Abstract
Small-cell lung cancer (SCLC) is an aggressive neuroendocrine subtype of lung cancer for which there is no effective treatment1,2. Using a mouse model in which deletion of Rb1 and Trp53 in the lung epithelium of adult mice induces SCLC3,4, we found that the Hedgehog signaling pathway is activated in SCLC cells independently of the lung microenvironment. Constitutive activation of the Hedgehog signaling molecule Smoothened (Smo) promoted the clonogenicity of human SCLC in vitro and the initiation and progression of mouse SCLC in vivo. Reciprocally, deletion of Smo in Rb1 and Trp53-mutant lung epithelial cells strongly suppressed SCLC initiation and progression in mice. Furthermore, pharmacological blockade of Hedgehog signaling inhibited the growth of mouse and human SCLC, most notably following chemotherapy. These findings show a crucial cell-intrinsic role for Hedgehog signaling in the development and maintenance of SCLC and identify Hedgehog pathway inhibition as a therapeutic strategy to slow the progression of disease and delay cancer recurrence in individuals with SCLC.
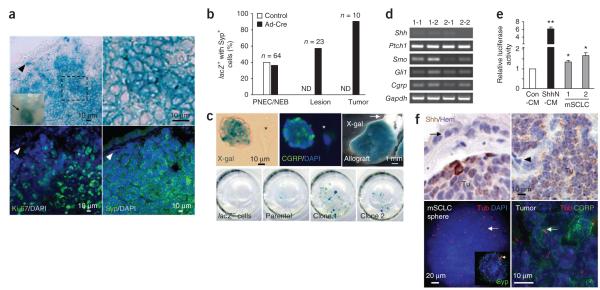
Activation of Hedgehog signaling has been reported in a subset of human SCLC cell lines and tumors5–8 without changes in Hedgehog pathway gene copy numbers9. Furthermore, we sequenced exons from 16 Hedgehog pathway genes in human SCLC and found no evidence for recurrent mutations (Supplementary Table 1). These observations raised questions about the potential roles of Hedgehog signaling in SCLC development as well as the relative contributions of cell-autonomous and non–cell-autonomous Hedgehog activity10. To investigate the Hedgehog pathway in SCLC in vivo, we first crossed mice carrying conditional alleles of Rb1 and Trp53 to Ptch1lacZ/+ (Ptch1 is also known as Ptc1) mice in which LacZ activity parallels Hedgehog pathway activation11. X-gal staining revealed that the vast majority of mouse SCLC (mSCLC) stained positive for lacZ activity in vivo 9–12 months after exposure to adenovirus expressing Cre (Ad-Cre) (Fig. 1a). The X-gal staining intensity was similar to that found in the cerebellum of Ptch1lacZ/+ mice (Supplementary Fig. 1a,b). We found no recurrent copy number changes in Hedgehog pathway genes in mouse tumors (Supplementary Table 2), which argues against mutations directly activating Hedgehog signaling in this model. Non-SCLC mouse lung tumors induced by oncogenic Kras12 were largely negative for LacZ activity (Supplementary Fig. 1c,d). A subset of stromal cells underlying the bronchiolar epithelium also stained positive for X-gal, both in wild-type and Rb1- and Trp53-mutant mice, indicating that this result was not caused by tumor-suppressor loss (Fig. 1a, Supplementary Fig. 2 and data not shown). We found reporter activity in ~60% of the small lesions and weak X-gal staining in ~35–40% of neuroendocrine cells, which are considered candidates for the cell of origin of SCLC13,14 (Fig. 1b and Supplementary Fig. 2a,b). Otherwise, only subsets of tracheal epithelial cells stained positive for LacZ activity (Supplementary Fig. 2c).
Figure 1.
