Abstract
Aims
To determine how excessive daytime sleepiness (EDS) and impaired cognition contribute to health-related quality of life (HRQL) in heart failure (HF).
Methods and results
Adults with chronic HF were enrolled into a prospective cohort study. Data were obtained from 280 subjects enrolled from three sites in the northeastern USA; 242 completed the 6-month study. At baseline, cohorts with and without EDS were identified using the Epworth Sleepiness Scale. Each EDS group was further subdivided into those with and without impaired cognition using a battery of five neuropsychological tests. Two disease-specific measures, the Kansas City Cardiomyopathy Questionnaire (KCCQ) and the Functional Outcomes of Sleep Questionnaire (FOSQ), were used to measure HRQL. General linear modelling of square-transformed variables was used to test the hypothesis that cohort membership was a significant predictor of HRQL. At 6 months the remaining sample was 62.5 [standard deviation (SD) 12] years old, mostly male (63%), white (65%), and functionally compromised [72% New York Heart Association (NYHA) class III/IV]. The cohort with both EDS and impaired cognition had the lowest KCCQ overall summary score (60.5 ± 22.5) compared with the cohort without EDS or impaired cognition (74.6 ± 17.4, P ≤ 0.001). A similar effect was seen on the FOSQ (16.0 ± 2.8 vs. 18.5 ± 2.2, P < 0.001).
Conclusion
Impaired cognition alone did not explain poor HRQL, but the addition of EDS poses a significant risk for poor HRQL. Interventions designed to influence EDS may improve HRQL in this population.
Keywords: Heart failure, Quality of life, Sleep, Cognition
Introduction
Poor health-related quality of life (HRQL) is more common in adults with heart failure (HF) than in age- and gender-matched controls.1 Individuals with HF have been noted to have worse HRQL than those with cancer or other serious illnesses.2 In advanced HF, HRQL that fails to improve within 1 month after hospital discharge predicts a shortened time to subsequent hospitalization or death.3
A variety of factors have been identified as being associated with the poor HRQL of HF, including symptom burden and cognitive impairment.4 Although poor sleep quality has been shown to be significantly correlated with impairments in HRQL in other patient populations,5 it has only recently been recognized as a contributor to poor HRQL in HF. Redeker and Hilkert found that self-reported sleep quality and lack of sleep continuity were associated with impaired HRQL in stable systolic HF patients.6 In another study, HF patients who had difficulties maintaining sleep, initiating sleep, and early morning awakenings had reduced HRQL.7
Numerous individual contributors to poor HRQL have been identified, but these contributors rarely occur in isolation. A recent study explored the contribution of impaired cognition to poor HRQL in adults with HF.8 They found that HF severity, age, depressive symptoms, and total recall memory explained 55% of the variance in HRQL, but the individual contribution of memory to the amount of variance in HRQL explained was minimal (1%). However, in previous work, we found that impaired cognition was commonly associated with poor sleep in adults with HF.9 Therefore, the purpose of this study was to determine how excessive daytime sleepiness (EDS) and impaired cognition together contribute to poor HRQL in HF.
Background
Heart failure patients commonly report difficulties initiating and maintaining sleep.7 A majority report poor sleep quality,10 and many have poor sleep continuity.11 Half of HF patients report insomnia-related symptoms, including EDS.12 In one sample, the most frequently reported problems were inability to sleep flat (51%), restless sleep (44%), trouble falling asleep (40%), and awakening early (39%).13
Numerous factors impair sleep and cause EDS, including stress,14 insomnia,12 and nocturia. Ageing assuredly contributes; as we age, sleep becomes fragmented, with an increase in superficial sleep [non-rapid eye movement (NREM)] stage 1 and 2 and a decrease in deep, slow wave sleep (NREM stage 3 and 4) and REM sleep. In adults with HF, sleep-disordered breathing is common but not consistently associated with either EDS or poor HRQL.15 Regardless of the cause, poor sleep dulls cognitive processing, impairing information processing, memory, vigilance, judgement, motivation, and decision-making.16 The impact varies depending on the severity of the sleep interruption. However, even modest sleep restriction (6 h/night) causes clear impairment in cognition after 7 days; mild sleep fragmentation (e.g. nocturia) takes longer to impair cognition.17
Methods
This was a pre-planned analysis from a prospective cohort comparison study. The methods used in this study have been described in detail previously and are summarized here.18 Subjects were enrolled from three sites in the northeastern USA. Institutional review board approval was obtained at each site and all subjects gave informed consent. Data were obtained at enrolment, and at 3 and 6 months during face-to-face visits. In all, 280 subjects were enrolled and 242 completed the 6-month follow-up. Reasons for attrition included death (n = 6), too ill to continue (n = 7), refusal to continue or withdrawal (n = 5), and loss to follow-up (n = 20).
