Abstract
Background and Aims
Unrelated plants pollinated by the same group or guild of animals typically evolve similar floral cues due to pollinator-mediated selection. Related plant species, however, may possess similar cues either as a result of pollinator-mediated selection or as a result of sharing a common ancestor that possessed the same cues or traits. In this study, visual and olfactory floral cues in Lysimachia species exhibiting different pollination strategies were analysed and compared, and the importance of pollinators and phylogeny on the evolution of these floral cues was determined. For comparison, cues of vegetative material were examined where pollinator selection would not be expected.
Methods
Floral and vegetative scents and colours in floral oil- and non-floral oil-secreting Lysimachia species were studied by chemical and spectrophotometric analyses, respectively, compared between oil- and non-oil-secreting species, and analysed by phylogenetically controlled methods.
Key Results
Vegetative and floral scent was species specific, and variability in floral but not vegetative scent was lower in oil compared with non-oil species. Overall, oil species did not differ in their floral or vegetative scent from non-oil species. However, a correlation was found between oil secretion and six floral scent constituents specific to oil species, whereas the presence of four other floral compounds can be explained by phylogeny. Four of the five analysed oil species had bee-green flowers and the pattern of occurrence of this colour correlated with oil secretion. Non-oil species had different floral colours. The colour of leaves was similar among all species studied.
Conclusions
Evidence was found for correlated evolution between secretion of floral oils and floral but not vegetative visual and olfactory cues. The cues correlating with oil secretion were probably selected by Macropis bees, the specialized pollinators of oil-secreting Lysimachia species, and may have evolved in order to attract these bees.
Keywords: Colour hexagon, oil secretion, correlated evolution, flower and vegetative scent, headspace analysis, GC-MS, Lysimachia, multidimensional scaling, oil-bee Macropis, phylogeny, spectral photometry
INTRODUCTION
Many flowering plant species rely on animal pollinators for their sexual reproduction, and adaptation of flowers to a specific guild of pollinators often promotes high efficiency in pollination (Baker and Hurd, 1968; Endress, 1994). Specific floral traits including size, shape, colour, scent and reward properties of plants pollinated by the same guild of animals may converge as a result of pollinator-mediated selection (Fenster et al., 2004; Harder and Johnson, 2009). This is because pollinators within a guild are suggested to have similar sensory preferences, whereas different pollinators are suggested to have different sensory preferences (Schiestl and Dötterl, 2012). Groups of plants pollinated by, for example, moths, bats and carrion flies, respectively, are each well known for a particular suite of floral traits even where the individual plants within each group are not closely related (von Helversen et al., 2000; Andersson et al., 2002; Raguso, 2004). This convergence, though often most important, is, however, not the only factor that explains floral phenotypes, and should not be explained in isolation without considering evolutionary relationships (Armbruster, 1997; Levin et al., 2003; Raguso et al., 2003; Theis and Lerdau, 2003; Steiner et al., 2011). Independently of the type of pollinator, closely related plants often share specific traits (Levin et al., 2003).
Visual (e.g. colour, shape) and olfactory (scent) floral cues play a central role in attracting pollinators and are often used by pollinators to discriminate between rewarding and non-rewarding plant species (Goulson et al., 2001; Wright and Schiestl, 2009). The interplay of olfactory and visual cues is complex, but studies have shown that olfactory and visual cues are often additive/synergistic (e.g. Kunze and Gumbert, 2001; Raguso and Willis, 2005; Burger et al., 2010), though pollinators may also/additionally be attracted by single floral cues (Dötterl and Schäffler, 2007; Dötterl et al., 2011; Klahre et al., 2011). In addition to floral traits, vegetative cues may also contribute to pollinator attraction or, in extreme cases, take over the signalling function from the flowers (Dufaÿ et al., 2003). In most cases, however, flower visitors respond specifically to floral cues (Ayasse et al., 2000; Plepys et al., 2002; Huber et al., 2005), whereas the importance of vegetative material for pollinator attraction seems typically to be small. Instead, volatiles released from vegetative tissues are well known to deter potential herbivores (Lin et al., 1987) and also to attract parasitoids of herbivores following herbivore damage of leaves (Turlings et al., 1990; Pichersky and Gershenzon, 2002). Pollinator-mediated selection may therefore be of only minor importance in the evolution of olfactory and visual (colour) traits of vegetative plant parts, which is known to be the case for morphological traits (Conner and Sterling, 1996; Armbruster et al., 1999). So far, however, no quantitative studies are available comparing the influence of pollinator-mediated selection on both olfactory and visual traits between floral and vegetative organs. Variation in scent within and among species has not yet been compared explicitly between vegetative and floral tissue. Instead, in studies focusing on pollination, vegetative volatiles are typically used as a control for floral scents (Raguso and Pichersky, 1995; Levin et al., 2003) or floral and vegetative scents are pooled and analysed as one data set (e.g. Honda et al., 1998; Füssel et al., 2007; Jhumur et al., 2008).
Lysimachia is a good model to study the importance of pollinators and phylogeny on olfactory and visual cues of floral as well as vegetative organs. The phylogeny of this genus is well known, and species of this genus exhibit different pollination strategies. About 40 % of the species of a few clades secrete floral fatty oils, and such species/clades are intermingled with species/clades of non-oil-secreting species (Hao et al., 2004; Anderberg et al., 2007). Oil species are involved in a highly specialized pollination system with Macropis oil bees (Vogel, 1986). These bees collect floral rewards, i.e. oil and also pollen, for their offspring only from Lysimachia species, and Lysimachia oil species are only/mainly pollinated by these bees. Non-oil-secreting Lysimachia species offer nectar/pollen as reward and were suggested to be pollinated by generalist bees or, in the case of a single cleistogamous species (L. minoricensis), reproductive success is expected to be independent of pollinators (Vogel, 1986). Species of the oil floral type have yellow (for L. vulgaris, see also Arnold et al., 2010) flowers. Non-oil species have yellow, red, white or purple flowers (Vogel, 1986; Arnold et al., 2010). Very little is known about olfactory floral cues in Lysimachia, and scent data are available for only one of the oil species (Dötterl and Schäffler, 2007). Recently, we demonstrated that both olfactory and visual cues of a Lysimachia species are involved in the attraction of a Macropis oil bee (Dötterl et al., 2011).
Oil-secreting Lysimachia species and Macropis bees are distributed in the Holarctic region including North America and Europe, but the highest diversity occurs in China (Vogel, 1986). In a specific region, Macropis bees collect oil and pollen not only from their native Lysimachia hosts, which may be from the same or from different clades, but also from introduced non-native Lysimachia oil flowers regardless of their clade membership. As an example, in Europe, both M. fulvipes and M. europaea visit Lysimachia species that occur natively in Europe (e.g. L. punctata and L. vulgaris), but are, according to Anderberg (2007), members of different clades (see also Fig. 4). Further, Vogel (1986) as well as Simpson and Neff (1983) mentioned that American Macropis bees collect floral rewards on introduced European L. punctata and L. nummularia. Both species belong to a different clade compared with oil plants native to North America (e.g. L. ciliata; Table 1, Fig. 4). Similarly, the European M. fulvipes visits the Asian L. congestiflora as well as the American L. ciliata in a flight cage (I. Schäffler, unpubl. data), both of which also belong to different clades compared with the native host plants (Table 1, Fig. 4). It seems that Macropis bees are not specialized on a specific Lysimachia oil species; instead, they seem to be attracted by any Lysimachia oil species independent of the geographic origin and clade membership.
Fig. 4.
Phylogenetic tree of 14 studied Lysimachia species (sequences available from GenBank) with the oil-secreting species shown in bold. The clade membership of the single species is as in Table 1. The pattern of occurrence of (E)-2-dodecenal, 2-tridecanone, (E)-citral and 1-hydroxy-1-phenyl-2-propanone (a) can be explained by phylogeny, and that of (E)-cinnamaldehyde (b) by correlated evolution with oil secretion/pollination by Macropis (Supplementary Data Table S5).
Table 1.
Species studied, their abbreviations used throughout the text, clade membership, floral types (O, oil-secreting species; NO, non-oil-secreting species), human-perceived flower colour and geographic origin (native region: E, Europe; M, Mediterranean; NA, North America; C, China; H, Hawaii; J, Japan). The number of scent samples collected from flower and vegetative parts of the different species, the number of flowers used for colour measurements and the GenBank codes of sequences used for testing phylogenetic patterns and correlated evolution of scent compounds/colour and pollination by oil bees are given. Species are listed according to clade membership
| Number of samples |
||||||||
|---|---|---|---|---|---|---|---|---|
| Species | Species abbreviation | Clade membership* | Floral type | Floral colour | Native region | Scent: flower/vegetative | Colour | GenBank code |
| L. ciliata | Lci | Subgenus Lysimachia group A | O | Yellow | NA | 5/5 | 6 | AY839977 |
| L. nummularia | Lnu | Subgenus Lysimachia group B | O | Yellow | E | 5†/5‡ | 5 | AY839988 |
| L. punctata | Lpu | Subgenus Lysimachia group B | O | Yellow | E | 5‡/4‡ | 3 | AY839987 |
| L. nemorum | Lne | Subgenus Lysimachia group C | NO | Yellow | E | 4†/5‡ | 4 | AF213747 |
| L. vulgaris | Lvu | Subgenus Lysimachia group E | O | Yellow | E | 6‡ /5‡ | 3 | AY839960 |
| L. congestiflora | Lco | Subgenus Lysimachia group F | O | Yellow | C | 6†/5‡ | 4 | AY839963 |
| L. atropurpurea | Lat | Subgenus Palladia + Lysimachiopsis | NO | Purple | M | 5/3 | 8 | AY839954 |
| L. clethroides | Lcl | Subgenus Palladia + Lysimachiopsis | NO | White | C | 5/3 | 3 | AY839955 |
| L. decurrens | Lde | Subgenus Palladia + Lysimachiopsis | NO | White | C | 5/5 | – | |
| L. ephemerum | Lep | Subgenus Palladia + Lysimachiopsis | NO | White | M | 5/5 | 3 | AY839976 |
| L. fortunei | Lfo | SubgenusPalladia + Lysimachiopsis | NO | White | C,J | 5/5 | 3 | |
| L. lichiangensis | Lli | Subgenus Palladia | NO | White | C | 5/1 | 3 | |
| L. mauritiana | Lmau | Subgenus Palladia + Lysimachiopsis | NO | White | H | 5/5 | – | AY839956 |
| L. minoricensis | Lmi | Subgenus Palladia + Lysimachiopsis | NO | Off-white | M | 5/5 | 3 | AF213749 |
| L. thyrsiflora | Lth | Subgenus Naumburgia | NO | Yellow | E | 4/5 | 5 | AY839959 |
| L. arvensis | Lar | Anagallis s. str. | NO | Blue | E | 1†,2‡/2 | 1§ | AF213735 |
| L. maritima | Lmar | Glaux | NO | White-purple | E | 1,4‡/1 | – | AF213743 |
* According to Anderberg et al. (2007) or, in the case of Lde and Lfo, Hao et al. (2004), and in the case of Lli, Vogel (1986).