Cell-autonomous activation of the Hedgehog pathway in mouse SCLC. (a) X-gal staining and immunostaining for Ki-67 and the neuroendocrine marker synaptophysin (Syp) of lung tumors from Rb1lox/lox; Trp53lox/lox; Ptch1lacZ/+ mice infected with Ad-Cre. Arrowheads point to a LacZ+Ki-67−Syp− stromal cell. (b) Quantification of X-gal staining in Syp+ cells, lesions and tumors. ND, not detected. 20 μm 10 μm (c) Shown at the top left and in the center are X-gal staining and immunostaining for the neuroendocrine Syp marker CGRP of a mouse SCLC sphere in culture. The asterisk indicates a stromal cell. Shown at the top right is an X-gal–stained subcutaneous allograft derived from Rb1-Trp53 mutant Ptch1lacZ/+ tumor cells (the arrow indicates the skin of the recipient mouse). Shown at the bottom is an X-gal staining of representative clones (1 and 2) derived from parental Rb1-Trp53–mutant Ptch1lacZ/+ SCLC cells. (d) RT-PCR analysis for Shh, Ptch1, Smo and Gli1 in two subclones (1 and 2) each from two independent parental SCLC cell lines (1 and 2). We used Gapdh as a loading control. (e) Luciferase activity in Shh-LIGHT2 reporter cells co-cultured with mouse SCLC cells (n ≥ 3). We used conditioned media from either 293 cells (Con-CM) or 293 cells secreting active Sonic hedgehog (ShhN-CM) as controls. Data are relative to Con-CM values. (f) Shown at the top is Sonic hedgehog (Shh) immunostaining (brown) on sections of mouse SCLC (Tu); the arrow indicates normal airway epithelial cells, the arrowhead indicates tumor-associated stromal cells and the counterstain used was hematoxylin (Hem). At the bottom, immunostaining for polyglutamylated tubulin (Tub, red) marks the primary cilium in a SCLC sphere (left), a single cell (inset) and a primary tumor (right). PNEC/NEB, pulmonary neuroendocrine cells including neuroepithelial bodies. Mean ± s.e.m. are shown. *P < 0.01, **P < 0.001.
Rb1-Trp53-Ptch1lacZ/+ tumor cells expressed lacZ in culture (Fig. 1c) and in allografts (Fig. 1c), and seven of eight single-cell subclonal cultures derived from Rb1-Trp53-Ptch1lacZ/+ tumors retained LacZ activity (Fig. 1c and data not shown). These subclones expressed components of the Hedgehog pathway (Fig. 1d and Supplementary Fig. 3a,b). Thus, Rb1-Trp53 mutant mSCLC cells maintain Hedgehog activity cell autonomously and independently of the lung cellular microenvironment. Shh-LIGHT2 reporter cells, in which the luciferase reporter is induced when the Hedgehog pathway is active15, were cultured with conditioned medium from mSCLC cells but showed no induction of reporter activity (data not shown). However, culture of the reporter cells with the mSCLC cells resulted in mild luciferase induction (Fig. 1e), suggesting active Hedgehog ligands that may be retained in close proximity to the producing cells. Accordingly, immunohistochemistry analysis showed that mSCLC cells expressed Hedgehog ligands in vivo (Fig. 1f). Appropriate Hedgehog signaling depends on a functional primary cilium16,17. We found that ~12% of mSCLC spheres in culture and subsets of neuroendocrine tumor cells in vivo (Fig. 1f) had a primary cilium. Moreover, addition of conditioned medium containing active Sonic hedgehog to mSCLC cells grown in low serum enhanced their survival and increased expression of the Hedgehog pathway member and target Gli1 (Fig. 2a,b). Together, these data suggest that the Hedgehog pathway is active in mSCLC cells through an autocrine-juxtacrine loop and that one function of the pathway is to enhance survival.
Figure 2.