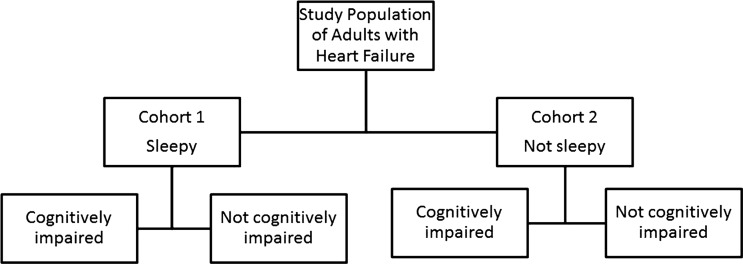
A prospective cohort design allowed us to have well-characterized cohorts for testing hypotheses about the effects of EDS and impaired cognition on HRQL. At baseline we identified a cohort with EDS and a control group without EDS. These groups were further divided into those with and without cognitive impairment, as described below. The result was four groups or cohorts for comparison (Figure 1). We hypothesized that cohort membership at enrolment would be a significant predictor of HRQL 6 months later.
Figure 1.
Graphic representation of the cohort allocation.
Sample
We enrolled adults with chronic HF confirmation based on echocardiographic and clinical evidence. All participants had to be currently or previously symptomatic (stage C HF). Potential subjects had to be able to participate in the study (i.e. adequate vision, hearing, and English literacy). The Telephone Interview for Cognitive Status (TICS) was used to ensure that we did not enrol anyone with frank dementia; anyone with a TICS score below 24 was excluded.19 Otherwise eligible individuals were excluded if they lived in a long-term care setting or worked nights or rotating shifts, or if they had renal failure requiring dialysis, an imminently terminal illness, plans to move out of the area, or a history of serious drug or alcohol abuse within the past year. Individuals with major depression were excluded because EDS is a prominent symptom of depression so it would be difficult to separate out the two problems. Major depressive illness was screened first by a review of the medical record; patients identified as having major depressive illness were not invited to participate. In addition, everyone was screened with the 9-item Patient Health Questionnaire (PHQ-9).20 Anyone reporting five or more of the nine symptoms on more than half of the days in the past 2 weeks was excluded if one of the symptoms was depressed mood or anhedonia. Most of the data were collected during home visits and by abstraction from the medical record.
Measurement
Excessive daytime sleepiness was measured using the Epworth Sleepiness Scale.21 Respondents rate the likelihood of falling asleep in eight boring situations, such as sitting in a car as a passenger, using a 4-point Likert scale ranging from never dozing (0) to high chance of dozing (3). Test–re-test reliability (r = 0.82) and internal consistency (α = 0.88) have been established in addition to its single factor structure. Scores are summed, with higher scores indicating higher sleepiness, or categorized as sleepy or not sleepy. At a cut-off point of 11, the Epworth Sleepiness Scale has a sensitivity of 93.5% and a specificity of 100% for distinguishing pathological from normal sleepiness. However, adults with HF do not report EDS as commonly as others,22 perhaps because of the sympathetic stimulation associated with HF. So, to make sure that we capture some variability in EDS, we used a cut-off point score ≥6 to indicate EDS on the advice of the instrument author.
A battery of five neuropsychological tests measuring attention, memory, and executive function was administered at enrolment and used to allocate participants to cohorts.23 The battery included a test of simple attention (Psychomotor Vigilance Task), complex attention (Trail Making Test B), processing speed (Digit Symbol Substitution Test), working memory (Probed Recall Memory Task), and short-term memory (Letter Number Sequencing test). The number of tests on which subjects scored below their age-based norm was used as the measure of cognitive status. Specifically, anyone scoring >1.5 standard deviations (SDs) on two or more of the cognition tests was judged to have impaired cognition.24
Health-related quality of life was measured with two disease-specific measures, the Kansas City Cardiomyopathy Questionnaire (KCCQ) and the Functional Outcomes of Sleep Questionnaire (FOSQ). The KCCQ is a 23-item health status measure of physical limitation, symptom frequency, severity, and change over time, overall quality of life, social interference, and self-efficacy.25 Internal consistency of the instrument ranges from 0.78 to 0.95 for all domains except self-efficacy (α = 0.62). Higher scores indicate better HRQL.
The FOSQ is a 30-item self-report measure designed to assess the impact of EDS on HRQL.26 The FOSQ has established content validity, test–re-test reliability (r = 0.91), and internal consistency (α = 0.96). Higher scores indicate better HRQL.
Socio-demographic characteristics were self-reported at enrolment. Most clinical information (e.g. co-morbid illnesses, HF type and duration) was gathered from the medical record. Co-morbidity was scored using the Charlson Index; higher scores indicate more co-morbid illnesses.27 To decrease subject burden, depression was measured over time using the short form of the Patient Health Questionnaire (PHQ-2). Information on symptoms in response to activities was gathered by trained research assistants using a structured interview.28 A single cardiologist used the interview data to assign a New York Heart Association (NYHA) score for every subject. Length of time with HF was based on information collected from a historical search of each subject's medical record.
