Abstract
Background and Aims
Despite the considerable number of studies on the impacts of climate change on alpine plants, there have been few attempts to investigate its effect on regeneration. Recruitment from seeds is a key event in the life-history of plants, affecting their spread and evolution and seasonal changes in climate will inevitably affect recruitment success. Here, an investigation was made of how climate change will affect the timing and the level of germination in eight alpine species of the glacier foreland.
Methods
Using a novel approach which considered the altitudinal variation of temperature as a surrogate for future climate scenarios, seeds were exposed to 12 different cycles of simulated seasonal temperatures in the laboratory, derived from measurements at the soil surface at the study site.
Key Results
Under present climatic conditions, germination occurred in spring, in all but one species, after seeds had experienced autumn and winter seasons. However, autumn warming resulted in a significant increase in germination in all but two species. In contrast, seed germination was less sensitive to changes in spring and/or winter temperatures, which affected only three species.
Conclusions
Climate warming will lead to a shift from spring to autumn emergence but the extent of this change across species will be driven by seed dormancy status. Ungerminated seeds at the end of autumn will be exposed to shorter winter seasons and lower spring temperatures in a future, warmer climate, but these changes will only have a minor impact on germination. The extent to which climate change will be detrimental to regeneration from seed is less likely to be due to a significant negative effect on germination per se, but rather to seedling emergence in seasons that the species are not adapted to experience. Emergence in autumn could have major implications for species currently adapted to emerge in spring.
Keywords: Seed ecology, seed germination, climate change, adaptation, alpine plants
INTRODUCTION
Alpine climates have undergone significant change over the past century. In many parts of the Alps temperatures have risen by up to 2 °C between 1901 and 2000 (Beniston et al., 1997) which is well above the observed global increase in temperatures of 0·7 °C (Jones and Moberg, 2003). In this century, the warming in the Alps is projected to continue. For example, under A1FI and B1 scenarios (IPCC, 2007), the predicted average temperature change for the European mid-latitude mountains is between +2·9 °C and +5·3 °C for 2085 (Nogués-Bravo et al., 2007).
Alpine ecosystems are considered to be particularly sensitive to the effects of global warming, because they are characterized by species adapted to low temperatures (Körner, 1999). In response to climate warming in alpine habitats there are expected and partially documented reductions in the duration and depth of snow cover (Valt and Cianfarra, 2010) and rapid glacier retreat (Paul et al., 2004) – these changes are associated with changing distribution, phenology and physiology of several plant species (Grabher et al., 1994; Sandvik et al., 2004; Klanderud and Totland, 2005; Gottfried et al., 2012). Across the various mountain ranges, the species distributed at higher elevations are expected to be at the greatest level of threat (Engler et al., 2011).
Despite the many studies on the overall impact of climate change on plants (see Pautasso et al., 2010), the effects on plant regeneration has largely been neglected (Hedhly et al., 2009). However, climate has a large influence on plant recruitment (Lloret et al., 2004; Fay and Schultz, 2009; Dalgleish et al., 2010). For example, with shortening of winter (Dong et al., 2010), seeds may remain partially dormant in spring and need an extended time to germinate (Walck et al., 1997). The alteration of temperature and water supply due to global climate change could preclude, delay or enhance regeneration from seeds (Walck et al., 2011).
In alpine habitats, the upward migration of plant species is clearly one of the responses to climate warming (Lenoir et al., 2008) and seed dispersal and their ability to germinate and establish in new sites at higher altitude are thought to be the main vehicles for plant migration (Parolo and Rossi, 2008; Vittoz et al., 2009). Regeneration from seeds is therefore likely to play an important role in the responses of alpine species to climate change. However, the effect of climate warming on seed germination itself in alpine plants has not been extensively investigated (but see Milbau et al., 2009; Shevtsova et al., 2009). Even fewer studies have addressed such responses in pioneer species of the glacier forelands (Erschbamer, 2007).
Seed germination has long being considered rare in the alpine habitat (Körner, 1999), which is mostly characterized by vegetative reproduction by clonal plants (de Kroon and van Groenendael, 1997). However, a number of studies have found high rates of seedling establishment (Niederfriniger and Erschbamer, 2000; Forbis, 2003) and diversity of genotypes (Jonsson et al., 1996; Gabrielsen, 1998) within alpine plants, arguing the importance of sexual reproduction in the maintenance of plant populations. Consequently, factors that affect the ability of seeds to develop into adult plants are likely to affect the fate of plant populations and communities.
Early studies on germination ecology of alpine plants demonstrated that freshly collected seeds of most arctic and alpine species required relatively high temperatures for germination (Bliss, 1958; Amen, 1966, Billings and Mooney, 1968), indicating that seed germination might benefit from a warmer climate. However, if high temperatures promote better germination, why is it that alpine species tend to migrate to higher altitudes in response to climate warming? The requirement of high temperatures for germination has been considered an adaptation to prevent early spring or autumn germination when a high likelihood of frost may result in a low probability of seedling establishment (Cavieres and Arroyo, 2000). Indeed, although a substantial fraction of fresh seeds of alpine plants are non-dormant or only conditionally dormant, the prevailing low temperatures in autumn, combined with short day lengths, are likely to prevent germination (Schwienbacher et al., 2011). After exposure to winter, the temperature window for germination usually widens toward lower values as a result of the process of loss of physiological dormancy (Baskin and Baskin, 1998). Hence, despite the fact that seed dispersal occurs mostly at the end of summer/early autumn, seed germination and seedling establishment of alpine plants tends to occur rapidly after snowmelt, in early summer (Körner, 1999). Nevertheless, in a future warmer climate the possibility that an increase in temperature might stimulate germination in autumn of non-dormant or only conditionally dormant species cannot be ruled out.
In our study, in order to investigate how the germination of alpine plants can be affected by a warmer climate, seeds of eight glacier foreland species were subjected to different climatic scenarios, derived from measurements taken at soil surface level in our study area located in the Alps. The incubation temperatures simulated (a) the current seasonal temperatures at the species growing site, (b) those at 400 m lower in altitude and (c) an increase of 4 °C to the current spring, summer and autumn temperatures recorded at the species growing site. Furthermore, climate warming in alpine ecosystems is not only expected to result in a significant increase in temperature, but also in a reduction in snow cover duration and snowfall (IPCC, 2007), as already detected, for example, during the last 40 years in the Italian Alps (Valt and Cianfarra, 2010). Hence, further cycles investigating the effects of the absence of snow cover in winter were set up, using the climatic data provided by an automatic weather station (LSI-LASTEM) located in the study area at 2100 m a.s.l.
