Abstract
Pathological aggression, and the inability to control aggressive impulses, takes a tremendous toll on society. Yet aggression is a normal component of the innate behavior repertoire of most vertebrate animal species, as well as of many invertebrates. Progress in understanding the etiology of disorders of aggressive behavior, whether genetic or environmental in nature, therefore requires an understanding of the brain circuitry that controls normal aggression. Efforts to understand this circuitry at the level of specific neuronal populations have been constrained by the limited resolution of classical methodologies, such as electrical stimulation and electrolytic lesion. The availability of new, genetically based tools for mapping and manipulating neural circuits at the level of specific, genetically defined neuronal subtypes provides an opportunity to investigate the functional organization of aggression circuitry with cellular resolution. However these technologies are optimally applied in the mouse, where there has been surprisingly little traditional work on the functional neuroanatomy of aggression. Here we discuss recent, initial efforts to apply optogenetics and other state-of-the-art methods to the dissection of aggression circuitry in the mouse. We find, surprisingly, that neurons necessary and sufficient for inter-male aggression are located within the ventrolateral subdivision of the ventromedial hypothalamic nucleus (VMHvl), a structure traditionally associated with reproductive behavior. These neurons are intermingled with neurons activated during male-female mating, with ~20% overlap between the populations. We discuss the significance of these findings with respect to neuroethological and neuroanatomical perspectives on the functional organization of innate behaviors, and their potential implications for psychiatry.
Keywords: aggression, mating, violence, hypothalamus, optogenetics, channelrhodopsin, mouse
Introduction
Violence, and the inability to control violent impulses, takes a tremendous toll on society. Yet aggression, which evolved to obtain or protect food, mating partners, progeny and territory, is an innate and normal part of most animals’ ethological repertoire [1]. It is also one of the evolutionarily oldest “emotional” behaviors: virtually all types of organisms, from primates to fish to insects, show species-specific aggressive behavior [2]. However, despite its evolutionary conservation and importance for human health and well being, the neurobiology of aggressive behavior, and of associated affective states, such as anger and rage, are relatively understudied, by comparison to other emotional and affective behaviors such as fear and anxiety [3–5]. Surprisingly, aggression and impulsive violence research are not even among the mental health topics supported by the NIMH [6]. Furthermore, there is no separate category for disorders of anger or aggression listed in the DSM IV [7]. The reasons for this are unclear, but may include nosologic criteria, legal and public policy concerns. Whatever the explanation, these factors have caused government funding agencies and many researchers to steer clear of this area. Consequently, our understanding of the neural circuitry of aggression lags far behind that of other emotional behaviors, despite the profound societal importance of the problem. Yet such an understanding is essential to provide a framework for identifying the neural substrates and mechanisms of genetic and environmental influences on pathological aggressive behavior, and a rational route to new therapies [8, 9].
The study of the neurobiology of aggression has, until recently, utilized the traditional tools of functional neuroanatomy, and has applied them to the classical model organisms of behavioral psychology such as the rat and the hamster (reviewed in [10, 11]). Many of these studies have exploited the phenomenon of artificial brain stimulation-evoked attack (reviewed in [12]), first reported by Hess (in cats) in the late 1920s [13, 14]. Results obtained from such systematic studies, particularly in the rat [15, 16], have provided an important framework for thinking about the functional organization of attack circuitry (reviewed in [12, 17, 18]). However electrical stimulation or lesion experiments target fibers-of-passage as well as local neuronal cell bodies. While these approaches can provide an overview of potential afferent and efferent pathways involved in attack behavior [19], the spatial resolution with which the critical hypothalamic neurons involved in this circuitry can be localized is limited.
Recently, emerging new technologies for mapping and manipulating neural circuitry at the level of genetically defined cell types have created new opportunities to elucidate the brain pathways mediating attack behavior [20, 21]. These technologies are most powerfully applied in the laboratory mouse, the genetically tractable model organism whose nervous system is closest to our own in its anatomical organization. Surprisingly, however, until recently there was no published report of brain stimulation-evoked attack in the mouse, in the 75 years since the phenomenon was first described.
Here we focus on recent work applying optogenetics and pharmacogenetics to the understanding of attack circuitry, in the mouse. We review these studies in the context of a speculative theoretical framework originally suggested by the Dutch neuroethologist and Nobel laureate Niko Tinbergen [1], for understanding the control of behavioral decision-making, and consider the implications for understanding the possible etiology of certain types of pathological aggressive behavior.
Tinbergen’s hypothesis – the neural substrate of ethological hierarchies
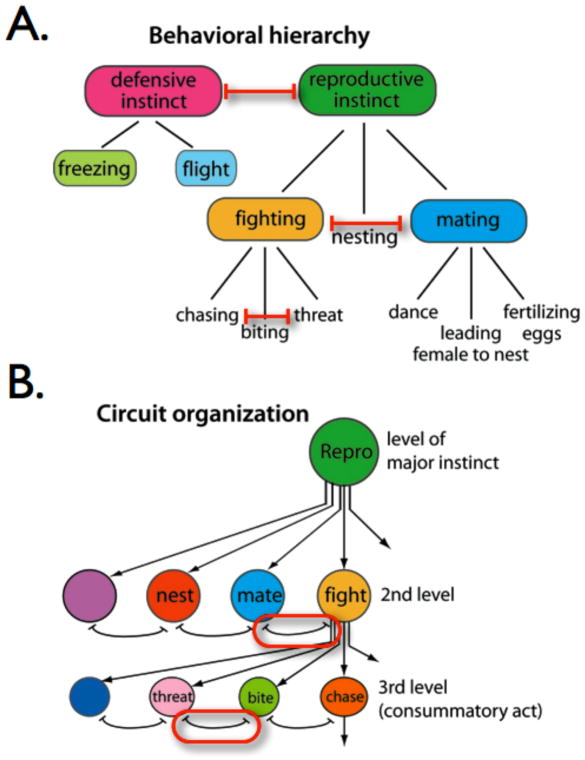
Based on his field observations of innate behavior in the stickleback fish, Tinbergen suggested that behavioral “decisions” are hierarchically organized [1] (Fig. 1A). According to this scheme, an individual animal would first choose whether to “enter” one of a few, broad behavioral hierarchies, such as the “defensive” or the “reproductive” hierarchy (Fig. 1A), based on environmental conditions (ambient temperature, time of year, etc.), the presence or absence of conspecifics of the same or opposite sex, predators, availability of nesting locations, etc. Having made, for example, the decision to enter the reproductive hierarchy, the animal would then choose from among a repertoire of innate reproductive behaviors, such as mating or nest-building. Interestingly, Tinbergen assigned aggression to the reproductive hierarchy as part of his ethologic taxonomy, although aggression is also employed as a defensive behavior by many animals [22]. Having chosen to engage in aggressive activity, the animal would then select between the expression of different specific aggressive actions, such as threat displays, chasing or biting (Fig. 1A).