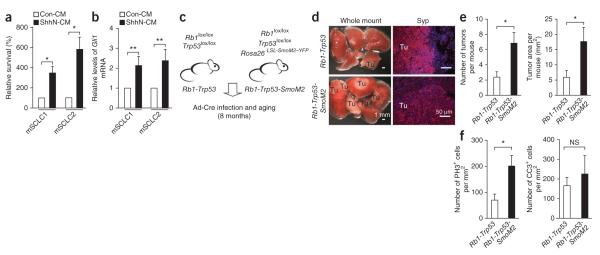
Constitutive Hedgehog signaling is sufficient to promote SCLC in mice. (a) Cell viability for two mouse SCLC cell lines (mSCLC1 and mSCLC2) treated with conditioned media from 293 cells (Con-CM) or 293 cells secreting active N-terminal Sonic hedgehog (ShhN-CM) for 4 days (n ≥ 3). (b) Quantitative RT-PCR analysis for Gli1 levels after 24 h of treatment (n ≥ 3). (c) Strategy to constitutively activate Smo (SmoM2) in Rb1-Trp53 mutant lung cells. (d) Whole-mount images of tumors (Tu) and immunostaining for synaptophysin (Syp) (red) counterstained with DAPI (blue). (e) We quantified tumor number and area in mice from both genotypes (n = 8 for Rb1-Trp53 and n = 9 for Rb1-Trp53-SmoM2 mice). (f) Quantification of cell proliferation and cell death by immunostaining for phospho histone 3 (PH3) and cleaved caspase 3 (CC3) in tumors. Mean ± s.e.m. are shown. NS, not significant. *P < 0.01, **P < 0.001.
We next crossed Rb1-Trp53 conditional mutant mice to Rosa26+/LSL-SmoM2–YFP (Rosa26 is also known as Gt(ROSA)26Sor) mice carrying a conditional, constitutively active mutant allele of Smo (SmoM2) fused to YFP (also known as Tg(Thy1-YFP)16Jrs)18 (Fig. 2c). We aged cohorts of Ad-Cre–infected Rb1lox/lox; Trp53lox/lox; Rosa26+/LSL-SmoM2–YFP (Rb1-Trp53-SmoM2) and Rb1lox/lox; Trp53lox/lox (Rb1-Trp53) mice for 8 months. All Rb1-Trp53-SmoM2 tumors analyzed expressed YFP, and analysis of Gli1 mRNA levels was indicative of a physiological activation of the Hedgehog pathway in these tumors (Supplementary Fig. 4). Rb1-Trp53-SmoM2 mice developed more mSCLCs than did their Rb1-Trp53 littermates (these mSCLCs were also associated with a greater tumor volume and higher mitotic index) but had comparable apoptotic cell death levels (Fig. 2d–f and Supplementary Fig. 5). We also determined that Hedgehog pathway activation could not replace loss of Rb1 or Trp53 using Rb1+/lox; Trp53lox/lox; Rosa26+/LSL-SmoM2–YFP or Rb1lox/lox; Trp53+/lox; Rosa26+/LSL-SmoM–YFP mice because one wild-type Trp53 allele was sufficient to prevent tumor development for up to 8–9 months after Ad-Cre exposure (data not shown), whereas retention of a wild-type Rb1 allele produced features of lung adeno-carcinoma but not SCLC (Supplementary Fig. 6 and data not shown). The inability of SmoM2 alone to initiate tumors in lung epithelium may be because of its weak activity and/or the ability of Trp53 to normally restrict full Hedgehog signaling activation19.
