Abstract
Context
Despite progress in tobacco control, secondhand smoke (SHS) exposure remains prevalent worldwide and is implicated in the initiation and maintenance of cigarette smoking.
Objective
To determine whether moderate SHS exposure results in brain α4β2* nicotinic acetylcholine receptor (nAChR) occupancy.
Design, Setting, and Participants
Positron emission tomography scanning and the radiotracer 2-[18F]fluoro-3-(2(S)azetidinylmethoxy) pyridine (also known as 2-[18F]fluoro-A-85380, or 2-FA) were used to determine α4β2* nAChR occupancy from SHS exposure in 24 young adult participants (11 moderately dependent cigarette smokers and 13 nonsmokers). Participants underwent two bolus-plus-continuous-infusion 2-FA positron emission tomography scanning sessions during which they sat in the passenger’s seat of a car for 1 hour and either were exposed to moderate SHS or had no SHS exposure. The study took place at an academic positron emission tomography center.
Main Outcome Measure
Changes induced by SHS in 2-FA specific binding volume of distribution as a measure of α4β2* nAChR occupancy.
Results
An overall multivariate analysis of variance using specific binding volume of distribution values revealed a significant main effect of condition (SHS vs control) (F1,22=42.5, P <.001) but no between-group (smoker vs nonsmoker) effect. Exposure to SHS led to a mean 19% occupancy of brain α4β2* nAChRs (1-sample t test, 2-tailed, P <.001). Smokers had both a mean 23% increase in craving with SHS exposure and a correlation between thalamic α4β2* nAChR occupancy and craving alleviation with subsequent cigarette smoking (Spearman ρ= −0.74, P =.01).
Conclusions
Nicotine from SHS exposure results in substantial brain α4β2* nAChR occupancy in smokers and nonsmokers. Study findings suggest that such exposure delivers a priming dose of nicotine to the brain that contributes to continued cigarette use in smokers. This study has implications for both biological research into the link between SHS exposure and cigarette use and public policy regarding the need to limit SHS exposure in cars and other enclosed spaces.
Despite substantial progress in tobacco control, secondhand smoke (SHS) exposure remains prevalent worldwide,1 where the home and other enclosed spaces are the predominant locations for such exposure.1,2 Secondhand smoke is reported to be more toxic than mainstream cigarette smoke3,4 and causes disease and premature death in children and adults who do not smoke.2 Exposure to SHS is associated with sudden infant death syndrome,5,6 low infant birth weight,7 chronic middle ear infections, and respiratory diseases in children2 as well as cancer and coronary heart disease in adults.8–10
In addition to direct health risks, prior research indicates that SHS exposure promotes the initiation and maintenance of smoking behavior through effects on the central nervous system. These studies demonstrate that young never-smokers exposed to SHS in a motor vehicle have an elevated risk of experiencing symptoms of nicotine dependence,11 children with higher nicotine intake from SHS are more likely to become cigarette smokers as teenagers,12 and adult smokers exposed to more sources of SHS are less likely to initiate or maintain abstinence.13 In addition, studies of laboratory rats demonstrate that long-term exposure to cigarette smoke leads to nicotine dependence14 and an upregulation of nicotinic acetylcholine receptor (nAChR) levels in brain.14,15 While these studies indicate that SHS exposure directly affects the central nervous system to promote smoking behavior, brain nAChR occupancy from SHS exposure has not yet (to our knowledge) been demonstrated.
The radiotracer 2-[18F]fluoro-3-(2(S)azetidinylmethoxy) pyridine (also known as 2-[18F]fluoro-A-85380, or 2-FA), developed for positron emission tomography (PET), binds in vivo with relative specificity and high affinity to the α4β2* nAChR16–21 (one of the most abundant nAChR subtypes in the mammalian brain22,23). Our group recently examined displacement of 2-FA during PET scanning to measure α4β2* nAChR occupancy from smoking regular and denicotinized cigarettes. Specifically, we found that the effective dose of a regular cigarette and the effective concentration of plasma nicotine needed to occupy 50% of available brain α4β2* nAChRs were only 13% (between 1 and 2 puffs) of a cigarette and 0.87 ng/mL (to convert to micromoles per liter, multiply by 0.006164), respectively.24 We also determined that smoking a full cigarette or smoking to satiety resulted in nearly complete saturation of α4β2* nAChRs. In a follow-up study,25 smoking a denicotinized cigarette (with the resulting intake of a trace amount of nicotine) resulted in 26% occupancy of α4β2* nAChRs. Results of these 2 studies24,25 indicate that nicotine alone is responsible for α4β2* nAChR occupancy from cigarette smoking. Because heavy exposures to SHS (peak air carbon monoxide level of 13 ppm) are associated with plasma nicotine concentrations greater than 2 ng/mL in humans,26 these prior findings strongly suggest that SHS exposure results in brain α4β2* nAChR occupancy. For the current study, we used the same general 2-FA PET scanning methods as in previous studies24,25,27 to examine α4β2* nAChR occupancy from moderate SHS exposure.
METHODS
PARTICIPANTS AND SCREENING METHODS
Tobacco-dependent cigarette smokers (n=11; mean of 15.2 cigarettes per day for an average of 13.0 years; mean [SD] age, 29.7 [7.5] years; 6 women, 5 men) and nonsmokers (n=13; >2 years without a cigarette and no history of nicotine dependence; mean [SD] age, 33.5 [8.5] years; 6 women, 7 men) were recruited through newspaper and Internet advertisements. Initial screening consisted of a telephone interview in which smoking, medical, psychiatric, and substance use histories were obtained. To maximize confidentiality until study enrollment, participant names were not recorded on the screening information sheet during the initial telephone interview. Individuals meeting study criteria who wished to participate were assessed in person using screening questions from the Structured Clinical Interview for DSM-IV Axis I Disorders–Patient Edition, Version 2.028 2 days prior to PET scanning. For smokers, the central inclusion criterion was current nicotine dependence, while for nonsmokers the central inclusion criteria were no history of nicotine dependence and at least 1 hour of SHS exposure per month. This latter criterion for non-smokers was included for ethical reasons to avoid exposing SHS-naive participants to SHS (nonsmoking participants reported having a family member or significant other who smoked, and none reported heavy SHS exposure of >1 hour per day). Exclusion criteria for both groups were pregnancy, use of a medication or history of a medical condition that might affect the central nervous system at the time of scanning, any history of mental illness or substance abuse or dependence, or use of the caffeine equivalent of more than 2 cups of regular coffee per day.