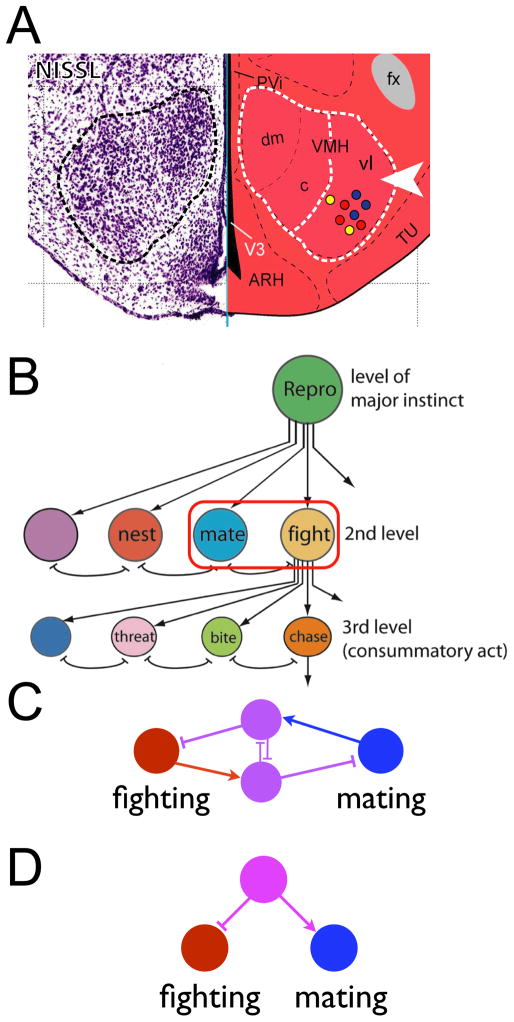
Figure 1. Tinbergen’s hypothesis for hierarchical organization of innate behavior.
(A) Ethological taxonomy illustrating hierarchical nature of behavioral decisions. Double-headed blunt red arrows indicate inhibitory interactions. Modified from Tinbergen (1951) [1] Fig. 89. (B) Hierarchical organization of brain circuit “nodes” postulated to control innate behaviors. Red circles highlight mutually inhibitory interactions between nodes. Adapted from Tinbergen (1951) [1] Fig. 98.
In his famous 1951 monograph entitled “The Study of Instinct” [1], Tinbergen proposed a bold hypothesis to explain the hierarchical organization of behavior he observed in the wild. Essentially, he argued that it reflects an underlying hierarchical organization of the neural circuits that mediate these innate behaviors (Fig. 1B). Central to this hypothesis is the concept of “organizing centers,” circuit nodes whose activation leads to the actuation of an innate behavioral program. Tinbergen was strongly influenced in his conception of such nodes by the work of Hess and others on brain stimulation-evoked attack, as well as the work of Paul Weiss on the hierarchical organization of motor behavior [23]. He suggested, moreover, that network-level reciprocal inhibition between such organizing centers could mediate the behavioral “decisions” observed at the organismal level (Fig. 1B, red circles). A hierarchical organization of such nodes would, therefore, canalize the process of behavioral decision-making into choices between progressively more specific alternatives. Implicit in this idea is the assumption that more closely related behaviors have a more closely related anatomical organization, thereby facilitating such reciprocal inhibition. There is evidence supporting this concept from studies of reciprocal inhibitory interactions between neighboring hypothalamic centers mediating brain stimulation-evoked “quiet biting” vs. “defensive rage” types of aggressive behavior in the cat [24, 25]. According to this principle, Tinbergen’s inclusion of aggression within the reproductive hierarchy predicts that its neural circuitry should be anatomically proximal to that mediating mating behavior.
It should be pointed out that while this conceptualization of behavioral circuits as a series of feed-forward networks with reciprocal inhibition at different nodes is attractive, it is not the only type of network organization that could mediate “behavioral decisions.” For example, an extensive body of work has shown that hypothalamic “nodes” involved in goal-directed behaviors (see below) are interconnected, both with each other and with other regions of the brain, by positive and negative feed-back “loops” including those involved in neuroendocrine and prefrontal cortical regulation [19, 26–32]. Such feedback loops provide an alternative, non-hierarchical neural substrate underlying “hierarchical” behavioral decision-making.
The medial hypothalamus: neural instantiation of Tinbergen’s hypothesis?
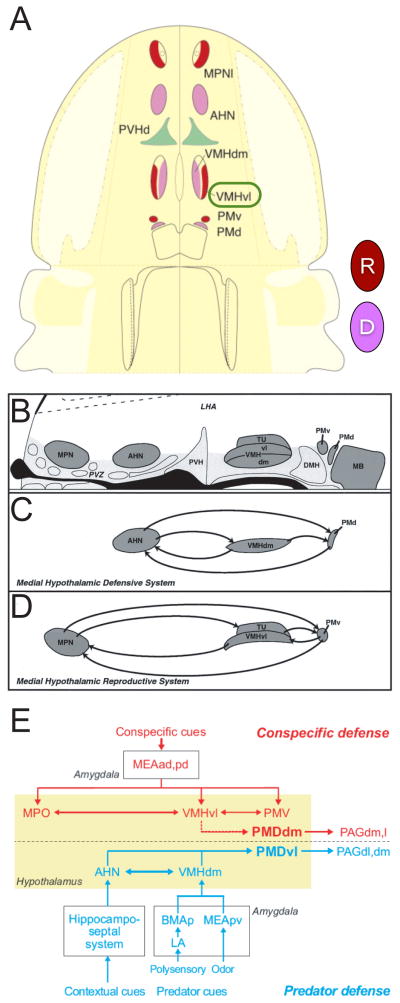
Evidence from functional neuroanatomical studies of the hypothalamus has been interpreted in the context of Tinbergen’s proposal. In particular, work by Canteras, Swanson and their colleagues has suggested a hodological organization for the “medial hypothalamic behavioral control column,” in which different anatomically distinct nuclei are arranged in pairs mediating reproductive or defensive behaviors, along the anterior-posterior axis of the brain (Fig. 2A, B; refs. [33–35]). According to this scheme, the anterior hypothalamic nucleus (AHN), dorso-medial VMH (VMHdm) and dorsal pre-mammillary nucleus (PMd) mediate defensive behaviors [36] (Fig. 2C), while the median preoptic nucleus (MPN), ventro-lateral subdivision of the ventromedial hypothalamic nucleus (VMHvl), tuberal nucleus (TU) and ventral portion of the pre-mammillary nucleus (PMv) mediate reproductive behaviors (Fig. 2D). Thus, these two sub-circuits could, collectively, be viewed as a neuroanatomical instantiation of Tinbergen’s reproductive and defensive “hierarchies” (although there is, so far, no evidence of anything intrinsically hierarchical about their organization) [34, 35, 37].