Specific research questions were: Is the current upward plant migration explained in terms of inhibition of seed germination in a warmer climate? Do germination responses to temperature change vary or are they consistent across species? Does an increase in temperature inhibit or promote germination in different season across the year? How is germination affected by subzero winter temperatures?
MATERIALS AND METHODS
Study species
Seeds were collected at the time of natural dispersal (Hay and Smith, 2003) on 1 September 2010 from the following eight species selected on occurrence and abundance in the 150-year ice-free moraine of the glacier Dosdé (46 °24′N; 10 °12′E; approx. 2500 m a.s.l.), in the Alps of Lombardy (Sondrio, northern Italy): Poa laxa Haenke subsp. laxa (Poaceae), Geum reptans L. (Rosaceae), Luzula alpinopilosa (Chaix) Breistr. (Juncaceae), Veronica alpina L. (Scrophulariaceae), Adenostyles leucophylla Rchb. (Asteraceae), Doronicum clusii (All.) Tausch (Asteraceae), Cerastium pedunculatum Gaudin ex Ser. (Caryophyllaceae) and Oxyria digyna Hill (Polygonaceae). These species are some of the most common taxa that colonize the glacier foreland in the Alps (Pirola and Credaro, 1994). Two days after harvest, seeds were sown on agar for the laboratory experiments. Hereafter, to facilitate reading, each species will be referred to by its genus name.
Germination phenology under simulated monthly temperatures
Laboratory treatments involved sowing three replicates of 30 seeds each on 1 % distilled water–agar held in 90-mm-diameter Petri dishes. Treatments were carried out in temperature and light-controlled incubators using a 12-h daily photoperiod (photosynthetically active radiation 40–50 µmol m−2 s−1).
Earlier studies on the effects of climate change on the germination of seeds from alpine/arctic plants, either through laboratory (Milbau et al., 2009) or field (Shevtsova et al., 2009) experiments, only considered increased spring/summer temperatures. However, in our study an alternative scenario of climate warming was chosen, based on the natural increase of temperatures with decreasing altitude at the study area. From the time of collection, seeds were exposed to temperature cycles simulating monthly changes occurring at the species growing site and, in order to simulate warmer conditions, to the temperatures occurring 400 m lower in altitude (Table 1A, temperature treatments ‘2500 m’ and ‘2100 m’, respectively). This difference of altitude was chosen to correspond to approx. 2·4 °C difference of mean annual temperature (considering a decrease of approx. 0·6 °C/100 m in the Alps; Körner, 1999) to reflect a less pessimistic scenario of temperature increase in the next century for mountain ecosystems (2·9–5·3 °C; Nogués-Bravo et al., 2007). Each cycle of temperature was set up considering the mean monthly diurnal (0800–2000 h) and nocturnal (2000–0800 h) temperatures, based on measurements taken at hourly intervals using Tiny Tag data loggers (Gemini, Chichester, West Sussex, UK) buried approx. 2 cm deep in the soil. Data loggers were buried for 1 year (1 September 2009 to 1 September 2010) at each of the two altitudinal sites (2500 and 2100 m a.s.l.) in south-west-facing open areas, chosen in order to be representative of the ecological niche of the target species. The first snow-free day after winter and the length of the growing season (daily mean temperature ≥2 °C) were derived from the temperature data. During the period of measurement (12 months), the mean temperature at 2100 m a.s.l. was approx. 2·6 °C higher than that recorded at 2500 m, in accordance with our expectation of temperature increase (approx. 2·4 °C, see above). In autumn 2009 snowfall began at both altitudes in mid-October, while in spring 2010 snow melted about 2 months earlier at 2100 m a.s.l. (end of April) than at 2500 m a.s.l. (end of June). Consequently, at 2500 m a.s.l. the growing season was about a 2 months shorter than that at 2100 m (Fig. 1).
Table 1.
Temperatures (°C) during the different weeks of the incubation treatments
| Current winter |
Subzero winter |
||||||
|---|---|---|---|---|---|---|---|
| Week of the experiments | Equivalent time of year | 2500 m | 2500 m + 4 °C | 2100 m | 2500 m | 2500 m + 4 °C | 2100 m |
| 1–4 | September | 12/5 | 16/9 | 17/6 | 12/5 | 16/9 | 17/6 |
| 5–12 | October–November | 0 | 0 | 0 | 0 | 0 | 0 |
| 13–28 | December–March | 0 | 0 | 0 | –7 | –7 | –7 |
| 29–32 | April | 0 | 0 | 0 | 0 | 0 | 0 |
| 33–36 | May | 0 | 0 | 15/3 | 0 | 0 | 15/3 |
| 37–40 | June | 0 | 0 | 21/7 | 0 | 0 | 21/7 |
| 41–44 | July | 20/8 | 24/12 | 24/10 | 20/8 | 24/12 | 24/10 |
| 45–48 | August | 16/6 | 20/10 | 19/8 | 16/6 | 20/10 | 19/8 |
Different styling indicates the simulated seasons of autumn (normal text), winter (italic) and spring/summer (bold).
Fig. 1.
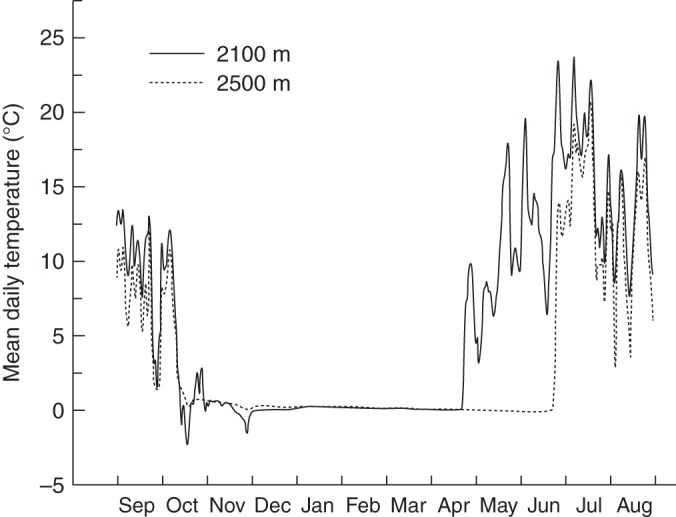
Mean daily temperatures (°C) about 2 cm deeep in the soil at the species growing site (2500 m a.s.l., dashed line) and at 400 m lower (2100 m a.s.l., continuous line), between September 2009 and September 2010.