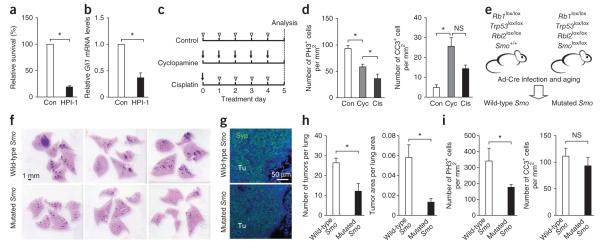
In determining whether Hedgehog signaling was required for the expansion of SCLC tumor cells, we found that treatment with cyclopamine, a Smo inhibitor20, decreased the survival of SCLC cells in low serum and also decreased Gli1 mRNA levels; a structural analog of cyclopamine, tomatidine, which does not inhibit Smo function, had minimal effects (Supplementary Fig. 7a,b). To rule out nonspecific activities of cyclopamine10, we treated mSCLC cell lines with HPI-1 (ref. 21) and GANT-61 (ref. 22), two inhibitors of Gli proteins; this treatment reduced Gli1 levels and cell survival compared to vehicle treatment (Fig. 3a,b and Supplementary Fig. 7c,d). We observed similar effects with the Smo inhibitor NVP-LDE225 (refs. 23,24) (Supplementary Fig. 7e). We observed decreased proliferation and increased apoptosis in mSCLC tumors that we treated short term with cyclopamine in vivo (Fig. 3c,d and Supplementary Fig. 7f); these levels were comparable to those seen with cisplatin, a platinum-based drug used to treat individuals with SCLC2 (Fig. 3d). vasculature marker PECAM-1 was similar in vehicle- and cyclopamine-treated tumors (data not shown). These observations suggest that the Hedgehog pathway is required for the maintenance of SCLC.
Figure 3.
Hedgehog pathway activity is necessary for the growth of mouse SCLC cells. (a,b) Cell viability (after 4 d) (a) and RT-quantitative PCR analysis of Gli1 levels (after 24 h) (b) for three independent SCLC cell lines treated with HPI-1 (10 μM) or vehicle control (Con) (n = 3). (c) The treatment protocol of mice having tumors with vehicle (n = 4 mice), cyclopamine (Cyc) (n = 4) or cisplatin (Cis) (n = 2). The arrow indicates the experimental treatment, and the arrowhead indicates the vehicle. (d) Quantification of PH3+ and CC3+ cells on tumor sections. (e) The experimental strategy to test the effects of deleting Smo in Rb1-Trp53-Rbl2 mutant lung cells. (f) Lung sections from mice infected with Ad-Cre and aged for 6 months before analysis. The counterstain is H&E, and the tumors appear dark purple. (g) Synaptophysin (Syp) immunostaining on tumors (Tu) counterstained with DAPI (blue). (h) Tumor numbers and tumor area in mutant mice (n = 6 for Rb1-Trp53-Rbl2 mice with wild-type Smo and n = 3 for Rb1-Trp53-Rbl2 mice with mutated Smo). (i) Quantification of cell proliferation and apoptotic cell death by immunostaining for phospho histone 3 (PH3) and cleaved caspase 3 (CC3) in tumors. Mean ± s.e.m. are shown. NS, not significant. *P < 0.01.
To further test whether inhibition of Hedgehog signaling intrinsically suppresses SCLC development, we used a mouse model in which loss of Rbl2 accelerates SCLC development4. We analyzed cohorts of Rb1lox/lox; Trp53lox/lox; Rbl2lox/lox; Smo+/+, Rb1lox/lox; Trp53lox/lox; Rbl2lox/lox; Smo+/lox and Rb1lox/lox; Trp53lox/lox; Rbl2lox/lox; Smolox/lox mice 6 months after Ad-Cre exposure (Fig. 3e). Rb1-Trp53-Rbl2 triple-knockout mice with mutated Smo developed fewer and smaller tumors than did their littermates that had wild-type Smo and those that were Staining for the vasculature marker PECAM-1 was similar in vehicle- and cyclopamine-treated tumors (data not shown). These observations suggest that the Hedgehog pathway is required for the maintenance of SCLC.
