Abstract
Aims
Prospective studies have shown that social isolation (i.e. lack of social contacts) predicts incident coronary heart disease (CHD), but it is unclear whether it predicts incident heart failure (HF) and what factors might mediate this association. HF patients may be more susceptible to social isolation as they tend to be older and may have disrupted social relationships due to life course factors (e.g. retirement or bereavement). We prospectively examined whether individuals with higher vs. low social isolation have a higher incidence of HF and determined whether this association is mediated by vital exhaustion.
Methods and results
We estimated incident HF hospitalization or death among 14 348 participants from Visit 2 (1990–1992) in the Atherosclerosis Risk in Communities (ARIC) study using Cox proportional hazard models which were sequentially adjusted for age, race/study community, gender, current smoking, alcohol use, and co-morbidities. We conducted mediation analyses according to the Baron and Kenny method. After a median follow-up of 16.9 person-years, 1727 (13.0%) incident HF events occurred. The adjusted hazard of incident HF was greater for those in the higher vs. low social isolation risk group (hazard ratio 1.21, 95% confidence interval 1.08–1.35). Our data suggest that vital exhaustion strongly mediates the association between higher social isolation and incident HF (the percentage change in beta coefficient for higher vs. low social isolation groups after adjusting for vital exhaustion was 36%).
Conclusion
These data suggest that greater social isolation is an independent risk factor for incident HF, and this association appears to be strongly mediated by vital exhaustion.
Keywords: Social isolation • Heart failure • Psychological distress • Prospective cohort study
Introduction
Heart failure (HF) is a complex disease which represents the final common pathway for many diseases of the heart. HF affects ∼6 million Americans, with US$37 billion in direct costs.1 It is a major cause of morbidity and mortality and thus is considered by policymakers as a high priority condition.2–5 As the population ages and therapeutic advances result in greater survival after acute cardiac events, the incidence of HF will continue to rise.6
Social relationships affect the aetiology and prognosis of cardiovascular diseases (CVDs),7 and extant research often operationalizes social relationships through the concepts of social support and integration. Social support refers to the quantity and quality of resources (e.g. emotional, tangible, and informational) available through social interactions with others. Social integration reflects the number and types of social ties to others (e.g. marital status, amount of family and friend contact, and group membership). Although related, social support and integration are distinct concepts with only moderate correlations.8 Social integration is thought to protect or enhance health by providing a source of generalized positive affect which reduces psychological distress, enhances one's motivation for self-care, suppresses neuroendocrine responses, and enhances immune function.9 Conversely, lack of social integration (i.e. social isolation) is considered a ‘stressor’, inducing a negative psychological state which can increase neuroendocrine responses, suppress immune function, and interfere with performance of health-promoting behaviours. Over time, social isolation may lead to vital exhaustion, which is defined by excessive fatigue, feelings of demoralization, and increased irritability, and is considered an adaptation to prolonged psychological distress.10 Vital exhaustion is conceptually related to depression, and some studies report a correlation coefficient as high as 0.76 between measures of vital exhaustion and depression.11 However, guilt and low self-esteem—key features of depression—are largely absent among exhausted persons.12 Previous studies show a positive association between vital exhaustion and incident cardiac events.13–16
Social support and social networks have also been shown to predict incident coronary heart disease (CHD) in five of eight long-term prospective studies reviewed.17 Although important, CHD causes only about half of the cases of incident HF in the general population under age 75,18 so it is worthwhile also to examine whether social relationships predict incident HF. However, few studies have specifically examined the association between social isolation and incident HF even though HF patients tend to be older and may have disrupted social relationships due to life-course factors, such as retirement and bereavement. Further, little is known about the potential mediators of this association. Vital exhaustion is one potential mediator of the association between social isolation and incident HF. Therefore, the objectives of this study were to: (i) prospectively examine whether individuals at higher vs. low risk for social isolation have higher HF incidence in the Atherosclerosis Risk in Communities (ARIC) study; and (ii) to determine whether the association between social isolation and incident HF is mediated by vital exhaustion.
Methods
The ARIC study is a prospective cohort study involving 15 792 adults aged 45–64 years at the baseline visit (1987–1989). Participants were recruited through population-based probability sampling from four US communities: Washington County, MD; suburban Minneapolis, MN; Forsyth County, NC; and Jackson, MS. Participants are contacted annually to obtain health updates and to ascertain vital status. Additionally, participants underwent three triennial re-examinations, the last occurring in 1998. Details of the ARIC study design and conduct have been previously published.19 Institutional review boards at each of the participating institutions approved the study.