According to current climate change scenarios, in the European mid-latitude mountains temperature is expected to rise about 2·9–5·3 °C (mean approx. 4 °C) in the coming 100 years (Nogués-Bravo et al., 2007), mostly because of an increase in summer temperatures (Casty et al., 2005; Giorgi, 2006). Here, in order to investigate the effects that such an increase might have on seed germination, a further cycle of simulated seasonal temperature was set up considering an increase of 4 °C to the temperatures recorded in summer and autumn (July–October) at the species growing site (Table 1A, temperature treatment ‘2500 + 4 °C’). Because of the insulation effect of the snow cover, winter temperature was kept at constant 0 °C, consistent with the temperature measurements (Fig. 1).
In order to simulate the effect of a strong reduction in snow cover during winter, three additional cycles of simulated seasonal temperatures were set up, considering the mean winter temperatures at about 2 m above the ground. In this case, winter temperature resulted from measurements recorded at hourly intervals during 2009 and 2010 using an automatic weather station (LSI-LASTEM) located in the study area, near to the study site at 2100 m a.s.l. The duration of winter was decided to be equivalent to the timing of snow cover at the site of measurements (October–April). During this period the mean air temperature was approx. 0 °C in October, November and April and approx. –7 °C between December and March. Before and after this period, seeds were exposed to the temperatures resulting from soil-surface measurements described above (Table 1).
In each case a 12 h/12 h thermo-period was used and illumination was provided during the warm phase, except during the cold stratification period (0 °C and/or –7 °C) which was conducted in complete darkness.
Germination phenology under different spring/summer scenarios in the absence of autumn
Since we expected a certain level of germination in autumn, the purpose was to assess the influence of autumn temperature on spring/summer emergence and to investigate the effects of the different spring/summer scenarios using a full population of ungerminated seeds. Hence, to serve as controls, the same six cycles of simulated seasonal temperatures listed in Table 1 were further investigated in the absence of autumn conditions.
Data analysis
During incubation seeds were checked for radicle emergence at weekly intervals until the end of each test and germinated seeds were removed. The final proportion of germinated seeds between each test has been compared using analysis of variance (ANOVA), followed by post-hoc Turkey HSD (Honest Significant Difference) multiple comparison test (SPSS 13·0). Data were arcsin transformed to improve normality and stabilize variances.
In addition to the germination percentage, the mean germination time (MTG) was calculated using the formula:
Where ni is the number of seeds that germinated within consecutive intervals of time, ti the time between the beginning of the test and the end of a particular interval of measurement, and N the total number of seeds that germinated.
RESULTS
Germination phenology under simulated monthly temperatures
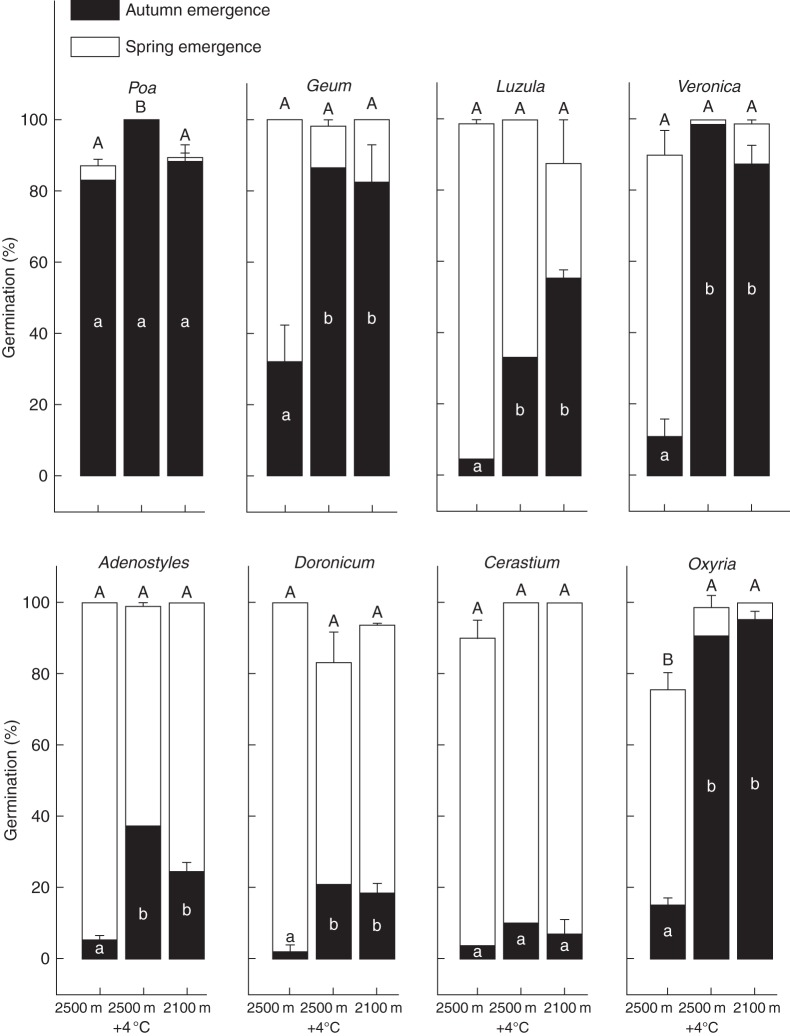
Under simulated autumn conditions (September, see Table 1), significant differences in germination percentage were observed either between species or temperatures tested (Table 2A). Furthermore, because of the strong species × temperature interaction, species and temperature specific analysis were also performed. At the temperature occurring at the current species growing site (12/5 °C; 2500 m a.s.l.) during the time of seed dispersal (September), seed germination was low (5–30 %) across all species except Poa, which showed >80 % germination (Fig. 2). However, an increase in autumn temperature resulted in a significant increase in germination in six out of the eight species tested. At 17/6 °C, simulating the temperature in September at 2100 m, about 70–80 % of seeds of Geum, Veronica and Oxyria had germinated by the time they were transferred to winter temperature (0 °C). At the same temperature, seed germination also increased in Adenostyles and Luzula to about 20 % and 60 %, respectively. However, seed germination remained low (<20 %) in Doronicum and Cerastium and high (>80 %) in Poa at all the autumn temperatures tested. Finally, except for Luzula, no differences in the final germination percentage were observed between 17/6 °C and 16/9 °C (2500 m + 4 °C).
Table 2.