During the initial visit, screening data were obtained to verify participant reports, including the Smoker’s Profile Form (to document smoking history), Fagerström Test for Nicotine Dependence29,30 (mean [SD] score for smokers, 3.6 [1.3], indicating moderate dependence), Hamilton Depression Rating Scale31 (mean [SD] score, 1.5 [2.8] for smokers and 0.7 [1.2] for non-smokers), and Hamilton Anxiety Rating Scale32 (mean [SD] score, 2.3 [3.3] for smokers and 1.2 [2.6] for nonsmokers). The exhaled carbon monoxide level was determined using a Micro Smokerlyzer (Bedfont Scientific Ltd, Kent, England) to verify smoking status (carbon monoxide level ≥8 ppm for active smokers and ≤4 ppm for nonsmokers). This study was approved by the local institutional review board, and participants provided written informed consent using forms approved by the institutional review board.
ABSTINENCE PERIOD AND PET PROTOCOL
After the initial screenings, participants underwent PET scanning following the same general procedure as in our prior reports,24,25 except that participants here underwent 2 PET scanning sessions (randomized order, 1 week apart) during which they sat in a car and either were or were not exposed to moderate SHS for 1 hour.
Prior to each PET session, participants from the cigarette smoker group began abstaining from smoking and other nicotine use at 6 PM two nights earlier so that nicotine from smoking would not compete with the radiotracer for receptor binding during the PET procedure. They reported to our laboratory at 1 PM the day after initiating abstinence (the day before PET scanning), and a brief clinical interview and exhaled carbon monoxide measurement were obtained. Participants were deemed to be compliant with the study protocol if they reported no smoking or nicotine use since 6 PM the previous night and had an exhaled carbon monoxide level of 8 ppm or less. Participants were seen the following day for PET scanning and were required to report continuous abstinence since 2 nights previously and have an exhaled carbon monoxide level of 4 ppm or less to undergo PET scanning. Participants were given financial incentives to maintain this abstinence.
At 12 PM on the scanning day, participants arrived at the Greater Los Angeles Veterans Affairs Healthcare System PET Center, and abstinence was verified as described earlier. Each participant then had an intravenous line placed at 12:45 PM in a room adjacent to the scanner. At 1 PM, bolus plus continuous infusion of 2-FA was initiated. The amount of 2-FA administered as a bolus was equal to the amount infused over 500 minutes.27,33
After initiation of the 2-FA bolus plus continuous infusion, participants remained seated in a room adjacent to the PET scanner for the next 3 hours to allow the radiotracer to reach a relatively steady state in the brain. At 4 PM, PET scanning commenced and continued for 1 hour. At 5 PM, participants had a 1-hour break in scanning, during which they sat in the passenger’s seat of a car immediately outside the PET center and were either exposed (one PET session) or not exposed (the other PET session) to SHS produced by a smoker seated in the driver’s seat. For the SHS condition, the smoker smoked a mean (SD) of 3.7 (0.8) cigarettes to maintain a target air carbon monoxide level of 7 ppm above the ambient level (as measured with a WolfSense EC-202 carbon monoxide monitor; GrayWolf Sensing Solutions, Shelton, Connecticut). Car windows were closed other than a small (<1 cm) opening on the passenger’s side of the car to allow space for the extension tubing delivering the infusion of the radiotracer. Participants were then scanned for 1 hour and subsequently had a 10-minute break. During this break, smokers smoked to satiety (mean [SD], 2.1 [0.8] cigarettes) to saturate α4β2* nAChRs and displace specifically bound radiotracer from the brain. This procedure allowed us to obtain a measure of nondisplaceable (ND) radioactivity (ie, the free and nonspecifically bound radioactivity). All participants then underwent PET scanning for 1 hour 40 minutes more with a 10-minute break. Scanning ended at roughly 9 PM.
The PET scans were obtained on a General Electric Advance NXi scanner (General Electric Medical Systems, Milwaukee, Wisconsin) with 35 slices in 3-dimensional mode, transaxial full-width-at-half-maximum resolution of 5.2 to 7.7 mm,34 and were obtained as series of 10-minute frames. Attenuation-correction scanning was performed with the germanium rotating-rod source built into the scanner for 5 minutes at the end of the scanning session, and this attenuation correction was applied to all scans. The 2-FA was prepared using a published method.35
Blood samples (5 mL) were drawn during PET scanning for determinations of free, unmetabolized 2-FA and nicotine levels in plasma. For plasma 2-FA levels, 9 venous blood samples were drawn at predetermined intervals from 3 to 8.5 hours after the initiation of 2-FA administration, and 2-FA levels were determined using previously published methods.36–38 For plasma nicotine levels, blood samples were drawn prior to and following the SHS break in scanning. Samples were centrifuged and venous plasma nicotine concentrations were determined in the laboratory of Peyton Jacob, PhD, at the University of California, San Francisco, using a modified version of a published gas chromatography–mass spectrometry method.39 Because maximum sensitivity was required, tandem mass spectrometry was used. The extraction was identical to the published method. Deuterium-labeled nicotine was used as the internal standard. The triple quadrupole (tandem) mass spectrometer was operated in the positive ion mode using chemical ionization with isobutane as the reagent gas. Quantification was achieved using selected reaction monitoring. The lower limit of quantification with this method is 0.1 ng/mL.
SYMPTOM RATING SCALE ADMINISTRATION
The Secondhand Smoke Rating Scale40 was administered before and after SHS exposure. This scale consists of analog ratings (range, 0–6) for common symptoms of SHS exposure (eye irritation, nose irritation, runny nose, nasal congestion, coughing, chest tightness, and heart palpitations). For the smoker group, cigarette craving was monitored at 4 points during the PET session (before and after SHS exposure and before and after cigarette smoking) with the Urge to Smoke Scale,41,42 an analog scale (range, 0–6) with 10 craving-related questions.
MAGNETIC RESONANCE IMAGING
A magnetic resonance imaging scan of the brain was obtained for each participant within a week of the PET scanning sessions, with the following specifications: 3-dimensional Fourier-transform spoiled-gradient-recalled acquisition with a repetition time of 30 milliseconds, an echo time of 7 milliseconds, a 30° flip angle, 2 acquisitions, and a 256×192 view matrix. The acquired volume was reconstructed as 90 contiguous 1.5-mm-thick transaxial slices.
PET IMAGE ANALYSIS
After decay and motion correction, each subject’s PET images were coregistered to his or her magnetic resonance imaging scan using PMOD version 2.9 software (PMOD Technologies Ltd, Zurich, Switzerland). Regions of interest (ROIs) were then drawn on magnetic resonance images using PMOD and transferred to the coregistered PET scans. The ROIs included the thalamus, brainstem, and cerebellum, which were chosen based on prior reports indicating high receptor binding of 2-FA in these regions.24,27,43–45 The ROI placement was visually inspected for each PET frame to minimize effects of coregistration errors and movement; this procedure was repeated if there was a noticeable problem.
