Abstract
Preeclampsia is a life-threatening hypertensive disease of pregnancy. The condition is characterized by the presence of autoantibodies that activate the major angiotensin receptor, AT1. Research conducted during the past decade has shown that these autoantibodies activate AT1 receptors on a variety of cell types and provoke biological responses that are relevant to the pathophysiology of preeclampsia. The introduction of these autoantibodies into pregnant mice results in hypertension, proteinuria and a variety of other features of preeclampsia including small fetuses and placentas. These findings demonstrate the pathophysiological role of these autoantibodies in preeclampsia. The biological properties of these autoantibodies can be blocked by a 7-amino acid peptide that corresponds to a specific sequence associated with the second extracellular loop of the AT1 receptor. The fact that autoantibodies from different individuals are directed to a common epitope provides obvious diagnostic and therapeutic opportunities. Research reviewed here raises the intriguing possibility that preeclampsia may be a pregnancy-induced autoimmune condition characterized by the presence of disease-causing angiotensin receptor activating autoantibodies
Introduction
Preeclampsia is a serious and common hypertensive complication of pregnancy that is a leading cause of maternal and neonatal mortality and morbidity. It is a multisystem disorder generally appearing after the 20th week of gestation and characterized by hypertension, proteinuria, vascular abnormalities, and often intrauterine growth retardation[1–3]. In severe cases preeclampsia is accompanied with the HELLP syndrome (Hemolysis, Elevated Liver enzymes and Low Platelets). Preeclampsia affects ~7% of first pregnancies and is a leading cause of maternal death and a major contributor to maternal and perinatal morbidity. The only effective treatment is delivery of the fetus and placenta, often resulting in serious complications of prematurity for the neonate. The resulting preterm births and the associated increased infant morbidity and mortality are especially disheartening consequences of preeclampsia. In fact, 15% of all preterm births are indicated early deliveries for preeclampsia. Preeclampsia also increases the risk of intrauterine growth restriction, resulting in low birth-weight babies at increased risk for long term disabilities. The underlying mechanisms responsible for the pathogenesis of preeclampsia remain poorly understood.
Numerous recent studies have shown that women with preeclampsia possess angiotensin receptor agonistic autoantibodies that bind to and activate the AT1 angiotensin receptor[4–12]. The introduction of these autoantibodies into pregnant mice induces clinical features of preeclampsia via AT1 receptor activation[6]. These findings suggest that preeclampsia may be an autoimmune condition in which AT1 receptor agonistic autoantibodies, termed AT1-AAs, contribute to many features of the disease. These autoantibodies recognize a common epitope on the AT1 receptor and their ability to activate AT1 receptors is blocked by a 7-amino acid (aa) peptide that corresponds to this epitope. Overall, the studies summarized here raise the intriguing possibility that preeclampsia is a pregnancy-induced autoimmune disease in which pathophysiological symptoms result from autoantibody-induced angiotensin receptor activation.
AT1-AAs activate AT1 receptors on a variety of cell types and provoke biological responses relevant to the pathophysiology of preeclampsia
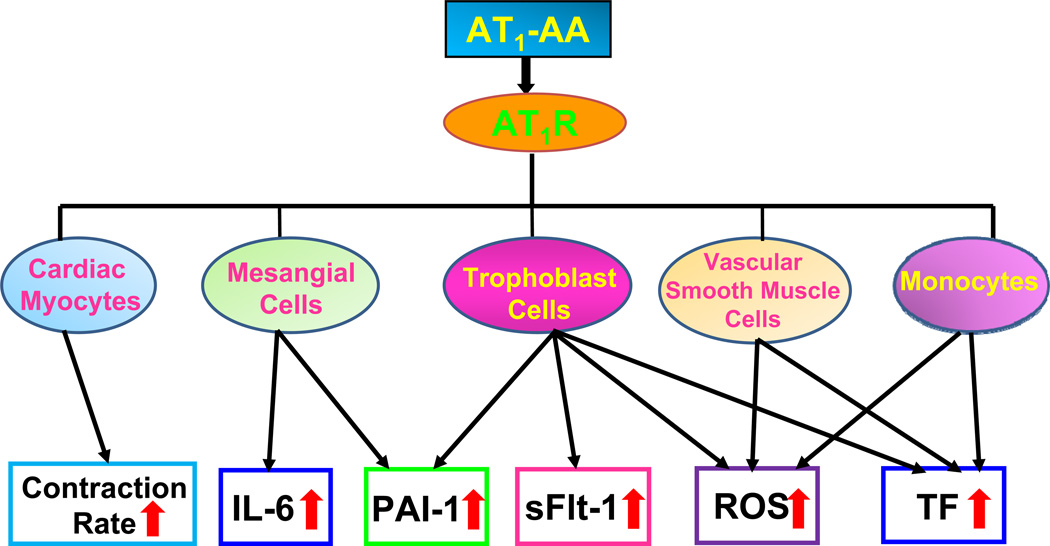
A growing body evidence indicate that AT1-AA activate AT1 receptors on a variety of cells and provoke biological responses that are relevant to the pathophysiology of preeclampsia (Fig. 1).
Figure 1. Autoantibodies from women with preeclampsia are functional mimics of Ang II and activate AT1 receptors on many cell types.
Autoantibody-induced AT1 receptor activation on cardiac myocytes leads to increased contraction rates. The activation of AT1 receptors on mesangial cells and trophoblast cells results in increased synthesis and secretion of soluble factors such as interleukin-6 (IL-6), plasminogen activator inhibitor-1 (PAI-1), and soluble fms like tyrosine kinase-1 (sFlt-1, a soluble form of the vascular endothelial growth factor-1 receptor), all of which are elevated in women with preeclampsia. In addition, AT1-AAs stimulate the synthesis of NADPH oxidase in several cell types, resulting in increased production of reactive oxygen species (ROS) and oxidative damage. Increased production of tissue factor (TF) by vascular smooth muscle cells and monocytes may contribute to hypercoagulation often associated with preeclampsia. We propose that AT1-AA activate AT1 receptors on many cell types and in this way contribute to pathophysiological changes associated with preeclampsia.
Cardiomyocytes
Angiotensin receptor agonistic autoantibodies (AT1-AAs) were originally detected by Wallukat et al [5] based on the ability of these autoantibodies to activate AT1 angiotensin receptors on cultured neonatal rat cardiac myocytes. Autoantibody-induced receptor activation stimulated an increase in the beating rate of cardiomyocytes, a feature that was blocked by losartan, an AT1 receptor antagonist. The autoantibody-induced chronotropic effect was also blocked by a seven amino acid (7-aa) peptide that corresponds to a sequence present on the second extracellular loop of the AT1 receptor. These remarkable findings were the first to show that preeclamptic women possess stimulatory autoantibodies against the AT1 receptor and that these autoantibodies are directed to a common epitope associated with the second extracellular loop.
Subsequent to the initial report by Wallukat et al.[5], numerous publications showed that AT1-AAs activate AT1 receptors on a variety of cells and provoke biological responses relevant to the pathophysiology of preeclampsia (Fig. 1). These in vitro studies with cultured cells are reviewed in the remainder of this section.