Figure 2. Behavioral control in the medial hypothalamus.
(A) Medial hypothalamic behavioral control column in schematic horizontal plane of section. Anterior is up. Nuclei associated with reproductive vs. defensive behaviors are indicated in red (“R”) or magenta (“D”), respectively. MPNl, medial preoptic nucleus (also called MPO), lateral part; AHN, anterior hypothalamic nucleus; PVHd, paraventricular hypothalamic nucleus, dorsal part; VMHdm, ventromedial hypothalamic nucleus, dorsomedial part; VMHvl, ventromedial hypothalamic nucleus, ventrolateral part; PMv, pre-mammillary nucleus, dorsal part; PMd, premammillary nucleus, ventral part. Modified from Swanson (2005) [34]. (B–D) Reproductive and defensive subcircuits in the medial hypothalamus. (B) Schematic saggital section showing nuclei indicated in (A); anterior is to the left. TU, tuberal nucleus; MB, mammillary body; DMH, dorso-medial hypothalamus; LHA, lateral hypothalamic area; PVZ, periventricular hypothalamic zone; other abbreviations as in (A). (C) Interconnections between nuclei assigned to the defensive subcircuit. (D) Interconnections between nuclei in the reproductive subcircuit, which includes VMHvl. Reproduced with permission from [33]. (E) Circuit diagram comparing roles of different hypothalamic nuclei in predator vs. conspecific defense, based on c-fos labeling and connectivity mapping. VMHvl is assigned to a subcircuit for conspecific defense. Reproduced with permission from [37]. It should be noted that lesions of the PAG impair defensive but not offensive aggression [18, 63].
How attack circuitry fits into this scheme, and its relationship to reproductive circuitry, has not been clear. As mentioned earlier, Tinbergen included aggression within his “reproductive” behavioral hierarchy [1]. But since aggression often has a defensive component [22], it was initially assumed that this behavior would map to “defensive” nuclei. Indeed, extensive studies in the hamster, as well as in other rodents [18] have implicated the AHN, the largest “defensive” nucleus (Fig. 2C), in aggression [10, 38]. A more recent c-fos mapping study in rats, however, revealed labeling in VMHvl, a structure traditionally assigned a reproductive role [39, 40] (Fig. 2A, D), in intruder males (which can exhibit defensive aggression) during the resident-intruder test [37]. Similar, although weaker, c-fos labeling was anecdotally reported in VMHvl in the resident male [37]. However no functional studies were performed to validate the behavioral relevance of this c-fos labeling. Such validation is important, because c-fos mapping not only reports increases in neuronal spiking activity, but also hormonal or neuromodulatory influences that can increase intracellular calcium or cAMP [41]; furthermore, it does not distinguish between the activation of inhibitory (GABAergic) vs. excitatory (glutamatergic) neurons. Based on their observations, the authors proposed a revised scheme in which VMHvl was included as part of a circuit mediating “conspecific defense,” while VMHdm was associated with predator defense (Fig. 2E; ref. [37]). However this scheme appears agnostic with respect to the role, if any, of VMHvl in offensive attack.
A role for at least a portion of VMHvl in offensive attack has been suggested by its overlap with the so-called “hypothalamic aggression/attack area” (HAA), a region defined by systematic brain stimulation-evoked aggression studies in the rat [19]. However, lesion studies have yielded conflicting results regarding the requirement for VMHvl in attack behavior [42, 43]. Interestingly, the boundaries of the HAA (as defined by electrical stimulation studies [44]) do not respect the anatomically defined boundaries of hypothalamic nuclei, but include both VMHvl and the adjacent “intermediate hypothalamic area” [15, 17, 19, 45] (IHA; Fig. 3A, red shaded region). Whether this more diffuse distribution indicates a fundamental difference in the anatomical organization of attack circuitry, compared to that mediating other innate behaviors [33–35], or rather reflects the limited spatial resolution of electrical stimulation techniques (due to the activation of fibers-of-passage), is not clear.
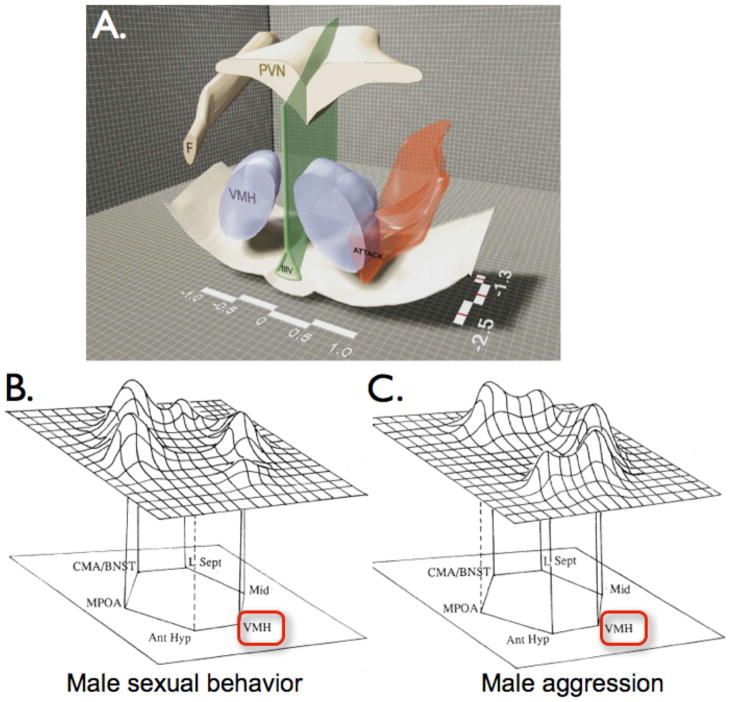
Figure 3. Hypothalamic regions involved in aggression.