Results of two two-way ANOVAs on the effects of autumn temperatures (Table 1, current winter) and species identity on germination percentage and on mean time to germinate
| Factor | d.f. | F-value | P-value |
|---|---|---|---|
| Germination percentage | |||
| Temperature (T) | 2, 80 | 109·12 | < 0·001 |
| Species (S) | 8, 80 | 94·847 | < 0·001 |
| S × T | 16, 80 | 10·734 | < 0·001 |
| Mean time to germinate | |||
| Temperature (T) | 2, 80 | 7·667604 | < 0·001 |
| Species (S) | 8, 80 | 10·52061 | < 0·001 |
| S × T | 16, 80 | 6·512982 | < 0·001 |
Significant values are highlighted in bold
Fig. 2.
Cumulative germination percentage (means ± s.e.) of each species under three temperature treatments at the end of autumn (September, black columns) and at the end of spring/summer (August, white columns). See Table 1 for the incubation temperatures. Winter germination has been omitted because no seeds germinated during incubation at this temperature. Different letters indicate significant differences of germination at P < 0·05 level (Tukey multiple comparison test).
When seeds were transferred to the winter temperature (0 °C) germination stopped in each of the treatments tested. However, germination resumed when seeds were transferred to the simulated spring/summer conditions and, regardless of the temperature treatment, almost all seeds had an emerged radicle during the first spring temperature experienced, whereupon no further germination occurred.
Considering the germination at the end of each temperature treatment (e.g. sum of autumn and spring/summer emergence), there were again significant differences across both the temperature (F2,144 = 3·41, P = 0·030) and the species tested (F7,144 = 5·62, P < 0·001). Post-hoc analysis highlighted that such variation was due to subtle differences which occurred only in Poa and Oxyria (Fig. 2). When ungerminated autumn-treated seeds were moved to the winter treatments simulating a strong absence of snow cover (4 months at –7 °C; Table 1), the final germination across either the species and/or the temperature tested was not significantly different (F1,144 = 3·41, P = 0·067) from that when winter was kept at constant 0 °C (data not shown). Germination at the end of each temperature treatment was, in several cases, strongly determined by different proportions of seeds germinating in autumn- and/or in spring/summer-simulated seasons and, therefore, it was not possible to infer reliable effects of the different spring/summer temperatures on seed germination. For this reason, the effect of spring/summer conditions on germination was analysed when autumn temperatures were excluded from the treatments (see below).
Germination phenology under different spring/summer scenarios in the absence of autumn
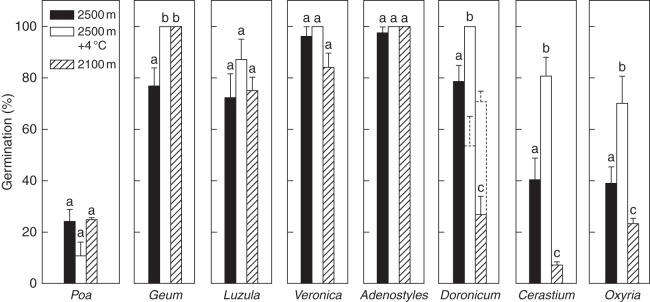
Because of the significant effect of autumn temperatures on seed germination, subsamples of seeds were sown under the same cycle of simulated monthly temperatures listed in Table 1, but in the absence of autumn in order to examine how germination could be affected by different spring/summer temperatures. In these cycles, seed germination started upon transfer from winter (0 °C) to spring/summer temperatures and occurred within the first temperature experienced, whereupon there was no further germination. Significant differences in germination percentage were observed both between species and temperatures tested (Table 3). Furthermore, because of the strong species × temperature interaction, species- and temperature-specific analyses were also conducted. Geum, Luzula, Veronica and Adenostyles showed similar high levels of germination (approx. >80 %) regardless of the temperature tested (Fig. 3). In contrast, seed germination was, in general, very low in Poa (approx. 20 %). In the remaining three species, temperature treatments simulating the conditions at 2100 m reduced germination and those at 2500 m + 4 °C increased germination (Fig. 3).
Table 3.
Results of two three-way ANOVAs on the effects of temperatures treatments (see Table 1) performed in the absence of autumn, species identity and winter condition (0 °C or –7 °C) on final germination percentage and on mean time to germinate
| Factor | d.f. | F-value | P-value |
|---|---|---|---|
| Germination percentage | |||
| Temperature cycle (T) | 2 | 20·476 | < 0·001 |
| Species (S) | 7 | 69·896 | < 0·001 |
| Winter (W) | 1 | 0·731 | 0·395 |
| T × S | 14 | 4·287 | < 0·001 |
| T × W | 2 | 6·752 | 0·002 |
| S × W | 7 | 1·752 | 0·106 |
| T × S × W | 14 | 2·150 | 0·015 |
| Mean time to germinate (MTG) | |||
| Temperature cycle (T) | 2, 144 | 16·557 | < 0·001 |
| Species (S) | 7, 144 | 38·28 | < 0·001 |
| Winter (W) | 1, 144 | 36·75 | < 0·001 |
| T × S | 14, 144 | 5·988 | < 0·001 |
| T × W | 2, 144 | 3·477 | 0·035 |
| S × W | 7, 144 | 5·448 | < 0·001 |
| T × S × W | 14, 144 | 3·426 | < 0·001 |
MTG was calculated from the beginning of spring/summer temperatures (e.g. May or July, according to the temperature treatment considered). Significant values are highlighted in bold.
Fig. 3.
Percentage germination (means ± s.e.) of each species at the end of the three temperature treatments listed in Table 1, performed in the absence of an autumn simulation. Also shown are the same results after subzero winter temperature (–7 °C, dashed columns), but only if significantly different from the winter-treated seeds performed at 0 °C. Winter germination has been omitted because no seeds had germinated during incubation at this temperature. Different letters indicate significant differences of germination at P < 0·05 level (Tukey multiple comparison test).
Finally, in general (all species combined) seeds germinated equally well regardless of whether winter was kept at constant 0 °C or –7 °C (Table 3). However, because of the significant winter × temperature interaction a temperature-specific analysis was carried out. This demonstrated that, in Doronicum, subzero winter temperature promoted an increase and a reduction in germination, respectively, at lower and higher spring/summer incubation temperatures (Fig. 3, dashed columns).