Vascular smooth muscle cells
Preeclampsia is associated with numerous abnormalities of circulatory function and vascular physiology. Among these are disseminated intravascular coagulation and increased production of reactive oxygen species (ROS). Dechend et al. explored the impact of AT1-AA-mediated AT1 receptor in contributing to hypercoagulation and the generation of reactive oxygen species by vascular smooth cells.
Tissue factor production (hypercoagulation)
Tissue factor (TF) is a 47-kDa transmembrane protein that initiates the extrinsic pathway of coagulation. Increased expression of TF is associated with preeclampsia and is believed to contribute to the hypercoagulation (i.e. increased fibrin deposition) associated with this condition. Dechend et al.[9] showed that AT1-AAs stimulate increased TF expression by human vascular smooth muscles cells. They also showed that AT1-AAs activate TF promoter/luciferase constructs after transfection into cells that contain AT1 receptors and that this activation required the presence of activating protein-1 (AP-1) binding sites. Increased TF synthesis by vascular smooth muscle cells and the activation of the TF/luciferase reporter were blocked by losartan. IgG from normotensive pregnant women had no effect in either assay. Thus, the studies of Dechend et al.[9] and Dorffel et al.[13] show that AT1-AAs activate AT1 receptors, initiating a signaling cascade resulting in increased TF expression that may contribute to the hypercoagulation seen in women with preeclampsia.
Reactive oxygen species (ROS)
The production of ROS is increased in preeclamptic women and may contribute to the oxidative stress associated with their condition. The identity of the ROS-producing enzymes in preeclampsia was also investigated by Dechend et al.[10], who recognized that reduced nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase is a major source of ROS in arteriosclerosis and reperfusion injury. They determined that IgG from preeclamptic women induces intracellular ROS in vascular smooth muscle cells via increased synthesis of NADPH oxidase. They suggested that AT1-AAs, through activation of NADPH oxidase, could contribute to ROS production in preeclampsia. They also showed that AT1-AAs activate nuclear factor κB (NFκB) a feature that could contribute to the enhanced inflammatory responses associated with preeclampsia.
Trophoblast cells
Placental abnormalities associated with preeclampsia include shallow trophoblast invasion, impaired spiral artery remodeling and increased production of ROS and antiangiogenic factors. Research reviewed in this section suggests that the action of AT1-AAs on trophoblast cells may contribute to these features.
Trophoblast invasion
Trophoblast invasion depends in part on the regulated production of plasmin from plasminogen, a process catalyzed by plasminogen activators. Plasminogen activator activity is negatively controlled by plasminogen activator inhibitors, of which PAI-1 is the most abundant. Xia et al. initially investigated the role of a local renin-angiotensin system in placental physiology and development and found that Ang II stimulated the synthesis and secretion of PAI-1 from trophoblast cells[14]. On the basis of these findings they tested IgG from women with preeclampsia for the ability to stimulate PAI-1 synthesis and secretion by human trophoblasts. They observed that IgG from women with preeclampsia, in contrast to IgG from normotensive pregnant women, stimulated an increase in the synthesis and secretion of PAI-1. Pharmacologic inhibition and peptide competition experiments indicate that the effect of IgG was mediated through activation of AT1 receptors. Xia et al. used an in vitro Matrigel cell invasion assay to show that the autoantibody-induced synthesis of PAI-1 resulted in reduced trophoblast invasion[4]. These results suggest that the action of AT1-AA on trophoblast cells may contribute to shallow trophoblast invasion and improper spiral artery remodeling.
Hypercoagulation
Elevated PAI-1 occurs in the maternal circulation of women with preeclampsia and is believed to contribute to the hypercoagulation and fibrinolytic imbalance associated with their condition. PAI-1 inhibits the conversion of plasminogen to plasmin, the serine protease responsible for degrading fibrin and fibrinogen in the serum. AT1-AAs may contribute to hypercoagulation by stimulating the synthesis and secretion of plasminogen activator inhibitor-1 (PAI-1) by trophoblast cells of the placenta. Because mesangial cells, cardiomyocytes, and vascular muscle cells also produce increased PAI-1 in response to Ang II it is likely that AT1-AAs also stimulate PAI-1 production from these cells. Trophoblast cells may also contribute to hypercoagulation by increased expression of TF[9]. Thus, IgG from women with preeclampsia may contribute to hypercoagulation in two ways: 1) by stimulating the synthesis of TF resulting in the activation of the coagulation cascade[9], and 2) by stimulating the synthesis of PAI-1, thereby reducing the production of plasmin and the degradation of fibrin clots[4].
ROS – NADPH oxidase
Trophoblast cells are also a source of ROS during pregnancy. Dechend et al. found that ROS production is increased in preeclamptic placentas, especially in and around the blood vessels and showed that this correlated with an increase in placental NADPH oxidase activity[10]. In addition, Dechend et al. found that autoantibody from women with preeclampsia stimulated the synthesis of NADPH oxidase and the production of ROS in cultured primary human trophoblasts[10].
Antiangiogenic factors
It is a commonly held view that “toxic factors” secreted by the placenta into the maternal circulation are responsible for systemic endothelial dysfunction, hypertension and multiorgan damage[15]. One such factor is soluble fms-related tyrosine kinase-1 (sFlt1), the soluble form of the vascular endothelial growth factor (VEGF) receptor that is significantly elevated in the plasma of women with preeclampsia and is believed to contribute to disease pathology by interference with VEGF signaling. Zhou et al. initially showed that Ang II stimulates sFlt1 synthesis and secretion by trophoblast cells during pregnancy[16]. Realizing that Ang II levels are not increased in women with preeclampsia over that occurring during normotensive pregnancies, they tested the possibility that AT1-AA contribute to increased production of sFlt1. They found that IgG from women with preeclampsia, in contrast to IgG from normotensive pregnant women, stimulates the synthesis and secretion of sFlt1 via AT1 receptor activation in pregnant mice, human placental explants and in human trophoblast cells[6][17]. Autoantibody induced sFlt1 production was blocked by losartan and by the 7aa epitope peptide and required the calcineurin/NFAT signaling pathway. Overall the studies of Zhou et al. suggest that Ang II is a significant regulator of sFlt1 synthesis and secretion during normal pregnancy and that the excessive accumulation of sFlt1 observed in women with preeclampsia is due to the additional activation of AT1 receptors mediated by AT1-AA[6].
Mesangial cells
Mesangial cells are modified smooth muscle cells that control blood flow through the glomerulus. Mesangial cells also produce a variety of cytokines when stimulated, and are capable of phagocytosis. These cells respond to the local renal renin-angiotensin system and secrete increased amounts of PAI-1 and IL-6 in response to Ang II. Bobst et al. have shown that IgG from women with preeclampsia stimulate an AT1 receptor-specific secretion of IL-6 and PAI-1 from human mesangial cells. Competition with the epitope peptide suggested that the stimulation was due to AT1-AA[11]. High levels of PAI-1 may contribute to the disseminated intravascular coagulation present in the glomerular structures of women with preeclampsia. The autoantibody-stimulated release of IL-6 by mesangial cells may contribute to the activation of the pro-inflammatory response associated with preeclampsia. These findings suggest that a maternal autoantibody with the ability to activate AT1 receptors may contribute to the development of renal damage seen in preeclamptic patients.
