Abstract
OBJECTIVE
The Fetal Inflammatory Response Syndrome (FIRS) is associated with the impending onset of preterm labor/delivery, microbial invasion of the amniotic cavity and increased perinatal morbidity. FIRS has been defined by an elevated fetal plasma interleukin-6 (IL-6), a cytokine with potent effects on the differentiation and proliferation of hematopoietic precursors. The objective of this study was to characterize the hematologic response of fetuses with FIRS.
STUDY DESIGN
Fetal blood sampling was performed in patients with preterm prelabor rupture of membranes and preterm labor with intact membranes (n=152). A fetal plasma IL-6 concentration >=11 pg/ml was used to define FIRS. Hemoglobin concentration, platelet count, total white blood cell (WBC) count, differential count and nucleated red blood cell (NRBC) count were obtained. Since blood cell count varies with gestational age, the observed values were corrected for fetal age by calculating a ratio between the observed and expected mean value for gestational age.
RESULTS
1) The prevalence of FIRS was 28.9% (44/152); 2) fetuses with FIRS had a higher median corrected WBC and corrected neutrophil count than those without FIRS (WBC median 1.4; range 0.3–5.6 vs. median 1.1; range 0.4–2.9 p=0.001; neutrophils median 3.6; range 0.1–57.5 vs. median 1.8; range 0.2–13.9 p<0.001); 3) neutrophilia (defined as a neutrophil count >95th centile of gestational age) was significantly more common in fetuses with FIRS than in those without FIRS [71% (30/42) vs. 35% (37/105); p<0 001]; 4) more than two thirds of fetuses with FIRS had neutrophilia, while neutropenia was present in only 4.8% (2/42); 5) FIRS was not associated with detectable changes in hemoglobin concentration, platelet, lymphocyte, monocyte, basophil or eosinophil counts and; 6) fetuses with FIRS had a median corrected NRBC count higher than those without FIRS. However, the difference did not reach statistical significance (NRBC median 0.07; range 0–1.3 vs. median 0.04; range 0–2.3 p=0.06);
CONCLUSION
The hematological response of the human fetus with FIRS is characterized by significant changes in the total white blood cell and neutrophil counts. The NRBC count in fetuses with FIRS tends to be higher than fetuses without FIRS.
Keywords: FIRS, preterm labor, preterm PROM, cordocentesis, neutrophil, nucleated red blood cells, infection, hypoxia, neutrophilia, neutropenia, white blood cell count
INTRODUCTION
The fetal inflammatory response syndrome (FIRS) was originally described in patients with spontaneous preterm labor and preterm prelabor rupture of membranes (PROM) and defined by fetal plasma interleukin (IL)-6 concentrations ≥11 pg/mL [1,2]. FIRS is also associated with an increase in fetal plasma concentrations of soluble tumor necrosis factor receptors-1 and 2, IL-1β, IL-8 and C-reactive protein [3–10]. FIRS is associated with the impending onset of preterm labor/delivery, microbial invasion of the amniotic cavity and increased perinatal morbidity after adjustment for gestational age [11–24].
A solid body of evidence supports the view that intra-amniotic infection/inflammation plays a major role in the pathophysiology of FIRS [15,25–31]. Fetuses with FIRS had a higher rate of severe neonatal morbidity (e.g. respiratory distress syndrome [1], suspected or proven neonatal sepsis [1,15], pneumonia [1], bronchopulmonary dysplasia [13,16,21,32,33], intraventricular hemorrhage [1], periventricular leukomalacia [12] and cerebral palsy [12,14,18,34–38], and necrotizing enterocolitis [1,9]. FIRS is considered the fetal counterpart of the systemic inflammatory response syndrome (SIRS) seen in adults [39,40]. Intra-amniotic infection/inflammation, which is characterized by changes in amniotic fluid concentrations of several proinflammatory cytokines [41–48], anti-inflammatory cytokines [49], chemokines [50–56], caspase-1 (a component of an inflammasome) [57], anti-microbial peptides [58–60], hemostatic factors [61,62], complement activation products [63,64], soluble pattern recognition receptor (pantraxin) [65], damage-associated molecular patterns and their receptors [66–70], protease-anti-protease [71], adipocytokines [72–75], surfactant proteins [76], angiogenic factors [77,78] and matrix degrading enzymes [25,27,28,79–87] is, therefore, a strong risk factor for short- and long-term neonatal complications [15,36,88–95].
Changes in hematologic parameters have been observed in patients with SIRS [96–101]. Indeed, either leukocytosis (white blood cell (WBC) count >12,000/µL) or leukopenia (WBC <4,000/µL) is one of the criteria for the diagnosis of SIRS. Either leukocytosis or leukopenia is associated with a poor outcome [102–104]. In adults, an increased total WBC or neutrophil count is an indicator of subclinical inflammation [103–107]. In neonates, neutrophilia is associated with respiratory distress syndrome [108–111], early-onset neonatal sepsis [112,113] and cerebral injury [95,114,115]. Similarly, an elevated nucleated red blood cell (NRBC) count has been observed in newborns with low umbilical arterial pH [116–118], intrauterine growth restriction [116,119–121], early-onset neonatal seizures [122–124], cerebral white matter injury [125–127] and infants who develop cerebral palsy [128,129]. Indeed, an abnormal NRBC count at birth is associated more strongly with short-term neonatal outcome (requirement of mechanical ventilation, need for vasopressure agents or neonatal mortality) than birth weight or gestational age regardless of being small or appropriate for gestational age [119].
