Abstract
Aims
The purpose of this study was to evaluate the cost-effectiveness of iron repletion using intravenous (i.v.) ferric carboxymaltose (FCM) in chronic heart failure (CHF) patients with iron deficiency with or without anaemia. Cost-effectiveness was studied from the perspective of the National Health Service in the UK.
Methods and results
A model-based cost-effectiveness analysis was used to compare iron repletion with FCM with no iron treatment. Using data from the FAIR-HF trial and publicly available sources and publications, per patient costs and clinical effectiveness of FCM were estimated compared with placebo. Cost assessment was based on study drug and administration costs, cost of CHF treatment, and hospital length of stay. The incremental cost-effectiveness ratio (ICER) of FCM use was expressed as cost per quality-adjusted life year (QALY) gained, and sensitivity analyses were performed on the base case. The time horizon of the analysis was 24 weeks. Mean QALYs were higher in the FCM arm (difference 0.037 QALYs; bootstrap-based 95% confidence interval 0.017–0.060). The ICER of FCM compared with placebo was €4414 per QALY gained for the FAIR-HF dosing regimen. Sensitivity analyses confirmed the base case result to be robust.
Conclusion
From the UK payers’ perspective, managing iron deficiency in CHF patients using i.v. FCM was cost-effective in this analysis. The base case ICER was clearly below the threshold of €22 200–€33 300/QALY gained (£20 000–£30 000) typically used by the UK National Institute for Health and Clinical Excellence and proved to be robust in sensitivity analysis. Improved symptoms and better quality of life contributed to this result.
Keywords: Chronic heart failure, Anaemia, Iron deficiency, Cost-effectiveness analysis, Healthcare costs
Introduction
Chronic heart failure (CHF) patients are often limited in their daily activities. Frequently reported symptoms are fatigue and dyspnoea, but also impaired physical working capacity, exhaustion, susceptibility to stress, and decreased mental and cognitive performance.1,2 Anaemia and iron deficiency are common findings in HF patients and may partially explain these symptoms. Anaemic HF patients are at risk of increased mortality, number of hospitalizations, and levels of natriuretic peptides, and reduced exercise capacity and impaired quality of life (QoL).3 Factors (e.g. renal dysfunction, haemodilution, and drugs) present in HF can contribute to the development of anaemia.3 A recent study found profound and general bone marrow dysfunction in CHF patients, another factor contributing to anaemia.4 Iron plays a key role in the uptake, transport, and storage of oxygen, which in CHF is often insufficiently supplied to the body. Iron deficiency in HF patients can exacerbate chronic diseases, and affects erythropoiesis and oxidative and immune mechanisms.3 In recent years, clinical research increasingly focused on iron therapy and administration of erythropoiesis-stimulating agents (ESAs) as treatment strategies for anaemia and iron deficiency in CHF patients.5 Intravenous (i.v.) iron repletion with ferric carboxymaltose (FCM) has been shown to improve symptoms and QoL in patients with CHF.6–8 FAIR-HF (see Appendix), a randomized, double-blind, placebo-controlled trial (n = 459) studied clinical and QoL benefits of treatment with FCM, an i.v. iron preparation, in iron-deficient CHF patients with New York Heart Association (NYHA) class II or III, a left ventricular ejection fraction of ≤40% (for patients in NYHA class II) or ≤45% (for patients in NYHA class III), a haemoglobin level at the screening visit of between 9.5 and 13.5 g/dL, and iron deficiency.9 Causes of HF in FAIR-HF patients were predominantly ischaemic [FCM n (%) 245 (80.6); placebo 123 (79.4)].6 Primary and secondary endpoints included NYHA functional class change from baseline and European Quality of Life-5 Dimensions (EQ-5D; EuroQol Group, Rotterdam, The Netherlands) questionnaire-based health-related quality of life (HRQoL).10 The FAIR-HF trial showed significantly better NYHA class changes from baseline in the FCM group compared with placebo, and FCM resulted in improved HRQoL [increased EQ-5D visual analogue scale (VAS) score change from baseline].6 This study evaluated the cost-effectiveness of iron repletion using i.v. FCM in CHF patients, from the perspective of the National Health Service (NHS) in the UK.
Methods
We performed a model-based cost-effectiveness analysis comparing a strategy of iron repletion with FCM with a standard strategy of no iron treatment in iron-deficient patients with or without anaemia. These strategies were generally equivalent to the strategies investigated in the FAIR-HF trial.9 Cost differences between the treatment strategies were also modelled and are reported in the Results section. A decision-tree model was used to facilitate sensitivity analysis. It allowed for performing deterministic as well as probabilistic sensitivity analyses.
The population basis for the clinical model inputs consisted of the 459 patients with NYHA functional class II and III at baseline, which formed the intention-to-treat (ITT) population of the FAIR-HF trial. Baseline characteristics are shown in Table 1. The FAIR-HF trial did not include British patients but was mostly performed in European countries (including Russia and Ukraine) with a predominantly Caucasian population. Therefore, the authors assume that clinical study results in a British population would not differ significantly from those of the actual trial.