† Cut flowers.
‡ Cut inflorescence or cut non-flowering stems.
§ Data from Arnold et al. (2010).
In the present study, we characterized qualitative and (semi-) quantitative floral and vegetative odours in Lysimachia oil and non-oil species. We also determined the flower and leaf colour in Lysimachia spp. by spectrophotometry and determined how the flower colours are perceived by bees, which have a different visual system to that of humans (e.g. instead of red they have an UV receptor; Backhaus, 1992).
Because specific Macropis bees visit Lysimachia oil plants belonging to different clades, we hypothesize that oil-secreting Lysimachia species evolved, independent of the phylogenetic relatedness, an oil-specific floral volatile compound or bouquet as well as a uniform bee colour. Lysimachia species that do not secrete floral oils are predicted to differ in their floral scent and colour from oil-secreting species. We expected correlated evolution between specific floral scent compounds/colours and secretion of floral oils.
In contrast to the visual and olfactory flower cues, potential leaf cues would be expected to vary independently of pollinator mode or floral type, and therefore we predicted that there would be no difference in leaf volatiles or spectral reflectance between oil and non-oil species.
MATERIALS AND METHODS
Plant material
Individual plants of five oil and 12 non-oil Lysimachia species (Table 1) were cultivated from seeds and plants obtained from Botanical Gardens or commercial suppliers, or collected in natural habitats (Supplementary Data Table S1). The classification of floral types follows Vogel (1986) and Klotz et al. (2002). Two of the species of our study, L. maritima and L. arvensis, have only recently been moved to Lysimachia from Glaux and Anagallis, respectively, based on molecular analyses (Banfi et al., 2005; Manns and Anderberg, 2009).
Volatile collection
Dynamic headspace scent samples from ‘flowers’ were collected from inflorescences in situ, from cut inflorescences or from individual cut flowers, whereas vegetative scents were collected from leaves or other non-floral plant parts, i.e. non-flowering shoots (Table 1). All samples were collected on sunny days between 10:00 and 16:00 h. When using cut material (to get more concentrated samples), we collected scent immediately after cutting. In two species, L. maritima and L. congestiflora, we collected scent in situ as well as from cut flowering plant parts and found that cutting did not influence floral scent emission when scent was collected immediately after cutting (I. Schäffler, unpubl. data). Floral or vegetative parts were enclosed within polyester oven bags (the size depending on the plant material; from 10 × 10 cm to 20 × 30 cm; Toppits®, Germany) for 10 min (flowers) and 60 min (vegetative parts), respectively, and the emitted volatiles were trapped on 1·5 mg of Tenax (mesh 60–80; Supelco, Bellefonte, PA, USA) and 1·5 mg of Carbotrap B (mesh 20–40, Supelco) in a quartz vial (Varian Inc.; length 15 mm, inner diameter 2 mm) for 2 min (4 min for vegetative parts: 2 min after 30 min and another 2 min after 60 min of enclosure) using a membrane pump (G12/01 EB, ASF Rietschle-Thomas, Puchheim, Germany). Although in all species the vegetative parts (especially the leaves) comprise a greater proportion of the plant than the corresponding floral material, the scent discharge from the vegetative material was low. For this reason we sampled the scent from vegetative parts for a longer period than for floral tissue. The flow rate was adjusted to 200 mL min−1 (Dötterl et al., 2005). Ambient controls were collected from empty bags (10 × 10 cm) following the procedure as described above.
Analysis of scent compounds
The dynamic headspace samples were analysed on a Varian Saturn 2000 mass spectrometer coupled to a Varian 3800 gas chromatograph equipped with a 1079 injector. The quartz vials used for scent collections were inserted into the injector by the use of the ChromatoProbe kit (Dötterl et al., 2005).
The injector split vent was opened and the injector heated to 40 °C to flush introduced air from the system. After 2 min, the split was closed and the injector heated to 200 °C at a rate of 200 °C min−1. This temperature was then held for 4·2 min, after which the split vent opened and the injector cooled down. A ZB-5 column (5 % phenyl polysiloxane) was used for the separation of compounds (60 m long, inner diameter 0·25 mm, film thickness 0·25 µm; Phenomenex). Helium carrier gas flow was 1·0 mL min−1. GC oven temperature was 40 °C for the first 7 min then increased by 6 °C min−1 to 250 °C and was held at the end temperature for 1 min. The MS-interface temperature was 260 °C and the ion trap worked at 175 °C. The mass spectra were taken at 70 eV (in EI mode) with a scanning speed of 1 scan s−1 from m/z 30 to 350. The GC-MS data were processed using the Saturn Software package 5·2·1. Component identification was carried out using the NIST 08, Wiley 7 and Adams (Adams, 2007) mass spectral databases, or the database provided in MassFinder 3, and confirmed by comparison of retention times with published data (Adams, 2007). Identification of individual components was confirmed by comparison of both mass spectrum and GC retention time with those of authentic standards. Compounds found in ambient control samples were excluded from the analyses.
To identify flower-specific compounds, we compared the ‘flower’ scent samples with the vegetative samples within species. Only compounds that were found in ‘flower’ but not in vegetative scent samples were treated as flower specific. We estimated total scent emission (absolute amount) by injecting known amounts of several standards compounds, and the mean response of these compounds (mean peak area) was used for quantification (Dötterl et al., 2005).
Statistical analyses of scent samples
Scents of plants pollinated by one or a few closely related bee species, i.e. specialized pollination systems, are known to contain unique compounds or unique blends (relative amount) of relatively widespread compounds (Schiestl et al., 1999; Burger et al., 2012). We therefore analysed our scent data using both qualitative (presence/absence of compounds) and semi-quantitative (relative amount of compounds with respect to total peak area) approaches.
For analyses of qualitative differences in flower-specific as well as vegetative scent among species, we calculated the qualitative Sørensen index using Primer 6·1·6 (Clarke and Gorley, 2006) to determine pairwise qualitative similarities among the individual samples. Based on the obtained similarity matrices (individual based matrix), we performed analyses of similarities (ANOSIM, 10 000 permutations) in Primer 6·1·6 to assess differences in scent among species. ANOSIM is a commonly used multivariate procedure roughly analogous to ANOVA/MANOVA that operates directly on a (dis)similarity matrix. It yields a test statistic R that is a relative measure of separation among a priori defined groups. It is based on differences of mean ranks among and within groups. An R value of ‘0’ indicates completely random grouping, whereas a value of ‘1’ indicates that samples within groups are more similar to each other than to any sample from a different group (Clarke and Gorley, 2006).
To test for qualitative differences in scent between oil and non-oil species, we used the overall compounds found per species (one list of compounds per species), calculated the Sørensen index to determine pairwise qualitative similarities among the species and, based on the obtained similarity matrix (species-based matrix), performed an ANOSIM as described above, but instead of testing for a species effect, we tested for an effect of presence/absence of oil.
For analyses of semi-quantitative differences in scent among species, we calculated the Bray–Curtis (semi)-quantitative similarity index using Primer 6·1·6 to assess pairwise semi-quantitative similarities among the individual samples, and again performed an ANOSIM (10 000 permutations) based on the obtained similarity matrix (individual based matrix). Semi-quantitative instead of quantitative data were used because the total amount of trapped volatiles strongly varied among as well as within species.
To test for semi-quantitative differences in scent between the oil and non-oil species, we determined the mean relative amount of compounds per species, calculated the pairwise semi-quantitative similarities (Bray–Curtis) to obtain a species-based matrix, and again performed an ANOSIM (10 000 permutations).
PERMDISP (Anderson et al., 2008) was used in Primer 6·1·6 to test for differences in within-group variability (dispersion) of vegetative and floral scent (based on qualitative as well as semi-quantitative species-based matrices) between oil and non-oil species (10 000 permutations).
Non-metric multidimensional scaling (NMDS), based on Bray–Curtis similarities, was used to display graphically the semi-quantitative differences in flower-specific as well as vegetative scents among species (based on the mean relative amount of compounds per species). Stress values indicate how well the two-dimensional plot represents relationships among samples in multidimensional space. Stress values <0·15 indicate a good fit (Smith, 2003).
To test if vegetative and flower-specific scents correlate (qualitatively and semi-quantitatively), i.e. plants have similar vegetative scents if they have similar flower scents, RELATE analyses (Mantel test) were performed in Primer 6·1·6 (Spearman; 10 000 permutations) using the qualitative as well as quantitative species-based similarity matrices.
Colour analysis, hexagon distance matrix and colour space
All Lysimachia species used for scent analyses, except L. decurrens, L. maritima and L. mauritiana, were also used to determine spectral reflection properties of the upper side of leaves and the apical petal parts (Table 1). Spectral reflection properties of red-coloured L. arvensis are from Arnold et al. (2010).
