Abstract
The presynaptic protein alpha-synuclein, associated with Parkinson's Disease (PD), plays a role in dopaminergic neurotransmission and is implicated in impulse control disorders (ICDs) such as drug addiction. In this study we investigated a potential causal relationship between alpha-synuclein and impulsivity, by evaluating differences in motor impulsivity in the 5-choice serial reaction time task (5-CSRTT) in strains of mice that differ in the expression of the alpha-synuclein gene. C57BL/6JOlaHsd mice differ from their C57BL/6J ancestors in possessing a chromosomal deletion resulting in the loss of two genes, snca, encoding alpha-synuclein, and mmrn1, encoding multimerin-1. C57BL/6J mice displayed higher impulsivity (more premature responding) than C57BL/6JOlaHsd mice when the pre-stimulus waiting interval was increased in the 5-CSRTT. In order to ensure that the reduced impulsivity was indeed related to snca, and not adjacent gene deletion, wild type (WT) and mice with targeted deletion of alpha-synuclein (KO) were tested in the 5-CSRTT. Similarly, WT mice were more impulsive than mice with targeted deletion of alpha-synuclein. Interrogation of our ongoing analysis of impulsivity in BXD recombinant inbred mouse lines revealed an association of impulsive responding with levels of alpha-synuclein expression in hippocampus. Expression of beta- and gamma-synuclein, members of the synuclein family that may substitute for alpha-synuclein following its deletion, revealed no differential compensations among the mouse strains. These findings suggest that alpha-synuclein may contribute to impulsivity and potentially, to ICDs which arise in some PD patients treated with dopaminergic medication.
Keywords: Alpha-synuclein, impulse control disorders, impulsivity, Parkinson's disease
Alpha-synuclein has received increasing interest because of its role in Parkinson's disease (PD) (Chartier-Harlin et al. 2004; Kruger et al. 1998; Singleton et al. 2003; Spillantini et al. 1998), in which mutations and haplotypes of the snca gene encoding alpha-synuclein are associated with development of, or increased risk for the disease (Kruger et al. 1998; Polymeropoulos et al. 1997; Satake et al. 2009; Zarranz et al. 2004). A premorbid low impulsive personality in PD patients (Menza 2000; Todes & Lees 1985), characterized by ‘caution, constraint and low novelty-seeking’ has been suggested. Conversely, some PD patients maintained on dopaminergic drugs develop impulse control disorders (ICDs) and uncontrolled drug use (Evans et al. 2009; Voon et al. 2009).
Although the normal biological functions of alpha-synuclein are not well understood, it is postulated to play a role in synaptic vesicular transport and synaptic plasticity (Nemani et al. 2010). Mice lacking alpha-synuclein show alterations in dopaminergic neurotransmission in striatal areas (Abeliovich et al. 2000; Anwar et al. 2011). Furthermore, a growing literature also suggests a role for alpha-synuclein in addiction (Boyer & Dreyer 2007); expression of alpha-synuclein is increased in brain reward areas of cocaine addicts (Mash et al. 2003; Qin et al. 2005) and following psychostimulant treatment in rodents (Brenz Verca et al. 2003; Fornai et al. 2005). The role of impulsivity in drug addiction is increasingly recognized (Dawe & Loxton 2004; Perry & Carroll 2008), and addictive behaviour may be viewed as an example of impaired impulse control (Ambermoon et al. 2010).
One form of impulsivity refers to the difficulty in inhibiting incorrect or inappropriate responses – motor (Evenden 1999; Robbins 2002) or waiting impulsivity (Robinson et al. 2009) – which can be assessed in rodents with the 5-CSRTT (Carli et al. 1983; Oliver et al. 2009; Robbins 2002; Walker et al. 2011). This task assesses attentional performance by the detection of brief visual stimulus presented pseudo-randomly across five spatial locations, requiring a nose-poke into the correct location to obtain a reward. The 5-CSRTT also provides information about aspects of inhibitory response control: premature responding into the holes provides a measure of impulsivity (Robbins 2002); perseverative responding after correct detections represents a measure of compulsivity (Dalley et al. 2011). In order to investigate a potential relationship between alpha-synuclein and impulsivity, we evaluated motor impulsivity in strains of mice that differ in the expression of alpha-synuclein. In the first experiment, we compared C57BL/6JOlaHsd mice, that display a spontaneous chromosomal deletion leading to the loss of two genes, the snca gene encoding alpha-synuclein, and the mmrn1, encoding multimerin-1 (Specht & Schoepfer 2001, 2004), with their C57BL/6J ancestral strain. Although mmrn1 has not been shown to be expressed in the brain (Specht & Schoepfer 2004), and is therefore unlikely to contribute to the behavioural phenotype of C57BL/6JOlaHsd mice, in order to ensure that the differences in impulsivity were indeed related to snca, and not adjacent genes affected by the chromosomal deletion, a second experiment was carried out in which wild type (WT) and mice with targeted deletion of alpha-synuclein (KO) were tested in the 5-CSRTT. Finally, we correlated levels of snca expression with impulsivity in a panel of BXD recombinant inbred mouse strains (Chesler et al. 2003).
Materials and methods
Subjects
For Experiment 1, male mice were obtained from Charles River Laboratories (Arbresle, France) (C57BL/6J; C; n = 12) and from Harlan Laboratories (Bicester, Oxon, UK) (C57BL/6JOlaHsd; H; n = 12).
For Experiment 2 mice carrying targeted null mutation of the gene encoding alpha-synuclein (Abeliovich et al. 2000) were produced in Cardiff University Transgenic Animal Unit by 11 generations of backcrosses with C57BL/6J mice (Charles River). At this stage heterozygous animals were intercrossed and the resulting WT and homozygous null mutant animals were used as breeders for production of experimental cohorts. Thus, experimental mice were the F2 offspring of homozygous knock-out (KO) or WT parents obtained by breeding heterozygous parents. Mouse genotyping was carried out as described previously (Robertson et al. 2004). A total of 31 (15 WT and 16 KO) male mice were shipped to the University of Sussex for testing.
