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J Neurosci. 2010;30:12950–12957. doi: 10.1523/JNEUROSCI.1158-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblois A, Wendel BJ, Perkel DJ. Striatal dopamine modulates basal ganglia output and regulates social context-dependent behavioral variability through D1 receptors. J Neurosci. 2010;30:5730–5743. doi: 10.1523/JNEUROSCI.5974-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London SE, Monks DA, Wade J, Schlinger BA. Widespread capacity for steroid synthesis in the avian brain and song system. Endocrinology. 2006;147:5975–5987. doi: 10.1210/en.2006-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, Fee MS. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature. 2008;456:189–194. doi: 10.1038/nature07448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav Neurosci. 2012;126:4–16. doi: 10.1037/a0026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Moore IT, Lent K, Brenowitz EA, Perkel DJ. Steroid hormones act transsynaptically within the forebrain to regulate neuronal phenotype and song stereotypy. J Neurosci. 2007;27:12045–12057. doi: 10.1523/JNEUROSCI.3289-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Eckersell CB, Holland K, Smith A. Induction of CCK mRNA levels in the limbic-hypothalamic circuit: time course and site-specific effects of estrogen. J Neurobiol. 1996;30:465–479. doi: 10.1002/(SICI)1097-4695(199608)30:4<465::AID-NEU3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Mooney R. Different subthreshold mechanisms underlie song selectivity in identified HVc neurons of the zebra finch. J Neurosci. 2000;20:5420–5436. doi: 10.1523/JNEUROSCI.20-14-05420.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R. Neural mechanisms for learned birdsong. Learn Mem. 2009;16:655–669. doi: 10.1101/lm.1065209. [DOI] [PubMed] [Google Scholar]
- Mooney R, Prather JF. The HVC microcircuit: the synaptic basis for interactions between song motor and vocal plasticity pathways. J Neurosci. 2005;25:1952–1964. doi: 10.1523/JNEUROSCI.3726-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J. Aromatase immunoreactivity in axon terminals of the vertebrate brain. An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinology. 1996;63:149–155. doi: 10.1159/000126951. [DOI] [PubMed] [Google Scholar]
- Nick TA, Konishi M. Neural auditory selectivity develops in parallel with song. J Neurobiol. 2005a;62:469–481. doi: 10.1002/neu.20115. [DOI] [PubMed] [Google Scholar]
- Nick TA, Konishi M. Neural song preference during vocal learning in the zebra finch depends on age and state. J Neurobiol. 2005b;62:231–242. doi: 10.1002/neu.20087. [DOI] [PubMed] [Google Scholar]
- Pasricha N, Joëls M, Karst H. Rapid effects of corticosterone in the mouse dentate gyrus via a nongenomic pathway. J Neuroendocrinol. 2011;23:143–147. doi: 10.1111/j.1365-2826.2010.02091.x. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc Biol Sci. 2005;272:2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan ML, Pytte CL, Vicario DS. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proc Natl Acad Sci U S A. 2006;103:1088–1093. doi: 10.1073/pnas.0510136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather JF, Peters S, Nowicki S, Mooney R. Precise auditory–vocal mirroring in neurons for learned vocal communication. Nature. 2008;451:305–310. doi: 10.1038/nature06492. [DOI] [PubMed] [Google Scholar]
- Rauske PL, Shea SD, Margoliash D. State and neuronal class-dependent reconfiguration in the avian song system. J Neurophysiol. 2003;89:1688–1701. doi: 10.1152/jn.00655.2002. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Plasticity in brain sexuality is revealed by the rapid actions of steroid hormones. J Neurosci. 2007;27:1114–1122. doi: 10.1523/JNEUROSCI.4282-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Estradiol interacts with an opioidergic network to achieve rapid modulation of a vocal pattern generator. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010;196:137–146. doi: 10.1007/s00359-009-0500-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, London SE, Schlinger BA. Birdsong and the neural production of steroids. J Chem Neuroanat. 