Table 1.
Baseline demographic and clinical characteristics of the FAIR-HF intention-to-treat population, according to study group
| Variable | Ferric carboxymaltose (n = 304) | Placebo (n = 155) |
|---|---|---|
| Age, years | 67.8 ±10.3 | 67.4 ±11.1 |
| Female sex | 159 (52.3) | 85 (54.8) |
| NYHA class | ||
| II | 53 (17.4) | 29 (18.7) |
| III | 251 (82.6) | 126 (81.3) |
| Left ventricular ejection fraction, % | 31.9 ±5.5 | 33.0 ±6.1 |
| Laboratory measurements | ||
| Haemoglobin, g/La | 119 ±12.6 | 119.5 ±13.8 |
| Serum ferritin, μg/L | 52.5 ±54.5 | 60.1 ±66.5 |
| Transferrin saturation, % | 17.7 ±12.6 | 16.7 ±8.4 |
Data presented are the mean value ± SD or number (%) of patients. Values were calculated from the study data by the authors. In the FAIR-HF trial a 2:1 randomization was used.9
NYHA, New York Heart Association.
aDue to missing values, the n for haemoglobin are 298 (ferric carboxymaltose) and 153 (placebo).
In FAIR-HF, patients were randomly assigned to receive either FCM or placebo (normal saline). During a correction phase, patients received weekly injections until iron repletion was achieved.6 The total iron dose required for iron repletion was calculated at baseline using the Ganzoni formula.6 FCM was administered as an i.v. bolus injection of 4 mL equivalent to 200 mg of iron until repletion was achieved. Subsequently, during a maintenance phase, an injection was given every 4 weeks. Patients were assessed for NYHA functional class and QoL at baseline and Weeks 4, 12, and 24. QoL was represented by health state utilities measured with the EuroQol Group 5-Dimension Self-Report Questionnaire (EQ-5D). The EQ-5D is an instrument designed for self-completion by respondents. The instrument comprises two parts: first, respondents report their health status according to a five-dimensional classification (mobility, self-care, usual activities, pain/discomfort, anxiety/depression). Each dimension is represented by a three-level ordered category item, which leads to a total of 243 possible health ratings that are valued using the standard UK time trade-off value set. Secondly, the respondents record their self-perceived health status using a graduated visual analogue scale (VAS), with grades from 0 to 100. The EQ-5D is a generic QoL instrument which has been validated and shown to be sensitive, internally consistent, and reliable in the general population and different patient groups.11–13
In the cost-effectiveness analysis, available outcome measures for the different strategies assessed were (i) cost; (ii) effectiveness, expressed as the number of quality-adjusted life years (QALYs) accrued during the study period; and (iii) the incremental cost-effectiveness ratio (ICER), expressed as cost per QALY gained. Costs are reported in Euros (€) and Pounds sterling (£). For the conversion of £ to €, an exchange rate of 1.11 was used (mean exchange rate for 2009; source: www.oanda.com). Costs and benefits were not discounted given the short time horizon of the study period. Where necessary, cost information was inflation corrected using the ‘Unit Costs of Health and Social Care’ inflation indices of the Personal Social Services Research Unit (PSSRU).14 Costs borne by the patient and society as a whole were not incorporated, as they are not relevant from the NHS perspective.
The time horizon of the analysis was 24 weeks, corresponding to the duration of the FAIR-HF trial. Extrapolation of the time horizon to a longer term, e.g. lifelong time horizon, was not considered adequate as the FAIR-HF trial provides no information on longer term survival or other long-term effects. In the FAIR-HF trial, the rates of death, hospitalization, and serious or any adverse events were similar in the two study groups.6
Two approaches to cost estimation were used, as there was no detailed medical resource use information available that would have allowed direct assessment of direct medical costs. (i) In the base case analysis, only CHF-related hospitalization costs were taken into account. (ii) In a univariate sensitivity analysis, the costs of CHF treatment were estimated using cost proportions observed for patients in different NYHA classes.
In both approaches, UK FCM costs and UK FCM administration costs15,16 were additionally taken into account. Costs for adverse events were not included because there were not any clinically relevant differences between the FAIR-HF study arms. Table 3 provides an overview of the different approaches used in the analysis. All data derivations from the FAIR-HF raw data were performed by the authors and checked for consistency with the publication by Anker et al.6 where applicable.
Table 3.