Data on sleep-disordered breathing were obtained from the medical record at the time of enrolment. If no documentation of recent polysomnography was found in the medical record, sleep was assessed in the home using Embletta (Medcare, Buffalo, NY, USA), a sensitive and specific screening device useful in quantifying the apnoea–hypopnoea index (AHI) in persons with suspected sleep-disordered breathing.29 Embletta data were scored by expert technicians. An AHI ≥5 was used to classify subjects as having sleep-disordered breathing.30 In addition, during each home visit, medicines were noted and later categorized to identify those known to cause daytime somnolence (e.g. sedative hypnotics).
Analysis
Graphical techniques were used to assess the distributional assumptions of the KCCQ overall summary score, the FOSQ total score, and the subscales of both measures. Transformations to normality were applied as necessary. Specifically, the KCCQ overall summary score and the FOSQ total score were squared in order to meet distribution assumptions for modelling. Linear mixed effects models were used to model the square-transformed total scores on both scales and transformed subscales that were approximately normally distributed. Back-transformed estimates were also calculated in order to interpret the effects on the original scale scores. Non-normal subscales were modelled via generalized estimating equations with an ordered, cumulative logit link. The primary analysis examined the cohort differences in the outcome scores. Other potential covariates such as NHYA class, income, depressive symptoms, perceived health, and medication adherence were included in the model via a backward stepwise technique. All models were adjusted for age, race, income, gender, data collection occasion, and data collection site. Analyses were performed using SAS 9.2.
Results
Descriptive details of the sample are shown in Table 1. Overall, the sample was predominantely male, white, well-educated, and financially comfortable. Most participants were overweight and functionally compromised. Significant demographic differences were evident among the cohorts defined by the presence or absence of cognitive impairment and EDS. The two cohorts with cognitive impairment were older, less likely to be white, more likely to have less than a high school education, and less likely to be employed than either cohort without cognitive impairment. The cohort with the highest perceived overall health had EDS but no cognitive impairment. The number of co-morbid illnesses was highest in those with cognitive impairment. Depression was highest in the cohort with both EDS and impaired cognition. No differences in the prevalence of sleep-disordered breathing or the number of medications known to cause daytime somnolence were identified. Table 2 describes how the HRQL total scores and subscales changed over the course of the 6-month study. Overall, HRQL scores improved from baseline to study completion among patients who completed the study.
Table 1.
Characteristics of the sample at 6 months by enrolment: sleepy and cognitive impairment cohorts
| Overall (n = 242) | +EDS +CI (n = 69) | +EDS no CI (n = 67) | No EDS + CI (n = 51) | No EDS no CI (n = 55) | P-valuea | |
|---|---|---|---|---|---|---|
| Age (years) | 62.5 ± 11.9 | 62.9 ± 12.5 | 60.4 ± 11.5 | 67.1 ± 10.8 | 60.4 ± 11.7 | 0.009 |
| Male | 152 (62.8) | 48 (69.6) | 35 (52.2) | 33 (64.7) | 36 (65.5) | 0.187 |
| Race/ethnicity | 0.016 | |||||
| White | 157 (64.9) | 39 (56.5) | 50 (74.6) | 27 (52.9) | 41 (74.6) | |
| Black | 77 (31.8) | 28 (40.6) | 15 (22.4) | 20 (39.2) | 14 (25.4) | |
| Otherb | 8 (3.3) | 2 (2.9) | 2 (3.0) | 4 (7.8) | 0 (0.0) | |
| Education | 0.011 | |||||
| Less than high school | 23 (9.5) | 8 (11.6) | 3 (4.5) | 10 (19.6) | 2 (3.6) | |
| High school | 80 (33.1) | 22 (31.9) | 23 (34.3) | 21 (41.2) | 14 (25.5) | |
| Some college | 139 (57.4) | 39 (56.5) | 41 (61.2) | 20 (39.2) | 39 (70.9) | |