Diffuse reflectance spectra were taken every nanometre from 300 to 700 nm using a Varian Cary 5 spectrophotometer (Varian Inc., USA) equipped with a praying mantis accessory (Harrick Scientific Products, Inc., Pleasantville, NY, USA) using the same method as described by Burger et al. (2010). Barium sulfate was used as white standard and the disconnected beam as black reference.
The mean reflections of the petals and of leaves (built from the replicate samples per species) were used to determine the loci of petal colours in the hexagon colour space (Chittka, 1992). The standard illumination function (D65) and the spectral sensitivities of the honeybee's photoreceptors were used from Chittka and Kevan (2005). Typically, bees do not differ substantially in their sensory systems (Peitsch et al., 1992) and therefore we used the spectral sensitivity functions described for the honeybees as representative approximation for Macropis bees (Chittka and Kevan, 2005). For comparison of the bee colours among the different Lysimachia species, the pairwise hexagon distances of colour loci among species, as well as the distance of each colour locus to its background (green leaves) was calculated (Chittka and Kevan, 2005). Behavioural experiments with bumble-bees trained to visit artificial flowers have demonstrated that colour distances <0·05 hexagon units are poorly discriminated, whereas distances >0·10 are easily discriminated by the bees (Dyer and Chittka, 2004).
Testing for correlated evolution
Phylogenetically controlled correlations between pollination type (oil-bee pollination vs. non-oil-bee pollination) and presence/absence of single scent compounds were analysed using correlated evolution of discrete binary traits implemented in the program BayesTraits (Pagel and Meade, 2006), using a consensus phylogeny obtained from MrBayes. Chloroplast ndhF gene sequences of 14 species (sequence data were not available for L. decurrens, L. fortunei and L. lichiangensis) were downloaded from GenBank (Table 1). A consensus tree was constructed using Bayesian analyses under a GTR model of sequence evolution with gamma-distributed rate variation in MrBayes 3·1·2 (Ronquist and Huelsenbeck, 2003) (Fig. 4). We ran three independent runs of four Markov chains for 10 million generations, sampling every 500 generations. Adequacy of sampling and run convergence were assessed using the effective sample size diagnostic in TRACER 1·5 (Rambaut and Drummond, 2007). Trees from the first million generations were discarded based on an assessment of convergence.
BayesTraits tests for correlated evolution in two binary traits by comparing the fit of two models, one in which the traits are allowed to evolve independently of one another on a phylogenetic tree (scent compound does not correlate with oil-bee pollination) and one in which traits evolve in a correlated fashion (scent compound correlates with oil-bee pollination). The method applies reversible-jump Markov chain Monte Carlo (RJ MCMC), which samples the posterior probability distributions of the parameter values of the model of trait evolution. The independent and dependent models can be compared with Bayes factors (BFs; Kass and Raftery, 1995), with the marginal likelihood of each model approximated by the harmonic mean of the likelihoods in the Markov chain. For this comparison, a BF value greater than ‘2’ and additionally a smaller harmonic mean for the independent model is taken as positive evidence that the dependent model is favoured (Pagel and Meade, 2006). For the analyses we used a uniform prior for the independent model and an exponential hyperprior (0 100) for the model of dependent evolution as suggested by Pagel and Meade (2006). The analyses were run for 11 000 000 iterations with burn-in at 1 000 000 iterations and sample frequency of 500 iterations. As harmonic means can be unstable, analyses for each model were repeated five times.
To test the correlated evolution of floral colours and pollinator mode (oil-bee vs. non-oil-bee pollination) we used a similar approach. We tested hexagon bee-green (the unique colour present in at least two oil species; see Fig. 3) vs. the rest of the colours. We use the same consensus phylogeny obtained from MrBayes. However, as colour data of L. maritima and L. mauritiana were not available, we marked this information as ambiguous in BayesTraits analysis.
Fig. 3.
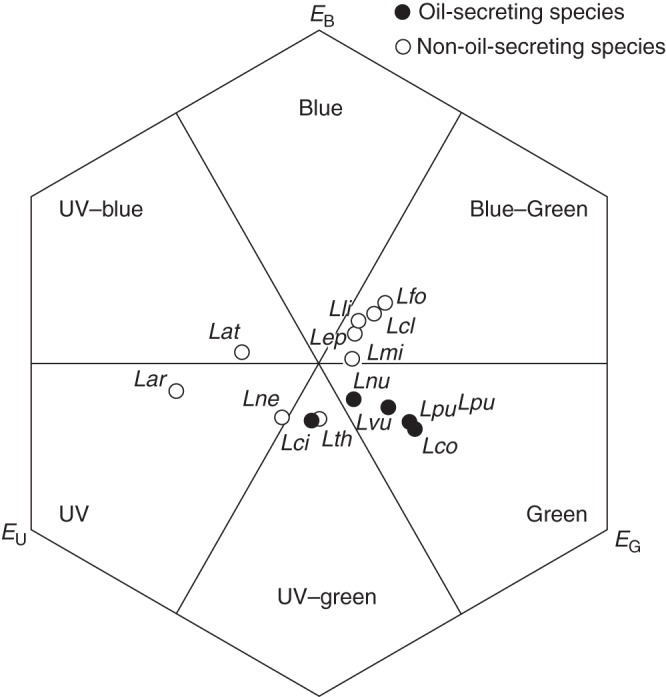
Petal colours of five oil and nine non-oil Lysimachia species displayed in a hexagon colour space. EU, EB, EG: excitation of the UV, blue, and green receptor, respectively. For species' abbreviations see Table 1. The pairwise interspecific distances in colour loci and the distances to the centre of the single species can be found in Supplementary Data Table S4.
Phylogenetic signal in scent compounds and colour
The ‘phylogenetic signals’ that affected each floral and vegetative compound (present in at least two oil species) and the presence of bee-green colour were assessed with independent Abouheif's tests (permutation: 1 000 000; adephylo package in R software; Abouheif, 1999; Jombart et al., 2010). The Abouheif's C statistic tested the null hypothesis that compounds did not experience phylogenetic autocorrelation (based on the topology of the tree). The test statistic C ranges from ‘–1’ to ‘1’. A value of ‘0’ indicates a random phylogenetic pattern, values ‘ > 0’ indicate phylogenetic autocorrelation, and values ‘ < 0’ indicate negative phylogenetic autocorrelation (non-random alternation). We performed the Abouheif's test additionally on oil-bee pollination to evaluate the phylogenetic constraint in the evolution of the pollination mode (oil-bee vs. non-oil-bee pollination).
RESULTS
Qualitative properties in flower specific and vegetative scents
We detected altogether 63 flower-specific scent compounds in the different species, 50 of which were (tentatively) identified (Table 2; for complete compound list see Supplementary Data Table S2). No flower-specific compounds were found in L. decurrens and L. ephemerum, but the other species emitted from one (L. mauritiana and L. nemorum) to 20 (L. punctata) flower-specific compounds. Aliphatics (26), aromatic compounds (17) and terpenoids (14) were the most common compounds present among the analysed species. Some of the species, such as L. punctata, emitted compounds from all three of these groups, whereas others had compounds from only two groups [e.g. aliphatics and aromatics (L. nummularia)], or one group [e.g. only aromatics (L. ciliata) or only terpenoids (L. nemorum)]. There was no single compound which occurred in all 15 species analysed, and different species emitted different sets of flower-specific compounds overall (ANOSIM: global R14,58 = 0·995; P < 0·001). Post-hoc comparisons among pairs of species revealed values of R >0·8 and P < 0·03 indicating that there were differences in scent among all the species which contained at least one compound. Variability (dispersion) in qualitative scent composition was lower in oil compared with non-oil species (PERMDISP: F1,13 = 16·5; P = 0·009). Oil and non-oil species overall did not differ significantly in flower scent composition (ANOSIM: global R1,13 = –0·053; P = 0·62).
Table 2.
Number of compounds, mean total absolute as well as percentage amount of flower-specific scent compounds (listed within classes according to Kovats retention index, RI)
| Species |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RI | Lci | Lnu | Lpu | Lne | Lvu | Lco | Lat | Lcl | Lfo | Lli | Lmau | Lmi | Lth | Lar | Lmar | |
| No. of compounds | 7 | 9 | 20 | 1 | 8 | 4 | 8 | 6 | 9 | 4 | 1 | 4 | 6 | 12 | 2 | |
| Amount of trapped scent, ng per flower per 12 min | 5 | 54 | 5 | Tr | Tr | Tr | 3 | 7 | 8 | 40 | Tr | Tr | Tr | 1 | Tr | |
| Aliphatics | ||||||||||||||||
| Methyl hexanoate* | 934 | – | – | – | – | – | – | – | – | – | – | – | – | 65 | – | – |
| Methyl 2-methylhexanoate† | 972 | – | – | – | – | – | – | – | – | – | – | – | 41 | – | – | – |
| Hexyl acetate* | 1008 | – | – | – | – | – | – | – | – | – | – | – | – | – | 30 | – |
| m/z: 74, 43, 55, 41, 39, 101 | 1067 | – | – | – | – | – | – | – | – | – | – | – | 15 | – | – | – |
| (Z)-3-Hexenyl propionate* | 1092 | – | – | – | – | – | – | – | – | – | – | – | – | – | 22 | – |
| 1,3-Diacetin‡ | 1232 | – | – | Tr | – | 28 | – | – | – | – | – | – | – | – | – | – |
| 1,2-Diacetin‡ | 1236 | – | – | Tr | – | 17 | – | – | – | – | – | – | – | tr | – | – |
| 2-Undecanone‡ | 1281 | – | 6 | Tr | – | – | 67 | – | – | – | – | – | – | – | 1 | – |
| 2-Tridecanone‡ | 1484 | – | 15 | 1 | – | – | – | – | – | – | – | – | – | – | – | – |
| Methyl dodecanoate* | 1507 | – | – | – | – | – | – | – | – | – | – | – | – | 27 | – | – |
| Aromatics | ||||||||||||||||
| Benzaldehyde‡ | 982 | 89 | 12 | 26 | – | – | – | – | – | 52 | 87 | – | – | – | – | – |
| Benzyl alcohol‡ | 1050 | 7 | Tr | – | – | 33 | 24 | – | 67 | 24 | 4 | – | – | – | – | – |
| Benzyl acetate‡ | 1104 | Tr | – | – | – | – | – | – | – | 1 | – | – | – | – | – | 70 |
| 1-Phenyl-1,2-propanedione‡ | 1171 | – | 57 | 43 | – | 11 | – | – | – | – | – | – | – | – | – | – |
| 3,5-Dimethoxytoluene* | 1268 | – | – | – | – | – | – | 12 | – | – | – | – | 38 | – | – | – |
| Terpenoids | ||||||||||||||||
| allo-Ocimene* | 1135 | – | – | – | – | – | – | – | – | – | – | – | – | – | 15 | – |
| 4-Oxoisophorone‡ | 1142 | – | – | – | – | – | – | – | 18 | 14 | – | 100 | – | – | – | – |
| m/z: 108, 93, 95, 67, 39, 79 | 1212 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 30 |
| m/z: 189, 133, 105, 91, 147, 79 | 1484 | – | – | – | 100 | – | – | – | – | – | – | – | – | – | – | – |
| N-containing compounds | ||||||||||||||||
| 1-Nitro-2-phenylethane* | 1313 | – | – | – | – | – | – | – | – | – | – | – | – | – | 10 | – |
| Unknowns | ||||||||||||||||
| m/z: 56, 41, 39, 42, 43, 69 | 1168 | – | – | – | – | – | – | 67 | – | – | – | – | – | – | – | – |
Data for oil-secreting species are highlighted in bold. Tr, trace amount (percentage <0·5 % or total absolute amount <0·5 ng). For species abbreviations see Table 1. Only compounds that contributed at least 10 % in any species are shown. A table with all the compounds detected in the individual samples can be found as Supplementary Data Table S2.