Mean time to germinate in autumn
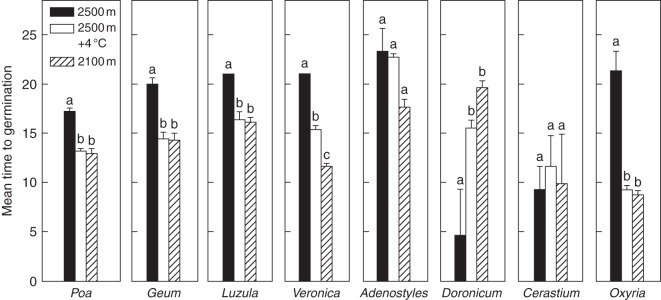
At the temperature recorded in the species growing site (12/5 °C; 2500 m a.s.l.) during the timing of seed dispersal (September), the MTG was about 20 d in Poa, Geum, Luzula, Veronica and Oxyria and about 5 d in Doronicum and Cerastium. The increase in autumn germination, following the two warmer autumn temperatures tested, resulted in a significantly reduced MTG in five out of the eight species tested (Table 2 and Fig 4). Indeed, when seeds were exposed to either 17/6 °C (mean autumn temperature at 2100 m a.s.l.) or 16/9 °C (mean autumn temperature at 2500 m a.s.l. + 4 °C) Geum, Luzula and Veronica reduced their MTG by about 25 % (approx. 5 d faster) and in Oxyria it was almost halved (approx. 10 d faster) compared with the test carried out at 12/5 °C (Fig. 4). In Poa, although the final proportion of germinated seeds was unchanged across the three autumn conditions tested (approx. 90–100 %), the MTG was again about 25 % faster at the warmer temperatures (approx. 4 d faster). In contrast, Doronicum showed an increase in MTG, while no significant changes were observed in Adenostyles and Cerastium across the autumn temperatures tested.
Fig. 4.
Mean time to germinate of each species under three autumn temperatures. See Table 1 for the incubation temperatures. Different letters indicate significant differences of MTG at P < 0·05 level (Tukey multiple comparison test).
Mean time to germinate in spring/summer
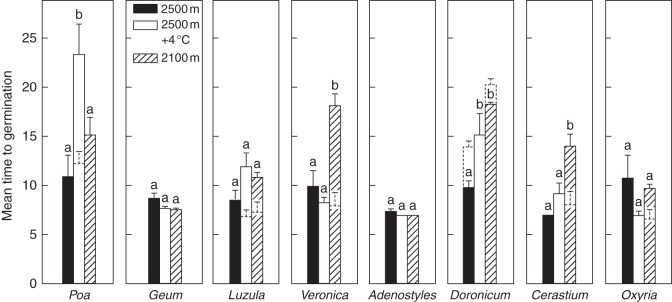
Because of the different number of ungerminated seeds remaining in spring due to the large variation in autumn germination across both species and the temperatures tested, MTG in spring/summer-simulated seasons was calculated only for the temperature cycles carried out in absence of autumn. Three-way analysis of variance showed a significant effect of both the temperature and the species tested on MTG (Table 3); MTG also differed depending on whether winter conditions were kept at 0 or –7 °C. Furthermore, because of the strong interaction among all the variables (temperatures, species identity and winter conditions), species-specific analyses were carried out. These demonstrated that winter conditions significantly affected the MTG in six out of the eight species (Fig. 5). In particular, subzero winter-treated seeds of Luzula, Veronica, Cerastium and Oxyria germinated faster compared with those that experienced 0 °C, but only under the spring/summer temperatures of the site at 2100 m a.s.l. Subzero winter temperature promoted faster germination even in Luzula and Poa when seeds of these species were exposed to the spring/summer temperatures occurring at 2500 m + 4 °C. In contrast, subzero winter-treated seeds of Doronicum showed a subtle but significant increase of MTG under the temperature treatments ‘2500 m’ and ‘2100 m’ (Fig. 5).
Fig. 5.
Mean time to germinate in spring/summer for each species and temperature treatment listed in Table 1 performed in the absence of autumn. Also shown are the same results after subzero winter temperature (–7 °C, dashed columns), but only if significantly different from the winter-treated seeds performed at 0 °C. Different letters indicate significant differences of MTG at P < 0·05 level (Tukey multiple comparison test).
Interestingly, winter-treated (at constant 0 °C) seeds of all species showed almost the same MTG (approx. 10 d), when moved to the spring/summer temperatures (F7,23 = 1·261 P = 0·329) simulating the condition of the site of species origin (2500 m temperature treatment). However, spring conditions resulted in a significant increase in MTG in Poa (+ 12 d) and Doronicum (+ 5 d) under the 2500 m + 4 °C temperature treatment, and MTG also increased in Veronica (+ 8 d), Doronicum (+ 8 d) and Cerastium (+ 7 d) when their seeds were exposed to the 2100-m temperature treatment (Fig. 5).
DISCUSSION
In alpine plants, spring germination is known to prevail due to temperatures being too low to stimulate emergence following autumn dispersal or due to a requirement for cold stratification over winter (Schwienbacher et al., 2011; Walck et al., 2011). Earlier studies investigating the effects of climate warming on seed germination of alpine/arctic species focused on laboratory and field manipulation of summer and/or winter temperatures (e.g. Milbau et al., 2009; Shevtsova et al., 2009) while, to the best of our knowledge, no similar experiments considered changes in autumn temperatures. The results reported here showed that, except for Poa, under the temperature treatment simulating the conditions of the species growing site, only a low proportion of seedlings emerged in autumn, while the majority are programmed to germinate in spring/summer (Fig. 2). Indeed, seed germination and seedling establishment of alpine plants tends to occur rapidly after snowmelt, in early summer (Körner, 1999). However, we have also demonstrated that, following an autumn warming, there was a significant increase in germination in six out of the eight species tested in this season, indicating that significant changes in the timing of seedling emergence might occur in some alpine species in a future warmer climate. Supporting this view, in a comparative study of germination ecology across several Papaver species, Karlsson and Milberg (2007) cautioned that, in cold temperate areas, global warming will lead to a shift from mostly spring to autumn emergence.