| Body mass index | 31.2 ± 12.0 | 30.1 ± 6.0 | 31.6 ± 9.2 | 33.0 ± 21 | 30.2 ± 8.0 | 0.553 |
| Financial status | 0.281 | |||||
| More than enough to make ends meet | 86 (35.5) | 22 (31.9) | 26 (38.8) | 21 (41.2) | 17 (30.9) | |
| Enough to make ends meet | 119 (49.2) | 35 (50.7) | 28 (41.8) | 22 (43.1) | 34 (61.8) | |
| Not enough to make ends meet | 37 (15.3) | 12 (17.4) | 13 (19.4) | 8 (15.7) | 4 (7.3) | |
| Employment status | 0.001 | |||||
| Employed | 68 (28.1) | 11 (15.9) | 28 (41.8) | 12 (23.5) | 17 (30.9) | |
| Disability/unemployed | 59 (24.4) | 26 (37.7) | 15 (22.4) | 5 (9.8) | 13 (23.6) | |
| Retired/homemaker | 115 (47.5) | 32 (46.4) | 24 (35.8) | 34 (66.7) | 25 (45.5) | |
| Perceived overall health | 0.002 | |||||
| Excellent/very good/good | 116 (47.9) | 25 (36.2) | 45 (67.2) | 22 (43.1) | 24 (43.6) | |
| Fair/poor | 126 (52.1) | 44 (63.8) | 22 (32.8) | 29 (56.9) | 31 (56.4) | |
| KCCQ Overall Summary Score | 69.4 ± 20.3 | 60.5 ± 22.5 | 72.8 ± 18.5 | 72.5 ± 17.7 | 74.6 ± 17.4 | <0.001 |
| FOSQ Total score | 17.4 ± 2.6 | 16.0 ± 2.8 | 17.6 ± 2.2 | 18.2 ± 2.1 | 18.5 ± 2.2 | <0.001 |
| Number of co-morbid conditions | 3.1 ± 2.1 | 3.5 ± 2.1 | 2.5 ± 2.1 | 3.8 ± 2.2 | 2.8 ± 1.7 | 0.003 |
| Patient Health Questionnaire (PHQ2) | 0.81 ± 1.28 | 1.2 ± 1.5 | 0.8 ± 1.2 | 0.5 ± 1.2 | 0.6 ± 1.0 | 0.022 |
| NYHA functional class | 0.083 | |||||
| Classes I and II | 86 (28.1) | 11 (15.9) | 20 (29.9) | 16 (31.4) | 21 (38.2) | |
| Class III | 143 (59.1) | 44 (63.8) | 39 (58.2) | 31 (60.8) | 29 (52.7) | |
| Class IV | 31 (12.8) | 14 (20.3) | 8 (11.9) | 4 (7.8) | 5 (9.1) | |
| Months with heart failure at study entry | 75.2 ± 73.7 | 66.0 ± 58.6 | 70.1 ± 62.3 | 70.8 ± 70.3 | 98.0 ± 101 | 0.373 |
| Sleep-disordered breathing | 137 (56.6) | 39 (56.5) | 41 (61.2) | 27 (52.9) | 30 (54.5) | 0.84 |
| Number of medicines known to cause daytime somnolence | 1.7 ± 1.1 | 1.8 ± 1.3 | 1.7 ±1.2 | 1.6 ± 1.0 | 1.5 ± 1.0 | 0.65 |
Mean ± standard deviation or n (%) are reported.
CI, cognitive impairment; EDS, excessive daytime sleepiness; FOSQ, Functional Outcomes of Sleep Questionnaire; KCCQ, Kansas City Cardiomyopathy Questionnaire; NYHA, New York Heart Association.
aComparison of groups via analysis of variance, Kruskal–Wallis, or χ2 tests.
bOther category combined with Black for analysis.
Table 2.
Mean ± standard deviation for health-related quality of life total scores and subscales over time
| Scale | Enrolment (n = 280) | 3 Months (n = 238) | 6 Months (n = 242) | P-valuea |
|---|---|---|---|---|
| KCCQ Overall Summary Score | 68.9 ± 21.5 | 72.8 ± 20.4 | 73.1 ± 20.5 | 0.036 |
| KCCQ Physical limitation score | 69.2 ± 23.8 | 72.7 ± 22.1 | 72.5 ± 23.2 | 0.194 |
| KCCQ Symptom frequency score | 74.4 ± 22.5 | 77.3 ± 21.6 | 78.0 ± 21.7 | 0.070 |
| KCCQ Symptom burden score | 73.6 ± 23.2 | 78.0 ± 20.7 | 78.6 ± 21.4 | 0.018 |
| KCCQ Symptom stability score | 57.5 ± 22.3 | 56.3 ± 20.4 | 51.4 ± 21.6 | 0.003 |
| KCCQ Total symptom score | 74.0 ± 22.0 | 77.7 ± 20.3 | 78.3 ± 20.4 | 0.038 |
| KCCQ Quality of life score | 65.1 ± 25.1 | 69.4 ± 24.3 | 71.4 ± 22.1 | 0.016 |
| KCCQ Social limitation score | 66.0 ± 28.2 | 70.8 ± 26.9 | 69.7 ± 28.0 | 0.117 |
| KCCQ Clinical summary score | 71.8 ± 20.9 | 75.2 ± 19.6 | 75.5 ± 20.2 | 0.056 |
| KCCQ Self-efficacy score | 87.5 ± 17.0 | 91.4 ± 12.3 | 90.3 ± 13.5 | 0.080 |
| FOSQ Total score | 17.3 ± 2.8 | 17.9 ± 2.4 | 17.8 ± 2.4 | 0.040 |
| FOSQ General productivity subscale score | 3.6 ± 0.6 | 3.7 ± 0.5 | 3.7 ± 0.5 | 0.400 |
| FOSQ Social outcome subscale score | 3.7 ± 0.6 | 3.8 ± 0.6 | 3.8 ± 0.5 | 0.010 |
| FOSQ Activity level subscale score | 3.3 ± 0.7 | 3.4 ± 0.7 | 3.4 ± 0.7 | 0.035 |
| FOSQ Vigilance subscale score | 3.4 ± 0.8 | 3.6 ± 0.6 | 3.6 ± 0.6 | 0.018 |
| FOSQ Intimacy subscale score | 4.2 ± 2.1 | 4.5 ± 2.1 | 4.6 ± 2.3 | 0.022 |
FOSQ, Functional Outcomes of Sleep Questionnaire; KCCQ, Kansas City Cardiomyopathy Questionnaire.
aKrusal–Wallis test for changes over time.