(A) Cutaway coronal view of hypothalamus illustrating distribution of Hypothalamic Attack Area in the rat (HAA; red-shaded region). F, fornix; PVN, paraventricular hypothalamic nucleus; IIIV, 3rd ventricle. Reproduced with permission from [45] (Fig. 1). (B, C) Proposed networks controlling male sexual (B) and aggressive (C) behavior, based on c-fos mapping studies in the hamster. Peak heights in the 3-dimensional landscape approximate relative levels of activity. Note that VMH (red box) is a component of both circuits, although the subdivision (VMHvl or VMHdm) is not indicated. Reproduced with permission from Newman (1999) [47], Figs. 2 and 3.
If indeed VMHvl and/or the adjacent IHA play a role in offensive attack, how can this be reconciled with the classical view of VMHvl as a “reproductive center”? Earlier c-fos mapping studies in hamsters had suggested that VMHvl, together with other “limbic” nuclei, were activated, although to quantitatively different extents, during both male-female mating and inter-male aggression [46], leading to the suggestion that aggressive and reproductive behaviors employ a common network comprised of shared nodes, whose different behavioral outputs depend on the relative levels of activity in the different nodes (Fig. 3B, C) [47]. While plausible, this model leaves open the important question of whether the same or different neurons in each of these nodes are involved in both aggressive and reproductive behavior. For example, it is possible that each of these limbic nuclei contains neurons involved in both aggression and mating, whose behavioral output depends on state-dependent modification of circuit dynamics, e.g., by steroid hormones, biogenic amines or other neuromodulators [48]. There is precedent for such functional plasticity in the crustacean stomatogastric ganglion, in which a network of ~30 neurons can produce a variety of behavioral outputs, depending on its state of modulation [49]. Alternatively, these nodes may instead contain intermingled populations of neurons, each dedicated to either aggressive or reproductive behavior. Because traditional c-fos labeling studies involve between-subject, rather than within-subject, comparisons of neuronal activity during different behaviors [46], they cannot distinguish between these alternatives.
VMH contains distinct but intermingled populations involved in mating and attack
With a view towards resolving the location of attack-promoting neurons and their relationship to reproductive circuitry, we recently undertook to characterize the role of hypothalamic neurons in fighting and mating behavior, both electrophysiologically and functionally, in the mouse [50]. Initial systematic, brain-wide c-fos mapping experiments indicated strong activation of common “limbic” nuclei in the male mouse brain during both male-female and male-male interactions. Application of a fos-based “catFISH” (cellular compartment analysis of temporal activity by Fluorescence In Situ Hybridization) method [51], which permits within-subject comparisons of neuronal activity patterns associated with different behaviors, indicated that several of these nuclei, including VMHvl, contain distinct neuronal subpopulations activated during mating and attack, with an overlap of ~20% [50].
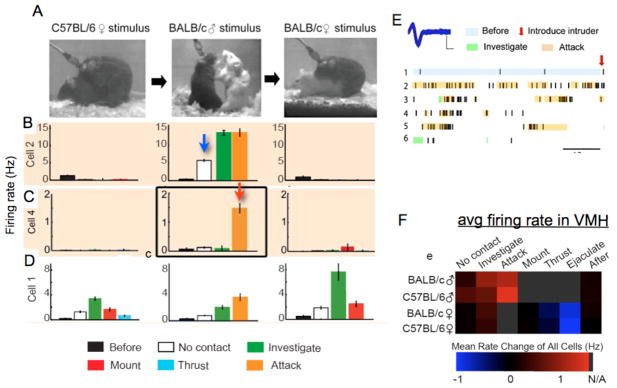
To confirm that the c-fos labeling was indeed representative of patterns of neuronal spiking activity, we performed chronic in vivo multi-electrode recordings of single unit activity in the VMHvl of male mice (residents) during sequential episodes of male-female and male-male social interactions (Fig. 4A). This analysis allowed recordings to be obtained from the same neuron in the same animal during sequential episodes of mating and fighting (Fig. 4B–D). In order to correlate these electrophysiological data with behavior, approximately 165 hrs of video recordings acquired during the electrophysiology experiments were manually scored, on a frame-by-frame basis, for the presence of a series of specific actions (investigation, mount, attack, etc.). These experiments confirmed that VMHvl indeed contains some neurons activated during male-male (aggressive) encounters, and others activated during male-female interactions, with a level of overlap (~25%) surprisingly close to that predicted from the c-fos mapping studies [50].
Figure 4. Distinct patterns of neuronal activity in VMHvl during mating vs. fighting.
(A) Photographs of the same male mouse with chronic recording device, during successive episodes of mating, fighting and mating again (left-to-right). (B–D) patterns of activity during indicated behaviors. Graphs in each row are from the same cell. Firing rates are mean±SEM over 0.5s bins. (E), recording from cell in (C), middle graph. Blue trace illustrates superimposition of multiple spikes. Scale bars, 200 μV (ordinate), 200 μs (abcissa). Raster plot is derived from 300 s of continuous recording. Colored shading marks duration of behavioral episodes determined by manual annotation. (F) Average firing rate in VMHvl compiled from all 104 recorded cells, during the indicated behavioral episodes. Squares shaded gray represent behaviors not applicable to the social encounter. Reproduced from [50], Figs. 2 and 3.
These electrophysiological recordings also revealed several surprising aspects of neuronal activity in VMHvl not predicted by c-fos analysis, due to its limited temporal resolution. First, activity patterns during aggressive behavior were heterogeneous: some neurons were activated as soon as the intruder was placed in the resident’s cage (Fig. 4B, white bar/blue arrow), and increased their activity as the encounter progressed to its consummatory (attack) phase, while others (a minority) were exclusively activated during attack itself (Fig. 4C, orange bar, red arrow; 4E). Second, not only were some neurons activated during social encounters, but others were specifically inhibited. In particular, neurons activated at the initiation of male-female encounters tended to become progressively more inhibited as the encounter proceeded to active copulation (intromission and ejaculation) (Fig. 4D, left panel). Third, many of the neurons activated during male-male aggressive encounters were also suppressed in the presence of females (Fig. 4B, left panel), suggesting that the presence of a female and/or engagement in copulatory behavior activates a mechanism that specifically inhibits attack circuitry in males. Overall, neuronal activity in VMHvl tended to escalate as male-male social encounters progressed towards their consummatory phase, while it decreased with such progression during male-female interactions (Fig. 4F) [50].