Study population
Because details of social isolation and vital exhaustion were only collected at the second clinic visit (1990–1992), we drew our sample from the 14 348 participants who returned for visit 2. We excluded 1008 participants with prevalent HF prior to visit 2, which was defined as: (i) answering ‘yes’ to the question, ‘Were any of the medications you took during the last 2 weeks for HF?’; (ii) having stage 3 or ‘manifest HF’ by Gothenburg criteria or missing information needed to define Gothenburg stage;20 or (iii) having an International Classification of Diseases (ICD) 428.x code for a HF hospitalization prior to visit 2. We excluded 39 participants with ethnicity other than white or black. Additionally, we excluded 44 black participants from the Washington County and suburban Minneapolis study communities since participants were mostly white in these field centres (i.e. the majority of the black participants were enrolled from the Jackson or Forsythe County sites). We further excluded 262 participants with incomplete data on social isolation, resulting in an analytic sample of 12 995.
Incident heart failure
We defined incident HF as the first occurrence of either (i) a hospitalization which included an ICD, 9th revision (ICD-9) discharge code of 428 (428.0–428.9) in any position (n = 1206); or (ii) a death certificate with a 428 ICD-9 code or ICD, 10th revision, I50 code in any position (n = 76). Incident HF was ascertained in the period following visit 2 until the date of HF hospitalization or death, the date of last contact if the subject was lost to follow-up, the date of death from other causes, or 31 December 2008, whichever came first.
Social isolation
We measured social isolation with the 10-item Lubben Social Network Scale, a well-validated measure21,22 which assesses the size of one's active social network and one's perceived support from family, friends, and neighbours.23 The total score is an equally weighted sum, with scores ranging from 0 to 50. Higher scores indicate a greater degree of social integration and support. A score ≤20 is considered ‘socially isolated’; 21–25 indicates ‘high risk’ for isolation; 26–30 indicates ‘moderate risk’ for isolation; and scores ≥31 indicate ‘low risk’ for isolation.23 We examined the distribution of scores for each of these four categories. We chose to combine the ‘socially isolated’, ‘moderate risk’, and ‘high risk’ categories into one group (i.e. moderate/high/isolated) and the ‘low’ risk category serves as the referent group.
Vital exhaustion
We assessed vital exhaustion using the 21-item Maastricht Questionnaire. We summed responses to obtain an overall vital exhaustion score which ranged from 0 to 42, with higher scores representing more exhaustion. A score of ≥14 is the suggested cut-off point for a clinical diagnosis; its Cronbach alpha has been reported as 0.89.10
Covariates
We included the following potential confounding variables in our models: demographic and behavioural characteristics, medical co-morbidities, and medication use. Race/study community, gender, educational levels, current alcohol use, and smoking status were self-reported at baseline. We defined hypertension as diastolic blood pressure of ≥90 mmHg, systolic blood pressure of ≥140 mmHg, or self-reported antihypertensive medication use during the previous 2 weeks. We further defined diabetes mellitus as self-reported history of physician-diagnosed diabetes or medication use for diabetes over the last 2 weeks, a fasting serum glucose level of ≥126 mg/dL, or non-fasting serum glucose of ≥200 mg/dL. We calculated and categorized body mass index (BMI) as weight (kg) divided by height (m) squared. CHD was defined as a history of myocardial infarction (MI), coronary revascularization procedure, coronary artery bypass surgery, or the development of any of these during follow-up. Detailed descriptions of the procedures used to ascertain cardiac events have been reported previously.19
Statistical analysis
We compared means, standard deviations (SDs), and percentages of participant characteristics overall and stratified by low and higher social isolation risk groups. We used analysis of variance and χ2 tests to examine differences in means for continuous variables or in proportions for categorical variables, respectively, by social isolation risk groups. We used Cox proportional hazards regression analyses to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between social isolation and time to incident HF after sequential adjustment for potential confounding variables. We fit three models. In Model 1, we adjusted for age, race/study community, and gender. Model 2 adjusted for variables in Model 1 plus current smoking and alcohol use. Model 3 included variables from Models 1 and 2 plus hypertension, diabetes, BMI, and prevalent CHD. We tested the proportional hazards assumption for groups of social isolation and all potential confounding variables by χ2 tests and graphical presentation based on Schoenfeld residuals. If the assumption was significantly violated with certain variables, we conducted further analyses stratified by those variables. The Kaplan–Meier method was used to estimate cumulative incidence of HF over time, stratified by social isolation group. We used the log-rank test to examine differences between the curves. We also performed a sensitivity analysis to examine whether incident CHD changed our results by adding CHD as a time-dependent variable in the proportional hazards model.