An increased NRBC count has been associated with chronic intrauterine hypoxia or stress [130–138], frequently seen in fetuses of mothers with preeclampsia [121,139–141], intrauterine growth restriction [116,119–121] or diabetes mellitus [142–144]. However, an experimental study indicates that the administration of recombinant IL-6 to animals can stimulate the erythroid progenitor cells in the bone marrow and eventually lead to the release of NRBC into circulation, suggesting that inflammation can trigger systemic elevation of NRBC count [145]. Similarly, in patients with preterm labor and preterm PROM, histologic chorioamnionitis or severe acute placental inflammation are associated with an increased neonatal NRBC count [129,146,147], suggesting that intrauterine infection may modulate hematopoiesis in neonates and/or fetuses.
The objective of this study was to determine if systemic inflammation in fetuses with FIRS is associated with a fetal hematologic response.
PATIENTS AND METHODS
Patients and eligibility
This retrospective cross-sectional study included women who were admitted at Hutzel Hospital with preterm labor and intact membranes or with preterm PROM between March 1992 and March 1996. They were offered amniocentesis for the diagnosis of MIAC and assessment of fetal lung maturity. Patients who consented to have an amniocentesis were asked to participate in a research management protocol that included the collection of additional amniotic fluid for research purposes and cordocentesis. Criteria for eligibility into this study included (1) preterm labor with intact membranes or with preterm PROM, (2) written informed consent to have amniocentesis and cordocentesis, and (3) availability of skilled medical staff to perform amniocentesis and cordocentesis. Exclusion criteria were: (1) clinical chorioamnionitis, (2) multiple gestation, (3) fetal distress, or (4) significant vaginal bleeding. Oligohydramnios was not an exclusion criterion.
Clinical definition
The diagnosis of preterm labor was made in the presence of regular uterine contractions (at least 3 in 30 minutes) and documented cervical change in patients with a gestational age of 20 to 36 6/7 weeks. Preterm PROM was diagnosed with sterile speculum examination with a combination of vaginal pooling and nitrazine and ferning tests. Intra-amniotic infection was defined as a positive microbiological culture in amniotic fluid. Clinical chorioamnionitis was diagnosed in the presence of a temperature elevation to 37.8°C or higher and two or more of the following criteria: uterine tenderness, malodorous vaginal discharge, fetal tachycardia (fetal heart rate >160 beats/min), and maternal leukocytosis (leukocyte count >15,000/µL) 55,56].
Since blood cell count varies with gestational age, the observed values were corrected for fetal age by calculating a ratio between the observed and expected mean value for gestational age. The reference ranges for each gestational age of fetal hemoglobin concentrations, nucleated red blood cell, white blood cell and platelet counts were obtained from previous studies [148–151]. The corrected eosinophil and basophil counts were not obtained since they do not change with gestational age [150]. Neutrophilia was defined as a neutrophil count >95th centile of gestational age and neutropenia was defined as a neutrophil count <5th centile of gestational age [150]. The results of nucleated red blood cell count were reported as count per 100 WBC.
All patients provided written informed consent prior to the performance of procedures and collection of samples. The collection and utilization of the samples for research was approved by the Human Investigation Committee of Wayne State University, (Detroit, MI) and the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/DHHS). Many of these samples have been used in previous studies.
Amniocentesis, cordocentesis and assays for IL-6
All patients had a detailed ultrasonographic examination before amniocentesis and cordocentesis were performed. Electronic fetal monitoring was performed before and after the procedure to evaluate fetal well-being. Amniocentesis and cordocentesis procedures were performed under ultrasound guidance with the “free-hand technique” as previously described [152]. Gram stain and microbial cultures for aerobic and anaerobic bacteria, and mycoplasmas were performed in amniotic fluid. The results of these tests were used for subsequent clinical management. Fetal blood was collected in ethylenediaminetetra-acetic acid (EDTA). Kleihauer-Betke stains were performed on fetal blood, and all specimens were found to be free of maternal blood. Fetal blood was analyzed for hemoglobin concentration, platelet and nucleated red blood cell count, as well as WBC and differential count, including neutrophils, monocytes, lymphocytes, basophils, and eosinophils. The hemoglobin concentration, platelet, and total nucleated cell count (WBC and nucleated red blood cell counts) were obtained with a Coulter S-Plus IV (Coulter Electronics, Hialeah, Fla., USA) analyzer. The blood films were stained by Giemsa-Wright method. The nucleated red cell and the differential leukocyte counts were determined by morphological classification of 100 cells.
Fetal plasma IL-6 concentrations were determined with commercially available enzyme-linked immunoassays (R&D Systems, Minneapolis, USA). The sensitivity of the assay was 0.06 pg/mL. The intra-assay and inter-assay coefficients of variation were 3.3% and 8.3%, respectively. The results of IL-6 concentrations in fetal plasma reported herein were neither available nor used for clinical decision-making.