Overview of different approaches to analysis and results
| Calculation |
Cost difference, € (£)a | QALY difference | ICER, € (£) | ||
|---|---|---|---|---|---|
| Base case analysis | |||||
| Cost via hospital days, EQ-5D questionnaire derived scores, all cases (LOCF) | 165 (149) | 0.037 | 4414 (3977) | ||
| Univariate sensitivity analysis | |||||
| Topic | Variable | Variation | Cost difference, € (£)a | QALY difference | ICER, € (£) |
| Costs | Cost of a hospital day varied by | –30% | 297 (268) | 0.037 | 7919 (7134) |
| +30% | 34 (31) | 905 (815) | |||
| Cost of an ambulatory injection | −30% | 102 (92) | 2722 (2452) | ||
| +30% | 229 (206) | 6108 (5503) | |||
| Calculation of costs via NYHA class approach | According to18 | 528 (476) | 14 096 (12 699) | ||
| According to18 | 546 (492) | 14 582 (13 137) | |||
| Drug costs varied by | –10% | 127 (114) | 3368 (3034) | ||
| +10% | 204 (184) | 5462 (4921) | |||
| Resources | Duration of hospitalization for CHF in the UK | –30% | 297 (268) | 7925 (7140) | |
| +30% | 34 (31) | 905 (815) | |||
| Proportional reduction of hospitalization days | Lower margin of CI | 23 (21) | 616 (555) | ||
| Upper margin of CI | 519 (468) | 13 855 (12 482) | |||
| Frequency of hospitalization in placebo group | Lower margin of CI | 316 (285) | 8443 (7606) | ||
| Upper margin of CI | –39 (–35) | FCM dominant | |||
| Mean dose of FCM received per patient | Lower margin of CI | 155 (140) | 4136 (3726) | ||
| Upper margin of CI | 175 (158) | 4693 (4228) | |||
| Mean number of push injections in FCM arm | Lower margin of CI | 160 (144) | 4264 (3841) | ||
| upper margin of CI | 171 (154) | 4565 (4113) | |||
| Utilities | QALY difference | Lower margin of CI | 165 (149) | 0.017 | 9673 (8714) |
| Upper margin of CI | 0.060 | 2738 (2467) | |||
| QALYs | Complete records only | 0.039 | 4208 (3791) | ||
| Computation of utilities | VAS scale-derived scores | 0.023 | 7201 (6487) | ||
Data shown are cost difference (€/£), QALY difference, and resulting ICER (€/£) of base case analysis and sensitivity analyses.
€, euros; £, pounds sterling; CHF, chronic heart failure; CI, confidence interval; FCM, ferric carboxymaltose; ICER, incremental cost-effectiveness ratio; LOCF, last observation carried forward; NYHA, New York Heart Association; QALY, quality-adjusted life year; VAS, visual analogue scale.
aPositive numbers indicate that FCM is more expensive than placebo.
Model inputs
Clinical data
The EQ-5D questionnaire (base case analysis) and VAS (sensitivity analysis) results from FAIR-HF,6 measured at baseline and at Weeks 4, 12, and 24, were converted into utility values as described above. QALYs were calculated by multiplying these utilities by the appropriate time periods for each individual. In order to achieve this, any changes in utility were assumed to occur in the middle of the periods defined by the assessment time points. Observations with missing values were imputed with the value of the last observation (last observation carried forward; LOCF), as was done in the FAIR-HF main clinical publication. Effectiveness was assessed as the number of QALYs accrued during the study. Clinical response to treatment, measured by change in NYHA class, was also assumed to be the same as in FAIR-HF.
Medical resource use
In the base case analysis, medical resources taken into account were drug (FCM), FCM administration, and hospitalization for CHF (Table 2). Concerning drug usage, there was no wastage, as FCM vials were fully administered in each case. Hospitalization costs were determined by multiplying UK-based hospital length of stay for CHF patients17by the average number of hospitalizations seen in placebo patients in the FAIR-HF trial and, for patients in the FCM group, by the proportional length of stay in the FCM arm (average length of stay across all patients and hospitalizations in the FCM group divided by average length of stay in the placebo group). For patients remaining hospitalized after 24 weeks an artificial discharge date (baseline plus 24 weeks) was assumed.
Table 2.
Model input parameters, ranges of variation, and distribution type, in sensitivity analyses
| Resource use | Unit | Value |
Range of variation in deterministic sensitivity analysis | Distribution type in probabilistic sensitivity analysis | |
|---|---|---|---|---|---|
| Mean dosea of FCM received per patient6 | mg | 1851.33 | 1802.12–1900.54 | Normal | |
| Mean number of push injections (200 mg)a in FCM arm6 | – | 9.46 | 9.21–9.72 | Normal | |
| UK hospital length of stay for CHF17 | Days | 11.8 | 8.26–15.34 | Triangular | |
| Relative length of stay b in FAIR-HF in FCM arm relative to placebo arm6 | – | 0.36 | 0.16–0.88c | Lognormal | |
| Length of stayd per hospitalization6 | Days | No iron: 2.95 | FCM: 1.07 | – | – |
| Frequencyd of hospitalizations6 | – | No iron: 0.17 | FCM: 0.08 | – | – |
| Unit cost | |||||
| FCM costs | |||||
| Costs FCM (PP; per 100 mg)22 | € (£) | 21.20 (19.10) | 18.03e (16.24) | – | |
| Administration costs | |||||
| Push injection (wages and material)15,16 | € (£) | 22.36 (20.14) | 15.64–29.06 (14.09–26.18) | Triangular | |
| Costs per hospital day for CHF21 | € (£) | 347.27 (312.86) | 243.09–452.57 (219.00–407.72) | Triangular | |
| Other variables used in sensitivity analysis | |||||
| QALY difference between armsf | – | 0.037 | 0.02–0.06c | Normal | |
Data presented are rounded values.