Although air warming might affect other environmental factors (biotic and abiotic), which in turn disturb the species ecological niche, such as soil moisture (Forbis, 2003), we decided to focus on temperature because this is considered to be one of the most important factors controlling seed dormancy and germination (Probert, 2000; Graee et al., 2008). Seed dormancy state and/or type might also play an important role in the response of species to climate change, promoting and/or inhibiting germination at different times of the year depending on the species. Our results illustrate different expressions of seed dormancy resulting in autumn or spring/summer germination in the species studied. For example, almost 90 % germination was observed in Poa at the autumn temperature of the seed collecting site (12/5 °C), indicating that seeds of this species are not dormant at the time of dispersal and are very likely programmed to germinate in this season, regardless of climate warming. Moreover, germination was very low (<20 %) when winter-treated seeds of Poa were moved to the all spring/summer temperatures tested (Fig. 3), suggesting that cold stratification might induce a secondary dormancy in the seeds of this species. A pronounced induction of secondary dormancy, when fully moistened seeds were kept for a prolonged period at temperatures not suitable for their germination, has been reported in other Poa species (Phaneendranath and Funk, 1981; Naylor and Abdalla, 1982). In freshly collected seeds of Geum, Veronica and Oxyri,a germination was low at current autumn temperatures but increased dramatically in warmer autumn scenarios (Fig. 2), suggesting that seeds of these species are weakly or conditionally dormant (sensu Baskin and Baskin, 1998). In contrast, the much lower or no increase in germination observed in Cerastium, Luzula, Doronicum and Adenostyles seeds indicates that the degree of dormancy appears to vary among the species: from non-deep (e.g. Geum) to deep (e.g. Cerastium). It follows that at the time of seed dispersal in early autumn, truly dormant seeds of alpine species might be less affected by the effects of autumn warming than non-dormant or weekly dormant species.
The effects of autumn emergence on seedling survival remain unknown. In general, the advantage or disadvantage of autumn emergence will depend on the capability of seedlings to cope with winter temperatures. In some species, such as woodland geophytes, autumn emergence represents an adaptation that presumably ensures seedlings are well placed to grow quickly when temperatures begin to rise in early spring (Mondoni et al., 2008). However, unlike seedlings of these species which are insulated from damaging frosts by a thick layer of leaf litter, in the alpine habitat night time needle ice and cryogenic processes in the soil during winter permit only a few seedlings to survive (Körner, 1999). Furthermore, although snow cover might provide shelter from freezing, seedlings resulting from anomalous autumn germination are unlikely to be programmed to withstand a long-lasting alpine winter season. On the other hand, the phenology of germination showed by Poa suggests that, in some species, seedling survival during winter is possible, at least in the absence of subzero winter conditions. Indeed, premature melting of snow and a decrease in snow cover driven by the global warming (IPCC, 2007) might impact frost penetration, melt-water and, hence, the thermal protection for seedlings (Schaberg et al., 2008). In situ sowing experiments at the Dosdè glacier forelands are underway in order to understand the extent to which autumn emergence might represent an advantage or disadvantage for seedling survival of these species.
In alpine habitats the length of the growing season is strongly controlled by the period of snow cover (Körner, 1999), which in turn depends on the amount of snowfall and the air temperature. For most regions with snow-pack, global warming will significantly reduce the duration and extent of snow cover during the year (Brown et al., 1994; McCabe and Wolock, 2010; Valt and Cianfarra, 2010). Accordingly, in our study area, at 2100 m, snow melted about 2 months earlier (end of April) than at 2500 m (end of June). Because of the different season, the mean soil surface temperature about 1 month after snowmelt was lower at 2100 m (15/3 °C) than at 2500 m (20/8 °C). Although counter-intuitive it follows that seeds of alpine plants might be exposed to lower rather than higher temperatures following snowmelt as a result of climate warming. Interestingly, this period is considered the most suitable for germination of alpine plants (see Introduction). Consequently future studies of the effects of climate change on plant regeneration in alpine habitats should focus on conditions occurring at lower altitudes rather than a generalized temperature increase.
Regarding the effects of spring/summer temperatures, it was observed that warming (temperature treatment 2500 m + 4 °C) and cooling (temperature treatment 2100 m) enhanced and reduced, respectively, the proportion of germinating seeds only in Doronicum, Cerastium and Oxyria (Fig. 3) compared with the temperature recorded at the current growing site (temperature treatment 2500 m). Interestingly, under the full cycle of temperature treatments (Fig. 2), the large proportion (approx. 90–80 %) of ungerminated autumn- (and winter-) treated seeds of Doronicum and Cerastium germinated to 80–100 % when moved to all spring/summer temperatures (Fig. 2). Similarly, while approx. 40 % germination was observed in winter-treated seeds of Oxyria when moved to the spring/summer temperature of the species growing site (Fig. 3), approx. 80 % germinated at the same temperature when seeds were first exposed to the autumn condition (Fig. 2), during which germination was approx. 15 %. These results indicate that, although autumn temperatures may not permit germination in autumn, physiological processes occur that foster spring germination in these species. In the other species, there were no significant differences among the spring/summer temperatures tested regardless of whether seeds experienced the autumn temperature or not and, except for Poa, all of them germinated to a high level (e.g. >80 %). In the majority of alpine plants, seed germination appears to be more sensitive to changes in autumn rather than spring/summer temperatures. Supporting this view, Milbau et al. (2009) found no significant differences in the germination percentage when winter-treated seeds of several subarctic species were exposed to different summer temperatures.
Milbau et al. (2009) reported that summer warming resulted in faster germination in the majority of the subarctic species studied, regardless of whether it was combined with subzero winter conditions or not. Here we have shown that an increase in spring/summer temperatures had no significant effect on germination time, except in Doronicum and Poa, which germinated even slower (Fig. 5); however, the combined effects of warming and subzero winter temperature accelerated germination in Luzula and Poa (Fig. 5). Furthermore, lower spring/summer incubation temperature delayed the germination in Veronica, Doronicum and Cerastium, but when this condition was preceded by subzero winter temperature, mean germination time was significantly reduced in half of the species tested (Luzula, Veronica, Cerastium and Oxyria), whereas in Doronicum germination percentage increased (Fig. 3). Therefore, because seeds of alpine plants might first experience lower temperatures just after the snowmelt in a future, warmer climate and, considering that, germination may still be high, or even accelerate, when lower spring/summer temperatures are preceded by subzero winter conditions, our findings support the conclusion drawn by Milbau et al. (2009) that at least some alpine/arctic plants might benefit in a warmer climate. A longer and warmer growing season should also improve the chances of seedling survival (Crawford, 2008a, b). However, drawing on evidence presented by Shevtsova et al. (2009), which showed a stronger reduction in germination and seedling survival of early-emerging tundra species under continuous summer heating, compared with late-emerging species, Walck et al. (2011) suggested that the sensitivity of seedlings to climate change may depend on the timing of emergence. Our results suggest that early spring emergence may increase the chances of young seedlings being exposed to freezing episodes and winter subzero temperatures will likely be detrimental for the survival of autumn-emerged seedlings. Returning to our research question it is therefore possible that, among other factors, the upward migration of alpine plants might not be a matter of inhibition of seed germination due to the temperature changing, but rather of precocious emergence which exposes seedlings to conditions (seasons) unfavourable for their survival. As a population of plants migrates up a mountain in response to climate warming, the conditions for recruitment and survival at the leading edge become more favourable as the conditions at the trailing edge become less favourable.