Statistical analysis
The Kolmogorov-Smirnov test and Shapiro-Wilk test were used to determine if the data was normally distributed. A two-tailed Mann-Whitney U test was used to compare continuous nonparametric variables. Comparisons between proportions were performed using Chi-square or Fisher’s exact tests. Correlation between fetal plasma IL-6 concentrations and fetal hematologic parameters was determined using Spearman’s rank correlation test. A p-value <0.05 was considered statistically significant. Analysis was performed with SPSS, version 15 (SPSS Inc., Chicago, USA)
RESULTS
Demographic and clinical characteristics
This study included 102 women, of which 67% (102/152) were presented with preterm labor and intact membranes and 33% (50/152) were presented with preterm PROM. Seventy nine percent (121/152) delivered at <37 weeks of gestation. The prevalence of intra-amniotic infection and FIRS was 27% (41/152) and 28.9% (44/152), respectively. FIRS was diagnosed more frequently in patients with preterm PROM than in those with preterm labor [preterm PROM 42% (21/50), vs. preterm labor 22% (23/102); p=0.01]. Patients with FIRS had a higher rate of intra-amniotic infection, clinical chorioamnionitis, and preterm delivery than those without FIRS (all p<0.05 see Table I). There was no significant difference in the frequency of exposure to antenatal steroids, tocolysis (magnesium sulfate, indomethacin or terbutaline) or antibiotics prior to cordocentesis between patients with and without FIRS (all p>0.05; see Table I). All patients except five (97%), had information on hemoglobin concentrations, platelet, NRBC and differential WBC counts.
Table I.
Clinical characteristics of the study population
| NO FIRS (n=108) |
FIRS (n=44) |
p | |
|---|---|---|---|
| Age (years) | 24.3 (15–38) | 24.5 (15–39) | 0.8 |
| GA at cordocentesis (weeks) | 30.7 (18.2–35.5) | 29.2 (20–36) | 0.02 |
| Preterm PROM | 29 (26.9%) | 21 (47.7%) | 0.01 |
| Intra-amniotic infection | 14 (13%) | 27 (61.4%) | <0.001 |
| Clinical chorioamnionitis | 6 (5.6%) | 10 (62.5%) | 0.002 |
| Antenatal steroids prior to cordocentesis | 29 (26.9%) | 13 (29.5%) | 0.7 |
| Tocolysis prior to cordocentesis | 71 (65.7%) | 23 (52.3%) | 0.1 |
| Antibiotics prior to cordocentesis | 27 (25%) | 13 (30.2%) | 0.5 |
| Preterm delivery | 79 (73.1%) | 42 (95.5%) | 0.002 |
| Delivery within 72 hours | 22 (20.4%) | 35 (79.5%) | <0.001 |
| GA at delivery (weeks) | 34.3 (24–41.8) | 30.1 (20–38.3) | <0.001 |
| Interval to delivery (hours) | 608.2 (2–3384) | 149.7 (1–1368) | <0.001 |
| Birthweight (grams) | 2327 (490–4337) | 1568 (360–3480) | <0.001 |
| Fetal blood IL-6 (pg/mL) | 4.8 (0.3–10.8) | 171.6 (11.5–900) | <0.001 |
Values were expressed as number (percent) or median (range)
FIRS: Fetal inflammatory response syndrome
GA: Gestational age; PROM: prelabor rupture of membranes
FIRS is associated with leukocytosis and neutrophilia
Fetuses with FIRS have a higher median total WBC count and corrected WBC count for gestational age than those without FIRS (total WBC: FIRS median 6.3 ×109L; range 1.3–23.1 ×109L vs. without FIRS median 5.4 ×109L; range 2–12.6 ×109L; p=0.008 and corrected WBC: FIRS median 1.4; range 0.3–5.6 vs. without FIRS median 1.1; range 0.4–2.9; p=0.001; Table II and Figure 1).
TABLE II.