* Identification based on mass spectrum and retention index.
† Identification based on mass spectrum.
‡ Identification based on authentic standards.
In the vegetative scent samples, we detected 62 compounds [34 (tentatively) identified], mainly terpenoids (41), aliphatics (10) and aromatics (5) (Table 3; for complete compound list see Supplementary Data Table S3). Some of these compounds (e.g. benzaldehyde, benzyl alcohol and 4-oxoisophorone) were also listed as flower-specific compounds (Table 2; Supplementary Data Table S2), indicating that in some species they were found in samples collected from vegetative material, whereas in others they were only found in flower samples. Analogous to our finding that flower scents were species specific, the scent of vegetative parts also differed among species and the set of compounds emitted was species specific (ANOSIM: global R16,51 = 0·838; P < 0·001). In contrast to flower-specific scents, however, vegetative scents did not differ among all species (27 % of the post-hoc tests with P > 0·05), which mainly has to do with the small number of vegetative samples (n = 1) in L. arvensis, L. lichiangensis and L. maritima. The only non-significant post-hoc tests between species pairs that did not contain at least one of these three species were between L. clethroides and L. ephemerum, and L. clethroides and L. atropurpurea. Analogous to the floral scents, there was no compound which occurred in vegetative samples of all the species. In contrast to floral scents, variability in vegetative scent composition did not differ between oil and non-oil species (PERMDISP: F1,15 = 5·9; P = 0·07). Species of the oil and the non-oil group were not characterized by a specific set of vegetative scent compounds (ANOSIM: global R1,15 = –0·16; P = 0·87).
Table 3.
Number of compounds, mean total absolute as well as percentage amount of vegetative scent compounds (listed within classes according to Kovats retention index, RI)
| Species |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RI | Lci | Lnu | Lpu | Lne | Lvu | Lco | Lat | Lcl | Lde | Lep | Lfo | Lli | Lmau | Lmi | Lth | Lar | Lmar | |
| No. of compounds | 10 | 9 | 10 | 14 | 23 | 9 | 3 | 31 | 9 | 5 | 18 | 18 | 6 | 7 | 7 | 12 | 10 | |
| Amount of trapped scent, ng per leaf per 12 min | 2 | Tr | Tr | Tr | Tr | Tr | Tr | 3 | Tr | Tr | Tr | Tr | Tr | Tr | Tr | ND | ND | |
| Aliphatics | ||||||||||||||||||
| (Z)-3-Hexenol* | 850 | 13 | 5 | 3 | 12 | 5 | – | – | 4 | – | 39 | 3 | – | 18 | – | – | 1 | 28 |
| (Z)-3-Hexenyl acetate* | 1004 | 61 | 68 | 41 | 48 | 72 | 7 | – | 60 | – | – | 36 | – | – | 72 | 74 | 15 | 58 |
| Methyl octanoate† | 1123 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 10 | – | – |
| m/z: 67, 57, 82, 41, 39, 83 | 1225 | – | 1 | 3 | – | 2 | – | – | 1 | – | – | – | – | – | – | 1 | 16 | – |
| m/z: 67, 57, 82, 41, 39, 83 | 1230 | – | – | Tr | – | Tr | – | – | 1 | – | 20 | 3 | – | – | – | 3 | 20 | – |
| Aromatics | ||||||||||||||||||
| Benzyl alcohol* | 1049 | – | – | – | – | – | – | 49 | – | – | – | – | – | 72 | – | – | – | – |
| Methyl benzoate* | 1107 | – | – | – | – | – | – | 33 | – | – | – | – | – | – | – | – | – | – |
| Methyl salicylate* | 1208 | – | – | – | – | 2 | – | – | 2 | – | 16 | – | – | – | – | – | – | – |
| p-Anisaldehyde* | 1262 | – | – | – | – | – | – | 18 | – | – | – | – | – | – | – | – | – | – |
| Terpenoids | ||||||||||||||||||
| Camphene† | 946 | – | 16 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| (E)-β-Ocimene* | 1044 | 2 | 1 | 3 | 3 | 1 | – | – | 1 | 2 | – | 10 | 43 | – | – | – | 30 | 2 |
| (E)-4,8-Dimethyl-1,3,7-nonatriene* | 1109 | 3 | – | – | 8 | 4 | 46 | – | 6 | 60 | 24 | 19 | 8 | 7 | – | – | 2 | 1 |
| α-Copaene* | 1377 | – | – | – | 3 | 2 | 21 | – | 5 | – | – | 3 | 5 | – | – | – | – | – |
| β-Caryophyllene* | 1440 | 11 | – | 3 | 13 | 1 | 17 | – | 5 | 15 | Tr | 9 | 13 | – | – | – | 1 | – |
| (E, E)-α-Farnesene* | 1496 | 4 | – | 41 | 3 | 6 | – | – | Tr | 13 | – | 2 | 1 | – | – | 1 | – | – |
| Unknowns | ||||||||||||||||||
| m/z: 179, 69, 107, 39, 95, 40 | 1296 | – | – | – | – | – | – | – | – | – | – | – | – | – | 14 | – | – | – |
Data for oil-secreting species are highlighted in bold. Tr, trace amount (percentage <0·5 % or total absolute amount <0·5 ng). For species abbreviations see Table 1. Only compounds which contributed at least 10 % in any species are shown. A table with all the compounds detected in the individual samples can be found as Supplementary Data Table S3. ND, not determined.
* Identification based on authentic standards.
† Identification based on mass spectrum and retention index.
Vegetative and flower scent correlated based on the Sørensen similarity matrices (ρ13 = 0·443; P = 0·007) indicating that species emitting a similar set of floral scent compounds also emitted a similar set of vegetative scents.
Quantitative and semi-quantitative properties in flower-specific and vegetative scents
The total amount of scent trapped per flower and per 12 min ranged from <0·5 ng (e.g. L. vulgaris) to 54 ng (L. nummularia) (Table 2). The species differed in their semi-quantitative floral scent composition (ANOSIM: global R14,58 = 0·955; P < 0·001) (Fig. 1), and comparisons among all pairs of species were significant (R > 0·2, P < 0·04). The most abundant compound in the floral scent of L. punctata and L. nummularia was 1-phenyl-1,2-propanedione, in L. vulgaris and L. clethroides benzyl alcohol, in L. maritima benzyl acetate, and in L. arvensis hexyl acetate. Benzaldehyde was the compound with the highest relative amount in floral scents of L. ciliata, L. fortunei and L. lichiangensis, 2-undecanone in L. congestiflora, methyl hexanoate in L. thyrsiflora, 4-oxoisophorone in L. mauritiana, methyl 2-methylhexanoate in L. minoricensis, an unknown sesquiterpene in L. nemorum, and an unknown compound in L. atropurpurea. Variability in semi-quantitative floral scent composition was lower in oil compared with non-oil species (PERMDISP: F1,13 = 11·5; P = 0·02). Oil species did not differ overall in their semi-quantitative scent composition from non-oil species (ANOSIM: global R1,13 = –0·057; P = 0·64).
Fig. 1.