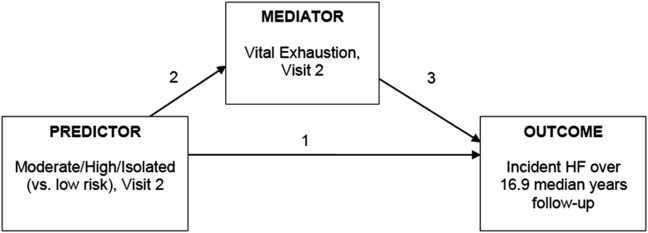
We conducted a mediation analysis using the Baron and Kenny method24 to examine whether vital exhaustion mediated the association between social isolation and incident HF (Figure 1). Mediation is commonly used in social psychology research and represents the generative mechanism through which the focal independent variable is able to influence the dependent variable of interest.24 In our analysis, vital exhaustion must meet the following criteria to be considered a mediator (Figure 1): (i) variations in the levels of the predictor variable (social isolation) significantly accounted for variations in the presumed mediator (vital exhaustion); (ii) variation in the mediator (vital exhaustion) significantly accounted for variations in the dependent variable (incident HF); and (ii) when paths in (i) and (ii) are controlled for, a previously significant relationship between the predictor and dependent variables was no longer significant, with the strongest demonstration of mediation occurring when the path articulated in (iii) was zero.24 We used four regression models to estimate the relative effect size needed to satisfy the four mediation criteria. We set statistical significance at P ≤ 0.05 for all regression models. One approach typically used to assess for mediation is to include the mediator in statistical models with other potential confounders. Therefore, we included variables from our fully adjusted model (Model 3) in the mediational model. We determined mediation with statistical significance and changes in the magnitude of unstandardized regression coefficients, β, between Pathways 1 and 4 (Figure 3). We considered a percentage change of ≥15% in β for social isolation when vital exhaustion is removed from the model as suggestive of mediation, while a percentage change of ≥30% was considered as strongly suggestive of mediation.25 Finally, we examined whether race/study community modified the association between social isolation risk and incident HF. We performed all analyses using SAS 9.2 (SAS Institute Inc., Cary, NC, USA) and R 2.13.0 (downloaded from http://www.R-project.org).
Figure 1.
Baron and Kenny mediational model of the conceptual relationships among social isolation (low risk vs. moderate/high/isolated), vital exhaustion, and incident heart failure (HF).
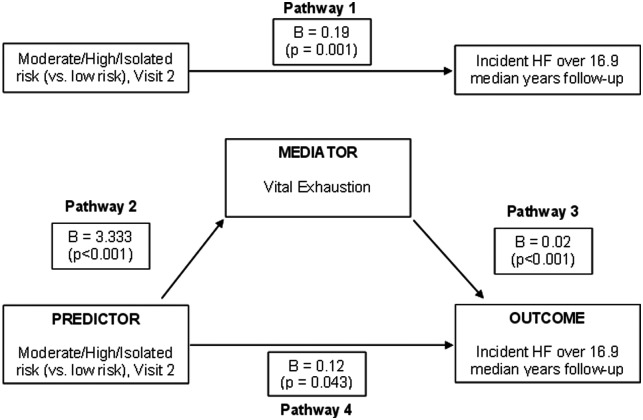
Figure 3.
The mediational model showing the unstandardized linear regression coefficients (β) for the direct and the mediated pathways by which social isolation (low risk vs. moderate/high/isolated) influences incident heart failure (HF).
Results
Table 1 lists baseline sample characteristics overall and stratified by higher vs. low social isolation risk groups. From 1990 to 2008 (median 16.9 person-years), 1727 (13.0%) incident HF events occurred. In our sample, 362 (2.8%) participants were ‘socially isolated’, 733 (5.6%) were ‘high risk’, 1803 (13.6%) were ‘moderate risk’, and 10 097 (76.2%) were ‘low risk’. Those in the moderate/high/isolated group were slightly older, less likely to be female, more likely to be black, more likely to have less than high school education, and more likely to be current smokers. The percentage of individuals with a vital exhaustion score of ≥14 (indicative of a clinical diagnosis) was higher for those in the higher social isolation risk group.