€, euros; £, pounds sterling; CHF, chronic heart failure; FCM, ferric carboxymaltose; PP, purchasing price; QALY, quality-adjusted life year.
aDue to missing data, seven patients were excluded from summary statistics.
bCumulative over the study period.
cBootstrap-based confidence intervals.
dRepresented in the model by the combined parameter ‘relative length of stay in FAIR-HF’.
eUsed in scenario analysis.
fQALY difference as a clinical study endpoint is shown here because it was used for probabilistic sensitivity analysis.
In the second, NYHA class-based approach to cost estimation, CHF-related medical resource use (other than for FCM and FCM administration) was assumed to be represented by the typical cost of a patient falling into a given NYHA class: cost proportions observed for CHF patients in different NYHA classes published by Levin and Szucs18,19 were multiplied by published total costs for CHF patients20 and patient days per NYHA class according to data from the FAIR-HF study arms.
Unit costs
Costs for a hospital day for CHF patients were calculated using 2008–2009 NHS reference costs21 (Table 2). For FCM, the UK purchasing price (PP) was used in the base case analysis.22 Ambulatory administration costs were calculated using information on wages from the PSSRU data on unit costs for medical services.15 Costs of materials were taken into account according to information from the Falkirk & District Royal Infirmary.16
Sensitivity analysis
In order to assess the impact of statistical uncertainty around key model inputs, we performed a series of univariate and probabilistic sensitivity analyses.
Univariate sensitivity analyses
In univariate sensitivity analysis, we varied (i) the mean duration of hospitalizations for CHF in the UK and (ii) the cost of a hospital day by ±30%; and (iii) drug costs by ±10% as no confidence intervals were available for these parameters. We further varied (iv) QALY difference; (v) proportional reduction of hospitalization days; and (vi) frequency of hospitalization in the placebo group on the basis of their confidence intervals. Further variations were: (vii) calculation of results considering only cases with complete data on utilities; (viii) calculation of costs via the NYHA class approach; and (ix) calculation of utilities using EQ-5D VAS scale scores.
Probabilistic sensitivity analyses
Probabilistic sensitivity analysis (second-order Monte Carlo simulation; PSA) was based on distributions corresponding to the ranges of variation used in the univariate sensitivity analyses assessing the impact of parameter uncertainty (Table 2). PSA was based on 10 000 sets of randomly drawn input parameters.
Technical implementation
The model was implemented and all Monte Carlo analyses were performed using TreeAge Pro 2011 Suite (TreeAge Software Inc., Williamstown, MA, USA). Further analyses were performed using Stata/IC 11 (StataCorp LP, College Station, TX, USA).
Results
Characteristics of the study patients
The clinical characteristics of the FAIR-HF ITT population are presented in Table 1. At baseline, there were 53 patients (17.4%) in NYHA functional class II and 251 (82.6%) in class III in the treatment group, and 29 patients (18.7%) in class II and 126 (81.3%) in class III in the placebo group.
Cost-effectiveness of intravenous iron therapy
In the base case analysis, mean QALYs were higher in the FCM arm (placebo, 0.298; FCM, 0.336); the difference was 0.037 QALYs [bootstrap-based 95% confidence interval (CI) 0.017–0.060] (cost and cost-effectiveness results are shown in Table 3). The ICER of FCM compared with placebo was €4414 (£3977) per QALY gained for the FAIR-HF dosing regimen. The FCM group yielded total costs of €852 (£768) and the placebo group of €687 (£619) over the study period. There were costs of €393 (£354) for the drug and €211 (£190) for administration compared with no costs on the placebo side. On the other hand, treatment with FCM saved €438 (£395) of costs for hospital treatment [FCM, €249 (£224); placebo, €687 (£619)], resulting in a net cost of the FCM strategy of €165 (£149) over 24 weeks.
Sensitivity analysis
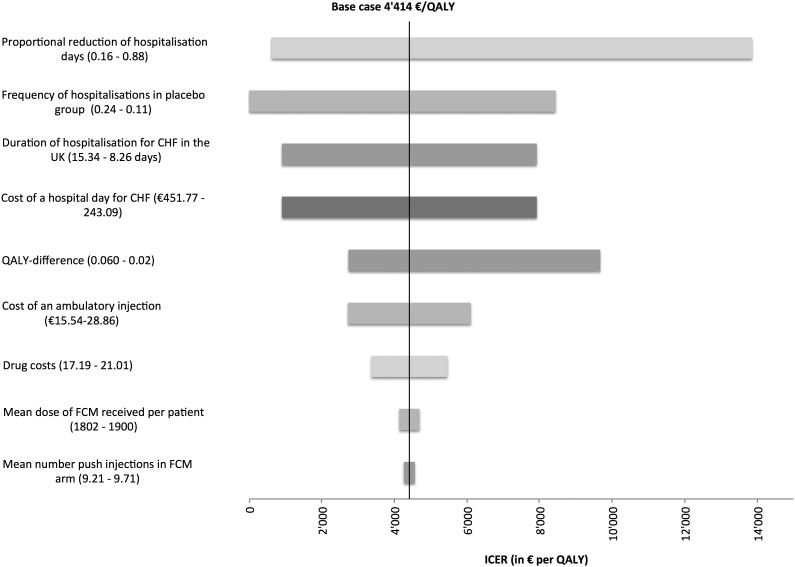
Univariate sensitivity analysis was carried out to characterize the robustness of the base case results. Results ranged from dominance (i.e. being both cost saving and more effective) of the i.v. iron strategy [€1045 (£941) saved; effectiveness 0.037 QALYs] to €13 855 (£12 482) per QALY gained. Frequency and duration of hospitalization, QALY difference, and cost of a hospital day were the most influential parameters. Univariate sensitivity analysis results are presented in Table 3 and summarized in a Tornado diagram (Figure 1).