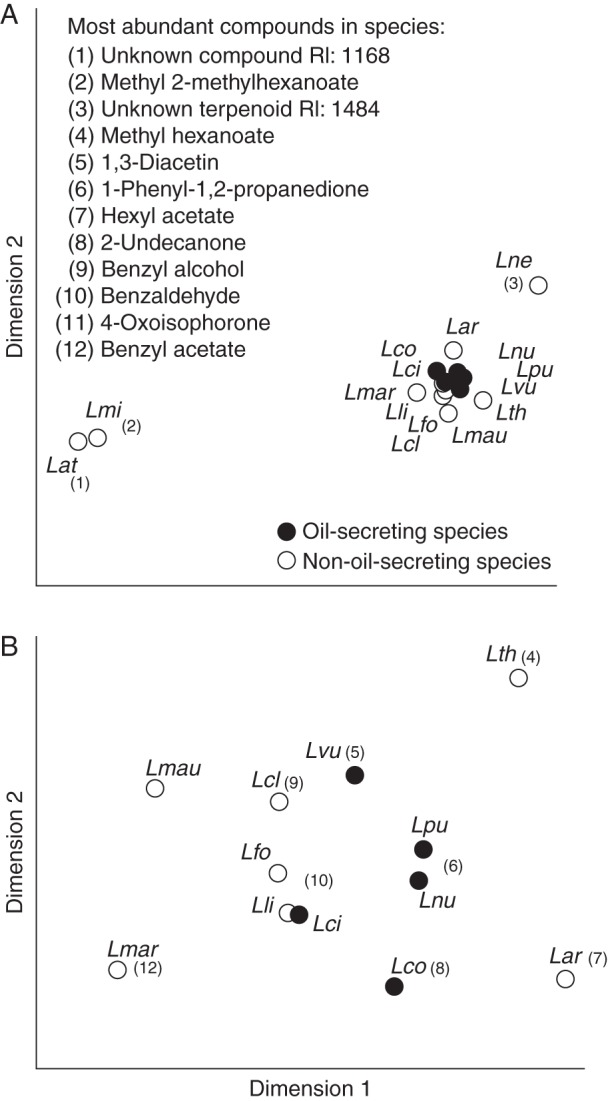
(A) Comparison of floral scent bouquets among five oil- and ten non-oil-secreting Lysimachia species based on semi-quantitative Bray–Curtis similarities (stress value = 0·01; see text for details). See Table 1 for species' abbreviations. In (B), the species L. atropurpurea (Lat), L. minoricensis (Lmi) and L. nemorum (Lne), which were outliers in (A), were excluded from the non-metric multidimensional scaling (NMDS) analysis (stress value = 0·06). The most abundant compounds in floral scents of the different species are indicated.
The total amount of scent trapped per leaf ranged from <0·5 ng (most of the species) to 3 ng (L. clethroides) per 12 min (Table 3). The species differed overall in their semi-quantitative vegetative scent composition (ANOSIM: global R16,51 = 0·608; P < 0·001) (Fig. 2); however, differences were not that prominent compared with the qualitative differences. More than 50 % of the post-hoc tests revealed non-significant values (data not shown). The most abundant compound in the vegetative scent samples of several species was (Z)-3-hexenyl acetate (Table 3, Fig. 2). (E)-4,8-Dimethyl-1,3,7-nonatriene was most abundant in L. congestiflora and L. decurrens, benzyl alcohol in L. atropurpurea and L. mauritiana, (Z)-3-hexenol in L. ephemerum, and (E)-β-ocimene in L. lichiangensis as well as L. arvensis. (E,E)-α-Farnesene was, besides (Z)-3-hexenyl acetate (see above), dominant in L. punctata. In contrast to floral scents, variability in vegetative scent composition did not differ between oil and non-oil species (PERMDISP: F1,15 = 4·5; P = 0·11). The oil and the non-oil species did not have different vegetative scents overall based on the relative amount of compounds (ANOSIM: global R1,15 = – 0·158; P = 0·90).
Fig. 2.
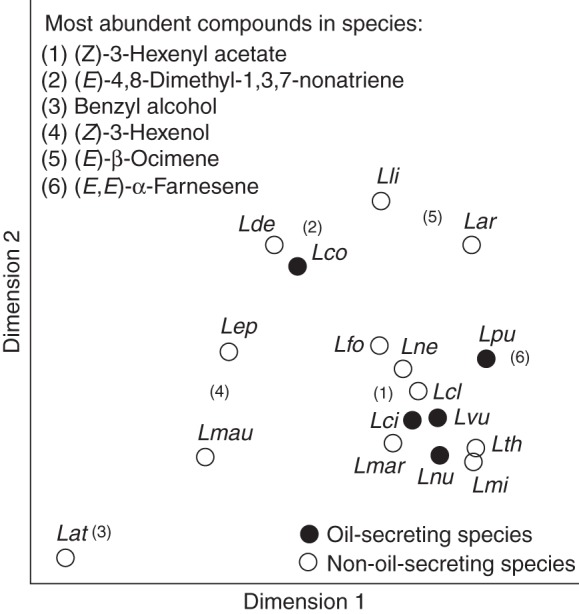
Comparison of vegetative scent bouquets among five oil and 12 non-oil Lysimachia species in a non-metric multidimensional scaling (NMDS) based on semi-quantitative Bray–Curtis similarities (stress value = 0·09. For species' abbreviations see Table 1. The most abundant compounds in scent of vegetative parts are indicated.
Vegetative and floral scent were not correlated, based on the semi-quantitative scent matrices (ρ13 = 0·161; P = 0·186).
The total amount of volatiles emitted from flowers was higher (2- to 2000-fold) than from leaves in 12 species (Tables 2 and 3). Only for L. vulgaris was the total amount of scent emitted higher in leaves (20-fold).
Leaf and floral colour properties
Leaves of oil and non-oil Lysimachia species had similar reflectance properties, and all leaves reflected most strongly at around 550 nm. One of the species, L. atropurpurea, also reflected to a lesser extent in the blue spectrum (450–500 nm; Supplementary Data Fig. S1). The yellow-coloured oil flowers reflected strongly at wavelengths of 500–700 nm, whereas L. ciliata additionally reflected strongly at 300–350 nm. Flowers of the non-oil species reflected at 400–700 nm (white coloured), 300–400 nm + 500–700 nm (yellow coloured), 300–400 nm + 600–700 nm (blue coloured) or 300–450 nm + 600–700 nm (purple coloured; see Supplementary Data Fig. S2).
Flowers that secrete oil appear bee-green or UV-green (only L. ciliata) to bees against their leaves as background and non-oil-secreting flowers appear blue-green, UV-blue, UV, UV-green or blue-green (Fig. 3). For details on flower reflectance properties, hexagon distances among colours and the distances of colour loci to the background, see Supplementary Data Fig. S2 and Table S4.
Phylogenetic signal and correlated evolution
Pollination systems showed a significant phylogenetic signal (C = 1; P < 0·05) and did not vary randomly on the tips of the phylogenetic tree. In addition, correlated evolution was found between oil-bee pollination and the pattern of occurrence of certain floral compounds (Supplementary Data Table S5). The aromatics 1-phenyl-1,2-propanedione and (E)-cinnamal-dehyde, the aliphatics 2-nonanone, monoacetin and 1,3-diacetin, and the monoterpene linalool showed BF values >2 and additionally a smaller harmonic mean for the independent model in all five replicates.
Floral compounds (E)-2-dodecenal, 2-tridecanone, (E)-citral and 1-hydroxy-1-phenyl-2-propanone exhibited a significant phylogenetic signal (Fig. 4; Supplementary Data Table S5).
The significant phylogenetic pattern of the vegetative compound terpinolene and the correlated evolution with oil-bee pollination of floral benzaldehyde and benzyl alcohol, and vegetative (E)-α-bergamotene is skewed due to missing sequence data from three non-oil species. Thus, it is not considered further here. These compounds occurred in at least one of the three non-oil species with no sequences available in GenBank (L. decurrens, L. fortunei and L. lichiangensis; Supplementary Data: compare Table S5 with Tables S2, S3).
There was correlated evolution between the petal colour bee-green and the oil-bee pollination system (harmonic means: correlated model –12·962, independent model –14·635; BF 3·346). Further, bee-green was not randomly distributed on the phylogenetic tree but showed a significant phylogenetic signal (C = 0·309, P = 0·03).
DISCUSSION
Contrary to our prediction, we found no specific compound or blend of compounds common in and unique to the floral scents of oil-secreting species. However, the occurrence of some compounds was correlated with oil secretion, suggesting that these compounds were selected by Macropis. As expected, we found no difference between the vegetative scents of plants bearing oil flowers vs. those with flowers having other types of food rewards (e.g. pollen or nectar) or being cleistogamous. There was no clear evidence for correlation between volatiles of vegetative parts and oil secretion. Independent of the pollination mode, leaf colours were similar among all species. In contrast, flower colours varied strongly among species. Flowers of most of the oil-secreting species were bee-green, a colour not found in any non-oil species. As a consequence, bee-green flower colour correlated with oil secretion, and the preponderance of this colour in oil species is likely due to selective pressures exerted by Macropis.
Floral scent characteristics and its evolution in Lysimachia
Most of the 63 compounds found in our analyses are widespread floral scent constituents (Knudsen et al., 2006), and several of the ones we found in oil-secreting species, such as benzaldehyde, benzyl alcohol and 2-tridecanone, are also known from other oil-secreting plants, i.e. South African/Neotropical orchids or a South African Iridaceae (Manning and Goldblatt, 2005; Kaiser, 2011; Steiner et al., 2011). The oil-secreting plants of South Africa, which are pollinated by melittid bees (Rediviva) closely related to Macropis, are, similarly to oil Lysimachia spp., typically dominated by aliphatics or aromatics. In contrast, terpenoids are very rarely found in abundant amounts (Table 2; Supplementary Data Table S2, Fig. S3), and this compound class may not play an important role for attracting melittid oil bees though it may be important to attract other bees; terpenoids are very widespread in other bee-pollinated plants (Dobson, 2006). In Lysimachia, the variability in floral scent within the oil as well as non-oil species is quite high; however, variability in oil species is lower than variability in non-oil species. This may indicate that Macropis exerts a stabilizing selective pressure on floral scent in oil-secreting Lysimachia species (Cresswell, 1998). We did not find overall differences in scent composition between oil and non-oil species (Table 2, Fig. 1; Supplementary Data Table S2), nor did we find any compound that was common in all oil species. Generally, almost 70 % of the flower-specific volatiles occurred only in one of the studied species and floral scent was generally species specific, which may be important for reproductive isolation among sympatric species (Knudsen, 1999; Waelti et al., 2008), such as L. punctata, L. vulgaris and L. nummularia. The remaining compounds occurred in at least two and up to seven species, and the pattern of occurrence of some of these compounds specific to plants with oil can be explained by phylogeny, whereas the presence of other compounds may be a result of pollinator-mediated selection.