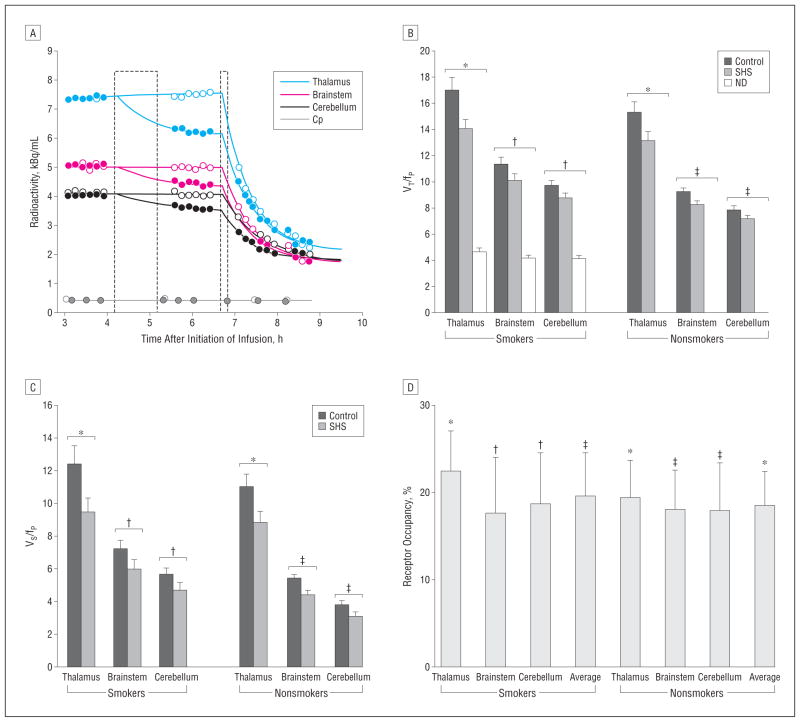
As an initial examination of the PET data, time-activity curves (Figure 1A) for each of the 3 brain ROIs were plotted to verify that a relatively steady state of radioactivity was present before and after the control condition (no SHS) and that displacement of radioactivity occurred from before to after the SHS condition. This initial examination of the raw data revealed little change in mean radioactivity across the thalamus, brainstem, and cerebellum from before to after the control condition (no SHS) for smokers (4%, 1%, and 1%, respectively) and non-smokers (1%, −3%, and −3%, respectively) (all within-group changes, nonsignificant), while there were significant mean displacements of radioactivity from before to after the SHS exposure for both smokers (−14%, −10%, and −8%, respectively; 2-tailed paired t tests, all P<.02) and nonsmokers (−13%, −13%, and −11%, respectively; 2-tailed paired t tests, all P <.005).
Figure 1.
Secondhand smoke (SHS) exposure leads to significant α4β2* nicotinic acetylcholine receptor occupancy. A, Representative time-activity curves from before to after SHS (solid symbols) and control (open symbols) exposure (first dashed box) and after cigarette smoking to satiety (second dashed box). Cp indicates concentration of free 2-[18F]fluoro-3-(2(S)azetidinylmethoxy) pyridine (also known as 2-[18F]fluoro-A-85380, or 2-FA) in plasma. Total volume of distribution (VT) (B) and specific binding volume of distribution (VS) (C), corrected for the free fraction of plasma 2-[18F]fluoro-3-(2(S)azetidinylmethoxy) pyridine (fP). Error bars indicate SEM; ND, nondisplaceable volume of distribution corrected for fP. *P <.001; †P <.02; and ‡P <.01 (paired t test). D, Occupancy of α4β2* nicotinic acetylcholine receptors from SHS exposure. Error bars indicate SEM; average, average of the 3 regions of interest. *P <.001; †P <.02; and ‡P <.01 (1-sample t test).
For the central analyses of the study, total and specific binding volumes of distribution (designated as VT/fP and VS/fP, respectively, based on the nomenclature suggested by Innis et al46) were determined for the control and SHS exposure conditions. The VT/fP value was determined from radioactivity values, measured from 50 to 80 minutes after the SHS exposure and control condition (no SHS exposure), as the ratio CT/(CP·fP), where CT is the total concentration of 2-FA in the ROIs, (CP·fP) is the concentration of free 2-FA in plasma, and fP is the fraction of free (unbound) 2-FA in plasma.44,46 The VS/fP value was then determined for each participant as the difference between VT/fP and the ND volume of distribution corrected for the free fraction of plasma 2-FA (VND/fP), such that VS/fP = VT/fP−VND/fP. For smokers, individual values of VND/fP were determined 95 minutes after smoking to satiety (average of the last 3 PET time frames) and were corrected for incomplete displacement of specifically bound radioactivity (11%), because of insufficient time (5%) and an insufficient smoking dose (6%), to lead to 100% displacement. Values for these corrections were based on data from previously published findings by our group.24 Because of ethical concerns and potential problems with nicotine tolerability, we did not administer pharmacological doses of nicotine to nonsmokers for calculation of VS/fP values in this study. Instead, the mean (SEM) region-respective VND/fP values obtained from the smoker group (4.6 [0.3], 4.1 [0.3], and 4.1 [0.3] for thalamus, brainstem, and cerebellum, respectively) were used in the calculations of VS/fP for the non-smoker group. Regional differences in nonspecific radioactivity were not significant, but this finding does not exclude the possibility of between-region differences in this measure.
STATISTICAL ANALYSIS
Overall repeated-measures multivariate analyses of variance were performed for both VT/fP and VS/fP, using these values for the 3 ROIs across the 2 conditions (no SHS vs SHS) as the repeated measures and group (smoker vs nonsmoker) as a between-subject factor. These multivariate analyses of variance were performed for the closely related values of VT/fP and VS/fP because VS/fP includes the VND/fP values obtained from smokers who smoked to satiety and we wanted to demonstrate condition differences (no SHS vs SHS) for data both without (VT/fP) and with (VS/fP) the correction for ND radioactivity.
To clarify results of these overall tests, percentage of α4β2* nAChR occupancy from SHS exposure was determined using VS/fP values from sessions without and with SHS exposure (for derivations, see the articles by Brody et al24 and Innis et al46). Specifically, percentage of occupancy was calculated as 100 × (VS/fP control − VS/fP SHS exposure)/(VS/fP control). A 1-sample t test was then performed for the entire study sample for the average percentage of occupancy of the 3 ROIs. One-sample t tests were also performed for the entire study group for the ROIs separately and for the ROIs for the smoker and nonsmoker groups separately. For completeness, percentages of α4β2* nAChR occupancy from SHS exposure were compared between smokers and nonsmokers and between regions using t tests.
For symptom rating scales, paired t tests were performed within the 2 study groups to determine whether SHS exposure resulted in increased SHS-related symptoms (a between-group unpaired t test was also run) and a paired t test was performed within the group of smokers who were not at maximal cigarette craving prior to testing (n=9) to determine whether SHS exposure resulted in a change in craving. Effect sizes for the regional occupancy and symptom rating scale analyses are reported as mean divided by standard deviation (Cohen d).