Neurons in VMHvl are necessary and sufficient for attack behavior
To move beyond such correlative studies to tests of the causal role of VMHvl neurons in attack behavior, we carried out genetically based loss- and gain-of-function manipulations of neuronal activity in this nucleus, by stereotaxic injection into VMHvl of recombinant adeno-associated viruses (rAAVs) containing optogenetic or pharmacogenetic effector genes (Fig. 5A, B). To test whether activating neurons in VMHvl was sufficient to promote attack, we expressed channelrhodopsin-2 (ChR2) under the control of a constitutive promoter [52]. These experiments demonstrated that, indeed, activation of VMHvl neurons elicited attack behavior time-locked to the onset of the light stimulus (Fig. 5C) [50]. This attack behavior could be directed at inappropriate targets, including castrated males, females, and even an inanimate object (rubber glove (Fig. 5D, arrowhead)). Interestingly, when activation of these neurons during a male-female encounter was delayed until intromission was underway, it became far more difficult to elicit attack towards the female. This inhibition was largely relieved once ejaculation had occurred. This observation is consistent with our electrophysiological data suggesting that mating causes an active inhibition of attack circuitry, so that the level of artificial stimulation required to override this inhibition is increased during the consummatory phase. Interestingly, although neuronal activation in VMHvl was detected during male-female encounters (by c-fos induction and electrophysiology), at no point did optogenetic activation induce or facilitate male-female mating behavior.
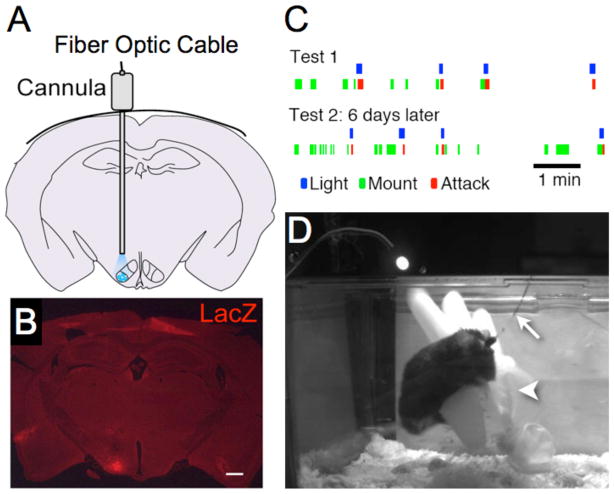
Figure 5. Optogenetic induction of attack behavior in mice.
(A) Schematic illustrating insertion of fiber optic cable through guide cannula and site of blue light illumination in VMHvl (blue shading). (B) Location of virally infected cells in VMHvl revealed by a co-injected rAAV::nuclear-lacZ virus. (C) Raster plot illustrating behavior of a male towards a conspecific female before, during and after optogenetic activation of VMHvl. Note abrupt switch from mounting behavior (green ticks) to attack (red ticks) upon illumination with 477 nm light (blue ticks). Reproduced from [50], Fig. 4. (D) Video frame taken from a trial in which optogenetically induced attack was directed toward a latex glove (arrowhead). Arrow indicates fiber-optic cable.
An outstanding issue in the field of brain-stimulated attack has been whether regions sufficient to elicit attack when artificially activated are also involved in normal attack behavior elicited using ecologically relevant stimuli. As mentioned earlier, efforts to address this question in VMH using conventional electrolytic lesions have yielded conflicting results [42, 43], and there is no report in which conventional inhibitory pharmacologic manipulations of VMH, such as injection of muscimol or ibotenic acid, inhibited attack (although Substance P antagonists and selective killing of NK1 receptor-expressing neurons have been reported to reduce levels of intense attack [53, 54]). Therefore, we addressed the question of whether neurons in VMHvl are necessary, as well as sufficient, for aggressive behavior. To do this, we used a pharmacogenetic method for inhibition of neuronal activity, based on expression of an ivermectin (IVM)-gated chloride channel from C. elegans (GluCl) [55, 56]. The results of these experiments indicated that attack behavior could indeed be reduced, and in some cases completely suppressed, by IVM administration to mice expressing GluCl in VMH, in a reversible manner [50]. Therefore, neurons whose somata are located within VMHvl are necessary as well as sufficient for attack behavior.
What did these experiments teach us that we did not already know from classical electrical brain stimulation-evoked attack experiments performed in other species? First of all, they reveal the power of optogenetic manipulations to evoke complex, innate social behaviors in the mouse, and also open up that powerful model organism to the study of attack circuitry using genetically based methods. Second, because optogenetic activation is restricted to neuronal cell bodies at the site of viral infection [50], and does not stimulate fibers-of-passage, the data provide new information on the location of the neurons responsible for attack behavior. Specifically, these neurons appear localized within VMHvl (Fig. 6A, red dots), rather than distributed broadly between VMHvl and the adjacent IHA (Fig. 3A). This conclusion is important for two reasons: 1) it suggests that attack, like other motivated behaviors encoded in the hypothalamus [33–35], is controlled by neurons whose distribution respects the cytoarchitectonically defined boundaries of hypothalamic nuclei; 2) surprisingly, it maps attack-promoting neurons to a structure traditionally associated with reproductive behavior. However, it is also possible that the difference in localization of attack neurons to VMHvl vs. the HAA reflects a species difference (mouse vs. rat) [12]; optogenetic studies of brain-stimulated aggression in the rat will be required to resolve this issue.
Figure 6. Attack neurons are localized to VMHvl and intermingled with neurons involved in mating.
(A) Schematic illustrating intermingling of cells involved in aggression (red dots), mating (blue dots) or both (yellow dots) within VMHvl (dashed white line, arrowhead). The left-hand panel shows the VMH as revealed by Nissl staining (dashed outline). Modified from the Allen Reference Atlas [64] (http://mouse.brain-map.org/atlas/index.html). (B) The intermingling of neurons involved in mating and offensive aggression within VMHvl (A) may permit reciprocal inhibitory interactions between neurons mediating these behaviors (red outline), as suggested by Tinbergen. Modified from [1], Fig. 98. (C, D) Inhibitory interactions between mating and fighting circuits may involve local inhibition (C; magenta neurons are inhibitory), or may reflect opposite-sign inputs to VMHvl (D). Circuit structure is hypothetical.