Importance of phylogeny
Lysimachia punctata and L. nummularia are oil plants that are members of the subgenus Lysimachia group B (Table 1), and both species emitted a few compounds, such as (E)-2-dodecenal and 2-tridecanone, that we did not find in any other species. The common occurrence of these compounds in L. punctata and L. nummularia can be explained by a shared common ancestor (Supplementary Data Table S5; see also Fig. 4). (E)-2-Dodecenal and 2-tridecanone are known as floral compounds of non-oil plant species (Kaiser, 2006, 2011; Knudsen et al., 2006; Balao et al., 2011), and recently they were also found in oil-secreting South African orchids (Steiner et al., 2011). (E)-2-Dodecenal occurred only in a few of these orchids, but 2-tridecanone was very widespread and in some of the species an abundant compound. Steiner et al. (2011) suggested that this compound may be biologically active for Rediviva, and electrophysiological analyses show that it is at least active in Macropis, since it elicited strong responses in antennae of M. fulvipes (Dötterl, 2008). This compound may therefore be involved in the attraction of Holarctic and South African oil bees. However, 2-tridecanone is well known as a feeding deterrent or repellent for insects (Williams et al., 1980) including generalist bee pollinators (Dobson et al., 1999), and may, therefore, prevent generalist pollinators from visiting oil plants. Plants secreting floral oil typically receive few, if any, visits from insects other than oil bees, even though some have flowers that offer large amounts of pollen (e.g. Lysimachia) as a pollinator reward (S. Dötterl, unpubl. res.). Taken together, 2-tridecanone may restrict the pollinator spectrum to the oil bees in L. punctata, L. nummularia and several South African orchids, and therefore act as a floral filter (Johnson et al., 2006; Balao et al., 2011).
Influence of pollinator-mediated selection
The apparent convergence of linalool, 1-monoacetin and 1,3-diacetin in sympatric, but distantly related oil species L. punctata and L. vulgaris (Table 1) suggests pollinator-mediated selection (Supplementary Data Table S5). These two oil species occur in Europe where they are visited by M. fulvipes and M. europaea. Linalool is among the most widespread floral scent compounds (Knudsen et al., 2006), occurs in many species pollinated by specialized or generalist bees (Dobson, 2006) and is known as an attractant for social as well as solitary bee species (Dötterl and Vereecken, 2010). It also may be involved in host plant finding for European Macropis bees, though on its own it may not be useful for discriminating between Lysimachia oil plants and other co-occurring plants. The acetylated glycerides 1-monoacetin and 1,3-diacetin, together with 1,2-diacetin (which was detected in L. vulgaris and non-oil L. thyrsiflora) and triacetin (detected in the scent of L. vulgaris), are described here for the first time as floral scents. They are structurally related to the ‘non-volatile’ floral oils in Lysimachia and other oil plants (Vogel, 1986; Seipold, 2004; Dumri, 2008), which often consist of mono-, di- or triacylglycerides (Neff and Simpson, 2005). Mono-, di- and triacetin occur, with the exception of 1,2-diacetin, only in oil species, and their occurrence seems to have to do with the presence of oil. They may be produced by biosynthetic pathways similar to those of the non-volatile floral oils. We are currently investigating whether these volatile acetylated glycerides are involved in attracting Macropis bees to Lysimachia oil flowers. The occurrence of 1,2-diacetin in non-oil L. thyrsiflora may have to do with its close relatedness to oil-secreting L. vulgaris. We do not yet know whether L. thyrsiflora produces and secretes trace amounts of floral oils despite being regarded as a non-oil plant.
(E)-Cinnamaldehyde, which occurs in Lysimachia species from three different clades and three different continents, and 1-phenyl-1,2-propanedione, which occurs in two different clades and three different European species, are other examples of correlated evolution with oil secretion (Supplementary Data Table S5; see also Fig. 4). (E)-Cinnamaldehyde is known from Cucurbitaceae flowers and attractive for Peponapis pruinosa, a bee specialized on Cucurbitaceae (Andrews et al., 2007), and we found in electroantennographic measurements that M. fulvipes as well as M. europaea can detect this compound (I. Schäffler and S. Dötterl, unpubl. res.).
1-Phenyl-1,2-propanedione was the most abundant compound in European L. punctata (see also Dötterl and Schäffler, 2007) and L. nummularia, both from the subgenus Lysimachia group B, and also was abundant in European L. vulgaris (subgenus Lysimachia group E) (Table 2). This aromatic compound is an uncommon floral volatile that is known to be emitted by only a few non-oil orchid species (Kaiser, 1993; Huber et al., 2005). Its reduced form, 1-hydroxy-1-phenyl-2-propanone, also a very rare floral scent compound (Knudsen et al. 2006), is probably emitted from the same three Lysimachia species, and could be selected by pollinators as well, although we detected it only in L. punctata and L. nummularia. However, 1-hydroxy-1-phenyl-2-propanone partly rearranges to 1-phenyl-1,2-propanedione during GC-MS analyses (S. Dötterl, unpubl. res.), and the amount of 1-phenyl-1,2-propanedione in L. vulgaris was at least 100-fold smaller than in L. punctata. 1-Hydroxy-1-phenyl-2-propanone, though present, may have been below the detection threshold after rearrangement of a large proportion to the diketone. The fact that 1-hydroxy-1-phenyl-2-propanone elicited strong responses in electrophysiological measurements with antennae of M. fulvipes could support the idea of pollinator-mediated selection; however, it failed to attract bees in behavioural experiments (Dötterl and Vereecken, 2010).
Scent from vegetative parts in Lysimachia
Scents from the vegetative parts of Lysimachia are species specific and, in contrast to floral scents, variability (dispersion) among oil species is comparable with that among non-oil species. These vegetative scents are mostly dominated by aliphatics and/or terpenoids (Fig. 5; Supplementary Data Fig. S3), which are well-known and widespread vegetative scents in plants (Kessler and Baldwin, 2001; Pichersky and Gershenzon, 2002). Therefore, it is unlikely that vegetative scents have played a major part in pollinator attraction in Lysimachia. There was also no obvious pattern to the distribution of scent compounds between oil and non-oil species. No compound occurred in more than one oil species and, at the same time, was absent from non-oil species. Furthermore, there was no evidence of convergence on a specific vegetative scent that could be considered characteristic for oil species, nor did oil species lack specific compounds that were abundant in several non-oil species. Some of the compounds, especially a group of non-identified terpenoids, occurred as minor (trace) compounds in a few non-oil species only (Supplementary Data Table S3), but the occurrence of these compounds can be explained best by the close relationship of the plant species emitting these compounds. Our analyses therefore do not reveal a vegetative scent compound which seems to be under pollinator-mediated selection and involved in attraction of Macropis.
Fig. 5.

Number of scent compounds per compound class in floral and vegetative scent of the plants studied. Species abbreviations' are as in Table 1; AL, aliphatics; AR, aromatics; T, terpenoids; C5, C5-branched chain compounds; N, N-containing compounds; UNK, unknown compounds.
Two of the non-oil species, L. mauritiana and L. atropurpurea, of the clade subgenus Palladia + Lysimachiopsis, differed in their vegetative scent pattern from the other species, and emitted only or mainly (relative amount) aromatics (Fig. 5; Supplementary Data Fig. S3). In both species, the most abundant compound was benzyl alcohol, but L. atropurpurea also emitted large amounts of methyl benzoate and p-anisaldehyde. These three compounds are known to elicit behavioural responses in generalist pollen-collecting bees and male fragrance-collecting euglossine bees (Dötterl and Vereecken, 2010), and it is possible that vegetative scents contribute to pollinator attraction in these generalist melittophilous Lysimachia species.
We found a correlation in qualitative scent pattern (set of compounds) between floral and vegetative scent data, i.e. species emitting a similar set of floral compounds also emitted a similar set of vegetative compounds, and species strongly differing in their floral scents also differed in their vegetative scents. One might predict that this correlation is due to shared biosynthetic pathways in floral and vegetative organs. However, several of the species emitted scents from different pathways in the different organs. As an example, some of the species emitted aliphatics only from vegetative parts (Fig. 5; Supplementary Data Fig. S3). Similarly, the occurrence of aromatics differed strongly between vegetative and floral parts. The correlation between floral and vegetative scents can, therefore, only partially be explained by shared pathways. Several species emitted aromatics only from their flowers (but see above), suggesting that these compounds are important for pollinator attraction. Indeed, recent meta-analyses suggest that aromatics evolved in flower scents in order to attract pollinators (Junker and Blüthgen, 2010; Schiestl, 2010).
Floral colour evolution in Lysimachia
Though flowers of all oil species are yellow to the human eye, we found differences in the colours that bees perceive (i.e. bee-colour) among the different species (Fig. 3). Nevertheless, there is evidence for correlated evolution between bee-green (yellow to the human eye) and oil secretion. Most of studied oil species are bee-green, though they belong to three different clades (subgenus Lysimachia group B, E and F, Table 1). Bee-green is not found in non-oil species, though it is known to be attractive for generalist bees (Giurfa et al., 1995), the suggested pollinators of these species. It also may be attractive to Macropis in general and, in the case of L. punctata, bee-green colours may have been responsible for attracting M. fulvipes to visual cues of the inflorescences (see also Dötterl et al., 2011). Nearly all species of the clade Palladia + Lysimachiopsis are bee blue-green, and this similarity in colour can be explained by the close relatedness of these species. Only one species of this clade, L. atropurpurea, evolved another colour and is bee UV-blue. Generally, these colours are known to elicit behavioural responses in bees (Menzel, 1985; Giurfa et al., 1995) and seem to be involved in attracting bee pollinators in these species.
The flower colour of one of our study species, cleistogamous L. minoricensis, was very similar to its leaf colour (distance to centre <0·1 hexagon units; Supplementary Data Table S4), and bees may, therefore, have difficulties in discriminating flowers from leaves. In this species, where pollinators are not required for reproductive success, selection may have favoured the evolution of cryptic flowers to prevent the attraction of florivores (Penet et al., 2009).