Table 1.
Characteristics of ARIC visit 2 participants (1990–1992) overall and by groups of social isolation (low risk vs. moderate/high/isolated)
| Overall sample (n = 12 995) | Moderate/high/isolated (n = 2898) | Low social isolation (n = 10 097) | P-value | |
|---|---|---|---|---|
| Mean age, years (SD) | 56.9 (5.7) | 57.1 (5.8) | 56.9 (5.7) | 0.047 |
| Female, % | 55.0 | 47.5 | 57.0 | <0.001 |
| Black, % | 24.0 | 27.4 | 22.6 | <0.001 |
| Education, % | < 0.001 | |||
| Less than high school | 21.1 | 24.4 | 19.8 | |
| High school graduate | 41.6 | 40.8 | 41.9 | |
| Beyond high school | 37.3 | 34.8 | 38.3 | |
| Current smoker, % | 22.2 | 28.8 | 20.2 | <0.001 |
| Current alcohol use, % | 57.2 | 58.1 | 57.3 | 0.418 |
| Coronary heart disease, % | 4.7 | 4.9 | 4.7 | 0.660 |
| Diabetes mellitus, % | 14.1 | 14.3 | 13.9 | 0.579 |
| Hypertension, % | 34.0 | 34.9 | 33.4 | 0.147 |
| Mean body mass index (SD) | 27.8 (5.3) | 27.6 (5.2) | 27.9 (5.3) | 0.003 |
| Mean vital exhaustion score (SD) | 10.1 (8.5) | 12.6 (9.9) | 9.4 (7.9) | <0.001 |
| % with vital exhaustion score ≥14 | 29.6 | 40.0 | 26.4 | <0.001 |
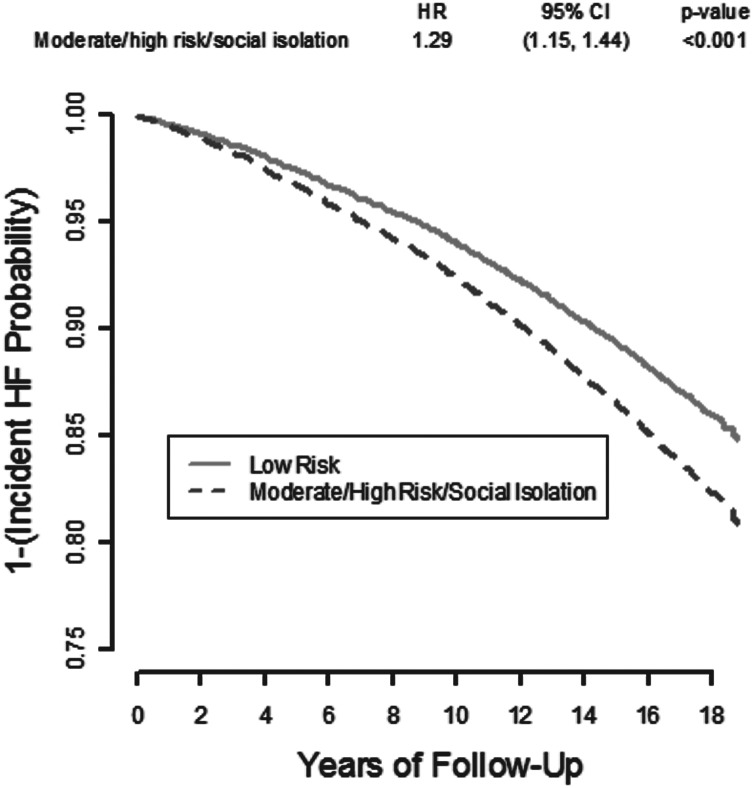
The Kaplan–Meier product limit estimates of the cumulative incidence of HF are depicted in Figure 2. The unadjusted hazard of developing incident HF is greater for those in the higher compared with the low social isolation groups (HR 1.29, 95% CI 1.15–1.24). Table 2 lists the adjusted HR of HF incidence by social isolation risk group. Those in the moderate/high/isolated group had significantly greater risk of developing HF in age-, gender-, race/study community-, and educational level-adjusted analyses (Model 1, HR 1.18, 95% CI 1.06–1.32). This association remained significant after additional adjustment for current smoking and alcohol use (HR 1.13, 95% CI 1.01–1.26), and for hypertension, diabetes, CHD, and BMI (HR 1.21, 95% CI 1.08–1.35). Of note, when we conducted the analysis using three categories of social isolation (low risk vs. moderate risk vs. high risk/socially isolated), we found qualitatively similar results, with the risk of incident HF increasing as the risk for social isolation increases (data not shown). The sensitivity analysis which included incident CHD as a time-dependent covariate indicated that the HR for incident HF changed by only 2%, from 1.21 (1.08–1.35) (Table 2, Model 3) to 1.23 (1.10–1.38), indicating that the effect of social isolation on incident HF was not sensitive to the development of CHD in our sample. Of note, race/study community did not modify the association between social isolation and incident HF (data not shown).