Experiment 3 used male mice from the BXD series of recombinant inbred strains, purchased from Jackson Laboratories (Bar Harbor, ME, USA). In addition to the C57BL/6J and DBA2/J parent lines, the following lines were used: BXD5, BXD11, BXD12, BXD18, BXD21, BXD29, BXD31, BXD32, BXD33 and BXD36. Eight male mice from each strain were imported, and accustomed to the University of Sussex facility for at least 1 month before testing in the 5-CSRTT. Since we found strain BXD11 to be highly aggressive, it was necessary to house this strain in individual cages. In order to maintain similar conditions across groups, all other strains were held under identical conditions. All mice (from Experiments 1, 2 and 3) were 8–12-weeks old at the start of 5-CSRTT training.
Mice were singly-housed, had free access to food and water and were maintained on a 12 h light/dark cycle (lights off at 1900 h) at a temperature of 19–21°C and 50% humidity. Mice were food restricted to reduce their body weights to 85% of their free-feeding weight. A total of five animals did not complete the 5-CSRTT experiment, one mouse from the C group and one mouse from the H group died during the experiment and their data for the 5-CSRTT were not included in the analysis. Two C animals and one H animal failed to learn the 5-CSRTT and were excluded from the experiment. All experiments were approved by the institutional ethics committee and were performed under United Kingdom legislation on animal experimentation [Animal (Scientific Procedures) Act, 1986].
Five-choice serial reaction time task apparatus
The test apparatus consisted of eight mouse operant chambers (Med Associates Inc., St. Albans, VT, USA). Each chamber was housed in a sound-attenuating outer cabinet, with a ventilator fan providing a constant low-level background noise. The left wall of the chamber was curved and contained five apertures fitted with infrared detectors to detect nose-poke responses. The apertures were illuminated by a yellow stimulus light located inside each aperture. The right wall of the chamber contained a receptacle hole with a round access opening where the liquid reinforcer was delivered. Condensed milk solution (30%) was used as a reinforcer (0.01 ml) and was delivered into a small cup by means of a dipper. Head entries into the food magazine were recorded by an infrared photo-cell beam crossing the entrance of the receptacle hole, which could be illuminated by a yellow stimulus light inside the aperture. A house-light was located at the top of the wall above the food magazine. The presentation of stimuli and the recording of the responses were controlled by a Smart Control Package 8IN/16out with an additional interface by MED-PC for Windows (Med Associates Inc., St. Albans, VT, USA).
Experiment 1: Performance of Five-choice serial reaction time task by C57BL/6J and C57BL/6JOlaHsd mice
Habituation to the reinforcer and to the 5-CSRTT boxes
During the first three sessions animals were placed in the boxes for 30 min and the liquid reinforcer was available in the magazine on a continuous schedule. The animal had to nose-poke in the magazine to receive the condensed milk solution (available for 10 seconds), after which the dipper was refilled. The house-light, the magazine light and the stimulus lights in the five holes were turned on during the entire session. During these initial sessions, a drop of condensed milk was placed at the front of each of the nose-poke holes to encourage the mice to approach the holes and nose-poke in them. Magazine head entries and number of reinforcers earned were recorded. After these habituation sessions training in a non-spatial version of the 5-CSRTT began.
Non-spatial 5-CSRTT training
During the non-spatial training sessions the stimulus lights in the five holes were illuminated simultaneously and continuously and a nose-poke in any hole produced the illumination of the food magazine and was reinforced by the delivery of the condensed milk solution (procedure adapted from Patel et al. 2006). These sessions lasted until the animal earned more than 50 reinforcers during two consecutive days and for a maximum of 10 sessions, after which the animal was moved on to the spatial 5-CSRTT training.
Spatial 5-CSRTT training
The session started with the illumination of the house-light and a free delivery of the liquid reinforcer accompanied by the illumination of the food magazine, which signalled the availability of the reinforcer. When the animal nose-poked into the magazine to obtain the reinforcer (dipper on for 3 seconds.) the first trial was initiated. After a fixed interval (inter-trial interval, ITI) one of the stimulus lights in the holes was turned on for a brief time and the animal was required to nose-poke within a certain period of time (limited hold, LH) into the correct hole in order to earn the reinforcer. After a correct response the animal had to nose-poke into the magazine to collect the reward and to initiate the next trial. An incorrect response occurred when the animal made a response in a different hole to the one that had been illuminated, and this was followed by a time out (TO) period during which the lights were turned off for 5 seconds. Responses made into the holes during this period restarted the TO. An error of omission occurred when the animal failed to respond into any of the holes after the completion of the LH and was also followed by a TO. Any response into the holes during the ITI, which means that the stimulus light had not yet been presented, was registered as a premature response and was followed by a TO. After a TO period the next trial was restarted by a nose-poke into the magazine. Perseverative responses, that is, further responses into the holes after a correct response and before the collection of the reward, were registered but had no programmed consequences.
At the beginning of the training, the stimulus duration (SD) was set to 30 seconds and the ITI was set to 2 seconds, but these parameters were adjusted according to the performance of each animal. When the animal produced two consecutive sessions achieving the performance criteria (see Table 1) the SD was reduced in the following pattern: 30, 20, 10, 5, 2.5 and 1.8 seconds (Stage 6, baseline) and the LH and the ITI were set at 5 seconds. Testing was carried out daily (5–6 days a week), and sessions lasted for 100 trials or 30 min, whichever came first. On achieving criterion at Stage 6, animals were further trained for a minimum of seven sessions until stable responding was reached, and the average of the last three sessions under Stage 6 was used to establish baseline performance levels.
Table 1.