Conclusions
Lysimachia species emit specific and highly variable floral and vegetative scents. Oil-secreting species have a lower variability in floral scents compared with non-oil species, but there is no corresponding difference in variability of vegetative scents between oil and non-oil species. Thus, as predicted, floral, but not vegetative, scent seems to be under stabilizing selection from Macropis pollinators. Overall vegetative and floral scent compositions do not differ between oil and non-oil species, and none of the compounds occurs in all oil or in all non-oil species. However, some floral compounds specific to a few oil species exhibit correlated evolution with oil bee pollination, and these compounds may be under selection by Macropis. Leaf colours are similar among all studied species, but flower colour differs among species. Most notable is the correlated evolution between the flower colour bee-green and oil secretion, even though most, but not all oil flowers have this colour. This relationship is not surprising, however, because non-oil species are never bee-green. Overall, the data suggest that floral, but not vegetative, scents and colours are under selection by Macropis oil bees and that this is congruent with the study of Dötterl et al. (2011) showing that M. fulvipes bees use both visual and olfactory inflorescence cues for host location, whereas vegetative cues are not attractive. Oil species, though all pollinated by Macropis bees, do not share a signature volatile compound or bee-colour, and this suggests that different Macropis species are effectively attracted by different scents or colours or that flowers of the oil species emit a compound or compounds that are commonly used by Macropis for finding oil hosts, but that have not yet been detected by our methods.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
This work was supported by Deutsche Forschungsgemeinschaft (grant number: DO 1250/3-1). We thank Kim Steiner and two anonymous reviewers for helpful comments on earlier versions of the manuscript, and Josef Breu and Bernd Putz for the use of the spectrophotometer. Several Botanical Gardens provided seeds.
LITERATURE CITED
- Abouheif E. A method for testing the assumption of phylogenetic independence in comparative data. Evolutionary Ecology Research. 1999;1:895–909. [Google Scholar]
- Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. Carol Stream, IL: Allured Publishing Corporation; 2007. [Google Scholar]
- Anderberg AA, Manns U, Källersjö M. Phylogeny and floral evolution of the Lysimachieae (Ericales, Myrsinaceae): evidence from ndhF sequence data. Willdenowia. 2007;37:407–421. [Google Scholar]
- Anderson MJ, Gorley RN, Clarke KR. PERMANOVA+ for PRIMER: guide to software and statistical methods. Plymouth, UK: PRIMER-E; 2008. [Google Scholar]
- Andersson S, Nilsson LAA, Groth I, Bergström G. Floral scents in butterfly-pollinated plants: possible convergence in chemical composition. Botanical Journal of the Linnean Society. 2002;140:129–153. [Google Scholar]
- Andrews ES, Theis N, Adler LS. Pollinator and herbivore attraction to Cucurbita floral volatiles. Journal of Chemical Ecology. 2007;33:1682–1691. doi: 10.1007/s10886-007-9337-7. [DOI] [PubMed] [Google Scholar]
- Armbruster WS. Exaptations link evolution of plant–herbivore and plant–pollinator interactions: a phylogenetic inquiry. Ecology. 1997;78:1661–1672. [Google Scholar]
- Armbruster WS, Di Stilio VS, Tuxill JD, Flores TC, Velásquez Runk JL. Covariance and decoupling of floral and vegetative traits in nine Neotropical plants: a re-evaluation of Berg's correlation-pleiades concept. American Journal of Botany. 1999;86:39–55. [PubMed] [Google Scholar]
- Arnold SEJ, Faruq S, Savolainen V, McOwan PW, Chittka L. FReD: the floral reflectance database – a web portal for analyses of flower colour. Plos One. 2010;5:e14287. doi: 10.1371/journal.pone.0014287. http://dx.doi.org/10.1371/journal.pone.0014287 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayasse M, Schiestl FP, Paulus HF, Löfstedt C, Hansson B, Ibarra F, Francke W. Evolution of reproductive strategies in the sexual deceptive orchid Ophrys sphegodes: how does flower-specific variation of odor signals influence reproductive success? Evolution. 2000;54:1995–2006. doi: 10.1111/j.0014-3820.2000.tb01243.x. [DOI] [PubMed] [Google Scholar]
- Backhaus W. Color-vision in honeybees. Neuroscience and Biobehavioral Reviews. 1992;16:1–12. doi: 10.1016/s0149-7634(05)80045-4. [DOI] [PubMed] [Google Scholar]
- Baker HG, Hurd PD. Intrafloral ecology. Annual Review of Entomology. 1968;13:385–414. [Google Scholar]
- Balao F, Herrera J, Talavera S, Dötterl S. Spatial and temporal patterns of floral scent emission in Dianthus inoxianus and electroantennographic responses of its hawkmoth pollinator. Phytochemistry. 2011;72:601–609. doi: 10.1016/j.phytochem.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Banfi E, Galasso G, Soldano A. Notes on systematics and taxonomy for the Italian vascular flora. 1. Atti della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano. 2005;146:219–244. [Google Scholar]
- Burger H, Dötterl S, Ayasse M. Host-plant finding and recognition by visual and olfactory floral cues in an oligolectic bee. Functional Ecology. 2010;24:1234–1240. [Google Scholar]
- Burger H, Dötterl S, Häberlein C, Schulz S, Ayasse M. An arthropod deterrent attracts specialised bees to their host plants. Oecologia. 2012;168:727–736. doi: 10.1007/s00442-011-2136-4. [DOI] [PubMed] [Google Scholar]
- Chittka L. The color hexagon – a chromaticity diagram based on photoreceptor excitations as a generalized representation of color opponency. Journal of Comparative Physiology A: Sensory, Neural and Behavioral Physiology. 1992;170:533–543. doi: 10.1007/BF00199332. [DOI] [PubMed] [Google Scholar]
- Chittka L, Kevan PG. Flower colour as advertisement. In: Dafni A, Kevan PG, Husband BC, editors. Practical pollination biology. Cambridge: Enviroquest, Ltd; 2005. pp. 157–196. [Google Scholar]
- Clarke KR, Gorley RN. Primer v6: user manual/tutorial. Plymouth, UK: Primer-E; 2006. [Google Scholar]
- Conner JK, Sterling A. Selection for independence of floral and vegetative traits: evidence from correlation patterns in five species. Canadian Journal of Botany. 1996;74:642–644. [Google Scholar]
- Cresswell JE. Stabilizing selection and the structural variability of flowers within species. Annals of Botany. 1998;81:463–473. [Google Scholar]
- Dobson HEM, Danielson EM, Wesep IDV. Pollen odor chemicals as modulators of bumble bee foraging on Rosa rugosa Thunb. (Rosaceae) Plant Species Biology. 1999;14:153–166. [Google Scholar]
- Dötterl S. Antennal responses of an oligolectic bee and its cleptoparasite to plant volatiles. Plant Signaling and Behavior. 2008;3:296–297. doi: 10.4161/psb.3.5.5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dötterl S, Schäffler I. Flower scent of oil-producing Lysimachia punctata as attractant for the oil-bee Macropis fulvipes. Journal of Chemical Ecology. 2007;33:441–445. doi: 10.1007/s10886-006-9237-2. [DOI] [PubMed] [Google Scholar]
- Dötterl S, Vereecken NJ. The chemical ecology and evolution of bee–flower interactions: a review and perspectives. Canadian Journal of Zoology. 2010;88:668–697. [Google Scholar]
- Dötterl S, Wolfe LM, Jürgens A. Qualitative and quantitative analyses of flower scent in Silene latifolia. Phytochemistry. 2005;66:203–213. doi: 10.1016/j.phytochem.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Dötterl S, Milchreit K, Schäffler I. Behavioural plasticity and sex differences in host finding of a specialized bee species. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 2011;197:1119–1126. doi: 10.1007/s00359-011-0673-2. [DOI] [PubMed] [Google Scholar]
- Dufaÿ M, Hossaert-McKey M, Anstett MC. When leaves act like flowers: how dwarf palms attract their pollinators. Ecology Letters. 2003;6:28–34. [Google Scholar]
- Dumri K. Chemical analyses of non-volatile flower oils and related bee nest cell linings. Halle-Wittenberg: PhD Thesis, Martin-Luther-Universität; 2008. [Google Scholar]
- Dyer AG, Chittka L. Fine colour discrimination requires differential conditioning in bumblebees. Naturwissenschaften. 2004;91:224–227. doi: 10.1007/s00114-004-0508-x. [DOI] [PubMed] [Google Scholar]
- Endress PK. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press; 1994. Special differentiations associated with pollinator attraction. [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution and Systematics. 2004;35:375–403. [Google Scholar]
- Füssel U, Dötterl S, Jürgens A, Aas G. Inter- and intraspecific variation in floral scent in the genus Salix and its implication for pollination. Journal of Chemical Ecology. 2007;33:749–765. doi: 10.1007/s10886-007-9257-6. [DOI] [PubMed] [Google Scholar]
- Giurfa M, Núñez J, Chittka L, Menzel R. Colour preferences of flower-naive honeybees. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 1995;177:247–259. [Google Scholar]
- Goulson D, Chapman JW, Hughes WOH. Discrimination of unrewarding flowers by bees; direct detection of rewards and use of repellent scent marks. Journal of Insect Behavior. 2001;14:669–678. [Google Scholar]
- Hao G, Yuan Y-M, Hu C-M, Ge X-J, Zhao N-X. Molecular phylogeny of Lysimachia (Myrsinaceae) based on chloroplast trnL-F and nuclear ribosomal ITS sequences. Molecular Phylogenetics and Evolution. 2004;31:323–339. doi: 10.1016/S1055-7903(03)00286-0. [DOI] [PubMed] [Google Scholar]