To further test whether inhibition of Hedgehog signaling intrinsically suppresses SCLC development, we used a mouse model in which loss of Rbl2 accelerates SCLC development4. We analyzed cohorts of Rb1lox/lox; Trp53lox/lox; Rbl2lox/lox; Smo+/+, Rb1lox/lox; Trp53lox/lox; Rbl2lox/lox; Smo+/lox and Rb1lox/lox; Trp53lox/lox; Rbl2lox/lox; Smolox/lox mice 6 months after Ad-Cre exposure (Fig. 3e). Rb1-Trp53-Rbl2 triple-knockout mice with mutated Smo developed fewer and smaller tumors than did their littermates that had wild-type Smo and those that were heterozygous for Smo (Fig. 3f–h and data not shown). Histopathological analysis confirmed that all tumors had features of SCLC (Fig. 3g and data not shown). This decrease in tumor number was associated with a lower mitotic index but not with a change in cell death levels at this time point (Fig. 3i). Thus, the Hedgehog pathway contributes to the maintenance of mSCLC tumors and participates in their initial development in vivo.
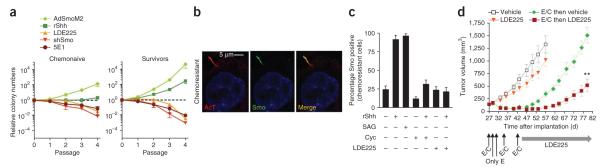
A major issue in the management of individuals with SCLC is disease recurrence following chemotherapy. We tested the possibility that signaling downstream of Smo has a role in this process by manipulating the activity of the Hedgehog pathway in suspension cultures of human LX22CL cells (derived from the primary xenograft line LX22)25. Inhibition of Hedgehog signaling resulted in fewer colonies, whereas activation of Smo signaling increased the cells’ long-term ability to grow in a clonogenic assay26 (Fig. 4a). LX22CL cells surviving a single carboplatin and etoposide treatment showed an increased sensitivity to manipulation of ligand-dependent Smo signaling in the same assay (Fig. 4a). In contrast to what we observed with mSCLC cells, only a small fraction of human chemonaive SCLC cells (<1%) expressed a primary cilium (data not shown). However, three rounds of treatment with carboplatin and etoposide produced a strong increase in the number of LX22CL cells with a primary cilium (~20%). We were able to modify Smo localization in the primary cilia of these cells by manipulating the Hedgehog pathway (Fig. 4b,c). Furthermore, LX22 primary SCLC xenografts had a marked increase in the expression of Sonic hedgehog and nuclear Gli2 following chemotherapy (Supplementary Fig. 8a,b). Although we did not detect cilia in chemo-naive xenografts (data not shown), approximately 10% of chemo-resistant LX22 tumor cells had primary cilia (Supplementary Fig. 8c). Thus, the increased sensitivity of chemoresistant SCLC cells to the inhibition of Smo signaling is directly correlated with Smo localization in the primary cilia of these cells, which is a sign of activation of the Hedgehog pathway.
Figure 4.
Hedgehog signaling is crucial for the growth of chemoresistant human SCLC cells. (a) Colony formation in chemonaive and chemosurviving LX22CL cells assessed by serial passage in methycellulose (n = 4). The clonal capacity following each treatment is shown relative to its respective control, to which we assigned a value of 1. Treatments and matching controls were as follows: infection with adenovirally expressed SmoM2 (AdSmoM2) or adenovirally expressed βGal (AdβGal) (green diamond); 0.2 μg ml−1 of recombinant human Sonic hedgehog or vehicle (green square); 100 nM NVPLDE225 or vehicle (orange triangle); transfection with a vector expressing shRNA molecules targeting human Smo or control shRNA (red triangle); or 5 μg ml−1 of the Hedgehog-neutralizing monoclonal antibody 5E1 or mouse IgG1 (brown circle). (b) Smo localization (green) in the primary cilia (AcT, red) of chemoresistant LX22CL cells counterstained with DAPI. (c) Smo expression in the primary cilia of chemoresistant LX22CL cells treated in vitro with recombinant Sonic hedgehog (rShh) (1 mg ml–1), the Smo agonist SAG (200 nM), cyclopamine (Cyc, 3 μM) or NVP-LDE225 200 nM (n = 3). (d) We treated nude mice subcutaneously implanted with LX22 tumors with vehicle (control, white square), NVP-LDE225 (80 mg per kg per day once a day, orange triangle), etoposide (12 mg per kg per day intraperitoneally on days 1, 2, 3 and 15 after the start of treatment) and carboplatin (E/C) (60 mg per kg per day intravenously on days 1, 8 and 15 after the start of treatment) alone (green diamond) or followed by NVP-LDE225 (red square) as indicated in the figure. The tumor volume (n = 8, two independent experiments) is shown. Mean ± s.e.m. are shown. **P < 0.001 compared to E/C then vehicle.