Monocytes
Monocytes from individuals with essential hypertension produce more proinflammatory cytokines and reactive oxygen species than monocytes from normotensive individuals and the adhesion to human umbilical vein endothelial cells is significantly increased. These features of activated monocytes are also displayed by monocytes from women with preeclampsia. Dorffel et al.[13] reported that AT1-AAs stimulated monocytes to produce increased amounts of TF, a feature that could contribute to increased adherence to endothelial cells. They found that monocyte adhesion to human aortic or umbilical vein endothelial cell layers was significantly higher following stimulation with AT1-AA and that the effect was inhibited by eposartan and by the 7aa epitope peptide as well as the presence of tissue factor antibody. Autoantibody from women with preeclampsia also stimulated increased production of ROS. These results suggest that AT1-AA may contribute to monocyte activation in women with preeclampsia.
Reporter cell lines
Calcium mobilization
Preeclampsia is associated with abnormalities in Ca2+ metabolism and increased intracellular Ca2+ levels in platelets, erythrocytes, and lymphocytes[18]. Haller et al. showed that basal intracellular free Ca2+ in platelets is substantially elevated in preeclamptic patients compared to women with uncomplicated pregnancy[18–19]. This phenomenon completely disappears 6 weeks after delivery, which suggests a relevant relationship to preeclampsia. Similar studies using lymphocytes and erythrocytes showed that the intracellular free Ca2+ concentration is increased in these cells of preeclamptic patients[18–20]. In addition, a more widespread dysregulation of cellular Ca2+ metabolism is also implicated in preeclampsia[21]. Earlier work by Xia et al. indicated that the calcineurin/NFAT signaling pathway is associated with the activation of AT1 receptors by AT1-AAs in human trophoblasts[4]. Realizing that the calcineurin/NFAT signaling pathway is dependent on increased intracellular calcium concentrations, Thway et al. hypothesized that AT1-AAs stimulate increased intracellular Ca2+ resulting in the activation of NFAT. Support for this hypothesis was obtained by showing that both Ang II and IgG from preeclamptic women but not IgG from normotensive pregnant women induced Ca2+ mobilization in a dosage-dependent way in a Chinese hamster ovary cell line. The specific mobilization of intracellular Ca2+ by AT1-AAs from women with preeclampsia was blocked by losartan and by the 7-aa epitope peptide[22]. AT1-AA-induced mobilization of intracellular Ca2+ resulted in the activation of the NFAT transcription factor. These results suggest that AT1-AA may account for increased intracellular free Ca2+ concentrations associated with preeclampsia.
Luciferase expression
Antibody-induced mobilization of intracellular calcium results in the activation of calcineurin, a phosphatase that dephosphorylates the cytoplasmic form of NFAT. Following dephosphorylation NFAT moves into the nucleus where it participates in the activation of genes controlled by NFAT response elements. This feature was exploited by the engineering of a Chinese hamster ovary cell line that carries an NFAT-luciferase reporter gene. These cells produce increased luciferase in response to Ang II or AT1-AA and serve as a bioassay to detect AT1 receptor activation[22][6].
AT1-AAs induce preeclampsia in pregnant mice: The adoptive transfer experiment
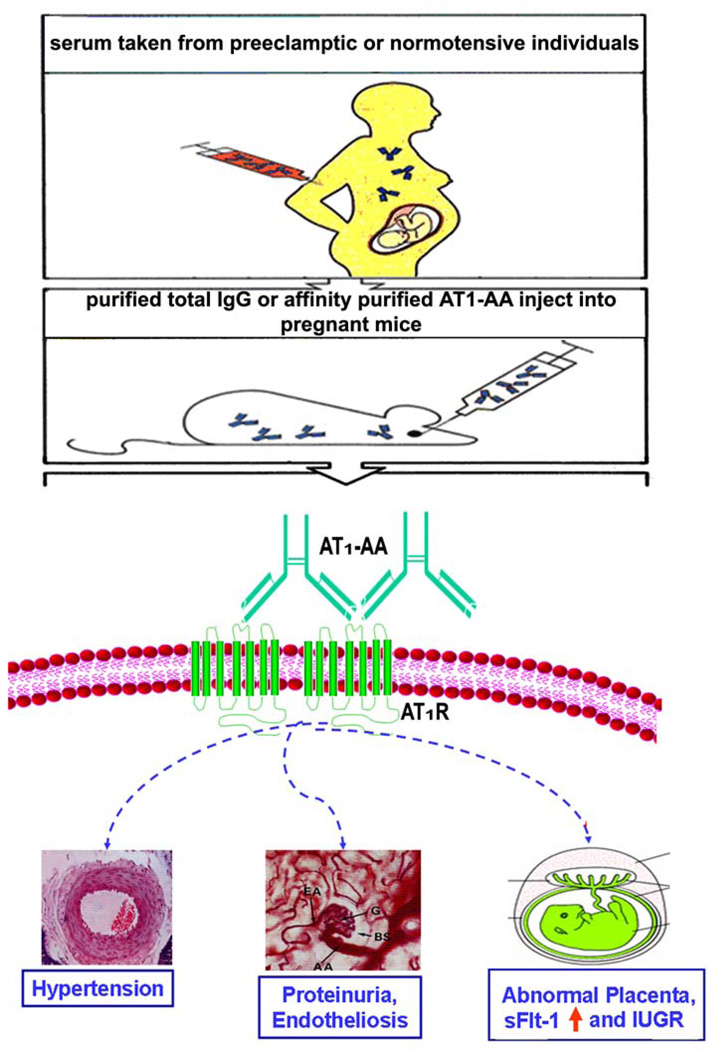
As reviewed above many features of preeclampsia can be explained by the ability of AT1-AAs to activate AT1 receptors on a variety of cells and provoke biological responses relevant to the pathophysiology of preeclampsia (Fig. 1). However, these studies were restricted to the use of in vitro cultured cell systems and therefore did not directly address the relevance of AT1-AA to hypertension and proteinuria, the defining features of preeclampsia. To formally examine the role of AT1-AAs in the pathophysiology of preeclampsia total IgG or affinity purified AT1-AA from women with preeclampsia was injected into pregnant mice and features of preeclampsia were monitored (Fig. 2).
Figure 2. Schematic illustration of the adoptive transfer experiment described here.
IgG or affinity purified AT1-AA were prepared from the sera taken from normotensive pregnant women or women with preeclampsia and introduced into pregnant mice (gestation day 13) by intraorbital injection. Some of the parameters monitored are shown.