- Harder LD, Johnson SD. Darwin's beautiful contrivances: evolutionary and functional evidence for floral adaptation. New Phytologist. 2009;183:530–545. doi: 10.1111/j.1469-8137.2009.02914.x. [DOI] [PubMed] [Google Scholar]
- von Helversen O, Winkler L, Bestmann HJ. Sulphur-containing ‘perfumes’ attract flower-visiting bats. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 2000;186:143–153. doi: 10.1007/s003590050014. [DOI] [PubMed] [Google Scholar]
- Honda K, Ômura H, Hayashi N. Identification of floral volatiles from Ligustrum japonicum that stimulate flower-visiting by cabbage butterfly. Pieris rapae. Journal of Chemical Ecology. 1998;24:2167–2180. [Google Scholar]
- Huber FK, Kaiser R, Sauter W, Schiestl FP. Floral scent emission and pollinator attraction in two species of Gymnadenia (Orchidaceae) Oecologia. 2005;142:564–575. doi: 10.1007/s00442-004-1750-9. [DOI] [PubMed] [Google Scholar]
- Jhumur U, Dötterl S, Jürgens A. Floral odors of Silene otites: their variability and attractiveness to mosquitoes. Journal of Chemical Ecology. 2008;34:14–25. doi: 10.1007/s10886-007-9392-0. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Hargreaves AL, Brown M. Dark, bitter-tasting nectar functions as a filter of flower visitors in a bird-pollinated plant. Ecology. 2006;87:2709–2716. doi: 10.1890/0012-9658(2006)87[2709:dbnfaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Jombart T, Balloux F, Dray S. adephylo: new tools for investigating the phylogenetic signal in biological traits. Bioinformatics. 2010;26:1907–1909. doi: 10.1093/bioinformatics/btq292. [DOI] [PubMed] [Google Scholar]
- Junker RR, Blüthgen N. Floral scents repel facultative flower visitors, but attract obligate ones. Annals of Botany. 2010;105:777–782. doi: 10.1093/aob/mcq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R. The scent of orchids. Amsterdam: Elsevier; 1993. [Google Scholar]
- Kaiser R. Meaningful scents around the world. Zürich: Wiley-VCH; 2006. [Google Scholar]
- Kaiser R. Scent of the vanishing flora. Zürich: Wiley-VCH; 2011. [Google Scholar]
- Kass RE, Raftery AE. Bayes factors. Journal of the American Statistical Association. 1995;90:773–795. [Google Scholar]
- Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- Klahre U, Gurba A, Hermann K, et al. Pollinator choice in petunia depends on two major genetic loci for floral scent production. Current Biology. 2011;21:730–739. doi: 10.1016/j.cub.2011.03.059. [DOI] [PubMed] [Google Scholar]
- Klotz S, Kühn I, Durka W. Schriftenreihe für Vegetationskunde 38. Bonn: Bundesamt für Naturschutz; 2002. BIOLFLOR – Eine Datenbank zu biologisch-ökologischen Merkmalen der Gefäßpflanzen in Deutschland. [Google Scholar]
- Knudsen JT. Floral scent chemistry in geonomoid palms (Palmae: Geonomeae) and its importance in maintaining reproductive isolation. Memoirs of the New York Botanical Garden. 1999;88:141–157. [Google Scholar]
- Knudsen JT, Eriksson R, Gershenzon J, Ståhl B. Diversity and distribution of floral scent. Botanical Review. 2006;72:1–120. [Google Scholar]
- Kunze J, Gumbert A. The combined effect of color and odor on flower choice behavior of bumble bees in flower mimicry systems. Behavioral Ecology. 2001;12:447–456. [Google Scholar]
- Levin RA, McDade LA, Raguso RA. The systematic utility of floral and vegetative fragrance in two genera of Nyctaginaceae. Systematic Biology. 2003;52:334–351. doi: 10.1080/10635150390196975. [DOI] [PubMed] [Google Scholar]
- Lin SYH, Trumble JT, Kumamoto J. Activity of volatile compounds in glandular trichomes of Lycopersicon species against two insect herbivores. Journal of Chemical Ecology. 1987;13:837–850. doi: 10.1007/BF01020164. [DOI] [PubMed] [Google Scholar]
- Manning JC, Goldblatt P. Radiation of pollination systems in the Cape genus Tritoniopsis (Iridaceae: Crocoideae) and the development of bimodal pollination strategies. International Journal of Plant Sciences. 2005;166:459–474. [Google Scholar]
- Manns U, Anderberg AA. New combinations and names in Lysimachia (Myrsinaceae) for species of Anagallis, Pelletiera and Trientalis. Willdenowia. 2009;39:49–54. [Google Scholar]
- Menzel R. Learning in honey bees in an ecological and behavioral context. In: Hölldobler B, Lindauer M, editors. Experimental behavioral ecology. Stuttgart: Fischer Verlag; 1985. pp. 55–74. [Google Scholar]
- Neff JL, Simpson BB. Rewards in flowers. Other rewards: oils, resins, and gums. In: Dafni A, Kevan PG, Husband BC, editors. Practical pollination biology. Cambridge: Enviroquest, Ltd; 2005. pp. 314–328. [Google Scholar]
- Pagel M, Meade A. Bayesian analysis of correlated evolution of discrete characters by reversible-jump Markov chain Monte Carlo. American Naturalist. 2006;167:808–825. doi: 10.1086/503444. [DOI] [PubMed] [Google Scholar]
- Peitsch D, Fietz A, Hertel H, Souza J, Ventura DF, Menzel R. The spectral input systems of hymenopteran insects and their receptor-based colour vision. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 1992;170:23–40. doi: 10.1007/BF00190398. [DOI] [PubMed] [Google Scholar]
- Penet L, Collin CL, Ashman TL. Florivory increases selfing: an experimental study in the wild strawberry. Fragaria virginiana. Plant Biology. 2009;11:38–45. doi: 10.1111/j.1438-8677.2008.00141.x. [DOI] [PubMed] [Google Scholar]
- Pichersky E, Gershenzon J. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Current Opinion in Plant Biology. 2002;5:237–243. doi: 10.1016/s1369-5266(02)00251-0. [DOI] [PubMed] [Google Scholar]
- Plepys D, Ibarra F, Löfstedt C. Volatiles from flowers of Platanthera bifolia (Orchidaceae) attractive to the silver Y moth, Autographa gamma (Lepidoptera: Noctuidae) Oikos. 2002;99:69–74. [Google Scholar]
- Raguso RA. Flowers as sensory billboards: progress towards an integrated understanding of floral advertisement. Current Opinion in Plant Biology. 2004;7:434–440. doi: 10.1016/j.pbi.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Raguso RA, Pichersky E. Floral volatiles from Clarkia breweri and C. concinna (Onagraceae) – recent evolution of floral scent and moth pollination. Plant Systematics and Evolution. 1995;194:55–67. [Google Scholar]
- Raguso RA, Willis MA. Synergy between visual and olfactory cues in nectar feeding by wild hawkmoths. Manduca sexta. Animal Behaviour. 2005;69:407–418. [Google Scholar]
- Raguso RA, Levin RA, Foose SE, Holmberg MW, McDade LA. Fragrance chemistry, nocturnal rhythms and pollination ‘syndromes’ in Nicotiana. Phytochemistry. 2003;63:265–284. doi: 10.1016/s0031-9422(03)00113-4. [DOI] [PubMed] [Google Scholar]
- Rambaut A, Drummond AJ. Tracer v1·5. 2007 http://tree.bio.ed.ac.uk/software/tracer/ [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Schiestl FP. The evolution of floral scent and insect chemical communication. Ecology Letters. 2010;13:643–656. doi: 10.1111/j.1461-0248.2010.01451.x. [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Dötterl S. The evolution of floral scent and olfactory preferences in pollinators: coevolution or pre-existing bias? Evolution. 2012 doi: 10.1111/j.1558-5646.2012.01593.x. in press. http://dx.doi.org/10.1111/j.1558-5646.2012.01593.x . [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Ayasse M, Paulus HF, Löfstedt C, Hansson B, Ibarra F, Francke W. Orchid pollination by sexual swindle. Nature. 1999;399:421–422. [Google Scholar]
- Seipold L. Blütenöle – Chemische Analyse, Biosynthese und Betrachtungen zur Entstehung von Ölblumen. Halle-Wittenberg: PhD Thesis, Martin-Luther-Universität; 2004. [Google Scholar]
- Simpson BB, Neff JL, Seigler DS. Floral biology and floral rewards of Lysimachia (Primulaceae) American Midland Naturalist. 1983;110:249–256. [Google Scholar]
- Smith KA. A simple multivariate technique to improve the design of a sampling strategy for age-based fishery monitoring. Fisheries Research. 2003;64:79–85. [Google Scholar]
- Steiner KE, Kaiser R, Dötterl S. Strong phylogenetic effects on floral scent variation of oil-secreting orchids in South Africa. American Journal of Botany. 2011;98:1663–1679. doi: 10.3732/ajb.1100141. [DOI] [PubMed] [Google Scholar]
- Theis N, Lerdau M. The evolution of function in plant secondary metabolites. International Journal of Plant Sciences. 2003;164:S93–S102. [Google Scholar]
- Turlings TCJ, Tumlinson JH, Lewis WJ. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science. 1990;250:1251–1253. doi: 10.1126/science.250.4985.1251. [DOI] [PubMed] [Google Scholar]
- Vogel S. Ölblumen und ölsammelnde Bienen, Zweite Folge: Lysimachia und Macropis. Mainz, Stuttgart, Akademie der Wissenschaft und der Literatur: Franz Steiner Verlag Wiesbaden GmbH; 1986. [Google Scholar]
- Waelti MO, Muhlemann JK, Widmer A, Schiestl FP. Floral odour and reproductive isolation in two species of Silene. Journal of Evolutionary Biology. 2008;21:111–121. doi: 10.1111/j.1420-9101.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- Williams WG, Kennedy GG, Yamamoto RT, Thacker JD, Bordner J. 2-Tridecanone: a naturally occurring insecticide from the wild tomato Lycopersicon hirsutum f. glabratum. Science. 1980;207:888–889. doi: 10.1126/science.207.4433.888. [DOI] [PubMed] [Google Scholar]
- Wright GA, Schiestl FP. The evolution of floral scent: the influence of olfactory learning by insect pollinators on the honest signalling of floral rewards. Functional Ecology. 2009;23:841–851. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