In addition to the determination of receptor occupancy from SHS exposure, exploratory analyses were performed to determine relationships between α4β2* nAChR occupancy and symptoms associated with SHS exposure. Specifically, Spearman rank correlation coefficients were determined between SHS-induced α4β2* nAChR occupancy and changes in SHS-related symptoms (both study groups) and cigarette craving (smoker group) during PET scanning. Statistical tests were performed using PASW/SPSS Statistics version 17.0 statistical software (SPSS Inc, Chicago, Illinois).
RESULTS
During the SHS exposure condition, the mean (SD) air carbon monoxide level was 7.4 (1.0) ppm, which was significantly higher than the control condition (0.5 [0.2] ppm) (2-sample t test, P<.001). This SHS exposure resulted in a mean increase in plasma nicotine concentration for the total study sample of approximately 0.2 ng/mL. In smokers, SHS exposure resulted in an increase in mean (SD) plasma nicotine concentration from 0.16 (0.03) ng/mL to 0.37 (0.10) ng/mL (paired t test, P=.046). In nonsmokers, an exact determination of the increase in plasma nicotine levels was not possible because almost all (11 of 13) of the plasma samples had nondetectable nicotine levels prior to exposure. The nonsmoker group had a mean (SD) plasma nicotine concentration of 0.17 (0.05) ng/mL following SHS exposure.
The overall multivariate analyses of variance for VT/fP and VS/fP revealed significant main effects of condition (control vs SHS exposure) (F1,22 = 42.4, P < .001 and F1,22=42.5, P <.001, respectively) but no interaction between condition and group (smoker vs nonsmoker) (F1,22=0.9, P =.36 for both measures), indicating that SHS exposure results in α4β2* nAChR occupancy and that smokers and nonsmokers do not differ in this effect. For all 3 ROIs studied, SHS exposure resulted in statistically significant decreases in VT/fP (Figure 1B) and VS/fP (Figure 1C) values compared with the control condition in both participant groups.
Based on calculations of percentage of α4β2* nAChR occupancy from VS/fP values, the central study result was that moderate SHS exposure led to a mean 19% occupancy across the 3 ROIs for the entire study sample (1-sample t test, 2-tailed, P<.001). For the smoker and non-smoker groups separately, the mean α4β2* nAChR occupancies across the 3 ROIs were 19% and 18%, respectively (for thalamus, brainstem, and cerebellum: 22%, 17%, and 19% for smokers, respectively, and 19%, 18%, and 18% for nonsmokers, respectively). All of these receptor occupancy values for the smoker and non-smoker groups were statistically significant (for thalamus, brainstem, and cerebellum: 1-sample t tests, 2-tailed, P =.02, P =.01, and P =.01, respectively, for smokers and P=.002, P=.008, and P=.001, respectively, for nonsmokers) (Figure 1D and Figure 2), with effect sizes (Co-hen d) for the ROIs in the study sample ranging from 0.9 to 1.3. There were no significant differences between smokers and nonsmokers in α4β2* nAChR occupancies from SHS exposure (P values ranged from .60 to .89, with corresponding effect sizes ranging from 0.06 to 0.22). There were no significant differences between regions in α4β2* nAChR occupancy from SHS exposure (paired t tests, P values of .34–.82; Cohen d values of 0.05–0.2). Average ROI receptor occupancies ranged up to 56%, indicating potential individual differences in susceptibility to the adverse effects of SHS exposure and/or variability inherent to the PET method.
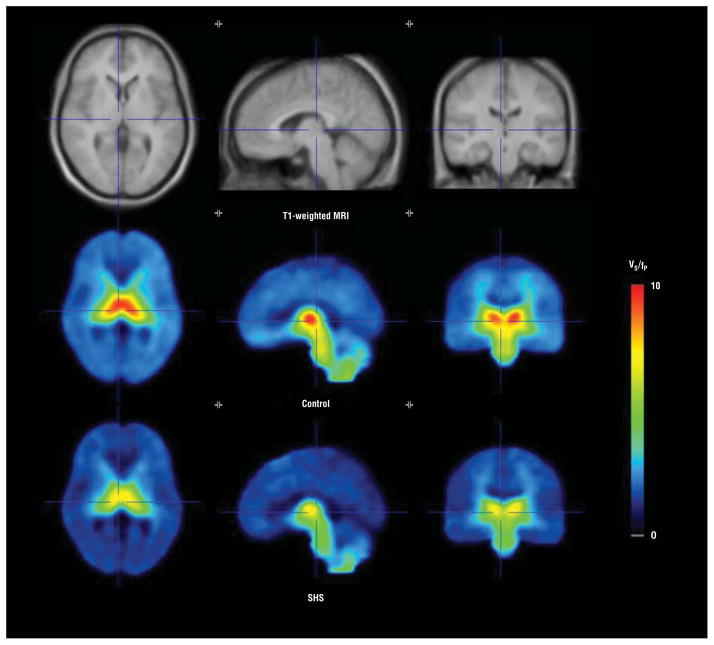
Figure 2.
One hour of moderate secondhand smoke (SHS) exposure leads to significant decreases in 2-[18F]fluoro-3-(2(S)azetidinylmethoxy) pyridine (also known as 2-[18F]fluoro-A-85380, or 2-FA) receptor binding in brain. Averages of spatially normalized images obtained from cigarette smokers in the study are shown. From left to right are transaxial, sagittal, and coronal slices through the thalamus. The top row shows the mean T1-weighted magnetic resonance images (MRIs). The middle and bottom rows display the mean specific binding volume of distribution (VS/fP) determined from positron emission tomography images following either the control condition (no SHS exposure) or moderate SHS exposure, respectively. Lower VS/fP is seen in the bottom row, demonstrating α4β2* nicotinic acetylcholine receptor occupancy from SHS exposure.
For the Secondhand Smoke Rating Scale, nonsmokers had a significant increase in symptom ratings from before to after SHS exposure (mean [SD] score increased from 1.2 [2.4] to 3.9 [2.6]; paired t test, P=.02), whereas smokers had a smaller nonsignificant increase in SHS symptoms (mean [SD] score increased from 2.8 [5.8] to 3.4 [4.8]; paired t test, P=.11). The difference between nonsmokers and smokers on change in this measure was not significant (2-sample t test; Cohen d=0.7). There were no significant correlations for the total sample (or the non-smoker or smoker groups) between SHS-induced α4β2* nAChR occupancy and SHS symptom severity.