Hematologic profile of the fetuses with and without FIRS
| NO FIRS (n=108) |
FIR S (n=44) |
p | |
|---|---|---|---|
| WBC (×109/L) | 5.4 (2–12.6) | 6.3 (1.3–23.1) | 0.008 |
| Corrected WBC | 1.1 (0.4–2.9) | 1.4 (0.3–5.6) | 0.001 |
| Neutrophil (×109/L) | 1.6α (0.1–7.2) | 2.8β (0.1–17) | 0.001 |
| Corrected Neutrophil | 1.8α (0.2–13.9) | 3.6β (0.1–57.5) | <0.001 |
| Neutrophilia | 37 (35%)α | 30 (71%)β | <0.001 |
| Neutropenia | 8 (7.7%)α | 2 (4.8%)β | 0.5 |
| Lymphocyte (×109/L) | 3.2α (1–6.1) | 2.8β (0.9–8.4) | 0.6 |
| Corrected Lymphocyte | 0.8α (0.3–1.8) | 0.7β (0.3–1.8) | 0.9 |
| Monocyte (×109/L) | 0.3α (0–0.9) | 0.3β (0–1.9) | 0.4 |
| Corrected Monocyte | 1.1α (0–4.7) | 1.2β (0–21.1) | 0.2 |
| Eosinophil (×109/L) | 0.1α (0–1.1) | 0.1β (0–0.6) | 0.4 |
| Basophil (×109/L) | 0α (0–0.2) | 0β (0–0.2) | 0.7 |
| NRBC/100 WBC | 0.3α (0–14) | 0.5β (0–14) | 0.01 |
| Corrected NRBC | 0.04α (0–2.3) | 0.07β (0–1.3) | 0.06 |
| Platelet (×109/L) | 249α (73–394) | 229β (28–368) | 0.1 |
| Corrected Platelet | 1.03α (0.3–1.6) | 0.9β (0.1–1.5) | 0.3 |
| Hemoglobin (g/dl) | 13.1α (0–15.8) | 12.9β (9.8–16) | 0.4 |
| Corrected hemoglobin | 0.9α (0–1.2) | 0.9β (0.7–1.2) | 0.7 |
Values were expressed as median (range) and number (percent)
FIRS: Fetal inflammatory response syndrome
WBC: White Blood Cell, NRBC: Nucleated Red Blood Cell
n=105,
n=42
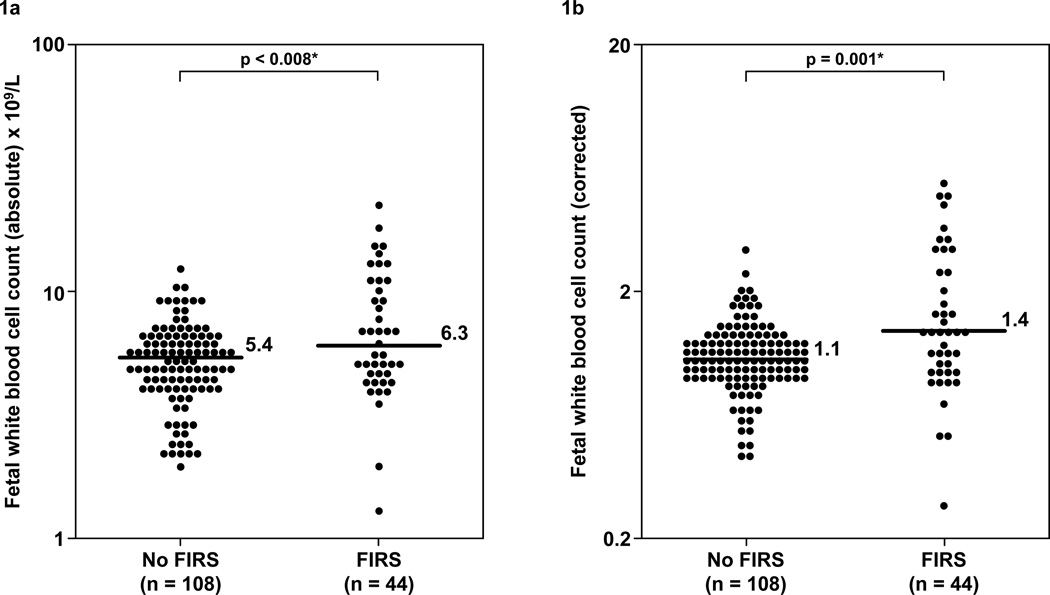
Figure 1. Comparison of the fetal blood absolute and corrected white blood cell counts between fetuses with FIRS and those without FIRS.
Fetuses with FIRS have a higher median absolute WBC count (FIRS: median 6.3 ×109/L; range 1.3–23.1 ×109/L vs. without FIRS: median 5.4 ×109/L; range 2–12.6 ×109/L; p=0.008, Figure 1a) and corrected WBC count (FIRS: median 1.4; range 0.3–5.6 vs. without FIRS median 1.1; range 0.4–2.9; p=0.001; Figure 1b) than those without FIRS. The y-axis is depicted in log scale.
Similarly, the median absolute and corrected neutrophil counts were higher in fetuses with FIRS than in those without FIRS (median absolute neutrophil count 2.8 ×109cells/ mL; range 0.1–17 ×109cells/ mL vs 1.6 ×109cells/ mL; range 0.1–7.2 ×109cells/ mL; median corrected neutrophil count 3.6; range 0.1–57.5 vs 1.8; range 0.2–13.9, all p<0.05; see Table II, Figure 2). Neutrophilia was significantly more common in fetuses with FIRS than in those without FIRS [71% (30/42), vs. 35% (37/104), p<0.001, Table II]. However, neither the prevalence of neutropenia [4.8% (2/42), vs 7.7% (8/105), p>0.05; Table II] nor the median lymphocyte, monocyte, eosinophil and basophil counts were significantly different between fetuses with and without FIRS (all p>0.05, Table II).