Figure 2.
Kaplan–Meier product limit estimate of the cumulative incidence of HF over time by degree of social isolation (low risk vs. moderate/high/isolated), ARIC visit 2 participants. CI, confidence interval; HF, heart failure; HR, hazard ratio.
Table 2.
Hazard ratios (95% confidence intervals) for the association between social isolation group (low vs. moderate/high/isolated) and incident heart failure in the ARIC visit 2 participants
| Regression models | Incident HF events (n = 1727; 13.0%) |
|
|---|---|---|
| HR (95% CI) | P-value | |
| Model 1a (n = 12 976) | ||
| Low social isolation (ref) | 1.00 | |
| Moderate/high/ isolated | 1.18 (1.06–1.32) | 0.003 |
| Model 2b (n = 12 967) | ||
| Low social isolation (ref) | 1.00 | |
| Moderate/high/isolated | 1.13 (1.01–1.26) | 0.029 |
| Model 3c (n = 12 759) | ||
| Low social isolation (ref) | 1.00 | |
| Moderate/high/isolated | 1.21 (1.08–1.35) | 0.001 |
CI, confidence interval; HF, heart failure; HR, hazard ratio.
aAdjusted for age, gender, race/study community, educational level.
bAdjusted for age, gender, race/study community, educational level, current smoking, alcohol use.
cAdjusted for age, gender, race/study community, educational level, current smoking, alcohol use, hypertension, diabetes, coronary heart disease, body mass index.
We examined whether the linear association between social isolation risk and time to incident HF was mediated through vital exhaustion (Figure 3). The β for the unadjusted association between higher social isolation and incident HF (Pathway 1) was 0.19 (P = 0.001). After adjusting for confounders and the potential mediator, vital exhaustion (Pathway 4), the β was 0.12 (P = 0.04). Therefore, the percentage change in β for higher vs. low social isolation risk after adjusting for vital exhaustion was 36%, which is strongly suggestive of mediation. Since social isolation and vital exhaustion were measured simultaneously and we could not assess directionality of the associations, we also ran the analysis considering social isolation as the potential mediator and vital exhaustion as the predictor. Although vital exhaustion was significantly associated with incident HF, our data do not support the claim that social isolation mediates the association between vital exhaustion and incident HF (data not shown).
Discussion
Few prospective studies have examined the association between social isolation and disease incidence. Most studies have examined mortality or have considered cross-sectional associations with morbid conditions.26–29 These studies have most often used measures of social isolation that captured numbers of close friends or relatives, marital status, or group affiliation. We extended the current literature by examining the association between social isolation and incident HF among a US population-based sample of >12 000 black and white subjects. Over a median follow-up of ∼17 years, there were 1727 (13.0%) incident HF events. The risk of incident HF was significantly greater among those with higher social isolation risk compared with those at low risk, even after multivariable adjustment. The association between higher social isolation risk and incident HF appears to be strongly mediated through vital exhaustion.
To our knowledge, no other population-based studies have evaluated the association between social isolation and incident HF. Our findings suggest that social isolation is an independent predictor of incident HF. Our HR of developing HF for those in the moderate/high/isolated risk group compared with those in at low risk is ∼1.21. Previous prospective cohort studies have examined the association between social support or social integration measures and incident CHD,17 and have demonstrated relative risks of incident CHD ranging from 1.5 to 3.8.30–33 While our estimate of effect is weaker than in previous studies, it is difficult to draw direct comparisons since these studies varied in the measures used to assess social relationships and the outcomes were different.