2010a;39:72–81. doi: 10.1016/j.jchemneu.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci U S A. 2010b;107:3852–3857. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong S, Maidment NT, Schlinger BA. Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. J Neurosci. 2011;31:10034–10038. doi: 10.1523/JNEUROSCI.0566-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong SM, Chao A, Schlinger BA. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. J Neurophysiol. 2012;107:1621–1631. doi: 10.1152/jn.00749.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha CJ, Coomaralingam L. Overlap and co-expression of estrogen synthetic and responsive neurons in the songbird brain: a double-label immunocytochemical study. Gen Comp Endocrinol. 2005;141:66–75. doi: 10.1016/j.ygcen.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol. 2000;423:619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Schlinger B, Brenowitz EA. Neural and hormonal control of birdsong. In: Pfaff DW, editor. Hormones, brain and behavior. Amsterdam: Elsevier; 2008. pp. 897–941. [Google Scholar]
- Siegel S, Castellan NJ. Nonparametric statistics for the behavioral sciences. New York: Mcgraw-Hill; 1988. [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. Reversal of neurosteroid effects at alpha4beta2delta GABAA receptors triggers anxiety at puberty. Nat Neurosci. 2007;10:469–477. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizemore M, Perkel DJ. Premotor synaptic plasticity limited to the critical period for song learning. Proc Natl Acad Sci U S A. 2011;108:17492–17497. doi: 10.1073/pnas.1104255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Aoki C, Shen H. Puberty, steroids and GABA(A) receptor plasticity. Psychoneuroendocrinology. 2009;34(Suppl 1):S91–S103. doi: 10.1016/j.psyneuen.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. New York: Freeman; 1995. [Google Scholar]
- Solis MM, Doupe AJ. Anterior forebrain neurons develop selectivity by an intermediate stage of birdsong learning. J Neurosci. 1997;17:6447–6462. doi: 10.1523/JNEUROSCI.17-16-06447.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Liu F, Brandon NJ, Penzes P. Estrogen receptor β activity modulates synaptic signaling and structure. J Neurosci. 2010;30:13454–13460. doi: 10.1523/JNEUROSCI.3264-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere LA, Pinaud R. Brain-generated estradiol drives long-term optimization of auditory coding to enhance the discrimination of communication signals. J Neurosci. 2011;31:3271–3289. doi: 10.1523/JNEUROSCI.4355-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere LA, Jeong JK, Pinaud R. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J Neurosci. 2009;29:5949–5963. doi: 10.1523/JNEUROSCI.0774-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere LA, Terleph TA, Jeong JK, Pinaud R. Bilateral multielectrode neurophysiological recordings coupled to local pharmacology in awake songbirds. Nat Protoc. 2010;5:191–200. doi: 10.1038/nprot.2009.224. [DOI] [PubMed] [Google Scholar]
- Volman SF. Development of neural selectivity for birdsong during vocal learning. J Neurosci. 1993;13:4737–4747. doi: 10.1523/JNEUROSCI.13-11-04737.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SA, Livingston FS, Mooney R. Androgens modulate NMDA receptor-mediated EPSCs in the zebra finch song system. J Neurophysiol. 1999;82:2221–2234. doi: 10.1152/jn.1999.82.5.2221. [DOI] [PubMed] [Google Scholar]
- Wong M, Moss RL. Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. J Neurosci. 1992;12:3217–3225. doi: 10.1523/JNEUROSCI.12-08-03217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Yazaki-Sugiyama Y, Mooney R. Sequential learning from multiple tutors and serial retuning of auditory neurons in a brain area important to birdsong learning. J Neurophysiol. 2004;92:2771–2788. doi: 10.1152/jn.00690.2004. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Zheng P. Neuroactive steroid regulation of neurotransmitter release in the CNS: action, mechanism and possible significance. Prog Neurobiol. 2009;89:134–152. doi: 10.1016/j.pneurobio.2009.07.001. [DOI] [PubMed] [Google Scholar]