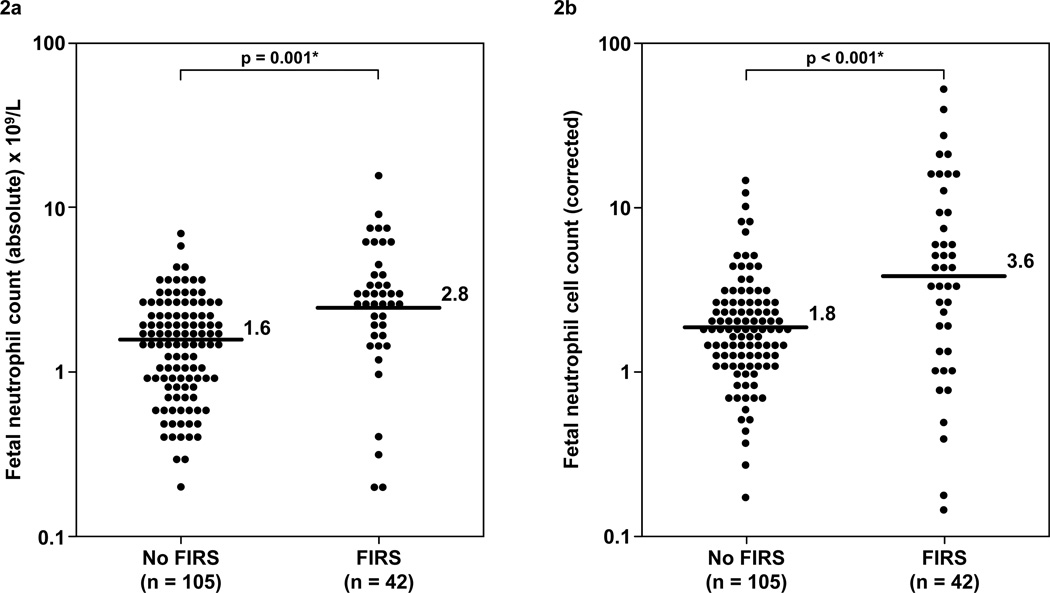
Figure 2. Comparison of the fetal blood absolute and corrected neutrophil counts between fetuses with FIRS and those without FIRS.
The median absolute and corrected neutrophil counts were higher in fetuses with FIRS than that of those without FIRS [median for absolute neutrophil count 2.8 ×109/L; range 0.1–17 ×109/L vs 1.6 ×109/L; range 0.1–7.2 ×109/L (Figure 2a); median for corrected neutrophil count 3.6; range 0.1–57.5 vs 1.8; range 0.2–13.9, all p<0.05; (Figure 2b)]. The y-axis is depicted in log scale.
Fetuses with FIRS had a tendency of enhanced erythropoiesis
Fetuses with FIRS have a median absolute fetal NRBC count higher than those without FIRS (FIRS: median 0.5/100WBC; range 0-14/100WBC vs. without FIRS: median 0.3/100WBC; range 0-14/100WBC; p=0.01). The median fetal NRBC count corrected for gestational age in FIRS was higher than that in fetuses without FIRS. However, the difference did not reach statistical significance (FIRS: median 0.07; range 0–1.3 vs. without FIRS: median 0.04; range 0–2.3; p=0.06; see Table II and Figure 3). The median hemoglobin concentration and platelet count were not significantly different between fetuses with and without FIRS (all p>0.05, Table II).
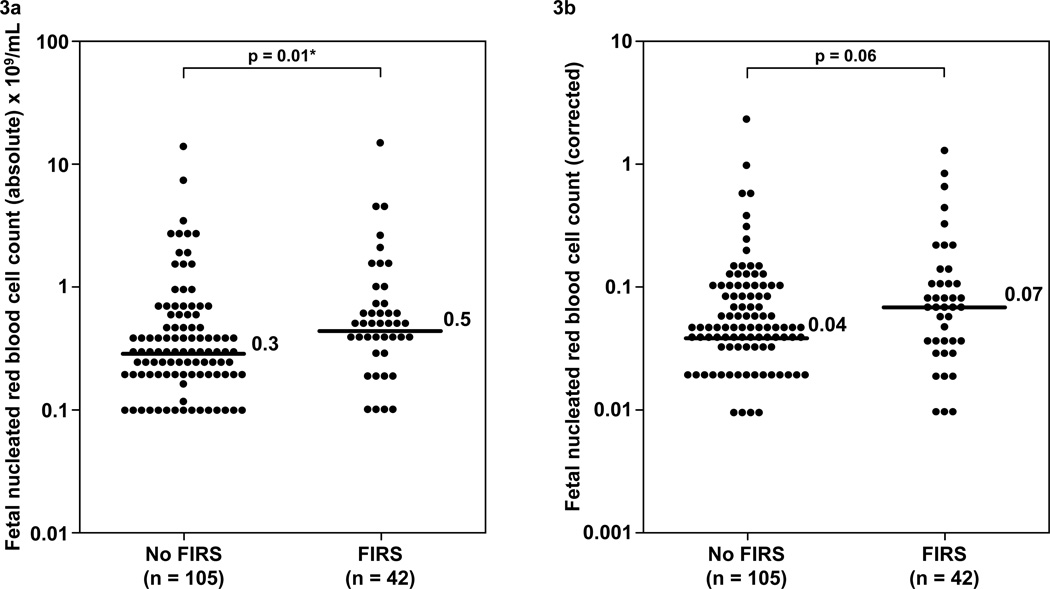
Figure 3. Comparison of the fetal blood absolute and corrected nucleated red blood cell counts between fetuses with FIRS and those without FIRS.
Fetuses with FIRS have a median absolute fetal NRBC count higher than those without FIRS. (FIRS: median 0.5 / 100WBC; range 0–14 / 100WBC vs. without FIRS: median 0.30 / 100WBC; range 0–14 / 100WBC; p=0.01, Figure 3a). The median fetal NRBC count corrected for gestational age in FIRS was higher than that in fetuses without FIRS. However, the difference did not reach statistical significance (FIRS: median 0.07; range 0–1.3 vs. without FIRS: median 0.04; range 0–2.3; p=0.06; Figure 3b). The y-axis is depicted in log scale.