Was Tinbergen right?
Our observations are consistent with several aspects of Tinbergen’s model for the organization of innate behavior. First, the localization of attack neurons within VMHvl, a structure traditionally associated with reproductive behavior (Fig. 6D), supports Tinbergen’s assignment of aggression (or at least of offensive attack), to his “reproductive” behavioral hierarchy (Fig. 1A). Although VMHvl has been associated with reproductive behavior (lordosis) primarily in female rats [39, 40], our observations confirm that even in males, this structure contains neurons that are specifically activated during mating encounters [46, 47]. The finding that neurons involved in mating or fighting behavior, as well as some involved in both, are present in VMHvl (Fig. 6A), supports Tinbergen’s idea of a close association between the neural substrates of these behaviors (Fig. 6B). Of course, this does not mean that all areas involved in male mating behavior also contain neurons involved in attack. For example, in male rats the MPN/MPO is strongly implicated in mating, but has yet to be implicated in fighting [46, 47, 57].
Second, our finding that VMHvl neurons involved in attack are inhibited during mating appears to fit, at least superficially, with Tinbergen’s suggestion that inhibitory interactions between neurons at a given hierarchical level may control behavioral decisions (Fig. 6E). Conceivably, neurons activated during mating in VMHvl could directly inhibit those involved in attack (Fig. 6B); alternatively this inhibition could arise elsewhere (Fig. 6C). The significance of neurons activated during both male-male and male-female encounters (Fig. 6A, yellow dots) is not yet clear. Since the majority of such neurons are activated at early stages of the social encounter, these dual-activated cells could, for example, signal the presence of a conspecific animal independent of its sex. Alternatively, they could be involved in the organization of motor programs common to both attack and mating, such as approach to a conspecific, ano-genital investigation, etc.
What is the functional role of the VMHvl neurons that are activated during male-female but not male-male encounters? While they could play a role in mating behavior, our perturbations failed to reveal any effect of either activation or silencing in VMHvl to promote such behavior. There are several possible explanations for this result. First, the failure to inhibit mating using IVM/GluCl could reflect redundant circuits controlling this behavior, such as the MPN [57]. Second, since most neurons initially activated by females are inhibited as the interaction proceeds to its consummatory phase (Fig. 4F), further inhibition by artificial means may not have any behavioral effect. By the same token, optogenetic stimulation of VMHvl would be expected to inhibit rather than to promote mating, since it is in the opposite direction as the changes in neuronal activity that normally occur. Third, these neurons may not play a role in the consummatory aspects of mating behavior (mounting/intromission/ejaculation), but instead may function in gender (or species) recognition, for example in response to olfactory (pheromonal) cues. This explanation would fit our observation that the activation of these neurons by females typically occurs early in the social encounter (i.e., prior to physical contact).
Last, but not least, these female-activated neurons may function to suppress attack behavior during male-female encounters (Fig. 6B). That such inhibition occurs is suggested by our observation that optogenetic stimulation of attack in VMHvl became progressively less effective as the mating encounter progressed to its consummatory phase [50]. One problem with assigning such an inhibitory role to female-activated VMHvl neurons, however, is that the spiking of many of these neurons was increasingly suppressed during the consummatory phase of mating. How can these female-specific neurons be responsible for suppressing attack neurons, if they themselves become inhibited during the epoch of mating behavior when stimulation-induced attack is most strongly suppressed? This paradox suggests that the female-activated neurons in VMHvl may not themselves inhibit attack neurons during mating (indeed, there are relatively few local GABAergic neurons in VMH [58]). But if so, then why are these neurons activated by females in the first place (early in the social encounter)? If, as mentioned earlier, these neurons play a role in gender recognition, then their initial activation may signal other regions of the brain that a female is present, eliciting negative feedback from these other areas that then suppresses activity in VMHvl as the mating encounter proceeds. In this way, such neurons might serve as “sentinels” to detect the presence of females, leading to an inhibition of attack circuitry in VMHvl.
Implications for psychiatric disorders
Our observations support Tinbergen’s view that aggression is localized in the brain within a behavioral network that also participates in the control of reproductive behavior. Indeed, they reveal an unexpectedly close anatomical association between neurons involved in reproductive and aggressive behavior: apparently, the circuitry for “sex and violence” is intimately linked in the brain. Why has such a linkage evolved? The observation that neurons involved in attack are apparently progressively inhibited during male-female mating suggests that part of the reason for the linkage may be to prevent inappropriate aggressive behavior during mating. Perhaps the imperative to “make love, not war” is hard-wired in our nervous system, to a greater extent than we have realized.
These considerations further raise the provocative question of whether defects in such wiring might underlie certain kinds of pathological disorders of violent aggression, particularly sexual violence [59]. For example, if (as our data show) the presence of a female normally transiently activates some neurons also involved in attack, and if the mechanisms that normally dampen activity in these attack neurons as mating proceeds are either absent, or not appropriately engaged, then pathological female-directed aggressive behavior could occur during a sexual encounter. The converse situation might also occur: the initiation of an aggressive drive normally directed at males could instead be pathologically “shunted” into sexual behavior, leading to rape or other forms of sexual violence. Such parallel activation of aggressive and reproductive drives of course occurs in nature: ungulates such as elk and deer, for example, exhibit the highest levels of inter-male aggression when they are in rut. However in such cases, the aggressive drive is directed towards other males, not towards females. If the mechanisms that normally shunt the expression of aggressive drive away from females and direct them towards other males are faulty in some individuals, it could result in pathological sexual violence. (It should be noted, however, that the role of VMH in attack is not exclusive to males; stimulation of this hypothalamic region in female rats elicits violent attack as well [60]).
While these notions are at present purely speculative, and based on research with experimental animals, our observations at least raise the possibility that the roots of some forms of pathological violence in humans may reflect faulty wiring within the complex circuitry of the brain. Whether such “circuitopathies” are caused by genetic or environmental factors (or by both), is an interesting but separate issue. The more important question, for the moment, is to determine whether such circuit-level abnormalities are actually involved in certain forms of sociopathic behavior in humans. Doing so will require the application of functional imaging and other emerging new technologies [2, 61, 62]. More importantly, it will require the will and persistence to investigate the neurobiological roots of violence, whether genetic or environmental in origin, and whatever the societal and legal implications.