Affinity purified AT1-AA from women with preeclampsia induce hypertension and proteinuria in pregnant mice
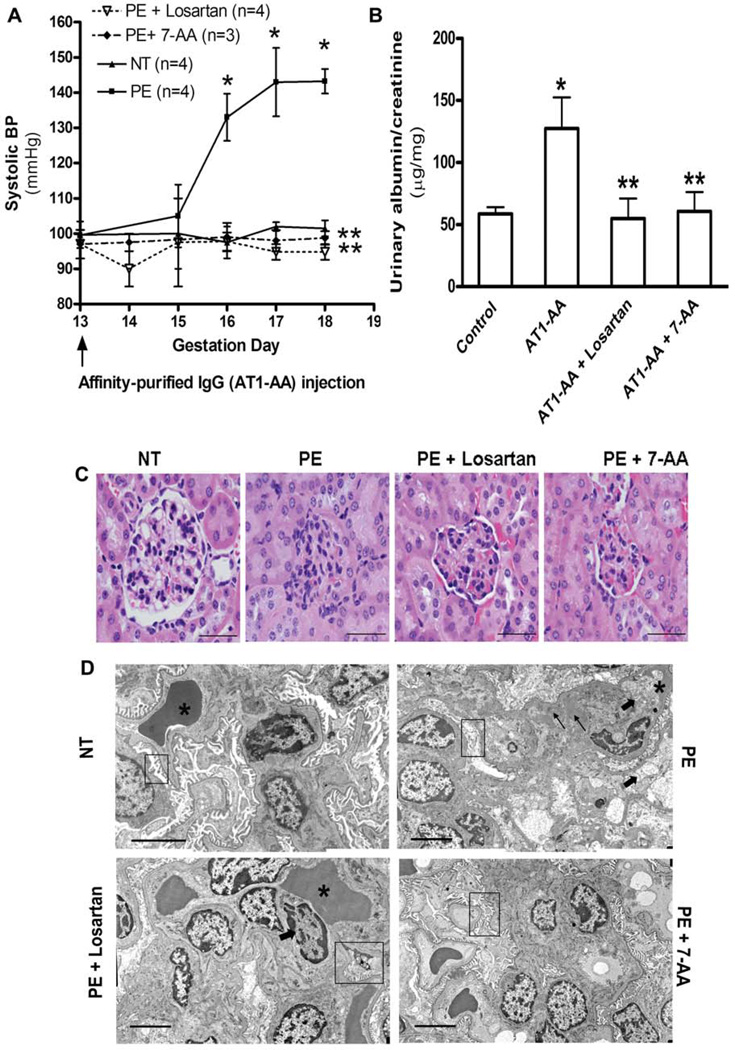
Injection of IgG or affinity purified AT1-AA isolated from preeclamptic women, in contrast to that from normotensive pregnant women, resulted in a pronounced increase in blood pressure that was evident by three days following antibody injection (Fig. 3A). Urinary protein also increased significantly by day 18 in the pregnant mice following injection with affinity purified AT1-AA from women with preeclampsia (Fig. 3B). The antibody-mediated increase in blood pressure and urinary protein was prevented by co-injection with losartan or the 7-aa epitope peptide. Thus, hypertension and proteinuria, the defining features of preeclampsia, appeared in pregnant mice following a single injection of affinity purified AT1-AA from women with preeclampsia[23].
Figure 3. Affinity-purified AT1-AAs induced an increase in blood pressure and proteinuria in pregnant mice.
A. Systolic blood pressure in pregnant mice after injection of affinity purified AT1-AAs (~20 µg) on gestation day 13 in the presence or absence losartan or a 7-aa epitope peptide. IgG from normotensive pregnant women was used as a control. B. The ratio of urinary albumin and creatinine on gestation day 18. C. H & E staining of kidneys from pregnant mice injected with IgG from normotensive women or IgG from women with preeclampsia in the presence or absence of losartan or the 7-aa epitope peptide. Note that the kidneys from mice treated with IgG from women with preeclampsia, in contrast to IgG from normotensive pregnant women were characterized by extensive endothelial swelling and occlusion of the capillary lumens. D. Electron microscopic examination of kidney sections from mice injected with IgG from normotensive women or with IgG from women with preeclampsia in the presence or absence of losartan or the 7-aa epitope peptide. Thick arrows show endothelial cell swelling causing marked narrowing or total occlusion of the capillary loop spaces (*). Thin arrows indicate focal subendothelial deposits. Boxes highlight the state of the fenestrations of the endothelial cells and the podocyte foot processes in the various kidney samples. Data from Zhou et al. [23], used with permission.
Kidney damage is induced in pregnant mice by injection of IgG from women with preeclampsia
Preeclampsia is also associated with impaired renal function and characteristic alterations in renal histology. The majority of the glomeruli seen in the mice injected with IgG from women with preeclampsia showed normal to smaller-sized glomeruli with endothelial swelling (Fig. 3C), causing narrowing and total obliteration or occlusion of the glomerular capillary spaces (a condition termed endotheliosis, a typical pathological feature seen in women with preeclampsia). Examination of kidneys from these mice by electron microscopy (Fig. 3D) confirmed the glomerular changes observed by light microscopy. These changes were not evident in mice after injection of IgG from normotensive pregnant women and were diminished when preeclampsia IgG was coinjected with either losartan or the 7-aa epitope peptide. Similar renal damages were observed in pregnant mice injected with affinity-purified AT1-AAs. Thus, these autoantibodies can induce renal histopathological changes characteristic of preeclampsia via AT1 receptor activation[23].
Injection of AT1-AAs into pregnant mice results in increased production of placental derived anti-angiogenic factors and smaller placentas
Soluble Flt1 (sFlt1) is a soluble form of the fms-like tyrosine kinase receptor-1 (also known as vascular endothelial growth factor receptor-1), and as discussed above is significantly elevated in the plasma of women with preeclampsia. Elevated sFlt1 is believed to contribute to disease pathology by interference with VEGF signaling[15; 24]. To evaluate the potential contribution of AT1-AA to increased production of sFlt1 in preeclampsia, pregnant mice were given injections of IgG from normotensive pregnant women or women with preeclampsia and the levels of sFlt1 in the plasma of injected mice was determined. The results show that sFlt1 levels were significantly greater in pregnant mice receiving injections of IgG from women with preeclampsia over that observed with pregnant mice injected with IgG from normotensive pregnant women. Smaller placentas were also seen in pregnant mice injected with IgG from women with preeclampsia, a feature that may result, in part, from the anti-angiogenic effects of autoantibody-induced sFlt1. Autoantibody-mediated induction of sFlt1 and the reduction in placental size were inhibited by co-injection with losartan or the 7-aa epitope peptide indicating that these outcomes were mediated by AT1-AA and required AT1 receptor activation[6].
Autoantibody-induced hypertension in non-pregnant mice
Autoantibody-induced hypertensive effects in pregnant mice may be mediated through the action of autoantibody-induced excessive sFlt1 production by the placenta[23]. To distinguish the hypertensive effects of AT1-AA from sFlt1, preeclampsia autoantibodies were introduced into non-pregnant mice and the effects on blood pressure and sFlt1 production were determined. As expected, the concentration of sFlt1 was very low in non-pregnant animals and was not elevated significantly following injection of preeclampsia autoantibodies. The blood pressure in antibody-injected non-pregnant mice achieved levels similar to that seen in antibody-injected pregnant mice, suggesting that AT1-AA can stimulate blood pressure independent of sFlt1. Because AT1-AAs are a biological mimic of Ang II it is not surprising that these autoantibodies induce hypertension.
Summary of adoptive transfer studies
The antibody injection model of preeclampsia provides strong experimental support for the hypothesis that preeclampsia is a pregnancy induced autoimmune condition in which AT1-AAs contribute to many features of the disease[23][25]. This important antibody-induced model of preeclampsia in mice provides important experimental opportunities to study the molecular mechanisms of AT1-AA induced pathophysiology.