Smokers had an increase in mean craving scores on the Urge to Smoke Scale (mean score increased from 3.0 to 3.7) from before to after SHS exposure (paired t test, 2-tailed, P=.02). Cohen d for this analysis was 0.9. In addition, there was a significant negative correlation between thalamic α4β2* nAChR occupancy from SHS exposure and change in craving from before to after cigarette smoking during the scanning session (Spearman ρ= −0.74, 2-tailed, P =.01).
COMMENT
Moderate SHS exposure leads to an increase in plasma nicotine concentration of approximately 0.2 ng/mL and a mean 19% brain α4β2* nAChR occupancy in young adults. These results are consistent with our finding that a plasma nicotine concentration of 0.87 ng/mL (from cigarette smoking) is associated with 50% occupancy of available α4β2* nAChRs.24 Because research examining heavy SHS exposure (in enclosed rooms with multiple smokers) demonstrates increases in plasma nicotine levels greater than 2 ng/mL,26 the present and prior studies indicate that such heavy exposures would result in greater than 70% α4β2* nAChR occupancy. Additionally, as prepubescent children and infants have a 1-minute ventilation per kilogram of bodyweight that is approximately 2 to 3 times higher than adults,47–49 increases in plasma nicotine concentration and occupancy of brain α4β2* nAChRs from similar levels of SHS exposure may be even greater for children than for adults.
The SHS exposure used here was moderate, being greater than, for example, the exposure in a well-ventilated moving car but less than the exposure in an enclosed space with multiple smokers. In addition, the plasma nicotine levels and brain α4β2* nAChR occupancies with SHS exposure are not as high as those found with primary cigarette smoking, where daily plasma nicotine levels of 10 to 50 ng/mL50 and brain α4β2* nAChR occupancy of approximately 95% from smoking to satiety24 have been reported. These differences between SHS exposure and primary cigarette smoking may explain why SHS exposure is not generally observed to be addictive in humans (although it may contribute to primary cigarette smoking behavior as noted earlier11–13).
In addition to the central study finding, SHS-related symptoms were numerically greater for nonsmokers than for smokers, which is consistent with prior research demonstrating greater sensitivity to SHS among nonsmokers.51,52 However, these symptoms did not correlate with brain α4β2* nAChR occupancy, suggesting that the SHS symptoms measured here (eg, coughing and runny nose) are mediated by local peripheral reactions53,54 and that nicotine binding to α4β2* nAChRs in the central nervous system does not mediate these symptoms. In addition, cigarette smoking leads to upregulation of α4β2* nAChRs14,15,55–58; therefore, while smokers and nonsmokers here had similar percentages of receptor occupancy from SHS exposure, the nonsmokers would be expected to have a smaller number of available receptors following SHS exposure than smokers, which could be an important factor for the substantial symptoms experienced by nonsmokers when exposed to SHS. These issues could be explored in future research with a larger sample size and a more detailed analysis of SHS-related symptoms.
The study findings of increased craving in smokers from before to after SHS exposure and a correlation between α4β2* nAChR occupancy and craving alleviation with smoking suggest that a moderate SHS exposure delivers a priming dose of nicotine, which increases craving, and that greater α4β2* nAChR occupancy from SHS exposure leads to greater craving alleviation when smokers subsequently smoke to satiety. This interpretation is consistent with prior research in laboratory animals demonstrating that a low dose of nicotine is sufficient to reinstate nicotine-seeking behavior in animals in which nicotine seeking has been extinguished.59,60 This mechanism may explain why adult smokers exposed to multiple sources of SHS have difficulty initiating and maintaining abstinence compared with smokers without such exposure.13
Although the sample size here was not large, examination of effect sizes indicates that the study had sufficient power for the central analyses. Specifically, the large effect sizes for all ROIs in the study sample were sufficient to have 80% power to detect α4β2* nAChR occupancy at the P <.05 threshold.61,62 Additionally, examination of individual data points revealed that occupancies were normally distributed and were not skewed by outliers. For the between-group occupancy analyses, group differences (and effect sizes) were small and indicated that large sample sizes (200–3000 participants) would be needed to detect whether significant group differences exist.
This study should be considered in the context of several limitations. First, participants were aware of whether they were being exposed to SHS during the PET sessions. Therefore, we cannot rule out the possibility that the stress of SHS exposure resulted in endogenous acetylcholine release; however, our prior research25 points to nicotine as being the predominant factor in α4β2* nAChR occupancy from cigarette smoke exposure. A future study using SHS produced by smoking denicotinized cigarettes could help clarify this point. Second, while the finding of a correlation between α4β2* nAChR occupancy and craving alleviation with smoking suggests that SHS exposure results in a priming dose of nicotine to the brain, it is also possible that SHS exposure affects craving through a conditioned response to the sensory characteristics of the smoke (or other smoking-related cues), which are independent of SHS-induced α4β2* nAChR occupancy and were not quantified in this study. Third, the limit of quantification for plasma nicotine levels allowed for only an approximation of the change from before to after SHS exposure because we did not have detectable levels for most of the non-smokers prior to SHS exposure. While a commonly used practice is to substitute the limit of quantification divided by 2 or the square root of 2 for these undetectable values,63 prior research indicates that this approach can lead to substantial error in the interpretation of data.64
Given prior research demonstrating that nicotine is likely primarily responsible for central α4β2* nAChR occupancy from smoking,24,25 this study demonstrates that nicotine inhaled from SHS crosses the blood-brain barrier and results in α4β2* nAChR occupancy—a factor that may contribute to a greater likelihood of an individual becoming a smoker as a teenager12 and the maintenance of cigarette smoking in adult smokers.13 In the United States, 47.8% of the population is protected from SHS exposure by laws that ban smoking in workplaces, restaurants, and bars, while an additional 31.6% are covered by laws banning smoking in at least one of these places.65 In addition to these bans on smoking in enclosed public spaces, recent laws have banned exposure of children to SHS in cars in 4 states in the United States and Puerto Rico, along with several areas internationally.66 The Royal College of Physicians67 and other prominent health care groups68–70 have recently called for wider applications of smoking bans in cars. Results of this study strongly support these public policy recommendations.
Acknowledgments
Funding/Support: This study was supported by grant 16RT-0098 from the Tobacco-Related Disease Research Program (Dr Brody), grant R01 DA20872 from the National Institute on Drug Abuse (Dr Brody), a Veterans Affairs Type I Merit Review Award (Dr Brody), grant DABT63-00-C-1003 from the Office of National Drug Control Policy (Dr London), and endowments from the Richard Metzner Chair in Clinical Neuropharmacology (Dr Brody), the Thomas P. and Katherine K. Pike Chair in Addiction Studies (Dr London), and the Marjorie Greene Family Trust (Dr London).
Role of the Sponsors: The funders of this study had no role in the design or conduct of the study; in the collection, management, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Drs Brody and Mandelkern had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Disclosure: Drs London and Mukhin received support from Philip Morris USA for research other than the project presented here.