Parameters used and criteria for progression during the successive stages of training in the 5-CSRTT (taken from Walker et al. 2011, with permission from the authors)
| Training stage | Stimulus duration (seconds) | Limited hold (seconds) | Inter-trial interval (seconds) | Criteria for progression |
|---|---|---|---|---|
| 1 | 30 | 30 | 2 | >30 Correct trials |
| 2 | 20 | 20 | 2 | >30 Correct trials |
| 3 | 10 | 10 | 5 | >50 Correct trials |
| 4 | 5 | 5 | 5 | >50 Correct trials, >75% accuracy, <25% omissions |
| 5 | 2.5 | 5 | 5 | >50 Correct trials, >75% accuracy, <25% omissions |
| 6 | 1.8 | 5 | 5 | >50 Correct trials, >75% accuracy, <25% omissions |
Following training under baseline conditions, mice were tested under conditions designed to increase premature responding. The effect of lengthening the ITI was tested: long ITI (10 seconds) sessions had a duration of 60 min. Long ITI challenge sessions were chosen because they have been shown to increase premature responding consistently in rats (Dalley et al. 2007) and mice (Oliver et al. 2009). Between each of the long ITI testing sessions mice performed a minimum of 3 days under baseline parameters (see Oliver et al. 2009; Walker et al. 2011 for further details of the 5-CSRTT protocol). The same procedure and time sequence was followed in all three 5-CSRTT experiments (except that in Experiment 2 a 2-month period elapsed between L1 and L2).
Experiment 2: Performance of five-choice serial reaction time task by C57BL/6J WT and alpha-synuclein KO mice
Training was identical to Experiment 1.
Experiment 3: Analysis of recombinant inbred strains
Male mice from 10 BXD recombinant inbred strains (see above) as well as C57BL/6J and DBA2/J parental strains were characterized in the 5-CSRTT. We used the %premature responding during baseline, and each of the three long ITI probe trials, to seek correlations with levels of snca expression in hippocampus, available from the WebQTL database (http://www.genenetwork.org/; Hippocampus consortium M430v2 (June 06) PDNN; Overall et al. 2009).
Quantitative real-time PCR
Brains were removed and stored at −80°C prior to microdissection. Bilateral punches from the prefrontal cortex and hippocampus were taken and used for PCR analysis. RNA was extracted (Trizol) and cleaned (RNeasy MinElute, Qiagen, Hilden, Germany), cDNA was synthesized (iScript Select, Bio-Rad, Hercules, CA, USA) and subjected to quantitative RT-PCR (SYBR Green, Quanti-tect, Qiagen, Hilden, Germany) using the following primer pairs: alpha-synuclein, GATCCTGGCAGTGAGGCTTA and GCTTCAGGCTCATAGTCTTGG; beta-synuclein, GGAACCAGAAGGGGAAAGTT and CTCTGGCTCGTATTCCTGGT; gamma-synuclein, GCCAAAGAGCAAGAGGAGAA and TGTCCCTTGAGCCTCTGTG; GAPDH, TGTCTCCTGCGACTTCAAC and AGCCGTATTCATTGTCATACC. For each sample (run in triplicate) the cycle threshold was determined, and fold change of genes (from WT and Charles River) analysed according to the method of Pfaffl (Pfaffl 2001) and assessed by one-way analysis of variance (ANOVA).
Statistical analysis
The statistical analysis was performed using the ‘Statistical Package for Social Sciences' (SPSS, version 18.0).
The variables considered in the analysis of the 5-CSRTT were
Accuracy (percentage of correct responses): correct responses/(correct responses + incorrect responses + omissions) × 100.
>Percentage of omissions: total omissions/(correct responses + incorrect responses + omissions) × 100.
Percentage of premature responding: premature responses/ (correct responses + incorrect responses + omissions + premature responses) × 100.
Correct latency: latency to nose-poke into the correct hole after the onset of the stimulus (s).
Magazine latency: latency to collect the reward after a correct response (s).
Perseverative responses: total number of responses made into the holes after a correct response and before the collection of the reward.
Two-way repeated measures ANOVA with strain or genotype as the between-subjects factor and session as the within-subjects factor was used for the analysis of each variable of the 5-CSRTT during the long ITI testing. Total number of sessions to achieve criteria of performance in the 5-CSRTT task was analysed by a non-parametric Kruskal-Wallis test. Independent samples t tests and paired samples t tests were used for post hoc analysis. Where sphericity assumptions were violated, the Greenhouse-Geisser correction was applied. The variables ‘accuracy’, ‘percentage omissions' and ‘premature responding’ were arcsine transformed [x′ = 2arcsine (√(x/100))] and ‘magazine latency’ and ‘perseverative responding’ were log_10 transformed in order to attain homogeneity of variance and permit valid parametric analysis. Level of gene expression data was analysed by one-way ANOVAs.
To investigate whether strain differences in premature responding may be attributable to strain differences in internal timing (or time estimation), we additionally analysed patterns of response timing from the onset of each trial. We argued that if premature responding is attributable to an overall tendency to perform at shorter time intervals, there would be a systematic shift in the pattern of responses to shorter time intervals. We thus analysed response latencies for all responses (including premature, correct and incorrect responses) by allocating responses to 1-second time bins, and performing ANOVA with the factors strain and time bin (repeated measure) for each long ITI session.
Results
Experiment 1. Comparison between C57BL/6J (C) and C57BL/6JOlaHsd (H) mice in the 5-CSRTT
Although there were no statistical differences in number of sessions required to achieve baseline performance criteria (H: 41.1 ± 2.87; C: 33.2 ± 2.6), a trend towards a significant effect of strain appeared (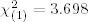 , P = 0.054) suggesting that C mice needed fewer sessions than H mice to learn the task. H mice showed higher levels of accuracy (t17 = 3.894, P = 0.001) but a trend towards more omission errors (t(17) = 1.913, P = 0.073) (see Fig. 1). No group differences appeared in impulsivity (%prematures), motivation (magazine latency) or compulsive responding (perseveratives) during baseline (t < 1.46, n.s.) but H mice showed significantly lower correct response latencies than C mice (t17 = 2.67, P < 0.05; see Fig. 1d).