Correlation between fetal blood IL-6 concentration and fetal blood hematologic parameters
The absolute and corrected fetal WBC counts were correlated with fetal plasma IL-6 concentrations (Spearman’s rho 0.2; p=0.004 and Spearman’s rho 0.3; p<0.001, respectively). Among the various WBC lineages, only the absolute and corrected neutrophil counts were correlated with an elevated fetal plasma IL-6 concentration (Spearman’s rho = 0.2 for both; p=0.01 and 0.006, respectively). There was a significant relationship between fetal plasma IL-6 concentration and both absolute and corrected NRBC counts (Spearman’s rho 0.3 for both; p<0.001 and p=0.001, respectively).
DISCUSSION
Principal findings of the study
1) Fetuses with FIRS had a higher median total WBC and neutrophil count than those without FIRS; 2) more than two thirds of fetuses with FIRS had neutrophilia, while neutropenia was present in only 4.8%; 3) FIRS was not associated with significant changes in hemoglobin concentration, platelet, lymphocyte, monocyte, basophil or eosinophil counts; and 4) there was a tendency for fetuses with FIRS to have an elevated NRBC count when compared to those without FIRS after adjustment for gestational age.
More than two thirds of fetuses with FIRS have neutrophilia
Neutrophils are part of the innate immune system and play a critical role in the acute inflammatory response and host defense against infection. During human fetal development, neutrophils first appear in the clavicular bone marrow at 12–13 weeks of gestation [153]. However, the lymphocyte is the predominant WBC type in the preterm fetus [154]. After 32 weeks of gestation, the proportion of neutrophils increases to become the predominant leukocyte at term [150,154]. It is interesting that while hematopoiesis in the fetal liver is characterized by proliferation of erythrocytes rather than neutrophils, the early bone marrow behaves differently – hypocellular for erythrocytes but rich in neutrophils [153].
The finding that fetuses with FIRS had elevated neutrophil counts is consistent with several previous studies from our group and others. Carroll and Nicolaides demonstrated that fetuses with bacteremia had higher leukocyte and neutrophil counts than those without bacteremia in patients with preterm PROM [155]. Fetuses with FIRS also have phenotypic evidence of monocyte-neutrophil activation in patients who delivered within 72 hours of cordocentesis [156]. Moreover, umbilical cord blood from patients with acute funisitis, the histological counterpart of FIRS [157], had phenotypic and metabolic changes on leukocytes [higher median mean channel brightness (MCB) of CD14, CD64, and CD66b on granulocytes and of CD64 on monocytes and increased basal intracellular reactive oxygen species and oxidative burst in monocytes) [158]. These findings suggest that fetuses with FIRS had a hematologic profile consistent with activation of the innate limb of the immune response.
More than two-thirds of fetuses with FIRS had neutrophilia, and only 4.8% had neutropenia. These observations seem to contradict the findings in septic preterm neonates who frequently present with neutropenia. The mechanism of fetal neutrophilia is not completely understood. However, it has been proposed that granulocyte colony stimulating factor (G-CSF), the primary physiologic regulator of neutrophil production and emergency signal of neutrophil release, may participate in these mechanisms [159–163]. Indeed, fetuses with FIRS had higher plasma concentrations of G-CSF than those without FIRS [162]. It is possible that fetal infection causes elevation of G-CSF and mobilization of neutrophils from bone marrow resulting in fetal neutrophilia. Subsequently, the neutrophil storage pool in these fetuses is depleted, and the neonates become neutropenic after birth. This hypothesis is consistent with a chronic nature of infection-related preterm birth [164–174].
Erythropoiesis and nucleated red blood cell count in FIRS
Erythropoiesis, a process of red blood cell production, is a component of hematopoiesis which is crucial from a developmental perspective. The development of an erythropoietic system allows growth of the higher organisms to a size not otherwise possible due to inadequate supply of oxygen by simple diffusion, a key mechanism for oxygen transport in lower organisms [175]. Humans have four principal sites of erythropoiesis: yolk sac, ventral aspect of the aorta, liver and bone marrow. Hepatic hematopoiesis diminishes with advancing gestational age, as the bone marrow becomes the primary site of erythropoiesis [176]. The main regulator of this process is erythropoietin. This growth factor maintains red blood cell production during fetal, neonatal and adult life by inhibiting apoptosis of erythroid progenitors and by stimulating their proliferation and differentiation into normoblasts.
In contrast to adults, the primary source of erythropoietin in fetal life is the liver, and it is not until approximately the 30th week of gestation that renal erythropoietin production begins [175,176]. This hematopoietic factor can be stimulated by hypoxia-inducible factors -1 and -2, IL-6 and tumor necrosis factor–α. Elevated erythropoietin has been reported in several pathologic conditions such as hypoxia [177–179], anemia [180,181], placental insufficiency [178,182] or infants of diabetic mothers [183,184]. In these conditions, erythropoietin is correlated with an elevated NRBC count in umbilical cord blood [185,186]. Recently, the administration of recombinant erythropoietin to mothers or fetuses ameliorated the fetal liver and white matter injury in endotoxin treated animals [187,188].