The mechanism by which social isolation may cause incident disease is not well understood, as previous studies have largely focused on the impact of social relationships on prevalent disease. There are two primary pathways through which social isolation can influence the development of CVD: health behaviours and neuroendocrine mechanisms.34,35 We conceptualized social isolation as a stressor which induces a negative psychological state (i.e. vital exhaustion—the result of prolonged psychological distress) which negatively impacts health-promoting behaviours and leads to incident HF. In our study, the effects of higher social isolation on incident HF persisted after controlling for known behavioural risk factors, such as current smoking and alcohol use. Therefore, our data suggest that the major effect of social isolation is not mediated solely by usual behavioural risk factors. Our findings suggest that vital exhaustion may be a mediating variable between social isolation and incident HF. Although the biological mechanism linking vital exhaustion to adverse cardiac events is not well understood, vital exhaustion is associated with several metabolic, haemodynamic, and immune responses of importance for the development and progression of CVD.36,37 It is also plausible that neuroendocrine mechanisms may mediate the relationship between social isolation and incident HF; however, we did not have the data to test these mechanisms empirically in our sample. Previous work suggests that lack of social support is aetiologically related to the development of a coronary artery lesion.34 Given that CHD is the cause of over half of the cases of incident HF in the general population under age 75,18 it was important to examine whether the association we found between social isolation and incident HF was sensitive to incident CHD. Our findings were robust to consideration of CHD as a time-dependent covariate.
Our study findings should be interpreted in light of its limitations. First, there may have been unmeasured confounders, such as depression, anxiety disorders, or health services utilization, that were not adjusted for in this analysis. The ARIC study did not include validated measures of depression (e.g. Centers for Epidemiologic Studies-Depression, Beck Depression Inventory, etc.) or anxiety disorders. Secondly, we cannot prove causality with an observational study. It is possible that social isolation could exert its effect on HF incidence through other pathways. Thirdly, both vital exhaustion and social isolation were measured simultaneously, making it difficult to determine definitively the directionality of the observed associations with incident HF. However, we looked for but found no evidence that social isolation mediates the relationship between vital exhaustion and incident HF. Finally, we used HF hospitalization or death to assess incident HF. This definition relies on coding practices and excludes milder HF events which do not result in hospitalization or death and less symptomatic cases of HF (stages B and C). It is possible that some of the participants with incident HF already had an outpatient diagnosis of HF before being hospitalized.
Despite these limitations, our study has several strengths. Foremost, we answered a novel research question; namely whether social isolation independently predicts incident HF. To our knowledge, we are the first to answer this question using population-level data. Secondly, we utilized the strengths of the ARIC study, including the population-based sampling method, a biracial cohort, a large sample size which allows for increased precision and simultaneous adjustment for multiple covariates, and a long duration of follow-up which allowed us to examine long-term risk. Finally, we used validated measures of social isolation and vital exhaustion.
In summary, high social isolation was independently predictive of incident HF hospitalization or death over a median follow-up of 17 years in a US community-based sample of blacks and whites. Vital exhaustion appears to mediate this association. Our results contribute to the literature on the effects of social relationships on physical and mental health. If, as our data suggest, greater social isolation is an independent risk factor for incident HF, future studies should investigate the potential risks and benefits of screening for social isolation in patients at risk for HF. We need to better understand the broader social context which shapes the development and maintenance of social network ties in order to inform interventions to reduce social isolation, since this and other studies suggest the critical role that social isolation plays in the development or worsening of CVD.
Funding
The National Center for Research Resources [a component of the National Institutes of Health (NIH), via the Clinical Translational Science Award (CTSA)-K12 Scholars Program and NIH Roadmap for Medical Research (grant no. KL2RR025746)]. (The CTSA is a national consortium with the goal of transforming how clinical and translational research is conducted, ultimately enabling researchers to provide new treatments more efficiently and quickly to patients.) The National Heart, Lung, and Blood Institute [grant no. K23HL107614 to C.W.C]; the National Center for Research Resources [salary support through a Mid-career Investigator Award in Patient-Oriented Research (K24 HL105493) and grant no. UL1RR025747 to G. C.-S., C.W.C. and L.L.].
Conflicts of interest: none declared.