Additional Contributions: Catherine Sugar, PhD, provided statistical consultation for the study. Josephine Ribe, BS, and Michael Clark, BS, provided technical support in performing PET and magnetic resonance imaging scans, respectively.
References
- 1.Warren CW, Jones NR, Eriksen MP, Asma S Global Tobacco Surveillance System (GTSS) Collaborative Group. Patterns of global tobacco use in young people and implications for future chronic disease burden in adults. Lancet. 2006;367(9512):749–753. doi: 10.1016/S0140-6736(06)68192-0. [DOI] [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services; Centers for Disease Control and Prevention; Coordinating Center for Health Promotion; National Center for Chronic Disease Prevention and Health Promotion; Office on Smoking and Health. Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Washington, DC: US Dept of Health & Human Services; 2006. [Google Scholar]
- 3.Schick S, Glantz S. Philip Morris toxicological experiments with fresh side-stream smoke: more toxic than mainstream smoke. Tob Control. 2005;14(6):396–404. doi: 10.1136/tc.2005.011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schick S, Glantz S. Scientific analysis of second-hand smoke by the tobacco industry, 1929–1972. Nicotine Tob Res. 2005;7(4):591–612. doi: 10.1080/14622200500185082. [DOI] [PubMed] [Google Scholar]
- 5.Dybing E, Sanner T. Passive smoking, sudden infant death syndrome (SIDS) and childhood infections. Hum Exp Toxicol. 1999;18(4):202–205. doi: 10.1191/096032799678839914. [DOI] [PubMed] [Google Scholar]
- 6.Klonoff-Cohen HS, Edelstein SL, Lefkowitz ES, Srinivasan IP, Kaegi D, Chang JC, Wiley KJ. The effect of passive smoking and tobacco exposure through breast milk on sudden infant death syndrome. JAMA. 1995;273(10):795–798. doi: 10.1001/jama.1995.03520340051035. [DOI] [PubMed] [Google Scholar]
- 7.Kharrazi M, DeLorenze GN, Kaufman FL, Eskenazi B, Bernert JT, Jr, Graham S, Pearl M, Pirkle J. Environmental tobacco smoke and pregnancy outcome. Epidemiology. 2004;15(6):660–670. doi: 10.1097/01.ede.0000142137.39619.60. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. State-specific prevalence of current cigarette smoking among adults, and policies and attitudes about secondhand smoke: United States, 2000. MMWR Morb Mortal Wkly Rep. 2001;50(49):1101–1106. [PubMed] [Google Scholar]
- 9.Witschi H, Joad JP, Pinkerton KE. The toxicology of environmental tobacco smoke. Annu Rev Pharmacol Toxicol. 1997;37:29–52. doi: 10.1146/annurev.pharmtox.37.1.29. [DOI] [PubMed] [Google Scholar]
- 10.Raupach T, Schäfer K, Konstantinides S, Andreas S. Secondhand smoke as an acute threat for the cardiovascular system: a change in paradigm. Eur Heart J. 2006;27(4):386–392. doi: 10.1093/eurheartj/ehi601. [DOI] [PubMed] [Google Scholar]
- 11.Bélanger M, O’Loughlin J, Okoli CT, McGrath JJ, Setia M, Guyon L, Gervais A. Nicotine dependence symptoms among young never-smokers exposed to secondhand tobacco smoke. Addict Behav. 2008;33(12):1557–1563. doi: 10.1016/j.addbeh.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becklake MR, Ghezzo H, Ernst P. Childhood predictors of smoking in adolescence: a follow-up study of Montreal schoolchildren. CMAJ. 2005;173(4):377–379. doi: 10.1503/cmaj.1041428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okoli CT, Browning S, Rayens MK, Hahn EJ. Secondhand tobacco smoke exposure, nicotine dependence, and smoking cessation. Public Health Nurs. 2008;25(1):46–56. doi: 10.1111/j.1525-1446.2008.00679.x. [DOI] [PubMed] [Google Scholar]
- 14.Small E, Shah HP, Davenport JJ, Geier JE, Yavarovich KR, Yamada H, Sabarinath SN, Derendorf H, Pauly JR, Gold MS, Bruijnzeel AW. Tobacco smoke exposure induces nicotine dependence in rats. Psychopharmacology (Berl) 2010;208(1):143–158. doi: 10.1007/s00213-009-1716-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yates SL, Bencherif M, Fluhler EN, Lippiello PM. Up-regulation of nicotinic acetylcholine receptors following chronic exposure of rats to mainstream cigarette smoke or alpha 4 beta 2 receptors to nicotine. Biochem Pharmacol. 1995;50 (12):2001–2008. doi: 10.1016/0006-2952(95)02100-0. [DOI] [PubMed] [Google Scholar]
- 16.Valette H, Bottlaender M, Dollé F, Guenther I, Coulon C, Hinnen F, Fuseau C, Ottaviani M, Crouzel C. Characterization of the nicotinic ligand 2-[F-18]fluoro-3-[2 (S)-2-azetidinylmethoxy]pyridine in vivo. Life Sci. 1999;64(5):PL93–PL97. doi: 10.1016/s0024-3205(98)00573-6. [DOI] [PubMed] [Google Scholar]
- 17.Horti AG, Scheffel U, Koren AO, Ravert HT, Mathews WB, Musachio JL, Finley PA, London ED, Dannals RF. 2-[18F]Fluoro-A-85380, an in vivo tracer for the nicotinic acetylcholine receptors. Nucl Med Biol. 1998;25(7):599–603. doi: 10.1016/s0969-8051(98)00031-6. [DOI] [PubMed] [Google Scholar]
- 18.Chefer SI, London ED, Koren AO, Pavlova OA, Kurian V, Kimes AS, Horti AG, Mukhin AG. Graphical analysis of 2-[18F]FA binding to nicotinic acetylcholine receptors in rhesus monkey brain. Synapse. 2003;48(1):25–34. doi: 10.1002/syn.10180. [DOI] [PubMed] [Google Scholar]
- 19.Deuther-Conrad W, Wevers A, Becker G, Schildan A, Patt M, Sabri O, Steinbach J, Brust P. Autoradiography of 2-[18F]F-A-85380 on nicotinic acetylcholine receptors in the porcine brain in vitro. Synapse. 2006;59(4):201–210. doi: 10.1002/syn.20232. [DOI] [PubMed] [Google Scholar]
- 20.Pavlova OA, Gundisch D, Koren AO, Horti AG, Chefer SI, Kimes AS, Mukhin AG, London ED. Neuroscience 2000 Abstracts. Washington, DC: Society for Neuroscience; 2000. In vitro characterization of 2-[18F]F-A-85380, a novel PET ligand for alpha4beta2 nicotinic receptors [abstract 235.16] [Google Scholar]
- 21.Valette H, Xiao Y, Peyronneau MA, Damont A, Kozikowski AP, Wei ZL, Kassiou M, Kellar KJ, Dollé F, Bottlaender M. 18F-ZW-104: a new radioligand for imaging neuronal nicotinic acetylcholine receptors: in vitro binding properties and PET studies in baboons. J Nucl Med. 2009;50(8):1349–1355. doi: 10.2967/jnumed.108.061374. [DOI] [PubMed] [Google Scholar]