Fetuses with FIRS tend to have higher NRBC counts than those without FIRS
One of the principal findings of this study is that the fetuses with FIRS had a higher absolute NRBC count than those without FIRS. However, when NRBC counts were adjusted for gestational age, the difference fell short of reaching statistical significance (p=0.06). Since NRBC counts in this study were expressed as numbers of NRBC per 100 WBC, the real magnitude of the changes in NRBC counts observed herein may have been underestimated. The increase in the total leukocyte count in FIRS may artificially lead to an underestimation of the number of NRB cells when using this ratio.
Elevated neonatal NRBC counts have been reported in patients with prolonged rupture of fetal membranes (>24 hours) [189], histologic chorioamnionitis [190], high acute placental inflammation score [146] and early-onset neonatal sepsis [122]. Since we have reported that FIRS is not associated with changes in the umbilical vein blood gas pH and PaO2 [191], hypoxia and metabolic acidosis is unlikely to cause the increased erythropoiesis observed herein. We propose that the increased number of NRBC represents the action of selected cytokines such as IL-6.
Interleukin-6 is a cytokine with a broad range of biological activities produced by macrophages, T cells and B cells [192,193]. This cytokine is a major mediator of the host response to infection and tissue damage that plays a central role in the defense mechanism, acute phase reaction, and hematopoiesis [192,193]. Studies in animals [145] and humans [194] indicate that IL-6 can stimulate erythropoiesis. Intrauterine administration of endotoxin to pregnant mice increases circulating NRBC in the fetal blood without significant elevations in erythropoietin concentrations [195]. Therefore, we propose that an elevated IL-6 in FIRS is responsible, at least in part, for the increased erythropoiesis reflected in the NRBC count.
Alternatively, the effect of IL-6 may be indirect. This cytokine can stimulate erythropoietin production and, through this mechanism, indirectly increase NRBC count. Evidence in support of this hypothesis includes: 1) umbilical venous blood erythropoietin and umbilical arterial blood IL-6 concentrations are higher in infants born to mothers with both histologic and clinical chorioamnionitis than those born to mothers without chorioamnionitis [196]; 2) neonates with acute funisitis have higher erythropoietin concentrations than those without acute funisitis [186]; and 3) in hospitalized adult patients, NRBC count is correlated with plasma erythropoietin as well as inflammation related cytokine (IL-3, IL-6 and IL-12p70) concentrations [197]. Patients with elevated NRBC counts have higher mortality rates than those with elevated erythropoietin or IL-6 concentration alone. Stachon et al. proposed that the NRBC count may be considered as a parameter which expresses the combined effects of hypoxia (erythropoietin) and inflammation (IL-6) [197].
Strength and limitations of the study
This is the first study describing the hematologic profiles of human fetuses with FIRS. The limitations of this study are: 1) a subset of patients received medication including antenatal steroids, tocolysis and antibiotics prior to the procedure. However, there was no significant difference in the frequency of these confounders between fetuses with and without FIRS; and 2) the NRBC count in the current study was reported as NRBC per 100WBC. This parameter has been criticized as being susceptible to under- or over-estimation of the absolute value of NRBC, especially in conditions where there is an increase or decrease in leukocyte counts, respectively [198]. Even if we wished to report the results as absolute numbers of NRBC per unit volume, an accurate adjustment of NRBC counts as a function of gestational age cannot be performed since there is no published reference standard for gestational age for the absolute number of NRBC per unit volume (e.g. cells/mm3 or cells /L) available.
Conclusion
The hematological response of the fetus with FIRS is characterized by significant changes in the total WBC and neutrophil counts. The NRBC count in fetuses with FIRS tends to be higher than that of fetuses without FIRS, and this cannot be attributed to the effect of hypoxia. In contrast to septic preterm neonates, the majority of fetuses with FIRS have neutrophilia. This study contributes to characterizing the hematologic parameters in fetuses with FIRS.