, P = 0.054) suggesting that C mice needed fewer sessions than H mice to learn the task. H mice showed higher levels of accuracy (t17 = 3.894, P = 0.001) but a trend towards more omission errors (t(17) = 1.913, P = 0.073) (see Fig. 1). No group differences appeared in impulsivity (%prematures), motivation (magazine latency) or compulsive responding (perseveratives) during baseline (t < 1.46, n.s.) but H mice showed significantly lower correct response latencies than C mice (t17 = 2.67, P < 0.05; see Fig. 1d).
Figure 1. Performance of C57BL/ 6J mice from Charles River (C, clear bars) and C57BL/6JOlaHsd mice from Harlan (H, dark bars) in the 5-CSRTT for the baseline (B: mean of the last three sessions on Stage 6) and the three long ITI sessions (L1, L2, L3).

The values represent the mean ± SE of accuracy of responding (a), percentage omissions (b), percentage of premature responses (c), latency to make a correct response (d), latency to collect the liquid reinforcer (e) and total perseverative responses (f). *P < 0.05, **P < 0.01, significant differences between sessions;  P < 0.05, significant differences between strains (post hoc tests).
P < 0.05, significant differences between strains (post hoc tests).
Following the introduction of the long ITI sessions (see Fig. 1), H mice continued to display higher accuracy than C mice (F1,17 = 7.79, P < 0.05) but all mice improved accuracy as the long ITI sessions were repeated (F2,34 = 20.036, P < 0.001). At the same time, in keeping with their baseline performance, H mice consistently showed more omissions than C mice (F1,17 = 5.58, P < 0.05), although both groups decreased the percentage of omissions across the long ITI sessions (F2,34 = 21.332, P < 0.001). The increase in accuracy and the decrease in omissions in consecutive long ITI sessions indicated that the animals adapted successfully to the new parameters of the task.
With regard to the impulsivity measure (%premature responding) strain differences emerged upon the introduction of the long ITI sessions (F2,34 = 5.069, P < 0.05; Fig. 1c). As expected, all animals increased premature responding above the levels shown in baseline sessions (due to the longer waiting time imposed by the new parameters) and post hoc analysis indicated that during the first long ITI session H mice were less impulsive (fewer premature responses) than C mice. Premature responding was also decreased upon successive presentations of the long ITI sessions.
Also consistent with their baseline performance, H mice showed shorter correct response latencies than C mice (F1,17 = 5.57, P < 0.05; Fig. 1d), especially during the first long ITI session (session × group, F2,34 = 3.33, P < 0.05).
Analysis of the overall pattern of response times (Fig. 3) showed that C mice emitted more responses than H mice (F1,17 = 9.242, P < 0.01), and there was a significant variation across time bins (F16,272 = 46.381, P < 0.001) but there was no difference between strains in terms of the pattern of responding (no statistically significant interaction of time bin × group appeared in the analysis: F16,272 = 1.632, n.s.), suggesting no internal timing differences between groups in the first long ITI session. For the second and third long ITI sessions the main effect of group disappeared and only an effect of time bin remained (F16,272 > 114.27, P < 0.001 for L1 and L2). Especially on the first long ITI sessions (see Fig. 3a,d), the data follow a bimodal distribution: premature responses are distributed during the first 10 seconds, while correct and incorrect responses contribute to the values shown after the 10 seconds ITI, showing the highest values immediately after the stimulus is presented.
Figure 3. Distribution of total responses (correct + incorrect + premature) during the total time in which a response can be made for each trial (ITI: 10 seconds + SD: 1.8 seconds + LH: 5 seconds; total = 16.8 seconds) of the long ITI sessions (L1, L2 and L3) in the 5-CSRTT.

Figures a, b and c show the performance of C57BL/6J Charles River mice (C, clear bars) vs. C57BL/6JOlaHsd Harlan mice (H, dark bars) and d, e and f show the performance of alpha-synuclein wild type (WT, clear bars) vs. knock-out mice (KO, dark bars). ITI: inter-trial interval, SD: stimulus duration, LH: Limited hold (time allowed for nose-pose).
No differences between groups or sessions were found in motivation (magazine latency; F < 0.81, n.s.; Fig. 1e), but C mice showed an increase in compulsive (perseverative) responding from the first to the third long ITI session, whereas H mice showed similar levels across sessions and less perseveration than C mice in the third long ITI session (F2,34 = 3.985, P < 0.05; Fig. 1f).
Experiment 2. Comparison of WT and alpha-synuclein KO mice in the 5-CSRTT
Mice did not differ in acquisition of the task as indicated by the similar number of training sessions to achieve performance criteria (WT: 36.87 ± 3.28; KO: 34.69 ± 2.76, 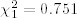 , n.s.,). No statistical differences between groups were found in any of the variables measured during baseline (see Fig. 2, all t < 1.53, n.s.).
, n.s.,). No statistical differences between groups were found in any of the variables measured during baseline (see Fig. 2, all t < 1.53, n.s.).
Figure 2. Performance of alpha-synuclein wild type (WT, clear bars) and knock-out (KO, dark bars) mice in the 5-CSRTT for the baseline (B: mean of the last three sessions on Stage 6) and the three long ITI sessions performed (L1, L2, L3).

The values represent the mean ± SE of accuracy of responding (a), percentage omissions (b), percentage of premature responses (c), latency to make a correct response (d), latency to collect the liquid reinforcer (e) and total perseverative responses (f). *p < 0.05, **p < 0.01, significant differences between sessions;  P < 0.05, significant differences between strains (post hoc tests).
P < 0.05, significant differences between strains (post hoc tests).
Figure 2 also shows the data from the three long ITI sessions. A 2-month period elapsed between the first long ITI (L1) and the second (L2); consequently two separate statistical analyses were performed. During the first long ITI session no statistical differences between groups emerged in any variable (all t < 1.9, n.s.). Analysis of the second and third long ITI sessions showed that both groups presented equal levels of accuracy and of percentage of omissions, although, during the third long ITI session both groups showed a decrease in omissions in comparison with the second long ITI (see Fig. 2b).