- 22.Lukas RJ, Changeux JP, Le Novère N, Albuquerque EX, Balfour DJ, Berg DK, Bertrand D, Chiappinelli VA, Clarke PB, Collins AC, Dani JA, Grady SR, Kellar KJ, Lindstrom JM, Marks MJ, Quik M, Taylor PW, Wonnacott S. International Union of Pharmacology, XX: current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol Rev. 1999;51(2):397–401. [PubMed] [Google Scholar]
- 23.Wu J, Liu Q, Yu K, Hu J, Kuo YP, Segerberg M, St John PA, Lukas RJ. Roles of nicotinic acetylcholine receptor beta subunits in function of human alpha4-containing nicotinic receptors. J Physiol. 2006;576(pt 1):103–118. doi: 10.1113/jphysiol.2006.114645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, Jou J, Allen V, Tiongson E, Chefer SI, Koren AO, Mukhin AG. Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Arch Gen Psychiatry. 2006;63(8):907–915. doi: 10.1001/archpsyc.63.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brody AL, Mandelkern MA, Costello MR, Abrams AL, Scheibal D, Farahi J, London ED, Olmstead RE, Rose JE, Mukhin AG. Brain nicotinic acetylcholine receptor occupancy: effect of smoking a denicotinized cigarette. Int J Neuropsychopharmacol. 2009;12(3):305–316. doi: 10.1017/S146114570800922X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarvis MJ, Russell MA, Feyerabend C. Absorption of nicotine and carbon monoxide from passive smoking under natural conditions of exposure. Thorax. 1983;38(11):829–833. doi: 10.1136/thx.38.11.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimes AS, Chefer SI, Matochik JA, Contoreggi CS, Vaupel DB, Stein EA, Mukhin AG. Quantification of nicotinic acetylcholine receptors in the human brain with PET: bolus plus infusion administration of 2-[18F]F-A85380. Neuroimage. 2008;39(2):717–727. doi: 10.1016/j.neuroimage.2007.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders–Patient Edition (SCID-I/P), Version 2.0. New York: Biometrics Research Dept, New York State Psychiatric Institute; 1995. [Google Scholar]
- 29.Fagerström KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3(3–4):235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- 30.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton M. Diagnosis and rating of anxiety. Br J Psychiatry. 1969;(3):76–79. special publication. [Google Scholar]
- 33.Carson RE. PET physiological measurements using constant infusion. Nucl Med Biol. 2000;27(7):657–660. doi: 10.1016/s0969-8051(00)00138-4. [DOI] [PubMed] [Google Scholar]
- 34.Dhawan V, Kazumata K, Robeson W, Belakhlef A, Margouleff C, Chaly T, Nakamura T, Dahl R, Margouleff D, Eidelberg D. Quantitative brain PET: comparison of 2D and 3D acquisitions on the GE Advance scanner. Clin Positron Imaging. 1998;1(2):135–144. doi: 10.1016/s1095-0397(98)00009-0. [DOI] [PubMed] [Google Scholar]
- 35.Dolle F, Valette H, Bottlaender M, Hinnen F, Vaufrey F, Guenther I, Crouzel C. Synthesis of 2-[F-18]fluoro-3-[2(S)-2-azetidinylmethoxy]pyridine, a highly potent radioligand for in vivo imaging central nicotinic acetylcholine receptors. J Labelled Comp Radiopharm. 1998;41(5):451–463. doi: 10.1002/(SICI)1099-1344 (199805)41:5<451::AID-JLCR111>3.0.CO;2-R. [DOI] [Google Scholar]
- 36.Shumway DA, Pavlova OA, Mukhin AG. A simplified method for the measurement of nonmetabolized 2-[18F]F-A-85380 in blood plasma using solid-phase extraction. Nucl Med Biol. 2007;34(2):221–228. doi: 10.1016/j.nucmedbio.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Sorger D, Becker GA, Patt M, Schildan A, Grossmann U, Schliebs R, Seese A, Kendziorra K, Kluge M, Brust P, Mukhin AG, Sabri O. Measurement of the alpha4beta2* nicotinic acetylcholine receptor ligand 2-[(18)F]fluoro-A-85380 and its metabolites in human blood during PET investigation: a methodological study. Nucl Med Biol. 2007;34(3):331–342. doi: 10.1016/j.nucmedbio.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Sorger D, Becker GA, Hauber K, Schildan A, Patt M, Birkenmeier G, Otto A, Meyer P, Kluge M, Schliebs R, Sabri O. Binding properties of the cerebral alpha4beta2 nicotinic acetylcholine receptor ligand 2-[18F]fluoro-A-85380 to plasma proteins. Nucl Med Biol. 2006;33(7):899–906. doi: 10.1016/j.nucmedbio.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Jacob P, III, Yu L, Wilson M, Benowitz NL. Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: absence of an isotope effect in the clearance of (S)-nicotine-3′,3′-d2 in humans. Biol Mass Spectrom. 1991;20(5):247–252. doi: 10.1002/bms.1200200503. [DOI] [PubMed] [Google Scholar]
- 40.Willes SR, Fitzgerald TK, Permutt T, Proud D, Haley NJ, Bascom R. Acute respiratory response to prolonged, moderate levels of side stream tobacco smoke. J Toxicol Environ Health A. 1998;53(3):193–209. doi: 10.1080/009841098159330. [DOI] [PubMed] [Google Scholar]
- 41.Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Jr, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59(12):1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- 42.Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schaap PN, Elins JL, Benowitz NL. Nicotine blood levels and subjective craving for cigarettes. Pharmacol Biochem Behav. 2000;66(3):553–558. doi: 10.1016/s0091-3057(00)00261-6. [DOI] [PubMed] [Google Scholar]
- 43.Kimes AS, Horti AG, London ED, Chefer SI, Contoreggi C, Ernst M, Friello P, Koren AO, Kurian V, Matochik JA, Pavlova O, Vaupel DB, Mukhin AG. 2-[18F]F-A-85380: PET imaging of brain nicotinic acetylcholine receptors and whole body distribution in humans. FASEB J. 2003;17(10):1331–1333. doi: 10.1096/fj.02-0492fje. [DOI] [PubMed] [Google Scholar]