Figure 1.
Tornado diagram of deterministic sensitivity analyses addressing the impact of parameter uncertainty. €, euros; CHF, chronic heart failure; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year.
Analysis of mean days spent in each NYHA class, for FCM (placebo), resulted in 6.5 days (1) for NYHA class I, 58 days (40) for NYHA class II, 100 days (119) for NYHA class III, and <1 day (3) for NYHA class IV. The approach of assessing CHF treatment costs based on the time spent in each NYHA class resulted in costs of €1907 (£1718) for FCM and €1361 (£1226) for placebo, with a cost difference of €546 (£492) and a resulting ICER of €14 582 (£13 137), if the proportions according to Levin18 were used. If the distribution according to Szucs19 was used, costs were €1415 (£1275) for FCM and €887 (£799) for placebo, with a cost difference of €528 (£476) and a resulting ICER of €14 096 (£12 699). None of the parameters tested resulted in an ICER less favourable than €22 200–€33 300/QALY gained (£20 000–£30 000), the threshold usually regarded as acceptable by the UK National Institute for Health and Clinical Excellence (NICE).23
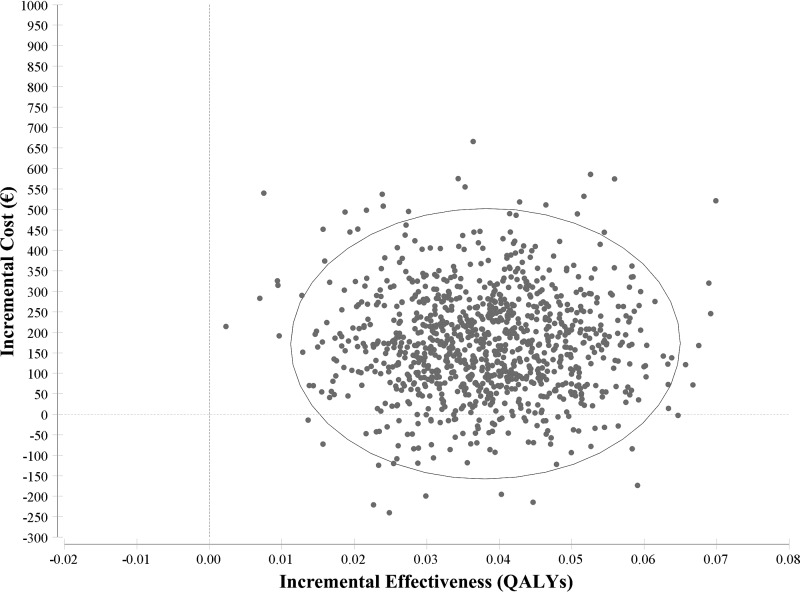
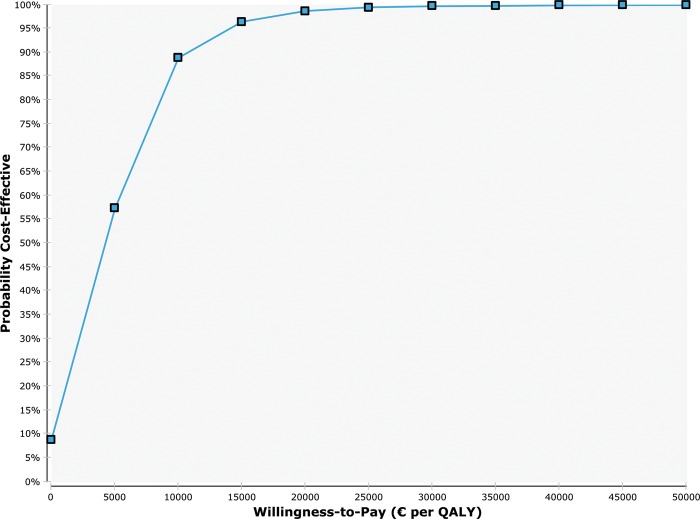
The PSA results showed mean costs of €877 (£790) for FCM and €686 (£618) for placebo [range: FCM, €608–€2050 (£548–£1847); placebo, €369–€1112 (£332–£1002)] and a mean effect of 0.038 QALYs (range –0.006 to 0.085). A total of 9866 scenarios (98.66%) were better than €22 200; 9968 scenarios (99.68%) were better than €33 300. A cost-effectiveness scatterplot and cost-effectiveness acceptability curve are shown in Figures 2 and 3, respectively. Generally, sensitivity analyses showed the results to be robust.