Acknowledgements
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The authors thank the staff and participants of the ARIC study for their important contributions. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
References
- 1.American Heart Association. Dallas, TX: American Heart Association; 2009. Heart Disease and Stroke Statistics—2009 Update. Available from: http://www.nanocorthx.com/Articles/HeartDiseaseStrokeStatistics.pdf . [Google Scholar]
- 2.Krumholz HM, Parent EM, Tu N, Vaccarino V, Wang Y, Radford MJ, Hennen J. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997;157:99–104. [PubMed] [Google Scholar]
- 3.Haldeman GA, Croft JB, Giles WH, Rashidee A. Hospitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995. Am Heart J. 1999;137:352–360. doi: 10.1053/hj.1999.v137.95495. [DOI] [PubMed] [Google Scholar]
- 4.Burns RB, McCarthy EP, Moskowitz MA, Ash A, Kane RL, Finch M. Outcomes for older men and women with congestive heart failure. J Am Geriatr Soc. 1997;45:276–280. doi: 10.1111/j.1532-5415.1997.tb00940.x. [DOI] [PubMed] [Google Scholar]
- 5.Medicare Payment Advisory Commission. Report to the Congress: New Approaches in Medicare. Washington, DC: Medicare Payment Advisory Commission; 2004. [Google Scholar]
- 6.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 7.Barth J, Schneider S, von Kanel R. Lack of social support in the etiology and the prognosis of coronary heart disease: a systematic review and meta-analysis. Psychosom Med. 2010;72:229–238. doi: 10.1097/PSY.0b013e3181d01611. [DOI] [PubMed] [Google Scholar]
- 8.Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98:310–357. [PubMed] [Google Scholar]
- 9.Cohen S, Gottlieb BH, Underwood LG. Social relationships and health. In: Cohen S, Underwood LG, Gottlieb BH, editors. Social Support Measurement and Intervention: A Guide for Health and Social Scientists. New York: Oxford University Press; 2000. pp. p3–25. [Google Scholar]
- 10.Appels A, Hoppener P, Mulder P. A questionnaire to assess premonitory symptoms of myocardial infarction. Int J Cardiol. 1987;17:15–24. doi: 10.1016/0167-5273(87)90029-5. [DOI] [PubMed] [Google Scholar]
- 11.Raikkonen K, Lassila R, Keltikangas-Jarvinen L, Hautanen A. Association of chronic stress with plasminogen activator inhibitor-1 in healthy middle-aged men. Arterioscler Thromb Vasc Biol. 1996;16:363–367. doi: 10.1161/01.atv.16.3.363. [DOI] [PubMed] [Google Scholar]
- 12.van Diest R, Appels A. Vital exhaustion and depression: a conceptual study. J Psychosom Res. 1991;35:535–544. doi: 10.1016/0022-3999(91)90048-s. [DOI] [PubMed] [Google Scholar]
- 13.Williams JE, Mosley TH, Jr, Kop WJ, Couper DJ, Welch VL, Rosamond WD. Vital exhaustion as a risk factor for adverse cardiac events (from the Atherosclerosis Risk In Communities [ARIC] study) Am J Cardiol. 2010;105:1661–1665. doi: 10.1016/j.amjcard.2010.01.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole SR, Kawachi I, Sesso HD, Paffenbarger RS, Lee IM. Sense of exhaustion and coronary heart disease among college alumni. Am J Cardiol. 1999;84:1401–1405. doi: 10.1016/s0002-9149(99)00585-8. [DOI] [PubMed] [Google Scholar]
- 15.Appels A, Falger PR, Schouten EG. Vital exhaustion as risk indicator for myocardial infarction in women. J Psychosom Res. 1993;37:881–890. doi: 10.1016/0022-3999(93)90177-h. [DOI] [PubMed] [Google Scholar]
- 16.Schuitemaker GE, Dinant GJ, van der Pol GA, Appels A. Assessment of vital exhaustion and identification of subjects at increased risk of myocardial infarction in general practice. Psychosomatics. 2004;45:414–418. doi: 10.1176/appi.psy.45.5.414. [DOI] [PubMed] [Google Scholar]
- 17.Hemingway H, Marmot M. Evidence based cardiology: psychosocial factors in the aetiology prognosis of coronary heart disease. Systematic review of prospective cohort studies. BMJ. 1999;318:1460–1467. doi: 10.1136/bmj.318.7196.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox KF, Cowie MR, Wood DA, Coats AJ, Gibbs JS, Underwood SR, Turner RM, Poole-Wilson PA, Davies SW, Sutton BC. Coronary artery disease as the cause of incident heart failure in the population. Eur Heart J. 2001;22:228–236. doi: 10.1053/euhj.2000.2289. [DOI] [PubMed] [Google Scholar]