With regard to impulsivity, the analysis revealed a session × group interaction in percentage of premature responding (F1,29 = 4.86, P < 0.05; Fig. 2c) due to a decrease in premature responding in snca KO mice compared to WT. Post hoc comparisons showed a significant decrease in premature responding in the third long ITI session compared to Session 2 in KO, but not WT mice (KO = t15 = 5.12, P < 0.0001); (WT = t14 = 1.78, n.s.) and that KO mice were significantly less impulsive than WT during the last long ITI session (t30 = 17.15, P < 0.0001). As for magazine latencies, a session × genotype interaction appeared in the analysis (F1,29 = 45.16, P < 0.05), indicating faster latencies in KO mice during L2 but no differences between groups in L3. No statistical differences in correct latency or perseverative responses (all F1,29 < 2.87, n.s.) were found.
Analysis of the overall pattern of response times in L1, L2 and L3 showed no statistically significant interaction between strain and time bin, suggesting that the decrease in premature responses seen in snca KO mice during L3 was not due to a different timing of responses. Only an effect of ‘time bin’ was found in the repeated measures ANOVA (F6,512 > 45.63, P < 0.001, for L1, L2 and L3; see Fig. 3) which is explained by the differential contribution of premature responses (before 10 seconds) and correct and incorrect responses (from 10 seconds onwards) along the x axis.
Experiment 3. Recombinant inbred strains
As loss of alpha-synuclein was associated with lower %premature responding, we tested the hypothesis that variation in impulsivity across 10 recombinant inbred BXD and parental strains would be associated with expression levels of snca. Premature responding during baseline conditions (R = 0.679; P = 0.028), as well as during the first (R = 0.713; P = 0.0188) and third (R = 0.857; P = 0.00068, see Fig. 4) long ITI correlated positively with alpha-synuclein mRNA levels in hippocampus (Overall et al. 2009), an area of high alpha-synuclein expression (Vivacqua et al. 2011).
Figure 4. Correlation of mRNA levels of alpha-synuclein in hippocampus (y axis) and percentage of premature responding in the third long ITI session (L3, x axis) for DBA2/J, C57BL/6J and BXD5, 11, 12, 21, 29, 31, 32 and 33 strains (no mRNA data available for BXD18 and BXD36 strains).

A highly significant positive correlation between level of expression of alpha-synuclein in hippocampus and percentage of premature responding was seen (r = 0.857, P = 0.00069).
Quantitative real-time PCR
No statistical differences in gene expression of gamma- and beta-synuclein in H and KO groups with comparison to the WT appeared in the analysis, indicating no differences in compensation following loss of snca in KO and H mice (Fig. 3; F < 1.6, n.s.). An effect of group on expression in PFC (F2,22 = 482.7, P < 0.0001, Fig. 5a) and hippocampus (F2,23 = 540.4, P < 0.0001, Fig. 5b) confirmed the absence of expression of snca in H and KO mice.
Figure 5. mRNA quantification of gene expression by means of quantitative RT-PCR.

Messenger RNA levels were normalized against GAPDH. Gene expression of gamma, beta and alpha-synuclein in the Prefrontal Cortex (PFC) (a) and Hippocampus (b) is shown as fold change in expression of Harlan (H) and alpha-synuclein knock-out mice (KO) vs. combined data of WT+Charles mice (±SEM).
Discussion
The present experiments reveal a role for alpha-synuclein in ‘waiting’ impulsivity, based on three pieces of evidence. In Experiment 1, H mice, with a spontaneous deletion of a section of chromosome 6 leading to loss of the snca gene encoding alpha-synuclein, showed lower impulsivity than its ancestral C strain when required to withhold a pre-potent response in the 5-CSRTT. Although both strains showed high levels of accuracy of responding throughout the experiment, in both baseline and challenge conditions H mice performed significantly more accurately than C animals. Together with this superior accuracy, H mice generally showed more omissions (i.e. less readiness to respond) than C mice. This effect appeared during each of the long ITI sessions, suggesting a different strategic approach to the task in the two sub-strains. We can speculate that H mice adopted a more conservative or inhibited approach, which might be described by a rule ‘when uncertain, do not respond, but when certain respond quickly’ resulting in more omissions, but higher accuracy and shorter correct latencies. The observation that H mice showed higher speed of responding (shorter correct latencies) rules out general motor impairment or a diminished behavioural activation in this strain. Following the same argument, the higher level of impulsive (premature) responses in C mice was not due to a greater readiness to respond because they showed slower correct latencies than H mice. At the same time, the differences between sub-strains in their latencies to make a correct response were not due to differences in general responding because latencies of incorrect and premature responses did not differ significantly between groups (data not shown) suggesting that this effect is specific to correct responses.
A potential explanation of increased premature responding might include differences in internal time perception (see Sanchez-Roige et al. in press, for further discussion of the potential contribution of timing to impulsivity in the task). To address this possibility, we analysed patterns of responding (including correct, incorrect and premature responses) during the total time allowed in each trial; differences in time estimation would result in time-shifts of all responses. No evidence was found of altered timing behaviour between C57BL/6J and C57BL/6JOlaHsd strains. It thus seems unlikely that the differences we observed in premature responding are attributable to differences in internal timing between strains.
C57BL/6J mice thus appear to have difficulties in waiting to obtain the information necessary for accurate responding, or show more inflexibility in the switch from baseline to long ITI sessions. It is unlikely that differences in motivation to obtain the reinforcer contribute to differences in impulsive responding as magazine latencies did not differ between groups either for the baseline or long ITI conditions. With regard to perseverative responding, C mice showed generally higher levels than H mice and their perseveration increased across successive sessions as compared with the more stable pattern of responses in the H mice. However, because the chromosomal deletion resulted in the loss of genetic material in addition to the snca gene, these findings cannot be viewed as conclusive, even though multimerin1 is not expressed in the CNS (Specht & Schoepfer 2004), and is therefore unlikely to have contributed to the impulsive phenotype.