- 44.Mukhin AG, Kimes AS, Chefer SI, Matochik JA, Contoreggi CS, Horti AG, Vaupel DB, Pavlova O, Stein EA. Greater nicotinic acetylcholine receptor density in smokers than in nonsmokers: a PET study with 2–18F-FA-85380. J Nucl Med. 2008;49(10):1628–1635. doi: 10.2967/jnumed.108.050716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitkovski S, Villemagne VL, Novakovic KE, O’Keefe G, Tochon-Danguy H, Mulligan RS, Dickinson KL, Saunder T, Gregoire MC, Bottlaender M, Dolle F, Rowe CC. Simplified quantification of nicotinic receptors with 2[18F]F-A-85380 PET. Nucl Med Biol. 2005;32(6):585–591. doi: 10.1016/j.nucmedbio.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 46.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 47.Martin RJ, Okken A, Katona PG, Klaus MH. Effect of lung volume on expiratory time in the newborn infant. J Appl Physiol. 1978;45(1):18–23. doi: 10.1152/jappl.1978.45.1.18. [DOI] [PubMed] [Google Scholar]
- 48.Tobin MJ, Chadha TS, Jenouri G, Birch SJ, Gazeroglu HB, Sackner MA. Breathing patterns, 1: normal subjects. Chest. 1983;84(2):202–205. doi: 10.1378/chest.84.2.202. [DOI] [PubMed] [Google Scholar]
- 49.Bennett WD, Zeman KL. Effect of body size on breathing pattern and fine-particle deposition in children. J Appl Physiol. 2004;97(3):821–826. doi: 10.1152/japplphysiol.01403.2003. [DOI] [PubMed] [Google Scholar]
- 50.Benowitz NL, Porchet H, Jacob PD, Wonnacott S, Russell MAH, Stolerman IP. Pharmacokinetics, Metabolism, and Pharmacodynamics of Nicotine: Nicotine Psychopharmacology: Molecular, Cellular and Behavioral Effects. Oxford, England: Oxford University Press; 1990. [Google Scholar]
- 51.Shephard RJ, Ponsford E, LaBarre R, Basu PK. Effect of cigarette smoke on the eyes and airway. Int Arch Occup Environ Health. 1979;43(2):135–144. doi: 10.1007/BF00378151. [DOI] [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention. Passive smoking: beliefs, attitudes, and exposures: United States, 1986. MMWR Morb Mortal Wkly Rep. 1988;37(15):239–241. [PubMed] [Google Scholar]
- 53.Zhang J, Liu Y, Shi J, Larson DF, Watson RR. Side-stream cigarette smoke induces dose-response in systemic inflammatory cytokine production and oxidative stress. Exp Biol Med (Maywood) 2002;227(9):823–829. doi: 10.1177/153537020222700916. [DOI] [PubMed] [Google Scholar]
- 54.Fidan F, Unlu M, Sezer M, Sahin O, Tokyol C, Esme H. Acute effects of environmental tobacco smoke and dried dung smoke on lung histopathology in rabbits. Pathology. 2006;38(1):53–57. doi: 10.1080/00313020500459615. [DOI] [PubMed] [Google Scholar]
- 55.Benwell ME, Balfour DJK, Anderson JM. Evidence that tobacco smoking increases the density of (-)-[3H]nicotine binding sites in human brain. J Neurochem. 1988;50(4):1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- 56.Mamede M, Ishizu K, Ueda M, Mukai T, Iida Y, Kawashima H, Fukuyama H, Togashi K, Saji H. Temporal change in human nicotinic acetylcholine receptor after smoking cessation: 5IA SPECT study. J Nucl Med. 2007;48(11):1829–1835. doi: 10.2967/jnumed.107.043471. [DOI] [PubMed] [Google Scholar]
- 57.Cosgrove KP, Batis J, Bois F, Maciejewski PK, Esterlis I, Kloczynski T, Stiklus S, Krishnan-Sarin S, O’Malley S, Perry E, Tamagnan G, Seibyl JP, Staley JK. Beta2-nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking. Arch Gen Psychiatry. 2009;66(6):666–676. doi: 10.1001/archgenpsychiatry.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, Leonard S. Effect of smoking history on [3H]nicotine binding in human postmortem brain. J Pharmacol Exp Ther. 1997;282(1):7–13. [PubMed] [Google Scholar]
- 59.Chiamulera C, Borgo C, Falchetto S, Valerio E, Tessari M. Nicotine reinstatement of nicotine self-administration after long-term extinction. Psychopharmacology (Berl) 1996;127(2):102–107. doi: 10.1007/BF02805981. [DOI] [PubMed] [Google Scholar]
- 60.Shaham Y, Adamson LK, Grocki S, Corrigall WA. Reinstatement and spontaneous recovery of nicotine seeking in rats. Psychopharmacology (Berl) 1997;130(4):396–403. doi: 10.1007/s002130050256. [DOI] [PubMed] [Google Scholar]
- 61.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum & Associates; 1988. [Google Scholar]
- 62.Lenth RV. [Accessed January 5, 2011.];Java Applets for Power and Sample Size [computer software] http://www.stat.uiowa.edu/~rlenth/Power.
- 63.Toxic Substances Control Program, Technical Services Branch, California Department of Health Services. . Scientific and Technical Standards for Hazardous Waste Sites. Sacramento: California Dept of Health Services; 1990. [Google Scholar]
- 64.Succop PA, Clark S, Chen M, Galke W. Imputation of data values that are less than a detection limit. J Occup Environ Hyg. 2004;1(7):436–441. doi: 10.1080/15459620490462797. [DOI] [PubMed] [Google Scholar]
- 65.American Nonsmokers’ Rights Foundation. [Accessed March 25, 2011.];Summary of 100% smokefree state laws and population protected by 100% US smokefree laws. http://www.no-smoke.org.
- 66.American Nonsmokers’ Rights Foundation. [Accessed March 25, 2011.];Smokefree cars. http://www.no-smoke.org.
- 67.Passive smoking is a major health hazard to children, says the RCP [press release] London, England: Tobacco Advisory Group, Royal College of Physicians; Mar 24, 2010. [Google Scholar]
- 68.Tapp D, Thomson G. Smokefree cars in New Zealand: rapid research among stakeholders on attitudes and future directions. N Z Med J. 2009;122(1303):54–66. [PubMed] [Google Scholar]
- 69.Mantziou V, Vardavas CI, Kletsiou E, Priftis KN. Predictors of childhood exposure to parental secondhand smoke in the house and family car. Int J Environ Res Public Health. 2009;6(2):433–444. doi: 10.3390/ijerph6020433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leatherdale ST, Smith P, Ahmed R. Youth exposure to smoking in the home and in cars: how often does it happen and what do youth think about it? Tob Control. 2008;17(2):86–92. doi: 10.1136/tc.2007.022475. [DOI] [PubMed] [Google Scholar]