This study has highlighted significant concerns for successful seedling survival of alpine plants under future climate change scenarios, which in turn might compromise the fate of these species. Facing global warming, alpine floras might benefit from the retreat of the nival zone, which provides new terrain for expansion (Crawford, 2008b) and/or the high topographic variability of the alpine landscapes, which has been suggested to result in thermally suitable ‘escape’ habitats for many species (Scherrer and Körner, 2011). Nevertheless, the species that live close the glaciers or at the peak of the mountain might disappear, because of the changed climatic conditions and/or the increased competition with new species migrating from lower altitudes.
In response to these threats, ex situ seed conservation through seed banks will play an important role, safeguarding alpine species and providing the propagating material for in situ conservation and habitat restoration (Cochrane et al., 2007). However, seeds of alpine populations and species have been shown to be shorter lived in dry storage when compared with lowland relatives (Mondoni et al., 2011), indicating that even long-term ex situ conservation cannot be guaranteed for these species. Human-mediated movement of several glacier foreland species to more suitable habitats may therefore become necessary in a future warmer climate (Vitt et al., 2010).
ACKNOWLEDGEMENTS
This work was funded by the Provincia Autonoma di Trento/EU VII Framework Programme (FP7), through the programme ‘People’ (Marie Curie Action – COFUND). The authors thank Prof. Claudio Smiraglia and Dr Guglielmina Diolaiuti (University of Milan), who provide the climatic data, and Simone Pedrini and Esther Sossai for the help in the field work. Further support was provided by the Centro Flora Autoctona (Lombardy Region) through the Lombardy Seed Bank and by the EV-K2-CNR SHARE project.
LITERATURE CITED
- Amen RD. The extent and role of seed dormancy in alpine plants. The Quarterly Review of Biology. 1966;41:271–281. [Google Scholar]
- Baskin CC, Baskin JM. Seeds: ecology, biogeography and evolution of dormancy and germination. London: Academic Press; 1998. [Google Scholar]
- Beniston M, Diaz HF, Bradley RS. Climatic change at high elevation sites: an overview. Climate Change. 1997;36:233–251. [Google Scholar]
- Billings WD, Mooney HA. The ecology of arctic and alpine plants. Biological Reviews. 1968;43:481–529. [Google Scholar]
- Bliss LC. Seed germination in arctic and alpine species. Arctic. 1958;11:180–188. [Google Scholar]
- Brown RD, Hughes MG, Robinson DA. Characterizing the long term viability of snow cover extent over the interior of North America. Annals of Glaciology. 1994;21:45–50. [Google Scholar]
- Casty C, Wanner H, Luterbacher JL, Esper J, Böhm R. Temperature and precipitation variability in the European Alps since 1500. International Journal of Climatology. 2005;25:1855–1880. [Google Scholar]
- Cavieres LA, Arroyo MTK. Seed germination response to cold stratification period and thermal regime in Phacelia secunda (Hydrophyllaceae): altitudinal variation in the Mediterranean Andes of Central Chile. Plant Ecology. 2000;149:1–8. [Google Scholar]
- Cochrane JA, Crawford AD, Monks LT. The significance of ex situ seed conservation to reintroduction of threatened plants. Australian Journal of Botany. 2007;55:356–261. [Google Scholar]
- Crawford RMM. Plants at the margin: ecological limits and climate change. Cambridge: Cambridge University Press; 2008a. [Google Scholar]
- Crawford RMM. Cold climate plants in a warmer world. Plant Ecology and Diversity. 2008b;1:285–297. [Google Scholar]
- Dalgleish HJ, Koons DN, Adler PB. Can life-history traits predict the response of forb populations to changes in climate variability? Journal of Ecology. 2010;98:209–217. [Google Scholar]
- Dong W, Jiang Y, Yang S. Response of the starting dates and the lengths of seasons in mainland China to global warming. Climatic Change. 2010;99:81–91. [Google Scholar]
- Engler R, Randin CF, Thuiller W, et al. 21st century climate change threatens mountain flora unequally across Europe. Global Change Biology. 2011;17:2330–2341. [Google Scholar]
- Erschbamer B. Winner and looser of climate change in a central alpine glacier foreland. Artic, Antarctic and Alpine Research. 2007;39:237–244. [Google Scholar]
- Fay PA, Schultz MJ. Germination, survival, and growth of grass and forb seedlings: effects of soil moisture variability. Acta Oecologica. 2009;35:679–684. [Google Scholar]
- Forbis TA. Seedling demography in an alpine ecosystem. American Journal of Botany. 2003;90:1197–1206. doi: 10.3732/ajb.90.8.1197. [DOI] [PubMed] [Google Scholar]
- Gabrielsen B. Sex after all: high levels of diversity detected in the arctic clonal plant Saxifraga cernua using RAPD markers. Molecular Ecology. 1998;7:1701–1708. [Google Scholar]
- Giorgi F. Climate change hot-spots. Geophysical Research Letters. 2006;33:L08707. [Google Scholar]
- Gottfried M, Pauli H, Futschik A, et al. Continent-wide response of mountain vegetation to climate change. Nature Climate Change. 2012;2:111–115. [Google Scholar]
- Grabherr G, Gottfried M, Pauli H. Climate effects on mountain plants. Nature. 1994;369:448. doi: 10.1038/369448a0. [DOI] [PubMed] [Google Scholar]
- Graee BJ, Alsos IG, Ejrnaes R. The impact of temperature regimes on development, dormancy breaking and germination of dwarf shrub seeds from arctic, alpine and boreal sites. Plant Ecology. 2008;198:275–284. [Google Scholar]
- Hay FR, Smith RD. Seed maturity: when to collect seeds from wild plants. In: Smith RD, Dickie JB, Linnington SH, Pritchard HW, Probert RJ, editors. Seed cconservation: turning science into practice. London: Royal Botanic Gardens, Kew; 2003. pp. 97–133. [Google Scholar]
- Hedhly A, Hormaza JI, Herrero M. Global warming and sexual plant reproduction. Trends in Plant Science. 2009;14:30–36. doi: 10.1016/j.tplants.2008.11.001. [DOI] [PubMed] [Google Scholar]
- IPCC Core Writing Team. Climate change 2007: synthesis report. In: Pachauri RK, Reiginger A, editors. Contribution of working groups I, II and III to the 4th assessment report of International Panel on Climate Change. Geneva: IPCC; 2007. [Google Scholar]