In the second experiment, WT and snca KO mice did not show differences in any of the variables measured in the training phases of the 5-CSRTT. However, like H mice, KO mice showed lower levels of premature responding than WT mice during the third long ITI session. Together these findings support the hypothesis that alpha-synuclein is involved in the regulation of impulsivity. Again, analysis of response timing failed to show differences in the pattern of responses between WT and KO mice, indicating that the differences in premature responses during L3 were not due to altered internal timing in the mutant mice.
Despite similarities in the two experiments, in Experiment 2 differences in impulsivity between groups did not appear until the third long ITI session. An informal comparison of performance during baseline conditions between the two experiments finds reduced levels of premature responding in Experiment 1 in comparison with Experiment 2 (see Figs. 1c and 2c), and equally, in the first long ITI session, premature responses were higher in Experiment 2. Thus, baseline differences in the two experiments could in principle account for the delay in the appearance of the less impulsive phenotype in KO mice in comparison with H mice. With regard to perseverative responding, a closer examination of the values during baseline finds higher values in C mice in comparison to H mice, which in turn resemble WT and KO mice.
Another potential explanation for the differences associated with loss of the snca gene in the two experiments could be different compensations within the synuclein family resulting from the different extents of the deletions in the mice. The chromosomal deletion in the H mice results in the loss of the coding regions of both the snca and mmrn1 genes together with regulatory regions of these, and potentially other genes (Specht & Schoepfer 2001, 2004), and unidentified microRNA-encoding sequences that might trans-regulate expression of other genes. In contrast, the snca KO mouse has been created by the deletion of the first two exons of the gene, encoding amino acids 1–41 and upstream untranslated regions (Abeliovich et al. 2000). The more localized disruption of the snca gene in the KO mice could trigger different compensations within the synuclein family compared to those taking place in the H mice, which could contribute to the differences in the two experiments. The synucleins are a family of three members, alpha-, beta- and gamma-synuclein that share considerable N-terminal amino acid sequence homology (Jakes et al. 1994; Nakajo et al. 1993). When one or more synucleins is deleted, the remaining members of the family are upregulated in some brain regions (Chandra et al. 2004; Robertson et al. 2004). That similar changes in gene regulation occur in response to targeted deletion of alpha- or gamma-synuclein, suggests a degree of functional overlap (Kuhn et al. 2007). We addressed this issue by investigating levels of expression of all three members of the synuclein family in prefrontal cortex and hippocampus of C and WT strains, as well as H and KO mice. We found no evidence for differential compensations at the gene expression level of gamma- or beta-synuclein in H or KO mice in comparison with the wild-types, suggesting that differences between H and KO mice are not attributable to differences in compensatory mechanisms within the synuclein family in prefrontal cortex or hippocampus. Nevertheless, the possibility of differential compensations within other brain areas such as the striatum (dorsal/ventral), or at a protein level, cannot be ruled out.
Third, we compared levels of impulsive responding across a series of mouse strains from the BXD series of recombinant inbred strains, and correlated levels of impulsivity with levels of expression of the snca gene in hippocampus, a brain area in which snca expression is prominent (Vivacqua et al. 2011), and which has been implicated in impulsive behaviour (Urcelay & Dalley 2011). Remarkably, the level of snca expression was associated with premature responding under baseline as well as long ITI conditions. Specifically, the correlation between snca expression in hippocampus and impulsivity levels was especially significant during the third long ITI session, which accounted for 73% of the variation in L3 impulsivity. While such correlative evidence can only be suggestive, taken with the convergent findings from Experiments 1 and 2, these results implicate snca expression in 5-CSRTT impulsivity.
Although the normal biological functions of alpha-synuclein are not well understood, it plays a role in synaptic vesicular transport and synaptic plasticity (Burre et al. 2010; Greten-Harrison et al. 2010; Murphy et al. 2000; Nemani et al. 2010). Mice lacking alpha-synuclein show alterations in dopaminergic neurotransmission in striatal areas (Abeliovich et al. 2000; Anwar et al. 2011; Nemani et al. 2010; Senior et al. 2008; Venda et al. 2010). Possible changes in dopamine function as a result of the deletion of the snca gene might give rise to the low impulsive phenotype seen in H and KO mice in the present investigation. In line with this, rats selected for high impulsivity in the 5-CSRTT show low levels of dopamine D2/3 receptors in ventral striatum (Dalley et al. 2007), and PD patients with a pathological gambling disorder had lower D2/3 receptor binding in ventral striatum (Steeves et al. 2009).
PD patients have been suggested to possess a premorbid low impulsive personality (Dagher & Robbins 2009; Ishihara & Brayne 2006; Menza 2000; Paulson & Dadmehr 1991; Todes & Lees 1985). Following manifestation of the condition, an association between PD and ICDs has been increasingly reported. These include repetitive, excessive and compulsive activities that interfere with normal life (Evans et al. 2005, 2009; Lawrence et al. 2007), manifested as pathological gambling, compulsive shopping, punding (repetitive purposeless behaviours) and hypersexuality, that appear in PD patients after dopamine replacement therapy (For reviews see Evans et al. 2009; Voon et al. 2007, 2009). As not all patients taking dopaminergic agonist treatment develop ICD, it has been speculated that dopaminergic medication might trigger ICD in individuals with specific impulsivity traits (Isaias et al. 2008; Voon et al. 2007, 2009). The results from the present investigation suggest that it is worth investigating whether alpha-synuclein may contribute to these differences in impulsivity present in PD patients, possibly through its modulation of the dopaminergic system (Dagher & Robbins 2009; Venda et al. 2010). Whether differences in levels of expression, or haplotypic variations in the gene encoding alpha-synuclein contribute to the propensity to develop ICDs remains to be investigated.
Acknowledgments
This work was funded by the IMAGEN consortium that receives research funding from the European Community's Sixth Framework Programme (LSHM-CT-2007-037286). This paper reflects only the authors' views and the Community is not liable for any use that may be made of the information contained therein. V.L.B. is supported by The Wellcome Trust grant 075615/Z/04/Z. The authors declare no conflicts of interest.