The final model for KCCQ overall summary scores across all visits is shown in Table 3. The two cohorts with EDS had significantly lower KCCQ overall scores compared with the cohort without either EDS or cognitive impairment; on average, the cognitively impaired EDS cohort had a 33 point lower KCCQ score (P < 0.001) and the non-impaired EDS cohort had a 25 point lower KCCQ score (P = 0.030). In addition, poor functional class moderated the relationship between cohort membership and HRQL. Subjects in NYHA classes III and IV had, on average, 36 and 49 point lower KCCQ total scores than a comparable subject in class I or II (both P < 0.001), based on a total possible score of 100. There were no significant interactions with cohorts or over time. In results not shown in the table, the cohort with both EDS and impaired cognition had significantly worse KCCQ subscale scores compared with the cohort without EDS or impaired cognition (P < 0.05), while the cohort with only EDS had significantly worse physical limitation scores (P = 0.036).
Table 3.
Final model for the Kansas City Cardiomyopathy Questionnaire overall summary score
| Variable | B estimate for transformed outcome | 95 % CI | B estimate on original scale | P-value |
|---|---|---|---|---|
| Cohort | 0.002 | |||
| Cognitively impaired + /sleepy + | –1066.8 | –1656.7 to –476.8 | –32.7 | <0.001 |
| Cognitively impaired–/sleepy + | –626.0 | –1216.6 to –35.5 | –25.0 | 0.038 |
| Cognitively impaired + /sleepy– | –254.8 | –892.8 to 383.2 | –16.0 | 0.432 |
| Cognitively impaired–/sleepy– | (ref) | |||
| NYHA functional class | <0.001 | |||
| Class I–II | (ref) | |||
| Class III | –1306.6 | –1602.3 to –1011.0 | –36.1 | <0.001 |
| Class IV | –2411.2 | –2858.0 to –1964.4 | –49.1 | <0.001 |
Model adjusted for age, race, income, gender, data collection occasion, depressive symptoms, perceived health, visit occasion, and data collection site.
Square-transformed Kansas City Cardiomyopathy Questionnaire total score was modelled.
CI, confidence interval; NYHA, New York Heart Association.
The FOSQ total score final model across all visits is shown in Table 4. Based on a total possible score of 20, the two cohorts with EDS had 8 and 7 point lower FOSQ total scores, respectively, compared with a similar subject in the cohort without EDS or impaired cognition (both P < 0.001). Again, NYHA moderated the relationship between cohort membership and HRQL; subjects in NYHA classes III and IV had, on average, 3 and 5 point lower scores than a comparable subject in class I or II (P = 0.017 and P < 0.001, respectively). There were no significant interactions with cohorts or over time. For the FOSQ subscale scores, both cohorts with EDS had significantly higher general productivity (with cognitive impairment, P < 0.001; without cognitive impairment, P = 0.016), activity level (both P < 0.001), and vigilance (both P < 0.001) subscale scores (results not shown) compared with the cohort without either EDS or cognitive impairment.
Table 4.
Final model for the Functional Outcomes of Sleep Questionnaire total score
| Variable | B estimate for transformed outcome | 95% CI | B estimate on original scale | P-value |
|---|---|---|---|---|
| Cohort | <0.001 | |||
| Cognitively impaired + /sleepy + | –62.7 | –83.3 to –42.2 | –7.9 | <0.001 |
| Cognitively impaired–/sleepy + | –44.8 | –65.2 to –24.4 | –6.7 | <0.001 |
| Cognitively impaired + /sleepy– | –14.4 | –36.6 to 7.8 | –3.8 | 0.202 |
| Cognitively impaired–/sleepy– | (ref) | |||
| NYHA functional class | 0.001 | |||
| Class I–II | (ref) | |||
| Class III | –11.9 | –21.3 to –2.5 | –3.4 | 0.017 |
| Class IV | –27.5 | –41.8 to –13.3 | –4.9 | <0.001 |
Model adjusted for age, race, income, gender, data collection occasion, depressive symptoms, perceived health, visit, and data collection site.
Square-transformed Functional Outcomes of Sleep Questionnaire was modelled.
CI, confidence interval; NYHA, New York Heart Association.
Discussion
Excessive daytime sleepiness contributes to HRQL, but when it is combined with cognitive impairment it is even more powerful as a predictor of poor HRQL in adults with HF. The double jeopardy of EDS and impaired cognition was especially prominent in subjects who were physically functionally impaired. These results suggest that NYHA class III and class IV patients with HF should be suspected of EDS and cognitive impairment. Also, those with cognitive impairment and/or EDS may well have poor HRQL. We found no differences in sleep-disordered breathing among the cohorts, illustrating that we cannot assume that HF patients with EDS are those with sleep apnoea. Further research is needed to determine if interventions can improve EDS and, if EDS improves, does HRQL improve as well.