Experimental induction of AT1-AA in animal models of preeclampsia
As reviewed above preeclampsia in humans is characterized by the presence of autoreactive antibodies, AT1-AA, that contribute to the pathophysiology of the disease[23]. These antibodies also appear in animal models of preeclampsia initiated by placental ischemia or the infusion of inflammatory cytokines[26]. Granger and colleagues developed a rat model of preeclampsia based on experimentally induced placental ischemia resulting from reduced uterine perfusion pressure (RUPP)[3; 27–28]. Such experimentally manipulated pregnant rats develop hypertension, proteinuria and other features of preeclampsia, including elevated levels of sFlt1, sEng, increased inflammatory cytokines such as TNF-α and IL-6, increased endothelin-1 production and endothelial dysfunction[29–34]. Remarkably, RUPP rats also develop AT1-AA[26]. RUPP-induced hypertension is inhibited by losartan but not by an ACE inhibitor, suggesting AT1-AAs contribute to hypertension associated with preeclampsia. Granger and colleagues also investigated the effect of TNF-α infusion. They found that low dose TNF-α infusion in pregnant rats also resulted in increased blood pressure and the appearance of AT1-AA[26]. Neither hypertension nor AT1-AA was induced by RUPP manipulation of, or TNF-α infusion into, non-pregnant rats, suggesting a role for pregnancy and the placenta in hypertension and the production of AT1-AAs. Factors contributing to the production of AT1-AAs and the autoimmune recognition of the AT1 receptor peptide epitope are not well understood at this time and may be associated with extensive vascular injury mediated by hypoxia in the placenta. The generation of these autoantibodies may be secondary to placental ischemia, vascular damage and increased maternal inflammatory response that is associated with preeclampsia[35]. Experimental induction of uteroplacental ischemia and the infusion of inflammatory cytokines appear to be promising experimental models to study the relationship between preeclampsia and autoimmunity.
Diagnostic and therapeutic opportunities provided by early detection and effective neutralization of AT1-AA
Preeclampsia is not currently considered an autoimmune condition. However, the research reviewed here implicating AT1-AA in the pathogenesis of preeclampsia could lead to a paradigm shift in our understanding of this complex disorder of pregnancy. If maternal circulating AT1-AAs play a significant role in preeclampsia pathogenesis, then the timely detection and removal or inhibition of these autoantibodies may provide significant benefits in the medical management of preeclampsia.
AT1-AA as a pre-symptomatic risk factor
For years, the diagnosis of preeclampsia has been made by the detection of sudden onset hypertension and proteinuria. The triggering factors in the pathogenesis of the disease remain elusive. An important component to the management of preeclampsia would be a sensitive and reliable diagnostic test to identify women at risk for preeclampsia at the earliest possible time. Important work by Walther et al.[12] showed that AT1-AAs are present before the 20th wk of gestation in women with impaired uterine perfusion as judged by Doppler sonography. AT1-AAs were not observed in second-trimester women with a normal Doppler ultrasound. Using the cardiomyocyte contraction assay they found that AT1-AAs were detected between the 18th and 22nd wk of gestation in women with reduced uterine perfusion pressure. When followed to term, these women fell into three groups: those who developed preeclampsia, those characterized by fetuses with intrauterine growth retardation, and those with otherwise normal outcomes. Thus, AT1-AAs track women showing reduced uterine perfusion pressure during the second trimester and may serve to identify women at risk for intrauterine growth retardation and/or preeclampsia. The authors of this study suggested, as we had done earlier[4], that AT1-AAs may be responsible for reduced trophoblast invasion and impaired placental development. The fact that AT1-AA can be detected many weeks before the symptoms of preeclampsia has significant implications regarding pre-symptomatic identification of women at risk for preeclampsia. The ability to conveniently and accurately detect AT1-AAs may have significant diagnostic and prognostic value in the management of preeclampsia. To facilitate the early detection of disease causing autoantibodies associated with preeclampsia research is needed to develop improved methods to conveniently and accurately detect and quantify AT1-AAs in the sera of pregnant women. A long range goal is to develop genetic tests to identify women at risk for preeclampsia prior to pregnancy.
Therapeutic possibilities based on blocking autoantibody-induced receptor activation
Currently there are no specific treatment options for preeclampsia, and severe cases often require premature delivery of the infant. The fact that IgG from women with preeclampsia causes preeclampsia-like features following injection into pregnant mice suggests that these autoantibodies are likely to contribute to symptoms of preeclampsia in the women from whom the IgG was obtained. If maternal circulating AT1-AAs contribute to the pathophysiology of preeclampsia as we hypothesize then blocking the action of these autoantibodies at the earliest possible time may provide significant therapeutic benefit. One approach to this is based on in vitro studies showing that AT1-AAs recognize a specific seven-amino acid sequence present on the second extracellular loop of the AT1 receptor and that the ability of AT1-AAs to activate AT1 receptors on various cell types can be blocked by the presence of this heptapeptide. Our recent studies show that this peptide can neutralize AT1-AAs and thereby prevent autoantibody-induced features of preeclampsia in pregnant mice[6]. Thus, the use of epitope peptide therapy to neutralize AT1-AA has the potential of being a safe and effective treatment of preeclampsia.
The role of the immune system in preeclampsia
Autoimmune disorders are estimated to affect approximately 5% of the United States population. Mechanisms leading to the development of autoimmune conditions are poorly understood[36][37][38]. The research presented here raises the intriguing possibility that preeclampsia is a pregnancy induced autoimmune disease[35]. Environmental and genetic factors contributing to the production of AT1-AA in preeclampsia are not known. We speculate that the adaptive immune response to foreign antigens and/or the inflammatory response accompanying placental ischemia are a key factors contributing to the production of AT1-AA. Each of these features could account for the well known fact that preeclampsia is most prevalent among first pregnancies. In addition preexisting conditions that increase the risk of preeclampsia, such as obesity and insulin dependent diabetes mellitus, are associated with immunological and/or inflammatory disturbances. A review of relevant literature linking adaptive and innate immunity with preeclampsia is provided below.
Adaptive immune response (Immunological tolerance)
A considerable amount of epidemiological evidence (Table 1) supports the view that increased exposure to paternal antigens present on the fetus or on sperm results in immunological tolerance to these paternal antigens and reduces the risk of preeclampsia[39–41].
Table 1.
Immunological tolerance to paternal antigens reduces the risk of preeclampsia
| 1. | Risk is reduced by repeated sexual exposure to paternal semen[63][44]. |
| 2. | Barrier contraception (e.g. condoms and diaphragms) nullifies the reduced risk resulting from repeated semen exposure[48]. |
| 3. | The decreased risk of preeclampsia by persistent oral sex[64]. |
| 4. | Risk is increased by the use of assisted reproductive technologies involving the use of donor gametes[65] |
| 5. | Highest risk is associated with donor embryos in which both gametes are donors[49]. |
| 6. | Multiparous women are at reduced risk compared to nulliparous women[42][66]. |
| 7. | The protective effect of multiparity is lost with change of partner[44]. |
| 8. | The increased risk associated with nulliparity is reduced by prior abortion, but only if the pregnancy involved the same partner[45][44]. |
It is well known multiparous women are at a reduced risk of preeclampsia compared to nulliparous women[42]. Thus, a prior normotensive pregnancy provides protection against preeclampsia in subsequent pregnancies with the same partner[43]. Likewise a previous spontaneous or induced abortion reduces the risk of preeclampsia in a subsequent pregnancy, but only if the subsequent pregnancy is with the same individual who contributed to the aborted pregnancy[44–45]. These epidemiological findings are consistent with the view that extended exposure to paternal antigens present on the fetus during the first pregnancy results in immunological tolerance that reduces the risk of preeclampsia in a subsequent pregnancy.