Acknowledgments
I thank Dayu Lin for her brilliant experimental and intellectual contributions to the work summarized in this review, Larry Swanson for introducing me to the medial hypothalamus and to Tinbergen’s The Study of Instinct, Menno Kruk for helpful information and insights into the field of brain stimulation-evoked attack and for helpful suggestions for improvement of the manuscript, Karl Deisseroth for training members of my laboratory in the application of optogenetic methods to the hypothalamus and Drs. David Spiegel and Daniel Auerbach for insights into the classification of aggressive disorders in DSM-IV. This work was supported by grants from the Weston-Havens Foundation, the Paul G. Allen Family Foundation, and the Howard Hughes Medical Institute. D.J.A. is an HHMI Investigator.
Footnotes
The author reports no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tinbergen N. The study of instinct. New York, NY: Clarendon Press/Oxford University Press; 1951. [Google Scholar]
- 2.Miczek KA, de Almeida RM, Kravitz EA, Rissman EF, de Boer SF, Raine A. Neurobiology of escalated aggression and violence. J Neurosci. 2007;27:11803–11806. doi: 10.1523/JNEUROSCI.3500-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 4.Paré D, Quirk GJ, LeDoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- 5.Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 6.Health NIoM. Mental Health Topics. [Website] [cited 2011; http://www.nimh.nih.gov/health/topics/index.shtml.
- 7.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 8.Haller J, Kruk MR. Normal and abnormal aggression: human disorders and novel laboratory models. Neurosci Biobehav Rev. 2006;30:292–303. doi: 10.1016/j.neubiorev.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Siegel A, Bhatt S, Bhatt R, Zalcman SS. The neurobiological bases for development of pharmacological treatments of aggressive disorders. Curr Neuropharmacol. 2007;5:135–147. doi: 10.2174/157015907780866929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- 11.Huhman KL. Social conflict models: can they inform us about human psychopathology? Horm Behav. 2006;50:640–646. doi: 10.1016/j.yhbeh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 12.Siegel A, Roeling TA, Gregg TR, Kruk MR. Neuropharmacology of brain-stimulation-evoked aggression. Neurosci Biobehav Rev. 1999;23:359–389. doi: 10.1016/s0149-7634(98)00040-2. [DOI] [PubMed] [Google Scholar]
- 13.Hess WR. Stammganglien-Reizversuche. Berichte der gesamten. Physiologie. 1928:42. [Google Scholar]
- 14.Hess WR, Brügger M. Das subkortikale Zentrum der affecktiven Abwehr-reaktion. Helv Physiol Acta. 1943;I:33–52. [Google Scholar]
- 15.Lammers JH, Kruk MR, Meelis W, van der Poel AM. Hypothalamic substrates for brain stimulation-induced attack, teeth-chattering and social grooming in the rat. Brain Res. 1988;449:311–327. doi: 10.1016/0006-8993(88)91046-3. [DOI] [PubMed] [Google Scholar]
- 16.Kruk MR, Van der Poel AM, Meelis W, Hermans J, Mostert PG, Mos J, et al. Discriminant analysis of the localization of aggression-inducing electrode placements in the hypothalamus of male rats. Brain Res. 1983;260:61–79. doi: 10.1016/0006-8993(83)90764-3. [DOI] [PubMed] [Google Scholar]
- 17.Kruk MR. Ethology and pharmacology of hypothalamic aggression in the rat. Neurosci Biobehav Rev. 1991;15:527–538. doi: 10.1016/s0149-7634(05)80144-7. [DOI] [PubMed] [Google Scholar]
- 18.Adams DB. Brain mechanisms of aggressive behavior: an updated review. Neurosci Biobehav Rev. 2006;30:304–318. doi: 10.1016/j.neubiorev.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Roeling TA, Veening JG, Kruk MR, Peters JP, Vermelis ME, Nieuwenhuys R. Efferent connections of the hypothalamic “aggression area” in the rat. Neuroscience. 1994;59:1001–1024. doi: 10.1016/0306-4522(94)90302-6. [DOI] [PubMed] [Google Scholar]
- 20.Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: optical technologies for probing neural signals and systems. Nat Rev Neurosci. 2007;8:577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- 22.Blanchard RJ, Wall PM, Blanchard DC. Problems in the study of rodent aggression. Horm Behav. 2003;44:161–170. doi: 10.1016/s0018-506x(03)00127-2. [DOI] [PubMed] [Google Scholar]
- 23.Weiss P. Comp Psychol Monogr. 1941. Self-differentiation of the basic patterns of coordination; p. 17. [Google Scholar]
- 24.Han Y, Shaikh MB, Siegel A. Medial amygdaloid suppression of predatory attack behavior in the cat: II. Role of a GABAergic pathway from the medial to the lateral hypothalamus. Brain Res. 1996;716:72–83. doi: 10.1016/0006-8993(95)01587-6. [DOI] [PubMed] [Google Scholar]
- 25.Cheu JW, Siegel A. GABA receptor mediated suppression of defensive rage behavior elicited from the medial hypothalamus of the cat: role of the lateral hypothalamus. Brain Res. 1998;783:293–304. doi: 10.1016/s0006-8993(97)01357-7. [DOI] [PubMed] [Google Scholar]
- 26.Canteras NS, Simerly RB, Swanson LW. Projections of the ventral premammillary nucleus. J Comp Neurol. 1992;324:195–212. doi: 10.1002/cne.903240205. [DOI] [PubMed] [Google Scholar]
- 27.Halasz J, Toth M, Kallo I, Liposits Z, Haller J. The activation of prefrontal cortical neurons in aggression--a double labeling study. Behav Brain Res. 2006;175:166–175. doi: 10.1016/j.bbr.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 28.Toth M, Fuzesi T, Halasz J, Tulogdi A, Haller J. Neural inputs of the hypothalamic “aggression area” in the rat. Behav Brain Res. 2010;215:7–20. doi: 10.1016/j.bbr.2010.05.050. [DOI] [PubMed] [Google Scholar]
- 29.Halasz J, Liposits Z, Meelis W, Kruk MR, Haller J. Hypothalamic attack area-mediated activation of the forebrain in aggression. Neuroreport. 2002;13:1267–1270. doi: 10.1097/00001756-200207190-00010. [DOI] [PubMed] [Google Scholar]
- 30.Kruk MR, Halasz J, Meelis W, Haller J. Fast positive feedback between the adrenocortical stress response and a brain mechanism involved in aggressive behavior. Behav Neurosci. 2004;118:1062–1070. doi: 10.1037/0735-7044.118.5.1062. [DOI] [PubMed] [Google Scholar]