Figure 2.
Cost-effectiveness scatterplot of 10 000 bootstrap replicates for incremental cost and incremental effectiveness. The circle is depicting 95% of observations. For each simulation run (represented as a dot), parameters were simultaneously and randomly sampled from the probability, cost, and outcome distributions for each strategy, to account for uncertainty in the base case parameter estimates. All simulation results fell in two quadrants of the cost-effectiveness plain: quadrant I (upper right), where the FCM strategy was both more costly and more effective than placebo, or quadrant II (lower right), where the FCM strategy was less costly and more effective. €, euros; FCM, ferric carboxymaltose; QALYs, quality-adjusted life years.
Figure 3.
For different willingness-to-pay values, the cost-effectiveness acceptability curve (CEAC) shows the probability that the ferric carboxymaltose (FCM) strategy (blue line) is cost-effective compared with placebo. The willingness-to-pay can be interpreted as the maximum amount one would be willing to pay for a gain of one quality-adjusted year of life. €, euros; QALY, quality-adjusted life years.
Discussion
As the cost burden of CHF reaches up to 1–2% of healthcare budgets, cost-effective treatments to improve symptoms and QoL are needed.24 The cost-effectiveness of iron repletion using FCM, compared with no iron treatment, in CHF patients with iron deficiency derived from our trial-based analysis (€4414/QALY gained in the base case) was clearly below the cost-effectiveness threshold typically regarded as acceptable by NICE, of €22 200–€33 300/QALY gained.23 Mean QALYs were higher in the FCM arm, and improved symptoms and better quality of life contributed to economic benefits seen with FCM. Sensitivity analyses showed ranges of QALY differences (ICERs) from 0.017 to 0.060 (FCM dominant: €13 855) for the univariate and QALYs of –0.006 to 0.085 for the probabilistic analysis.
To the authors‘ knowledge FAIR-HF is the first study that compared FCM with placebo in a population of CHF patients with iron deficiency. Clinical implications of FCM use compared with other i.v. and oral iron compounds have been studied in various indications. Cost-effectiveness analyses of FCM use are scarce across indications, and none is available for CHF patients.
The use of ESAs in CHF patients is still under debate, and further research is needed in this regard.5 If ESA treatment was established as a therapeutic option, combination treatment with i.v. iron might have fewer cost implications in the long run than ESA treatment alone, as required ESA doses might be reduced.25–29
One important limitation of the present analysis is the lack of exhaustive medical resource use information; data on some uses of medical resources such as co-medications, devices (e.g. pacemakers), and ambulatory treatments could not be accounted for in the analysis, as they were not recorded in the FAIR-HF trial. As there is no indication of an increased use of unmeasured resource items in patients treated with i.v. iron vs. patients with no iron treatment, the authors do not expect that other resource items, had they been included in the analysis, would diminish the cost difference between FCM and placebo. The strategy to assess treatment costs via hospital days is likely to produce conservative results, because other potential resource savings, relating, for example, to outpatient visits, were not included in the calculation. In an alternative approach to cost assessment, treatment costs for CHF patients were estimated using the time spent in each NYHA class, and resulted in ICERs that are almost three-fold compared with the approach of using hospital days to estimate costs. Although the level of healthcare expenditure has been shown to be associated with NYHA class, this approach apparently cannot appropriately reflect changes in hospitalization rate and duration, as observed in FAIR-HF. Moreover, clinical treatment regimens most probably will not immediately follow and be adapted to changes in NYHA class (particularly improvements), preventing a rapid translation into cost changes. While it is of interest to study the distribution of CHF costs across NYHA classes, reverse use of such information to predict costs in other situations does not appear to be a suitable option. The study by Szucs et al.19 is based on a random sample of CHF outpatients in Switzerland, whereas the study by Levin et al. is based on the CHARM-trial,30 which contains data from CHF patients in 26 countries; both studies date back to 1999.
Some model inputs used in this analysis were subject to substantial uncertainty. It may, for example, be questionable whether NHS-reported Healthcare Resource Group (HRG) costs really reflect actual hospitalization costs. In the NYHA-based analysis, the most uncertain variable represented UK yearly costs for CHF treatment.
When discussing the generalizability of the presented results, it has to be noted that in routine clinical practice, fewer FCM administrations may be required, as FCM allows injections or infusions of up to 1000 mg in 15 min, which may lead to improved ICERs. The extent to which this dosing regime may be achieved remains uncertain. Our analysis was based on average hospitalization rates and lengths of stay. In routine practice, wide value ranges may occur and resource use may be structured differently, which could lead to an under- or overestimation of costs.