The risk of preeclampsia among nulliparous women is reduced by extended periods of sexual activity prior to the first pregnancy[43][46]. Oral sexual activity also provides an opportunity for paternal antigen exposure and serves to reduce the risk of preeclampsia[47–48]. The protective effect of extended cohabitation and sexual contact is reduced by the use of barrier contraception such as condoms or diaphragms that reduce the opportunity for exposure to paternal antigens. These findings suggest that repeated exposure to paternal antigens present on sperm prior to the first pregnancy allows the maternal immune system to become adapted (i.e. tolerant) to paternal antigens and in this way allows for normal trophoblast invasion and placental development and a reduced risk of preeclampsia.
Pregnancies resulting from donor sperm fall into a special high risk category because they represent the first encounter with paternal antigens encoded by donor sperm[49]. The increased risk of preeclampsia associated with donor sperm does not apply to sperm obtained from a sexual partner that has provided prior antigen exposure. The highest risk of preeclampsia (~33%) is associated with the use of donor embryos derived entirely of donor gametes (i.e., both egg and sperm)[49]. In this case the embryo represents a complete allograft and provides the strongest maternal immunological response. Thus, pregnancies occurring without the opportunity to develop immunological tolerance to paternal antigens (or fetal alloantigens) are at a significantly increased risk of developing preeclampsia.
Maternal inflammatory response (activation of the innate arm of the immune system)
Another factor linking preeclampsia to the immune system is a systemic maternal inflammatory response representing widespread activation of the innate arm of the immune system[50–54]. Normal pregnancy is associated with a mild systemic maternal inflammatory response[55–56]. Preeclampsia, however, is characterized as an excessive maternal inflammatory response stemming from poor placentation resulting in placental ischemia, vascular damage and oxidative stress. A growing body of evidence demonstrates that leukocyte proliferation, complement activation coupled with the increased proinflammatory cytokine secretion (such as TNF-a, IL-6, IL-8 and IL-12) are seen in preeclamptic patients [50–61]. The increased occurrence of preeclampsia among primigravadas may be due in part to an increased risk of placental ischemia (and the associated endovascular damage and inflammatory response) because of the small size of the spiral arteries in the nulliparous uterus[62]. Prior to the first pregnancy the uterine spiral arteries are thick and muscular. This muscular layer must be fully infiltrated by endovascular trophoblast cells of fetal origin in order to produce adequate dilation and subsequent increased blood flow to the uteroplacental unit. The spiral artery diameters increase four to five fold as they become flaccid and non-muscular. The structural conversion of the small caliber spiral arteries in the non-pregnant uterus to the large caliber uteroplacental arteries is necessary to provide for adequate blood flow to the placenta. Following pregnancy, the uterine spiral arteries do not return to their original small virgin size but instead remain larger. Such permanent changes in the uterine spiral arteries are believed to allow for enhanced placental perfusion and in this way contribute to the higher birth weights associated with subsequent pregnancies[62]. It is possible that the enlarged uterine arteries following the first pregnancy provide for increased blood flow into the placenta, resulting in reduced risk of placental ischemia and endovascular damage. The improved placental perfusion in multigravadas results in a reduced risk of an inflammatory response and the production of AT1-AA. Thus, the reduced risk of preeclampsia in multigravadas may be due in part to the reduced risk of placental ischemia, vascular damage and oxidative stress and the resulting inflammatory response.
Concluding remarks
In 1999 Wallukat et al. reported their remarkable findings that sera from women with preeclampsia contain autoantibodies that react with AT1 receptors in a stimulatory fashion [5]. During the past decade their important findings have been confirmed and extended in numerous ways, showing that these autoantibodies activate AT1 receptors on a variety of cell types and provoke biological responses that are relevant to the pathophysiology of preeclampsia. The introduction of these autoantibodies into pregnant mice results in hypertension, proteinuria, increased production of placenta-derived soluble factors and other key features of preeclampsia[6]. On the basis of these findings we hypothesize that preeclampsia is a pregnancy-induced autoimmune condition characterized by the presence of disease-causing angiotensin receptor activating autoantibodies, AT1-AAs. We hypothesize further that these autoantibodies are responsible for widespread activation of AT1 receptors on a variety of maternal and placental cells (and probably in the fetus) and in this way contribute to many features of preeclampsia. The biological properties of these autoantibodies can be blocked by a 7-aa peptide that corresponds to a specific epitope associated with the second extracellular loop of the AT1 receptor. This fact suggests a common immunological origin for these autoantibodies in different individuals and provides obvious therapeutic opportunities.
Acknowledgement
These work was supported by NIH grants HL076558(to Y.X.) and HD034130 (to R.E.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 2.Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357:53–56. doi: 10.1016/s0140-6736(00)03577-7. [DOI] [PubMed] [Google Scholar]
- 3.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation. 2002;9:147–160. doi: 10.1038/sj.mn.7800137. [DOI] [PubMed] [Google Scholar]
- 4.Xia Y, Wen HY, Bobst S, Day MC, Kellems RE. Maternal autoantibodies from Preeclampsia patients Activate Angiotensin Receptors on Human Trophoblast Cells. J. Soc. Gyenocologic Investigation. 2003;10:82–93. doi: 10.1016/s1071-5576(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 5.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou CC, Ahmad S, Mi T, Abbasi S, Xia L, Day MC, Ramin SM, Ahmed A, Kellems RE, Xia Y. Autoantibody from women with preeclampsia induces soluble Fms-like tyrosine kinase-1 production via angiotensin type 1 receptor and calcineurin/nuclear factor of activated T-cells signaling. Hypertension. 2008;51:1010–1019. doi: 10.1161/HYPERTENSIONAHA.107.097790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dechend R, Llinas M, Caluwaerts S, Herse F, Lamarca B, Mueller DN, Luft FC, Pijnenborg R, Wallukat G, Granger JP. Agonistic autoantibodies to the AT1 receptor in rat models of preeclampsia: induced by chronic reduction in uterine perfusion pressure (RUPP) and low dose TNF-a infusion. Hypertension in pregnancy. 2006;25:70. (abstract) [Google Scholar]