These results build on those from Pressler et al. who found that impaired memory contributed only 1% of the variance in HRQL of HF patients.8 In our study, we found that impaired cognition, which includes memory, contributed significantly to HRQL but only when combined with EDS. This suggests that it is the symptom burden that affects HRQL more than changes in cognition, as suggested by Heo et al.4 Pressler et al. also found that HF severity was an important contributor to HRQL, consistent with our results showing NYHA functional class as a moderator of the relationship between cohort group and HRQL. Again, NYHA reflects symptom burden, which continues to be identified as an important determinant of poor HRQL.31,32
The finding that EDS contributes significantly to poor HRQL is consistent with previous results demonstrating that poor sleep quality impairs HRQL in adults with HF.6,7 However, neither of those investigative teams tested EDS as the symptom indicator of poor HRQL. Identifying a symptom of focus is important because screening for EDS is easy. As we showed in a previous study in which four different approaches to measuring EDS were compared, the measure of EDS that was most sensitive to daytime dysfunction was a single Likert item measured on a 10-point (1–10) scale. Patients with a score ≥4 were 2.4 times more likely to have daytime dysfunction than those with a score <4.33 Adding this single question to a screening form at clinic visits could help to identify patients needing intervention.
Few interventions for poor sleep have been tested in adults with HF, and only sleep apnoea has received any significant testing. However, results of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure (CANPAP) trial were disappointing. Bradley and colleagues tested the hypothesis that continuous positive airway pressure (CPAP) would improve survival in adults with HF and central sleep apnoea.34 After 3 months, the group randomized to CPAP had fewer episodes of apnoea and hypopnoea, lower norepinephrine levels, and greater increases in the mean nocturnal oxygen saturation, ejection fraction, and distance walked in 6 min, but long-term outcomes were not improved at 2 years. There were no differences between the control and CPAP groups in atrial natriuretic peptide levels, HRQL, or the number of hospitalizations. The CANPAP study was stopped early for low enrolment.
Few other sleep-promoting approaches have been tested in HF patients, and no other approach has been tested in anything other than a pilot study or a small clinical trial. Preliminary research suggests that exercise may improve total sleep time and HRQL;35 however, not all studies support this result.36 Further study is needed to understand the effects of exercise on sleep and the amount and type of exercise needed to have an effect. Fortunately, such a trial is underway testing the effect of a supervised exercise training programme in recently hospitalized HF patients attending a disease management programme.37
Daytime sleepiness may be easier to influence than cognitive impairment. Pressler reviewed studies of cognitive impairment in adults with HF and concluded that 25–50% of HF patients have impaired cognition.38 This impairment appears to differ from typical vascular dementia in that some studies have shown that cognition may improve over time in HF patients treated with angiotensin-converting enzyme (ACE) inhibitor therapy or aerobic exercise.39,40 Pressler et al. have had some preliminary success in improving memory in HF patients with cognitive impairment.41 HF patients were randomly assigned to a computerized plasticity-based cognitive training intervention called Brain Fitness. Compared with an active control intervention group receiving health education, the Brain Fitness participants improved significantly over time in delayed recall memory, and both groups improved over time in list learning, delayed recall memory, psychomotor speed, and performance of independent activities of daily living.
The observation that HRQL improved over time in all of the groups was surprising. Others have noted an increase in HRQL over time even without an intervention, which they attributed to passing through a high-risk period following hospitalization in one study.42 We provided no intervention, nor were our participants necessarily recently hospitalized, so the improvement may have been due to social desirability effects, better engagement in care, reactivity to the measures, or behavioural activation due to engagement in the study.
Limitations of this current study include limited generalizability, as many patients were ineligible for inclusion in the study if the cohort they qualified for was already full. This sample was also younger, better educated, and more financially comfortable than many HF patients in the general community. Strengths of the study include the relatively large proportion of minority participants and the thoroughness with which HRQL and cognitive impairment were measured.
In summary, in this study we demonstrated that EDS is associated with poor HRQL, and the combination of EDS and impaired cognition represents double jeopardy for HF patients. EDS is easily screened and could be used to identify patients at risk for poor HRQL. Testing of interventions designed to improve EDS in HF patients is needed.
Funding
The National Heart, Lung, and Blood Institute (RO1 HL084394-01A1) at the National Institutes of Health; the Philadelphia Veterans Affairs Medical Center; VISN 4 Mental Illness Research, Education, and Clinical Center (MIREC).
Conflict of interest: none declared.
Acknowledgements
The authors gratefully acknowledge Thomas A. Gillespie, MD, FACC for scoring the NYHA interviews.