- 31.Veening JG, Coolen LM, de Jong TR, Joosten HW, de Boer SF, Koolhaas JM, et al. Do similar neural systems subserve aggressive and sexual behaviour in male rats? Insights from c-Fos and pharmacological studies. Eur J Pharmacol. 2005;526:226–239. doi: 10.1016/j.ejphar.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 32.Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- 33.Canteras NS. The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol Biochem Behav. 2002;71:481–491. doi: 10.1016/s0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- 34.Swanson LW. Anatomy of the soul as reflected in the cerebral hemispheres: neural circuits underlying voluntary control of basic motivated behaviors. J Comp Neurol. 2005;493:122–131. doi: 10.1002/cne.20733. [DOI] [PubMed] [Google Scholar]
- 35.Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- 36.Canteras NS, Chiavegatto S, Ribeiro do Valle LE, Swanson LW. Severe reduction of rat defensive behavior to a predator by discrete hypothalamic chemical lesions. Brain Res Bull. 1997;44:297–305. doi: 10.1016/s0361-9230(97)00141-x. [DOI] [PubMed] [Google Scholar]
- 37.Motta SC, Goto M, Gouveia FV, Baldo MV, Canteras NS, Swanson LW. Dissecting the brain’s fear system reveals the hypothalamus is critical for responding in subordinate conspecific intruders. Proc Natl Acad Sci U S A. 2009;106:4870–4875. doi: 10.1073/pnas.0900939106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav Evol. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- 39.Pfaff DW, Sakuma Y. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J Physiol. 1979;288:203–210. [PMC free article] [PubMed] [Google Scholar]
- 40.Pfaff DW, Sakuma Y. Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. J Physiol. 1979;288:189–202. [PMC free article] [PubMed] [Google Scholar]
- 41.Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- 42.Olivier B. Ventromedial hypothalamus and aggressive behavior in rats. Aggressive Behavior. 1977;3:1. [Google Scholar]
- 43.Olivier B, Wiepkema PR. Behaviour changes in mice following electrolytic lesions in the median hypothalamus. Brain Res. 1974;65:521–524. doi: 10.1016/0006-8993(74)90241-8. [DOI] [PubMed] [Google Scholar]
- 44.van der Poel AM, van der Hoef H, Meelis W, Vletter G, Mos J, Kruk MR. A locked, non-rotating, completely embedded, moveable electrode for chronic brain stimulation studies in freely moving, fighting rats. Physiol Behav. 1983;31:259–263. doi: 10.1016/0031-9384(83)90130-0. [DOI] [PubMed] [Google Scholar]
- 45.Hrabovszky E, Halasz J, Meelis W, Kruk MR, Liposits Z, Haller J. Neurochemical characterization of hypothalamic neurons involved in attack behavior: glutamatergic dominance and co-expression of thyrotropin-releasing hormone in a subset of glutamatergic neurons. Neuroscience. 2005;133:657–666. doi: 10.1016/j.neuroscience.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 46.Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66:721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- 47.Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 48.Pfaff DW, Kieffer BL, Swanson LW. Mechanisms for the regulation of state changes in the central nervous system: an introduction. Ann N Y Acad Sci. 2008;1129:1–7. doi: 10.1196/annals.1417.031. [DOI] [PubMed] [Google Scholar]
- 49.Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- 50.Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Imaging neural activity with temporal and cellular resolution using FISH. Curr Opin Neurobiol. 2001;11:579–584. doi: 10.1016/s0959-4388(00)00252-x. [DOI] [PubMed] [Google Scholar]
- 52.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halasz J, Zelena D, Toth M, Tulogdi A, Mikics E, Haller J. Substance P neurotransmission and violent aggression: the role of tachykinin NK(1) receptors in the hypothalamic attack area. Eur J Pharmacol. 2009;611:35–43. doi: 10.1016/j.ejphar.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 54.Halasz J, Toth M, Mikics E, Hrabovszky E, Barsy B, Barsvari B, et al. The effect of neurokinin1 receptor blockade on territorial aggression and in a model of violent aggression. Biol Psychiatry. 2008;63:271–278. doi: 10.1016/j.biopsych.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 55.Slimko EM, McKinney S, Anderson DJ, Davidson N, Lester HA. Selective electrical silencing of mammalian neurons in vitro by the use of invertebrate ligand-gated chloride channels. J Neurosci. 2002;22:7373–7379. doi: 10.1523/JNEUROSCI.22-17-07373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lerchner W, Xiao C, Nashmi R, Slimko EM, van Trigt L, Lester HA, et al. Reversible silencing of neuronal excitability in behaving mice by a genetically targeted, ivermectin-gated Cl- channel. Neuron. 2007;54:35–49. doi: 10.1016/j.neuron.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 57.Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 58.Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, et al. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 59.Saper CB. Animal behaviour: the nexus of sex and violence. Nature. 2011;470:179–181. doi: 10.1038/470179a. [DOI] [PubMed] [Google Scholar]
- 60.Kruk MR, Van der Laan CE, Mos J, Van der Poel AM, Meelis W, Olivier B. Comparison of aggressive behaviour induced by electrical stimulation in the hypothalamus of male and female rats. Prog Brain Res. 1984;61:303–314. doi: 10.1016/S0079-6123(08)64443-X. [DOI] [PubMed] [Google Scholar]
- 61.Lee TM, Chan SC, Raine A. Hyperresponsivity to threat stimuli in domestic violence offenders: a functional magnetic resonance imaging study. J Clin Psychiatry. 2009;70:36–45. doi: 10.4088/jcp.08m04143. [DOI] [PubMed] [Google Scholar]
- 62.Dolan MC. What imaging tells us about violence in anti-social men. Crim Behav Ment Health. 2010;20:199–214. doi: 10.1002/cbm.771. [DOI] [PubMed] [Google Scholar]
- 63.Mos J, Lammers JHCM, van der Poel AM, Bermond B, Meelis W, Kruk MR. Effects of midbrain central gray lesions on spontaneous and electrically induced aggression in the rat. Aggressive Behavior. 1983;9:133–155. [Google Scholar]
- 64.Dong HW. The Allen references atlas: A digital color brain atlas of the C57Bl/6J male mouse. Hoboken, N.J: John Wiley & Sons, Inc; 2008. [Google Scholar]