References
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Ambermoon P, Carter A, Hall WD, Dissanayaka NN, O'Sullivan JD. Impulse control disorders in patients with Parkinson's disease receiving dopamine replacement therapy: evidence and implications for the addictions field. Addiction (Abingdon, England) 2010;106:283–293. doi: 10.1111/j.1360-0443.2010.03218.x. [DOI] [PubMed] [Google Scholar]
- Anwar S, Peters O, Millership S, Ninkina N, Doig N, Connor-Robson N, Threlfell S, Kooner G, Deacon RM, Bannerman DM, Bolam JP, Chandra SS, Cragg SJ, Wade-Martins R, Buchman VL. Functional alterations to the nigrostriatal system in mice lacking all three members of the synuclein family. J Neurosci. 2011;31:7264–7274. doi: 10.1523/JNEUROSCI.6194-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer F, Dreyer JL. Alpha-synuclein in the nucleus accumbens induces changes in cocaine behaviour in rats. The European journal of neuroscience. 2007;26:2764–2776. doi: 10.1111/j.1460-9568.2007.05878.x. [DOI] [PubMed] [Google Scholar]
- Brenz Verca MS, Bahi A, Boyer F, Wagner GC, Dreyer JL. Distribution of alpha- and gamma-synucleins in the adult rat brain and their modification by high-dose cocaine treatment. The European journal of neuroscience. 2003;18:1923–1938. doi: 10.1046/j.1460-9568.2003.02913.x. [DOI] [PubMed] [Google Scholar]
- Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science (New York, NY) 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behavioural brain research. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- Chandra S, Fornai F, Kwon HB, Yazdani U, Atasoy D, Liu X, Hammer RE, Battaglia G, German DC, Castillo PE, Sudhof TC. Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions. Proc Natl Acad Sci USA. 2004;101:14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Wang J, Lu L, Qu Y, Manly KF, Williams RW. Genetic correlates of gene expression in recombinant inbred strains: a relational model system to explore neurobehavioral phenotypes. Neuroinformatics. 2003;1:343–357. doi: 10.1385/NI:1:4:343. [DOI] [PubMed] [Google Scholar]
- Dagher A, Robbins TW. Personality, addiction, dopamine: insights from Parkinson's disease. Neuron. 2009;61:502–510. doi: 10.1016/j.neuron.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science (New York, N.Y.) 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Dawe S, Loxton NJ. The role of impulsivity in the development of substance use and eating disorders. Neuroscience and Biobehavioral Reviews. 2004;28:343–351. doi: 10.1016/j.neubiorev.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Evans AH, Lawrence AD, Potts J, Appel S, Lees AJ. Factors influencing susceptibility to compulsive dopaminergic drug use in Parkinson disease. Neurology. 2005;65:1570–1574. doi: 10.1212/01.wnl.0000184487.72289.f0. [DOI] [PubMed] [Google Scholar]
- Evans AH, Strafella AP, Weintraub D, Stacy M. Impulsive and compulsive behaviors in Parkinson's disease. Mov Disord. 2009;24:1561–1570. doi: 10.1002/mds.22505. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Fornai F, Lenzi P, Ferrucci M, Lazzeri G, di Poggio AB, Natale G, Busceti CL, Biagioni F, Giusiani M, Ruggieri S, Paparelli A. Occurrence of neuronal inclusions combined with increased nigral expression of alpha-synuclein within dopaminergic neurons following treatment with amphetamine derivatives in mice. Brain Res Bull. 2005;65:405–413. doi: 10.1016/j.brainresbull.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Greten-Harrison B, Polydoro M, Morimoto-Tomita M, Diao L, Williams AM, Nie EH, Makani S, Tian N, Castillo PE, Buchman VL, Chandra SS. alphabetagamma-Synuclein triple knockout mice reveal age-dependent neuronal dysfunction. Proc Natl Acad Sci USA. 2010;107:19573–19578. doi: 10.1073/pnas.1005005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaias IU, Siri C, Cilia R, De Gaspari D, Pezzoli G, Antonini A. The relationship between impulsivity and impulse control disorders in Parkinson's disease. Mov Disord. 2008;23:411–415. doi: 10.1002/mds.21872. [DOI] [PubMed] [Google Scholar]
- Ishihara L, Brayne C. What is the evidence for a premorbid Parkinsonian personality: a systematic review. Mov Disord. 2006;21:1066–1072. doi: 10.1002/mds.20980. [DOI] [PubMed] [Google Scholar]
- Jakes R, Spillantini MG, Goedert M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994;345:27–32. doi: 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nature Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Kuhn M, Haebig K, Bonin M, Ninkina N, Buchman VL, Poths S, Riess O. Whole genome expression analyses of single- and double-knock-out mice implicate partially overlapping functions of alpha- and gamma-synuclein. Neurogenetics. 2007;8:71–81. doi: 10.1007/s10048-007-0079-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Blackwell AD, Barker RA, Spagnolo F, Clark L, Aitken MR, Sahakian BJ. Predictors of punding in Parkinson's disease: results from a questionnaire survey. Mov Disord. 2007;22:2339–2345. doi: 10.1002/mds.21702. [DOI] [PubMed] [Google Scholar]
- Mash DC, Ouyang Q, Pablo J, Basile M, Izenwasser S, Lieberman A, Perrin RJ. Cocaine abusers have an overexpression of alpha-synuclein in dopamine neurons. J Neurosci. 2003;23:2564–2571. doi: 10.1523/JNEUROSCI.23-07-02564.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menza M. The personality associated with Parkinson's disease. Curr Psychiatry Rep. 2000;2:421–426. doi: 10.1007/s11920-000-0027-1. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Rueter SM, Trojanowski JQ, Lee VM. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000;20:3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajo S, Tsukada K, Omata K, Nakamura Y, Nakaya K. A new brain-specific 14-kDa protein is a phosphoprotein. Its complete amino acid sequence and evidence for phosphorylation. Eur J Biochem/FEBS. 1993;217:1057–1063. doi: 10.1111/j.1432-1033.1993.tb18337.x. [DOI] [PubMed] [Google Scholar]
- Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, Chaudhry FA, Nicoll RA, Edwards RH. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65:66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver YP, Ripley TL, Stephens DN. Ethanol effects on impulsivity in two mouse strains: similarities to diazepam and ketamine. Psychopharmacology. 2009;204:679–692. doi: 10.1007/s00213-009-1500-0. [DOI] [PubMed] [Google Scholar]
- Overall RW, Kempermann G, Peirce J, Lu L, Goldowitz D, Gage FH, Goodwin S, Smit AB, Airey DC, Rosen GD, Schalkwyk LC, Sutter TR, Nowakowski RS, Whatley S, Williams RW. Genetics of the hippocampal transcriptome in mouse: a systematic survey and online neurogenomics resource. Front Neurosci. 2009;3:55. doi: 10.3389/neuro.15.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Stolerman IP, Asherson P, Sluyter F. Attentional performance of C57BL/6 and DBA/2 mice in the 5-choice serial reaction time task. Behav Brain Res. 2006;170:197–203. doi: 10.1016/j.bbr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Paulson GW, Dadmehr N. Is there a premorbid personality typical for Parkinson's disease? Neurology. 1991;41:73–76. doi: 10.1212/wnl.41.5_suppl_2.73. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology. 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science (New York, N.Y.) 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Qin Y, Ouyang Q, Pablo J, Mash DC. Cocaine abuse elevates alpha-synuclein and dopamine transporter levels in the human striatum. Neuroreport. 2005;16:1489–1493. doi: 10.1097/01.wnr.0000175617.39054.ba. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robertson DC, Schmidt O, Ninkina N, Jones PA, Sharkey J, Buchman VL. Developmental loss and resistance to MPTP toxicity of dopaminergic neurones in substantia nigra pars compacta of gamma-synuclein, alpha-synuclein and double alpha/gamma-synuclein null mutant mice. J Neurochem. 2004;89:1126–1136. doi: 10.1111/j.1471-4159.2004.02378.x. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Economidou D, Theobald DE, Mar AC, Murphy ER, Robbins TW, Dalley JW. Behavioural characterisation of high impulsivity on the 5-choice serial reaction time task: specific deficits in ‘waiting’ versus ‘stopping’. Behav Brain Res. 2009;196:310–316. doi: 10.1016/j.bbr.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Sanchez-Roige S, Peña-Oliver Y, Stephens DN. Measuring Impulsivity in mice: the 5-Choice serial reaction time task. Psychopharmacology (in press) [DOI] [PubMed]
- Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, Kawaguchi T, Tsunoda T, Watanabe M, Takeda A, Tomiyama H, Nakashima K, Hasegawa K, Obata F, Yoshikawa T, Kawakami H, Sakoda S, Yamamoto M, Hattori N, Murata M, Nakamura Y, Toda T. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nat Genet. 2009;41:1303–1307. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- Senior SL, Ninkina N, Deacon R, Bannerman D, Buchman VL, Cragg SJ, Wade-Martins R. Increased striatal dopamine release and hyperdopaminergic-like behaviour in mice lacking both alpha-synuclein and gamma-synuclein. The Eur J Neuroscience. 2008;27:947–957. doi: 10.1111/j.1460-9568.2008.06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, et al. alpha-Synuclein locus triplication causes Parkinson's disease. Science (New York, N.Y.) 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Specht CG, Schoepfer R. Deletion of the alpha-synuclein locus in a subpopulation of C57BL/6J inbred mice. BMC Neurosci. 2001;2:11. doi: 10.1186/1471-2202-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht CG, Schoepfer R. Deletion of multimerin-1 in alpha-synuclein-deficient mice. Genomics. 2004;83:1176–1178. doi: 10.1016/j.ygeno.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves TD, Miyasaki J, Zurowski M, Lang AE, Pellecchia G, Van Eimeren T, Rusjan P, Houle S, Strafella AP. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: a [11C] raclopride PET study. Brain. 2009;132:1376–1385. doi: 10.1093/brain/awp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todes CJ, Lees AJ. The pre-morbid personality of patients with Parkinson's disease. J Neurol, Neurosurg, Psychiatry. 1985;48:97–100. doi: 10.1136/jnnp.48.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcelay GP, Dalley JW. Linking ADHD, Impulsivity, and Drug Abuse: A Neuropsychological Perspective. Curr Topics Behav Neurosci. 2011 doi: 10.1007/7854_2011_119. DOI: 10.1007/7854_2011_1119. [DOI] [PubMed] [Google Scholar]
- Venda LL, Cragg SJ, Buchman VL, Wade-Martins R. alpha-Synuclein and dopamine at the crossroads of Parkinson's disease. Trends Neurosci. 2010;33:559–568. doi: 10.1016/j.tins.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivacqua G, Casini A, Vaccaro R, Fornai F, Yu S, D'Este L. Different sub-cellular localization of alpha-synuclein in the C57BL\6J mouse's central nervous system by two novel monoclonal antibodies. J Chem Neuroanat. 2011;41:97–110. doi: 10.1016/j.jchemneu.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Voon V, Potenza MN, Thomsen T. Medication-related impulse control and repetitive behaviors in Parkinson's disease. Curr Opin Neurol. 2007;20:484–492. doi: 10.1097/WCO.0b013e32826fbc8f. [DOI] [PubMed] [Google Scholar]
- Voon V, Fernagut PO, Wickens J, Baunez C, Rodriguez M, Pavon N, Juncos JL, Obeso JA, Bezard E. Chronic dopaminergic stimulation in Parkinson's disease: from dyskinesias to impulse control disorders. Lancet Neurol. 2009;8:1140–1149. doi: 10.1016/S1474-4422(09)70287-X. [DOI] [PubMed] [Google Scholar]
- Walker SE, Pena-Oliver Y, Stephens DN. Learning not to be impulsive: disruption by experience of alcohol withdrawal. Psychopharmacology. 2011;217:433–442. doi: 10.1007/s00213-011-2298-0. [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]