References
- 1.Lesman-Leegte I, Jaarsma T, Coyne JC, Hillege HL, Van Veldhuisen DJ, Sanderman R. Quality of life and depressive symptoms in the elderly: a comparison between patients with heart failure and age- and gender-matched community controls. J Card Fail. 2009;15:17–23. doi: 10.1016/j.cardfail.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Bekelman DB, Rumsfeld JS, Havranek EP, Yamashita TE, Hutt E, Gottlieb SH, Dy SM, Kutner JS. Symptom burden, depression, and spiritual well-being: a comparison of heart failure and advanced cancer patients. J Gen Intern Med. 2009;24:592–598. doi: 10.1007/s11606-009-0931-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moser DK, Yamokoski L, Sun JL, Conway GA, Hartman KA, Graziano JA, Binanay C, Stevenson LW. Improvement in health-related quality of life after hospitalization predicts event-free survival in patients with advanced heart failure. J Card Fail. 2009;15:763–769. doi: 10.1016/j.cardfail.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heo S, Doering LV, Widener J, Moser DK. Predictors and effect of physical symptom status on health-related quality of life in patients with heart failure. Am J Crit Care. 2008;17:124–132. [PubMed] [Google Scholar]
- 5.Gooneratne NS, Dean GE, Rogers AE, Nkwuo JE, Coyne JC, Kaiser LR. Sleep and quality of life in long-term lung cancer survivors. Lung Cancer. 2007;58:403–410. doi: 10.1016/j.lungcan.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redeker NS, Hilkert R. Sleep and quality of life in stable heart failure. J Card Fail. 2005;11:700–704. doi: 10.1016/j.cardfail.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Brostrom A, Stromberg A, Dahlstrom U, Fridlund B. Sleep difficulties, daytime sleepiness, and health-related quality of life in patients with chronic heart failure. J Cardiovasc Nurs. 2004;19:234–242. doi: 10.1097/00005082-200407000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Pressler SJ, Subramanian U, Kareken D, Perkins SM, Gradus-Pizlo I, Sauve MJ, Ding Y, Kim J, Sloan R, Jaynes H, Shaw RM. Cognitive deficits and health-related quality of life in chronic heart failure. J Cardiovasc Nurs. 2010;25:189–198. doi: 10.1097/JCN.0b013e3181ca36fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riegel B, Vaughan Dickson V, Goldberg LR, Deatrick JA. Factors associated with the development of expertise in heart failure self-care. Nurs Res. 2007;56:235–243. doi: 10.1097/01.NNR.0000280615.75447.f7. [DOI] [PubMed] [Google Scholar]
- 10.Redeker NS, Stein S. Characteristics of sleep in patients with stable heart failure versus a comparison group. Heart Lung. 2006;35:252–261. doi: 10.1016/j.hrtlng.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Ferrier K, Campbell A, Yee B, Richards M, O'Meeghan T, Weatherall M, Neill A. Sleep-disordered breathing occurs frequently in stable outpatients with congestive heart failure. Chest. 2005;128:2116–2122. doi: 10.1378/chest.128.4.2116. [DOI] [PubMed] [Google Scholar]
- 12.Redeker NS, Jeon S, Muench U, Campbell D, Walsleben J, Rapoport DM. Insomnia symptoms and daytime function in stable heart failure. Sleep. 2010;33:1210–1216. doi: 10.1093/sleep/33.9.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erickson VS, Westlake CA, Dracup KA, Woo MA, Hage A. Sleep disturbance symptoms in patients with heart failure. AACN Clin Issues. 2003;14:477–487. doi: 10.1097/00044067-200311000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Vgontzas AN, Bixler EO, Chrousos GP, Vgontzas AN, Bixler EO, Chrousos GP. Obesity-related sleepiness and fatigue: the role of the stress system and cytokines. Ann NY Acad Sci. 2006;1083:329–344. doi: 10.1196/annals.1367.023. [DOI] [PubMed] [Google Scholar]
- 15.Carmona-Bernal C, Ruiz-Garcia A, Villa-Gil M, Sanchez-Armengol A, Quintana-Gallego E, Ortega-Ruiz F, Baron-Esquivias G, Capote F. Quality of life in patients with congestive heart failure and central sleep apnea. Sleep Med. 2008;9:646–651. doi: 10.1016/j.sleep.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010;136:375–389. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altena E, Van Der Werf YD, Strijers RL, Van Someren EJ. Sleep loss affects vigilance: effects of chronic insomnia and sleep therapy. J Sleep Res. 2008;17:335–343. doi: 10.1111/j.1365-2869.2008.00671.x. [DOI] [PubMed] [Google Scholar]
- 18.Riegel B, Moelter ST, Ratcliffe SJ, Pressler SJ, De Geest S, Potashnik S, Fleck D, Sha D, Sayers SL, Weintraub WS, Weaver TE, Goldberg LR. Excessive daytime sleepiness is associated with poor medication adherence in adults with heart failure. J Card Fail. 2011;17:340–348. doi: 10.1016/j.cardfail.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandt J, Folstein MF. Telephone Interview for Cognitive Status. Psychological Assessment Resources, Inc; 2003. [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–381. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 22.Arzt M, Young T, Finn L, Skatrud JB, Ryan CM, Newton GE, Mak S, Parker JD, Floras JS, Bradley TD. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166:1716–1722. doi: 10.1001/archinte.166.16.1716. [DOI] [PubMed] [Google Scholar]