- Jones PD, Moberg A. Hemispheric and large-scale surface air temperature variations: an extensive revision and an update to 2001. Journal of Climate. 2003;16:206–223. [Google Scholar]
- Jonsson BO, Jonsdottir IS, Cronberg N. Clonal diversity and allozyme variation in populations of the arctic sedge Carex bigelowii (Cyperaceae) Journal of Ecology. 1996;84:449–459. [Google Scholar]
- Karlsson LM, Milberg P. A comparative study of germination ecology of four Papaver taxa. Annals of Botany. 2007;99:935–946. doi: 10.1093/aob/mcm045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klanderud K, Totland O. Simulated climate change altered dominance hierarchies and plant community diversity in alpine biodiversity hot-spot. Ecology. 2005;86:2047–2054. [Google Scholar]
- Körner C. Alpine plant life: functional plant ecology of high mountain ecosystems. Berlin: Springer-Verlag; 1999. [Google Scholar]
- de Kroon H, van Groenendael J. The ecology and evolution of clonal plants. Leiden: Backhuys Publishers; 1997. [Google Scholar]
- Lenoir J, Gégout JC, Marquet PA, De Ruffray P, Brisse H. A significant upward shift in plant species optimum elevation during the 20th century. Science. 2008;320:1768–1771. doi: 10.1126/science.1156831. [DOI] [PubMed] [Google Scholar]
- Lloret F, Peñuelas J, Estiarte M. Experimental evidence of reduced diversity of seedlings due to climate modification in a Mediterranean-type community. Global Change Biology. 2004;10:248–258. [Google Scholar]
- McCabe GJ, Wolock DM. Long-term variability in Northern Hemisphere snow and associations with warmer winters. Climate Change. 2010;99:141–153. [Google Scholar]
- Milbau A, Graae BJ, Shevtsova A, Nijs I. Effects of a warmer climate on seed germination in the subarctic. Annals of Botany. 2009;104:287–296. doi: 10.1093/aob/mcp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondoni A, Probert R, Rossi G, Hay F, Bonomi C. Habitat-correlated seed germination behaviour in populations of wood anemone (Anemone nemorosa L.) from northern Italy. Seed Science Research. 2008;18:213–222. [Google Scholar]
- Mondoni A, Probert RJ, Rossi G, Vegini E, Hay FR. Seeds of alpine plants are short lived: implications for long-term conservation. Annals of Botany. 2011;107:171–179. doi: 10.1093/aob/mcq222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor REL, Abdalla AF. Variation in germination behaviour. Seed Science Technology. 1982;10:67–76. [Google Scholar]
- Niederfriniger S, Erschbamer B. Germination and establishment of seedlings on a glacier foreland in the central Alps, Austria. Artic, Antarctic and Alpine Research. 2000;32:270–277. [Google Scholar]
- Nogués-Bravo D, Araújo MB, Errea MP, Martìnez-Rica JP. Exposure of global mountain systems to climate warming during the 21st Century. Global Environmental Change. 2007;17:420–428. [Google Scholar]
- Parolo G, Rossi G. Upward migration of vascular plants following a climate warming trend in the Alps. Basic and Applied Ecology. 2008;9:100–107. [Google Scholar]
- Paul F, Kääb A, Maisch M, Kellenberger T, Haeberli W. Rapid disintegration of Alpine glaciers observed with satellite data. Geophysical Research Letters. 2004;31:1–4. [Google Scholar]
- Pirola A, Credaro V. Osservazioni sul dinamismo della vegetazione di morena recente nel Gruppo del Bernina. Fitosociologia. 1994;27:139–149. [Google Scholar]
- Pautasso M, Dehnen-Schmutz K, Holdenrieder O, et al. Plant health and global change: some implications for landscape management. Biological Reviews. 2010;85:729–755. doi: 10.1111/j.1469-185X.2010.00123.x. [DOI] [PubMed] [Google Scholar]
- Phaneendranath BR, Funk CR. Effect of storage conditions on viability, after-ripening and induction of secondary dormancy of Kentucky bluegrass seed. Journal of Seed Technology. 1981;6:9–22. [Google Scholar]
- Probert RJ. The role of temperature in the regulation of seed dormancy and germination. In: Fenner M, editor. Seeds: the ecology of regeneration in plant communities. Wallingford, UK: CAB International; 2000. pp. 261–292. [Google Scholar]
- Sandvik SM, Heegaard E, Elven E, Vandvik V. Responses to long term environmental warming in alpine snow bed vegetation at Finse, south-western Norway. Ecoscience. 2004;11:150–159. [Google Scholar]
- Schaberg PG, Hennon PE, D'Amore DV, Hawley GJ. Influence of simulated snow cover on the cold tolerance and freezing injury of yellow-cedar seedlings. Global Change Biology. 2008;14:1282–1293. [Google Scholar]
- Scherrer D, Körner C. Topographically controlled thermal-habitat differentiation buffers alpine plant diversity against climate warming. Journal of Biogeography. 2011;38:406–416. [Google Scholar]
- Schwienbacher E, Navarro-Cano JA, Neuner G, Erschbamer B. Seed dormancy in alpine species. Flora. 2011;206(845– 856) doi: 10.1016/j.flora.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevtsova A, Graae BJ, Jochum T, et al. Critical periods for impact of climate warming on early seedling establishment in subarctic tundra. Global Change Biology. 2009;15:2662–2680. [Google Scholar]
- Valt M, Cianfarra P. Recent snow cover variability in the Italian Alps. Cold Regions Science and Technology. 2010;64:146–157. [Google Scholar]
- Vitt P, Havens K, Kramer AT, Sollenberger D, Yates E. Assisted migration of plants: changes in latitudes, changes in attitudes. Biological Conservation. 2010;143:18–27. [Google Scholar]
- Vittoz P, Dussex N, Wassef J, Guisan A. Diaspore traits discriminate good from weak colonisers on high-elevation summits. Basic and Applied Ecology. 2009;10:508–515. [Google Scholar]
- Walck JL, Hidayati SN, Dixon KW, Thompson K, Poschlod P. Climate change and plant regeneration from seed. Global Change Biology. 2011;17:2145–2161. [Google Scholar]
- Walck JL, Baskin JM, Baskin CC. A comparative study of the seed germination biology of a narrow endemic and two geographically-widespread species of Solidago (Asteraceae). 1 Germination phenology and effect of cold stratification on germination. Seed Science Research. 1997;7:47–58. [Google Scholar]