The effect of FCM was seen in a closely followed patient group with high NYHA classes( ∼20% NYHA II and 80% NYHA III) and may be different in other collectives. Compared with a UK routine practice population, the FAIR-HF population was older, consisted of more females, and had lower diastolic and systolic blood pressure and higher co-morbidity.31 The impact of such differences is not currently predictable. The results of FAIR-HF stem from mostly pan-European study sites. There were no sites in the UK, but given the large European contribution one can assume that transferability of results to the UK is not substantially affected by geographical clinical variation. An analysis of anaemic vs. non-anaemic subgroups was not performed, as sample sizes would have been inadequate to perform a reliable analysis.
Study data cover a period of 24 weeks of treatment. Extrapolation of the time horizon to a longer term, e.g. a lifelong time horizon, was not considered adequate as the FAIR-HF trial provides no information on long-term survival or other long-term effects. The cost-effectiveness of longer term iron treatment remains unknown.
Given that the trial only collected very limited resource use data, the results do have an approximate character to some extent. In order to gain more in-depth knowledge about use of FCM in CHF patients, e.g. whether treatment and cost effects are sustained over a longer period, trials covering longer time periods and gathering further resource use should be the focus of further research. In conclusion, it can be noted that over the study period, treatment with FCM in iron-deficient CHF patients with or without anaemia improves symptoms and is likely to be cost-effective in routine clinical practice, from the perspective of the UK NHS.
Funding
Vifor Pharma Ltd, Switzerland. The authors accept full responsibility for the conduct and publication of the study and had access to the study data.
Conflict of interest: F.S.G. and P.R.B. report research funding from Vifor Pharma Ltd, Switzerland. M.S. reports speaker's honoraria and research funding from Vifor Pharma Ltd, Switzerland. P.G.B. and C.M. are employees of Vifor Pharma, Glattbrugg, Switzerland. C.M. holds shares of Galenica. T.D.S. reports no conflict of interest. P.P. reports receiving consulting fees from Vifor Pharma and Amgen, as well as honoraria and research support from Vifor Pharma. S.D.A. reports receiving consulting fees from Vifor Pharma, Amgen, Takeda, and Noxxon, honoraria for lectures from Vifor Pharma and Amgen, and research support from Vifor Pharma.
Appendix
FAIR-HF: (clinicaltrials.gov: NCT00520780). Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009;361:2436–2448.
References
- 1.Bodnar LM, Cogswell ME, McDonald T. Have we forgotten the significance of postpartum iron deficiency? Am J Obstet Gynecol. 2005;193:36–44. doi: 10.1016/j.ajog.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Grondin MA, Ruivard M, Perreve A, Derumeaux-Burel H, Perthus I, Roblin J, Thiollieres F, Gerbaud L. Prevalence of iron deficiency and health-related quality of life among female students. J Am Coll Nutr. 2008;27:337–341. doi: 10.1080/07315724.2008.10719709. [DOI] [PubMed] [Google Scholar]
- 3.van Veldhuisen DJ, Anker SD, Ponikowski P, Macdougall IC. Anemia and iron deficiency in heart failure: mechanisms and therapeutic approaches. Nat Rev Cardiol. 2011;8:485–493. doi: 10.1038/nrcardio.2011.77. [DOI] [PubMed] [Google Scholar]
- 4.Westenbrink BD, Voors AA, de Boer RA, Schuringa JJ, Klinkenberg T, van der Harst P, Vellenga E, van Veldhuisen DJ, van Gilst WH. Bone marrow dysfunction in chronic heart failure patients. Eur J Heart Fail. 2010;12:676–684. doi: 10.1093/eurjhf/hfq061. [DOI] [PubMed] [Google Scholar]
- 5.Lipsic E, van der Meer P. Erythropoietin, iron, or both in heart failure: FAIR-HF in perspective. Eur J Heart Fail. 2010;12:104–105. doi: 10.1093/eurjhf/hfp196. [DOI] [PubMed] [Google Scholar]
- 6.Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Bart B, Banasiak W, Niegowska J, Kirwan B-A, Mori C, von Eisenhart Rothe B, Pocock SJ, Poole-Wilson PA, Ponikowski P. Ferric carboxymaltose in patients with heart failure iron deficiency. N Engl J Med. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 7.Okonko DO, Grzeslo A, Witkowski T, Mandal AK, Slater RM, Roughton M, Foldes G, Thum T, Majda J, Banasiak W, Missouris CG, Poole-Wilson PA, Anker SD, Ponikowski P. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency. FERRIC-HF: a randomized, controlled, observer-blinded trial. J Am Coll Cardiol. 2008;51:103–112. doi: 10.1016/j.jacc.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 8.Usmanov RI, Zueva EB, Silverberg DS, Shaked M. Intravenous iron without erythropoietin for the treatment of iron deficiency anemia in patients with moderate to severe congestive heart failure and chronic kidney insufficiency. J Nephrol. 2008;21:236–242. [PubMed] [Google Scholar]