- 23.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th. New York: Oxford University Press; 2004. [Google Scholar]
- 24. WAIS-III Technical Manual: Harcourt Assessment Co.; 2002. [Google Scholar]
- 25.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 26.Weaver TE, Laizner AM, Evans LK, Maislin G, Chugh DK, Lyon K, Smith PL, Schwartz AR, Redline S, Pack AI, Dinges DF. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–843. [PubMed] [Google Scholar]
- 27.Charlson M, Pompei P, Ales K, MacKenzie C. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Kubo S, Schulman S, Starling R, Jessup M, Wentworth D, Burkhoff D. Development and validation of a patient questionnaire to determine New York Heart Association Classification. J Card Fail. 2004;10:228–235. doi: 10.1016/j.cardfail.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Ng SSS, Chan T, To K, Ngai J, Tung A, Ko F, Hui D. Validation of Embletta portable diagnostic system for identifying patients with suspected obstructive sleep apnoea syndrome (OSAS) Respirology. 2010;15:336–342. doi: 10.1111/j.1440-1843.2009.01697.x. [DOI] [PubMed] [Google Scholar]
- 30.Ruehland WR, Rochford PD, O'Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009;32:150–157. doi: 10.1093/sleep/32.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Opasich C, Gualco A, De Feo S, Barbieri M, Cioffi G, Giardini A, Majani G. Physical and emotional symptom burden of patients with end-stage heart failure: what to measure, how and why. J Cardiovasc Med (Hagerstown) 2008;9:1104–1108. doi: 10.2459/JCM.0b013e32830c1b45. [DOI] [PubMed] [Google Scholar]
- 32.Bekelman DB, Hutt E, Masoudi FA, Kutner JS, Rumsfeld JS. Defining the role of palliative care in older adults with heart failure. Int J Cardiol. 2008;125:183–190. doi: 10.1016/j.ijcard.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Riegel B, Hanlon AL, Zhang X, Fleck D, Sayers SL, Goldberg LR, Weintraub WS. What is the best measure of daytime sleepiness in adults with heart failure? J Am Acad Nurs Pract. 2012 doi: 10.1111/j.1745-7599.2012.00784.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradley TD, Logan AG, Kimoff RJ, Series F, Morrison D, Ferguson K, Belenkie I, Pfeifer M, Fleetham J, Hanly P, Smilovitch M, Tomlinson G, Floras JS CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–2033. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 35.Gary R, Lee SY. Physical function and quality of life in older women with diastolic heart failure: effects of a progressive walking program on sleep patterns. Prog Cardiovasc Nurs. 2007;22:72–80. doi: 10.1111/j.0889-7204.2007.05375.x. [DOI] [PubMed] [Google Scholar]
- 36.Chien CL, Lee CM, Wu YW, Chen TA, Wu YT. Home-based exercise increases exercise capacity but not quality of life in people with chronic heart failure: a systematic review. Aust J Physiother. 2008;54:87–93. doi: 10.1016/s0004-9514(08)70041-2. [DOI] [PubMed] [Google Scholar]
- 37.Mudge AM, Denaro CP, Scott AC, Atherton JJ, Meyers DE, Marwick TH, Adsett JA, Mullins RW, Suna JM, Scuffham PA, O'Rourke PK. Exercise training in recently hospitalized heart failure patients enrolled in a disease management programme: design of the EJECTION-HF randomized controlled trial. Eur J Heart Fail. 2011;13:1370–1375. doi: 10.1093/eurjhf/hfr139. [DOI] [PubMed] [Google Scholar]
- 38.Pressler SJ. Cognitive functioning and chronic heart failure: a review of the literature (2002–July 2007) J Cardiovasc Nurs. 2008;23:239–249. doi: 10.1097/01.JCN.0000305096.09710.ec. [DOI] [PubMed] [Google Scholar]
- 39.Zuccala G, Onder G, Marzetti E, Monaco MR, Cesari M, Cocchi A, Carbonin P, Bernabei R. Use of angiotensin-converting enzyme inhibitors and variations in cognitive performance among patients with heart failure. Eur Heart J. 2005;26:226–233. doi: 10.1093/eurheartj/ehi058. [DOI] [PubMed] [Google Scholar]
- 40.Tanne D, Freimark D, Poreh A, Merzeliak O, Bruck B, Schwammenthal Y, Schwammenthal E, Motro M, Adler Y. Cognitive functions in severe congestive heart failure before and after an exercise training program. Int J Cardiol. 2005;103:145–149. doi: 10.1016/j.ijcard.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 41.Pressler SJ, Therrien B, Riley PL, Chou CC, Ronis DL, Koelling TM, Smith DG, Sullivan BJ, Frankini AM, Giordani B. Nurse-enhanced memory intervention in heart failure: the MEMOIR study. J Card Fail. 2011;17:832–843. doi: 10.1016/j.cardfail.2011.06.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mulligan K, Mehta PA, Fteropoulli T, Dubrey SW, McIntyre HF, McDonagh TA, Sutton GC, Walker DM, Cowie MR, Newman S. Newly diagnosed heart failure: change in quality of life, mood, and illness beliefs in the first 6 months after diagnosis. Br J Health Psychol. 2012 doi: 10.1111/j.2044-8287.2011.02047.x. in press. [DOI] [PubMed] [Google Scholar]