To test the idea that chemotherapy and Smo inhibition might cooperate, we treated mice bearing LX22 xenografts with carboplatin and etoposide chemotherapy followed by oral administration of NVPLDE225. NVP-LDE225 monotherapy in chemonaive tumors had little effect on tumor growth. Notably, however, NVP-LDE225 treatment was highly effective in preventing the recurrence of residual tumors following chemotherapy (Fig. 4d). The histopathology of these xenografts following treatment was unchanged (Supplementary Fig. 8d).
Direct mutations affecting key regulators of the Hedgehog signaling pathway are potent drivers of tumors8,27 that are sensitive to pharmacological inhibition of the Hedgehog pathway28,29. SCLC cells do not carry recurrent mutations in Hedgehog pathway genes but are nevertheless sensitive to Hedgehog pathway inhibition, largely in a cell-autonomous manner. Such a mode of action for Hedgehog signaling may be relevant to several cancer types30–37 (Supplementary Discussion). Our study also reveals a previously unsuspected role for the Hedgehog pathway during SCLC initiation in addition to its role in the maintenance of tumors, suggesting that both early and advanced SCLC lesions may be responsive to Hedgehog pathway inhibitors. Future experiments should investigate the exact mode of action of Hedgehog molecules on SCLC cells, including juxtacrine and autocrine mechanisms. The cell-intrinsic activation of Hedgehog signaling in SCLC raises the possibility that metastasis of SCLC cells is largely independent of their microenvironment34. Finally, our data indicate that treatment of individuals with SCLC with Hedgehog pathway inhibitors may cooperate with chemotherapy and/or radiation therapy regimens to inhibit the growth of primary and metastatic SCLC and to reduce tumor recurrence in affected individuals.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/naturemedicine/.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank A. Berns (The Netherlands Cancer Institute) and M. Scott (Stanford University) for the Trp53lox and Ptch1lacZ/+ mice, respectively, J. Whitsett (Cincinnati Children’s Hospital) for the antibodies to surfactant protein C, C. Janke (Orsay Curie Institute) for antibodies to polyglutamylated tubulin and C.-M. Fan (Carnegie Institution of Washington) for the adenovirally expressed SmoM2 virus, as well as T. Oro, James Kim, Jynho Kim and P. Beachy for helpful discussions throughout the course of this study. We thank R. Toftgård (Karolinska Institute) and K. McGovern at Infinity Pharmaceuticals for their generous gift of the GANT-61 and cyclopamine, respectively, R. Rohatgi for help with the immunoblot analysis and B. Schaffer for help with the cell culture. This work was supported by the Lucile Packard Foundation for Children’s Health (J.S.), the Damon Runyon Cancer Research Foundation (J.S.), the American Lung Association (J.S. and K.-S.P.), the Francis Family Foundation (K.-S.P.), the American Cancer Society (J.S.), the Tobacco-Related Disease Research Program of California (J.F.C.), US National Institutes of Health (NIH) National Cancer Institute R01 CA136574 (J.K.C.), the Flight Attendant Medical Research Institute (YCSA 072033) (C.D.P.), NIH 5T32 CA009302-33 (C.O.), the National Health and Medical Research Council of Australia project grants 546024 and 546098 and the Victorian Cancer Agency (D.N.W., L.G.M. and A.S.), the Deutsche Krebshilfe (107954) (R.K.T.), the German Ministry of Science and Education as part of the Nationales Genomforschungsnetz (NGFN-plus) program (01GS08100) (R.K.T.), the Max Planck Society (MIFA NEUR8061) (R.K.T.), the Deutsche Forschungsgemeinschaft (DFG) through Sonderforschungsbereiche (TP6) (R.K.T.), the Ministry for Innovation, Science, Research and Technology of the State of Nordrhein-Westfalen (MIWT, 4000-12 09) (R.K.T.) and by an anonymous foundation to R.K.T.