- 8.Dechend R, Gratze P, Wallukat G, Shagdarsuren E, Plehm R, Brasen JH, Fiebeler A, Schneider W, Caluwaerts S, Vercruysse L, Pijnenborg R, Luft FC, Muller DN. Agonistic autoantibodies to the AT1 receptor in a transgenic rat model of preeclampsia. Hypertension. 2005;45:742–746. doi: 10.1161/01.HYP.0000154785.50570.63. [DOI] [PubMed] [Google Scholar]
- 9.Dechend R, Homuth V, Wallukat G, Kreuzer J, Park JK, Theuer J, Juepner A, Gulba DC, Mackman N, Haller H, Luft FC. AT(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation. 2000;101:2382–2387. doi: 10.1161/01.cir.101.20.2382. [DOI] [PubMed] [Google Scholar]
- 10.Dechend R, Viedt C, Muller DN, Ugele B, Brandes RP, Wallukat G, Park JK, Janke J, Barta P, Theuer J, Fiebeler A, Homuth V, Dietz R, Haller H, Kreuzer J, Luft FC. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation. 2003;107:1632–1639. doi: 10.1161/01.CIR.0000058200.90059.B1. [DOI] [PubMed] [Google Scholar]
- 11.Bobst SM, Day MC, Gilstrap LC, 3rd, Xia Y, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human mesangial cells and induce interleukin-6 and plasminogen activator inhibitor-1 secretion. American Journal of Hypertension. 2005;18:330–336. doi: 10.1016/j.amjhyper.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Walther T, Wallukat G, Jank A, Bartel S, Schultheiss HP, Faber R, Stepan H. Angiotensin II type 1 receptor agonistic antibodies reflect fundamental alterations in the uteroplacental vasculature. Hypertension. 2005;46:1275–1279. doi: 10.1161/01.HYP.0000190040.66563.04. [DOI] [PubMed] [Google Scholar]
- 13.Dorffel Y, Wallukat G, Bochnig N, Homuth V, Herberg M, Dorffel W, Pruss A, Chaoui R, Scholze J. Agonistic AT(1) receptor autoantibodies and monocyte stimulation in hypertensive patients. Am J Hypertens. 2003;16:827–833. doi: 10.1016/s0895-7061(03)00982-8. [DOI] [PubMed] [Google Scholar]
- 14.Xia Y, Wen HY, Kellems RE. Angiotensin II inhibits human trophoblast invasiveness through AT-1 receptor activation. Journal of Biological chemistry. 2002;277:24601–24608. doi: 10.1074/jbc.M201369200. [DOI] [PubMed] [Google Scholar]
- 15.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou CC, Ahmad S, Mi T, Xia L, Abbasi S, Hewett PW, Sun C, Ahmed A, Kellems RE, Xia Y. Angiotensin II induces soluble fms-Like tyrosine kinase-1 release via calcineurin signaling pathway in pregnancy. Circ Res. 2007;100:88–95. doi: 10.1161/01.RES.0000254703.11154.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karumanchi SA, Lindheimer MD. Preeclampsia pathogenesis: "triple a rating"-autoantibodies and antiangiogenic factors. Hypertension. 2008;51:991–992. doi: 10.1161/HYPERTENSIONAHA.107.100735. [DOI] [PubMed] [Google Scholar]
- 18.Haller H OT, Hauck U, Distler A, Philipp T. Increased intracellular free calcium and sensitivity to angiotensin II in platelets of preeclamptic women. Am J Hypertens. 1989;2:238–243. doi: 10.1093/ajh/2.4.238. [DOI] [PubMed] [Google Scholar]
- 19.Haller H, Ziegler E-M, Homuth V, Drab M, Eichhorn J, Nagy Z, Busjahn A, Vetter K, Luft FC. Endothelial Adhesion Molecules and Leukocyte Integrins in Preeclamptic Patients. Hypertension. 1997;29:291–296. doi: 10.1161/01.hyp.29.1.291. [DOI] [PubMed] [Google Scholar]
- 20.Hojo M, Suthanthiran M, Helseth G, August P. Lymphocyte intracellular free calcium concentration is increased in preeclampsia. Am J Obstet Gynecol. 1999;180:1209–1214. doi: 10.1016/s0002-9378(99)70618-6. [DOI] [PubMed] [Google Scholar]
- 21.Hardwick JS, Kuruvilla FG, Tong JK, Shamji AF, Schreiber SL. Rapamycin-modulated transcription defines the subset of nutrient- sensitive signaling pathways directly controlled by the Tor proteins. Proc Natl Acad Sci U S A. 1999;96:14866–14870. doi: 10.1073/pnas.96.26.14866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thway TM, Shlykov SG, Day MC, Sanborn BM, Gilstrap LC, 3rd, Xia Y, Kellems RE. Antibodies from preeclamptic patients stimulate increased intracellular Ca2+ mobilization through angiotensin receptor activation. Circulation. 2004;110:1612–1619. doi: 10.1161/01.CIR.0000142855.68398.3A. [DOI] [PubMed] [Google Scholar]
- 23.Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14:855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maynard S, Epstein FH, Karumanchi SA. Preeclampsia and angiogenic imbalance. Annu Rev Med. 2008;59:61–78. doi: 10.1146/annurev.med.59.110106.214058. [DOI] [PubMed] [Google Scholar]
- 25.Parikh SM, Karumanchi SA. Putting pressure on pre-eclampsia. Nat Med. 2008;14:810–812. doi: 10.1038/nm0808-810. [DOI] [PubMed] [Google Scholar]
- 26.Lamarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the Angiotensin Type I Receptor in Response to Placental Ischemia and Tumor Necrosis Factor {alpha} in Pregnant Rats. Hypertension. 2008 doi: 10.1161/HYPERTENSIONAHA.108.120576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunction. Hypertension. 2001;38:718–722. doi: 10.1161/01.hyp.38.3.718. [DOI] [PubMed] [Google Scholar]
- 28.Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, Bennett W. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med. 2006;122:383–392. doi: 10.1385/1-59259-989-3:381. [DOI] [PubMed] [Google Scholar]
- 29.Roberts L, LaMarca BB, Fournier L, Bain J, Cockrell K, Granger JP. Enhanced endothelin synthesis by endothelial cells exposed to sera from pregnant rats with decreased uterine perfusion. Hypertension. 2006;47:615–618. doi: 10.1161/01.HYP.0000197950.42301.dd. [DOI] [PubMed] [Google Scholar]
- 30.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension. 2005;46:1022–1025. doi: 10.1161/01.HYP.0000175476.26719.36. [DOI] [PubMed] [Google Scholar]
- 31.LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension. 2005;46:82–86. doi: 10.1161/01.HYP.0000169152.59854.36. [DOI] [PubMed] [Google Scholar]
- 32.Gadonski G, LaMarca BB, Sullivan E, Bennett W, Chandler D, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of interleukin 6. Hypertension. 2006;48:711–716. doi: 10.1161/01.HYP.0000238442.33463.94. [DOI] [PubMed] [Google Scholar]