- 19.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 20.Eriksson H, Caidahl K, Larsson B, Ohlson LO, Wlin L, Wilhelmsen L, Svardsudd K. Cardiac and pulmonary causes of dyspnoea–validation of a scoring test for clinical-epidemiological use: the study of men born in 1913. Eur Heart J. 1987;8:1007–1014. doi: 10.1093/oxfordjournals.eurheartj.a062365. [DOI] [PubMed] [Google Scholar]
- 21.Lubben J, Blozik E, Gillmann G, Iliffe S, von Renteln Kruse W, Beck JC, Stuck AE. Performance of an abbreviated version of the Lubben Social Network Scale among three European community-dwelling older adult populations. Gerontologist. 2006;46:503–513. doi: 10.1093/geront/46.4.503. [DOI] [PubMed] [Google Scholar]
- 22.Chi I, Boey KW. Validation of Measuring Instruments of Mental Health Status of the Elderly in Hong Kong. Hong Kong: University of Hong Kong; 1992. Department of Social Work & Social Administration. [Google Scholar]
- 23.Lubben J. Assessing social networks among elderly populations. Fam Community Health. 1988;11:42–52. [Google Scholar]
- 24.Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 25.Pollitt RA, Daniel M, Kaufman JS, Lynch JW, Salonen JT, Kaplan GA. Mediation and modification of the association between hopelessness, hostility, and progression of carotid atherosclerosis. J Behav Med. 2005;28:53–64. doi: 10.1007/s10865-005-2563-y. [DOI] [PubMed] [Google Scholar]
- 26.House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 27.House JS, Robbins C, Metzner HL. The association of social relationships and activities with mortality: prospective evidence from the Tecumseh Community Health Study. Am J Epidemiol. 1982;116:123–140. doi: 10.1093/oxfordjournals.aje.a113387. [DOI] [PubMed] [Google Scholar]
- 28.Berkman LF. The role of social relations in health promotion. Psychosom Med. 1995;57:245–254. doi: 10.1097/00006842-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Ruberman W. Psychosocial influences on mortality of patients with coronary heart disease. JAMA. 1992;267:559–560. [PubMed] [Google Scholar]
- 30.Medalie JH, Goldbourt U. Angina pectoris among 10,000 men. II. Psychosocial and other risk factors as evidenced by a multivariate analysis of a five year incidence study. Am J Med. 1976;60:910–921. doi: 10.1016/0002-9343(76)90921-9. [DOI] [PubMed] [Google Scholar]
- 31.Berkman LF, Breslow L. Health and Ways of Living: The Alameda County Study. New York: Oxford University Press; 1983. [Google Scholar]
- 32.Vogt TM, Mullooly JP, Ernst D, Pope CR, Hollis JF. Social networks as predictors of ischemic heart disease, cancer, stroke and hypertension: incidence, survival and mortality. J Clin Epidemiol. 1992;45:659–666. doi: 10.1016/0895-4356(92)90138-d. [DOI] [PubMed] [Google Scholar]
- 33.Orth-Gomer K, Rosengren A, Wilhelmsen L. Lack of social support and incidence of coronary heart disease in middle-aged Swedish men. Psychosom Med. 1993;55:37–43. doi: 10.1097/00006842-199301000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Knox SS, Uvnas-Moberg K. Social isolation and cardiovascular disease: an atherosclerotic pathway? Psychoneuroendocrinology. 1998;23:877–890. doi: 10.1016/s0306-4530(98)00061-4. [DOI] [PubMed] [Google Scholar]
- 35.Cohen S, Underwood LG, Gottlieb BH, editors. Social Support Measurement and Intervention: A Guide for Health and Social Scientists. New York: Oxford University Press; 2000. [Google Scholar]
- 36.von Kanel R, Frey K, Fischer J. Independent relation of vital exhaustion and inflammation to fibrinolysis in apparently healthy subjects. Scand Cardiovasc J. 2004;38:28–32. doi: 10.1080/14017430310015884. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe T, Sugiyama Y, Sumi Y, Watanabe M, Takeuchi K, Kobayashi F, Kono K. Effects of vital exhaustion on cardiac autonomic nervous functions assessed by heart rate variability at rest in middle-aged male workers. Int J Behav Med. 2002;9:68–75. doi: 10.1207/s15327558ijbm0901_05. [DOI] [PubMed] [Google Scholar]