Footnotes
Note: Supplementary information is available on the Nature Medicine website.
AUTHOR CONTRIBUTIONS A.N.K. analyzed the histopathology of all mouse lung tumors and edited the manuscript. A.S. performed and analyzed the immunohistochemistry. D.N.W. and L.G.M. edited the manuscript and analyzed the immunohistochemistry. L.G.M. performed experiments with Smo inhibitors and chemotherapy in culture and in xenografts. C.A.O. and J.K.C. generated HPI-1 for cell culture experiments. J.K.C. edited the manuscript. M.R.M. and T.S. designed, performed and analyzed the experiments related to the primary cilia in mouse cells. M.P., M.L.S. and R.K.T. designed and performed the experiments related to the genomic analysis of mousec and human tumors. K.-S.P. and K.B. quantified the proliferation and survival phenotypes in tumors treated with cyclopamine. K.-S.P. and J.F.C. analyzed gene and protein expression levels in tumor cells. K.-S.P. performed all the other experiments involving mouse cells. K.-S.P. and J.S. designed the experiments for the analysis of mouse SCLC cells in culture and in vivo, and generated the corresponding figures. C.D.P. designed and analyzed the research performed by A.M. and W.L.D. on the human SCLC cells in vitro. J.F., S.Buonamici, S. Bennett, J.Y., R.G., B.O., M.D., A.M., W.L.D. and T.J.B. designed and performed in vivo xenograft experiments and analyzed the data. K.-S.P., J.S., C.D.P. and S.B. wrote and edited the manuscript.
COMPETING FINANCIAL INTERESTS The authors declare competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/naturemedicine/.
References
- 1.Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2008;113:5–21. doi: 10.1002/cncr.23542. [DOI] [PubMed] [Google Scholar]
- 2.Rudin CM, Hann CL, Peacock CD, Watkins DN. Novel systemic therapies for small cell lung cancer. J. Natl. Compr. Canc. Netw. 2008;6:315–322. doi: 10.6004/jnccn.2008.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meuwissen R, et al. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell. 2003;4:181–189. doi: 10.1016/s1535-6108(03)00220-4. [DOI] [PubMed] [Google Scholar]
- 4.Schaffer BE, et al. Loss of p130 accelerates tumor development in a mouse model for human small-cell lung carcinoma. Cancer Res. 2010;70:3877–3883. doi: 10.1158/0008-5472.CAN-09-4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vestergaard J, et al. Hedgehog signaling in small-cell lung cancer: frequent in vivo but a rare event in vitro. Lung Cancer. 2006;52:281–290. doi: 10.1016/j.lungcan.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Chi S, et al. Activation of the hedgehog pathway in a subset of lung cancers. Cancer Lett. 2006;244:53–60. doi: 10.1016/j.canlet.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 7.Watkins DN, et al. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313–317. doi: 10.1038/nature01493. [DOI] [PubMed] [Google Scholar]
- 8.Teglund S, Toftgard R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim. Biophys. Acta. 2010;1805:181–208. doi: 10.1016/j.bbcan.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Voortman J, et al. Array comparative genomic hybridization-based characterization of genetic alterations in pulmonary neuroendocrine tumors. Proc. Natl. Acad. Sci. USA. 2010;107:13040–13045. doi: 10.1073/pnas.1008132107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yauch RL, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 11.Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 12.Jackson EL, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutherland KD, et al. Cell of origin of small cell lung cancer: inactivation of Trp53 and rb1 in distinct cell types of adult mouse lung. Cancer Cell. 2011;19:754–764. doi: 10.1016/j.ccr.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Park KS, et al. Characterization of the cell of origin for small cell lung cancer. Cell Cycle. 2011;10:2806–2815. doi: 10.4161/cc.10.16.17012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taipale J, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 16.Milenkovic L, Scott MP, Rohatgi R. Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. J. Cell Biol. 2009;187:365–374. doi: 10.1083/jcb.200907126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J, Kato M, Beachy PA. Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proc. Natl. Acad. Sci. USA. 2009;106:21666–21671. doi: 10.1073/pnas.0912180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao J, et al. A novel somatic mouse model to survey tumorigenic potential applied to the Hedgehog pathway. Cancer Res. 2006;66:10171–10178. doi: 10.1158/0008-5472.CAN-06-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stecca B, Ruiz i Altaba AA. GLI1-p53 inhibitory loop controls neural stem cell and tumour cell numbers. EMBO J. 2009;28:663–676. doi: 10.1038/emboj.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper MK, Porter JA, Young KE, Beachy PA. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science. 1998;280:1603–1607. doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- 21.Hyman JM, et al. Small-molecule inhibitors reveal multiple strategies for Hedgehog pathway blockade. Proc. Natl. Acad. Sci. USA. 2009;106:14132–14137. doi: 10.1073/pnas.0907134106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lauth M, Bergstrom A, Shimokawa T, Toftgard R. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc. Natl. Acad. Sci. USA. 2007;104:8455–8460. doi: 10.1073/pnas.0609699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buonamici S, et al. Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci. Transl. Med. 2010;2:51ra70. doi: 10.1126/scitranslmed.3001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan S, et al. Discovery of NVP-LDE225, a potent and selective Smoothened antagonist. ACS Med Chem Lett. 2010;1:130–134. doi: 10.1021/ml1000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniel VC, et al. A primary xenograft model of small-cell lung cancer reveals irreversible changes in gene expression imposed by culture in vitro. Cancer Res. 2009;69:3364–3373. doi: 10.1158/0008-5472.CAN-08-4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peacock CD, et al. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc. Natl. Acad. Sci. USA. 2007;104:4048–4053. doi: 10.1073/pnas.0611682104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scales SJ, de Sauvage FJ. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol. Sci. 2009;30:303–312. doi: 10.1016/j.tips.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Berman DM, et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297:1559–1561. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]
- 29.Rudin CM, et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N. Engl. J. Med. 2009;361:1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez P, et al. Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proc. Natl. Acad. Sci. USA. 2004;101:12561–12566. doi: 10.1073/pnas.0404956101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stecca B, et al. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc. Natl. Acad. Sci. USA. 2007;104:5895–5900. doi: 10.1073/pnas.0700776104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr. Biol. 2007;17:165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varnat F, et al. Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Mol. Med. 2009;1:338–351. doi: 10.1002/emmm.200900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arimura S, et al. Reduced level of smoothened suppresses intestinal tumorigenesis by down-regulation of Wnt signaling. Gastroenterology. 2009;137:629–638. doi: 10.1053/j.gastro.2009.04.059. [DOI] [PubMed] [Google Scholar]
- 35.Thayer SP, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan Z, et al. Frequent requirement of hedgehog signaling in non-small cell lung carcinoma. Oncogene. 2007;26:1046–1055. doi: 10.1038/sj.onc.1209860. [DOI] [PubMed] [Google Scholar]
- 37.Singh S, et al. Hedgehog-producing cancer cells respond to and require autocrine Hedgehog activity. Cancer Res. 2011;71:4454–4463. doi: 10.1158/0008-5472.CAN-10-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.