- 33.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension. 2007;50:1142–1147. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert JS, Gilbert SA, Arany M, Granger JP. Hypertension produced by placental ischemia in pregnant rats is associated with increased soluble endoglin expression. Hypertension. 2009;53:399–403. doi: 10.1161/HYPERTENSIONAHA.108.123513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Opar A. Common reproductive disorders may have immunological basis. Nat Med. 2008;14:1172. doi: 10.1038/nm1108-1172. [DOI] [PubMed] [Google Scholar]
- 36.Mostarica-Stojkovic M. Mechanisms of the induction of autoimmunity] Srp Arh Celok Lek. 2005;133(Suppl 1):9–15. doi: 10.2298/sarh05s1009m. [DOI] [PubMed] [Google Scholar]
- 37.Pearce SH, Merriman TR. Genetic progress towards the molecular basis of autoimmunity. Trends Mol Med. 2006;12:90–98. doi: 10.1016/j.molmed.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Barak Y. The immune system and happiness. Autoimmun Rev. 2006;5:523–527. doi: 10.1016/j.autrev.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Saftlas AF, Olson DR, Franks AL, Atrash HK, Pokras R. Epidemiology of preeclampsia and eclampsia in the United States, 1979–1986. Am J Obstet Gynecol. 1990;163:460–465. doi: 10.1016/0002-9378(90)91176-d. [DOI] [PubMed] [Google Scholar]
- 40.Saito S, Shiozaki A, Nakashima A, Sakai M, Sasaki Y. The role of the immune system in preeclampsia. Mol Aspects Med. 2007;28:192–209. doi: 10.1016/j.mam.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Dekker G, Robillard PY. Pre-eclampsia: Is the immune maladaptation hypothesis still standing? An epidemiological update. J Reprod Immunol. 2007;76:8–16. doi: 10.1016/j.jri.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 42.Campbell DM, MacGillivray I, Carr-Hill R. Pre-eclampsia in second pregnancy. Br J Obstet Gynaecol. 1985;92:131–140. doi: 10.1111/j.1471-0528.1985.tb01064.x. [DOI] [PubMed] [Google Scholar]
- 43.Robillard PY, Hulsey TC, Alexander GR, Keenan A, de Caunes F, Papiernik E. Paternity patterns and risk of preeclampsia in the last pregnancy in multiparae. J Reprod Immunol. 1993;24:1–12. doi: 10.1016/0165-0378(93)90032-d. [DOI] [PubMed] [Google Scholar]
- 44.Dekker G. The partner's role in the etiology of preeclampsia. J Reprod Immunol. 2002;57:203–215. doi: 10.1016/s0165-0378(02)00039-6. [DOI] [PubMed] [Google Scholar]
- 45.Dekker GA, Robillard PY, Hulsey TC. Immune maladaptation in the etiology of preeclampsia: a review of corroborative epidemiologic studies. Obstet Gynecol Surv. 1998;53:377–382. doi: 10.1097/00006254-199806000-00023. [DOI] [PubMed] [Google Scholar]
- 46.Skjaerven R, Wilcox AJ, Lie RT. The interval between pregnancies and the risk of preeclampsia. N Engl J Med. 2002;346:33–38. doi: 10.1056/NEJMoa011379. [DOI] [PubMed] [Google Scholar]
- 47.Robillard PY, Hulsey TC, Perianin J, Janky E, Miri EH, Papiernik E. Association of pregnancy-induced hypertension with duration of sexual cohabitation before conception. Lancet. 1994;344:973–975. doi: 10.1016/s0140-6736(94)91638-1. [DOI] [PubMed] [Google Scholar]
- 48.Klonoff-Cohen HS, Savitz DA, Cefalo RC, McCann MF. An epidemiologic study of contraception and preeclampsia. Jama. 1989;262:3143–3147. [PubMed] [Google Scholar]
- 49.Salha O, Sharma V, Dada T, Nugent D, Rutherford AJ, Tomlinson AJ, Philips S, Allgar V, Walker JJ. The influence of donated gametes on the incidence of hypertensive disorders of pregnancy. Hum Reprod. 1999;14:2268–2273. doi: 10.1093/humrep/14.9.2268. [DOI] [PubMed] [Google Scholar]
- 50.Schiessl B. Inflammatory response in preeclampsia. Mol Aspects Med. 2007;28:210–219. doi: 10.1016/j.mam.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16:206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 52.Visser N, van Rijn BB, Rijkers GT, Franx A, Bruinse HW. Inflammatory changes in preeclampsia: current understanding of the maternal innate and adaptive immune response. Obstet Gynecol Surv. 2007;62:191–201. doi: 10.1097/01.ogx.0000256779.06275.c4. [DOI] [PubMed] [Google Scholar]
- 53.Matthiesen L, Berg G, Ernerudh J, Ekerfelt C, Jonsson Y, Sharma S. Immunology of preeclampsia. Chem Immunol Allergy. 2005;89:49–61. doi: 10.1159/000087912. [DOI] [PubMed] [Google Scholar]
- 54.a.S CW, Redman IL. Placenta Stress and Pre-eclampsia: A Revised View. Placenta. 2009;23:S38–S42. doi: 10.1016/j.placenta.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 55.Sacks G, Sargent I, Redman C. Innate immunity in pregnancy. Immunol Today. 2000;21:200–201. doi: 10.1016/s0167-5699(00)01615-7. [DOI] [PubMed] [Google Scholar]
- 56.Sacks G, Sargent I, Redman C. An innate view of human pregnancy. Immunol Today. 1999;20:114–118. doi: 10.1016/s0167-5699(98)01393-0. [DOI] [PubMed] [Google Scholar]
- 57.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–86. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 58.Sargent IL, Germain SJ, Sacks GP, Kumar S, Redman CW. Trophoblast deportation and the maternal inflammatory response in preeclampsia. J Reprod Immunol. 2003;59:153–160. doi: 10.1016/s0165-0378(03)00044-5. [DOI] [PubMed] [Google Scholar]
- 59.Girardi G, Bulla R, Salmon JE, Tedesco F. The complement system in the pathophysiology of pregnancy. Mol Immunol. 2006;43:68–77. doi: 10.1016/j.molimm.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 60.Redman CW, Sargent IL. Microparticles and immunomodulation in pregnancy and pre-eclampsia. J Reprod Immunol. 2007;76:61–67. doi: 10.1016/j.jri.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 61.Sacks GP, Scott D, Tivnann H, Mire-Sluis T, Sargent IL, Redman CW. Interleukin-12 and pre-eclampsia. J Reprod Immunol. 1997;34:155–158. doi: 10.1016/s0165-0378(97)00028-4. [DOI] [PubMed] [Google Scholar]
- 62.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 63.Robillard PY, Hulsey TC. Association of pregnancy-induced-hypertension, pre-eclampsia, and eclampsia with duration of sexual cohabitation before conception. Lancet. 1996;347:619. doi: 10.1016/s0140-6736(96)91315-x. [DOI] [PubMed] [Google Scholar]
- 64.Koelman CA, Coumans AB, Nijman Doxiadis HW, II, Dekker GA, Claas FH. Correlation between oral sex and a low incidence of preeclampsia: a role for soluble HLA in seminal fluid? J Reprod Immunol. 2000;46:155–166. doi: 10.1016/s0165-0378(99)00062-5. [DOI] [PubMed] [Google Scholar]
- 65.Wang JX, Knottnerus AM, Schuit G, Norman RJ, Chan A, Dekker GA. Surgically obtained sperm, and risk of gestational hypertension and preeclampsia. Lancet. 2002;359:673–674. doi: 10.1016/S0140-6736(02)07804-2. [DOI] [PubMed] [Google Scholar]
- 66.Robillard PY, Dekker GA, Hulsey TC. Primipaternities in families: is the incidence of pregnancy-induced hypertensive disorders in multigravidas an anthropological marker of reproduction? Aust N Z J Obstet Gynaecol. 1998;38:284–287. doi: 10.1111/j.1479-828x.1998.tb03067.x. [DOI] [PubMed] [Google Scholar]