- 9.Anker SD, Colet JC, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Mori C, von Eisenhart Rothe B, Pocock S, Poole-Wilson PA, Ponikowski P. Rationale and design of Ferinject Assessment in patients with IRon deficiency and chronic Heart Failure (FAIR-HF) study: a randomized, placebo-controlled study of intravenous iron supplementation in patients with and without anaemia. Eur J Heart Fail. 2009;11:1084–1091. doi: 10.1093/eurjhf/hfp140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 11.Hurst NP, Jobanputra P, Hunter M, Lambert M, Lochhead A, Brown H. Validity of Euroqol — a generic health status instrument–in patients with rheumatoid arthritis. Economic and Health Outcomes Research Group. Br J Rheumatol. 1994;33:655–662. doi: 10.1093/rheumatology/33.7.655. [DOI] [PubMed] [Google Scholar]
- 12.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–43. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 13.Dorman PJ, Waddell F, Slattery J, Dennis M, Sandercock P. Is the EuroQol a valid measure of health-related quality of life after stroke? Stroke. 1997;28:1876–1882. doi: 10.1161/01.str.28.10.1876. [DOI] [PubMed] [Google Scholar]
- 14.Curtis L. Unit Costs of Health and Social Care. Canterbury: University of Kent; 2009. Personal Social Services Research Unit (PSSRU) [Google Scholar]
- 15.Curtis L. Unit Costs of Health and Social Care. Canterbury: University of Kent; 2007. Personal Social Services Research Unit (PSSRU) [Google Scholar]
- 16.Falkirk & District Royal Infirmary. Disposables costs collected from the Falkirk & District Royal Infirmary. 2006 [Google Scholar]
- 17.The NHS Information Centre. Hospital Episode Statistics for England. 2008–09 Inpatient statistics 2009; Available from: http://www.hesonline.nhs.uk. (accessed August 2010). [Google Scholar]
- 18.Levin L, Jørgensen E, Eriksson B, Swedberg K, Paulsson T. The cost-effectiveness of candesartan in the treatment of chronic heart failure (HF). An assessment of the low left ventricular ejection fraction (low-lvef) trials in the Candesartan in Heart failure—Assessment of moRtality and Morbidity (CHARM) trial programme. Value in Health. 2008;11:A16. [Google Scholar]
- 19.Szucs T. Gesundheitsökonomische Aspekte der chronischen Herzinsuffizienz. Schweiz Aïrztezeitung. 2003;84:2431–2503. [Google Scholar]
- 20.Stewart S, Jenkins A, Buchan S, McGuire A, Capewell S, McMurray JJJV. The current cost of heart failure to the National Health Service in the UK. Eur J Heart Fail. 2002;4:361–371. doi: 10.1016/s1388-9842(01)00198-2. [DOI] [PubMed] [Google Scholar]
- 21.Department of Health. NHS reference costs. 2008–2009 2010; available from: http://www.dh.gov.uk. (accessed August 2010). [Google Scholar]
- 22.Joint Formulary Committee. British National Formulary. 2011 doi: 10.7861/clinmedicine.9-4-349. ted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Institute for Health and Clinical Excellence. Guide to the methods of technology appraisal. 2008 available from: http://www.nice.org.uk/ (accessed August 2010). [PubMed] [Google Scholar]
- 24.Berry C, Murdoch DR, McMurray JJ. Economics of chronic heart failure. Eur J Heart Fail. 2001;3:283–291. doi: 10.1016/s1388-9842(01)00123-4. [DOI] [PubMed] [Google Scholar]
- 25.Lipsic E, Van Der Meer P, Van Veldhuisen DJ. Erythropoiesis-stimulating agents and heart failure. Cardiovasc Ther. 2010;29:e52–e59. doi: 10.1111/j.1755-5922.2010.00240.x. [DOI] [PubMed] [Google Scholar]
- 26.O'Meara E, de Denus S. Management of anemia and iron deficiency in heart failure. Curr Treat Options Cardiovasc Med. 2010;12:532–548. doi: 10.1007/s11936-010-0095-4. [DOI] [PubMed] [Google Scholar]
- 27.Geisler BP. Treating anemia in heart failure patients: a review of erythropoiesis-stimulating agents. Expert Opin Biol Ther. 2010;10:1209–1216. doi: 10.1517/14712598.2010.500282. [DOI] [PubMed] [Google Scholar]
- 28.Bhandari S, Brownjohn A, Turney J. Effective utilization of erythropoietin with intravenous iron therapy. J Clin Pharm Ther. 1998;23:73–78. doi: 10.1046/j.1365-2710.1998.00147.x. [DOI] [PubMed] [Google Scholar]
- 29.Fishbane S, Frei GL, Maesaka J. Reduction in recombinant human erythropoietin doses by the use of chronic intravenous iron supplementation. Am J Kidney Dis. 1995;26:41–46. doi: 10.1016/0272-6386(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 30.Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759–766. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 31.Davies MK, Hobbs FDR, Davis RC, Kenkre JE, Roalfe AK, Hare R, Wosornu D, Lancashire RJ. Prevalence of left-ventricular systolic dysfunction and heart failure in the Echocardiographic Heart of England Screening study: a population based study. Lancet. 2001;358:439–444. doi: 10.1016/s0140-6736(01)05620-3. [DOI] [PubMed] [Google Scholar]