Supplementary Material
Acknowledgment
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 2.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50:652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 3.Romero R, Maymon E, Pacora P, Gomez R, Mazor M, Yoon BH, et al. Further observations on the fetal inflammatory response syndrome: a potential homeostatic role for the soluble receptors of tumor necrosis factor alpha. Am J Obstet Gynecol. 2000;183:1070–1077. doi: 10.1067/mob.2000.108885. [DOI] [PubMed] [Google Scholar]
- 4.Romero R, Chaiworapongsa T, Espinoza J, Gomez R, Yoon BH, Edwin S, et al. Fetal plasma MMP-9 concentrations are elevated in preterm premature rupture of the membranes. Am J Obstet Gynecol. 2002;187:1125–1130. doi: 10.1067/mob.2002.127312. [DOI] [PubMed] [Google Scholar]
- 5.Yoon BH, Romero R, Shim JY, Shim SS, Kim CJ, Jun JK. C-reactive protein in umbilical cord blood: a simple and widely available clinical method to assess the risk of amniotic fluid infection and funisitis. J Matern Fetal Neonatal Med. 2003;14:85–90. doi: 10.1080/jmf.14.2.85.90. [DOI] [PubMed] [Google Scholar]
- 6.Santana C, Guindeo MC, Gonzalez G, Garcia-Munoz F, Saavedra P, Domenech E. Cord blood levels of cytokines as predictors of early neonatal sepsis. Acta Paediatr. 2001;90:1176–1181. doi: 10.1080/080352501317061602. [DOI] [PubMed] [Google Scholar]
- 7.Veleminsky M, Jr, Stransky P, Veleminsky M, Sr, Tosner J. Relationship of IL-6, IL-8, TNF and sICAM-1 levels to PROM, pPROM, and the risk of early-onset neonatal sepsis. Neuro Endocrinol Lett. 2008;29:303–311. [PubMed] [Google Scholar]
- 8.Mestan K, Yu Y, Thorsen P, Skogstrand K, Matoba N, Liu X, et al. Cord blood biomarkers of the fetal inflammatory response. J Matern Fetal Neonatal Med. 2009;22:379–387. doi: 10.1080/14767050802609759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satar M, Turhan E, Yapicioglu H, Narli N, Ozgunen FT, Cetiner S. Cord blood cytokine levels in neonates born to mothers with prolonged premature rupture of membranes and its relationship with morbidity and mortality. Eur Cytokine Netw. 2008;19:37–41. doi: 10.1684/ecn.2008.0118. [DOI] [PubMed] [Google Scholar]
- 10.Petrakou E, Mouchtouri A, Levi E, Lipsou N, Xanthou M, Fotopoulos S. Interleukin-8 and monocyte chemotactic protein-1 mRNA expression in perinatally infected and asphyxiated preterm neonates. Neonatology. 2007;91:107–113. doi: 10.1159/000097127. [DOI] [PubMed] [Google Scholar]
- 11.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179:186–193. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 12.Yoon BH, Romero R, Yang SH, Jun JK, Kim IO, Choi JH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174:1433–1440. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- 13.Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim BI, et al. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1999;181:773–779. doi: 10.1016/s0002-9378(99)70299-1. [DOI] [PubMed] [Google Scholar]
- 14.Leviton A, Paneth N, Reuss ML, Susser M, Allred EN, Dammann O, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Developmental Epidemiology Network Investigators. Pediatr Res. 1999;46:566–575. doi: 10.1203/00006450-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000;183:1124–1129. doi: 10.1067/mob.2000.109035. [DOI] [PubMed] [Google Scholar]
- 16.Yoon BH, Romero R, Shim JY, Lim JH, Cho EJ, Hanna J, Mi-Young P. "Atypical" chronic lung disease of the newborn is linked to fetal systemic inflammation. Am J Obstet Gynecol. 2002;187:S129. [Google Scholar]
- 17.Leviton A. Preterm birth and cerebral palsy: is tumor necrosis factor the missing link? Dev Med Child Neurol. 1993;35:553–558. doi: 10.1111/j.1469-8749.1993.tb11688.x. [DOI] [PubMed] [Google Scholar]
- 18.Dammann O, Kuban KC, Leviton A. Perinatal infection, fetal inflammatory response, white matter damage, and cognitive limitations in children born preterm. Ment Retard Dev Disabil Res Rev. 2002;8:46–50. doi: 10.1002/mrdd.10005. [DOI] [PubMed] [Google Scholar]
- 19.Kim YM, Romero R, Chaiworapongsa T, Espinoza J, Mor G, Kim CJ. Dermatitis as a component of the fetal inflammatory response syndrome is associated with activation of Toll-like receptors in epidermal keratinocytes. Histopathology. 2006;49:506–514. doi: 10.1111/j.1365-2559.2006.02542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romero R, Espinoza J, Goncalves LF, Gomez R, Medina L, Silva M, et al. Fetal cardiac dysfunction in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2004;16:146–157. doi: 10.1080/14767050400009279. [DOI] [PubMed] [Google Scholar]
- 21.Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996;97:210–215. [PubMed] [Google Scholar]
- 22.Yanowitz TD, Jordan JA, Gilmour CH, Towbin R, Bowen A, Roberts JM, et al. Hemodynamic disturbances in premature infants born after chorioamnionitis: association with cord blood cytokine concentrations. Pediatr Res. 2002;51:310–316. doi: 10.1203/00006450-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Yoon BH, Kim YA, Romero R, Kim JC, Park KH, Kim MH, et al. Association of oligohydramnios in women with preterm premature rupture of membranes with an inflammatory response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1999;181:784–788. doi: 10.1016/s0002-9378(99)70301-7. [DOI] [PubMed] [Google Scholar]
- 24.Madsen-Bouterse SA, Romero R, Tarca AL, Kusanovic JP, Espinoza J, Kim CJ, et al. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol. 2010;63:73–92. doi: 10.1111/j.1600-0897.2009.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park JS, Romero R, Yoon BH, Moon JB, Oh SY, Han SY, et al. The relationship between amniotic fluid matrix metalloproteinase-8 and funisitis. Am J Obstet Gynecol. 2001;185:1156–1161. doi: 10.1067/mob.2001.117679. [DOI] [PubMed] [Google Scholar]
- 26.Lee SE, Romero R, Jung H, Park CW, Park JS, Yoon BH. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2007;197:294–296. doi: 10.1016/j.ajog.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Moon JB, Kim JC, Yoon BH, Romero R, Kim G, Oh SY, et al. Amniotic fluid matrix metalloproteinase-8 and the development of cerebral palsy. J Perinat Med. 2002;30:301–306. doi: 10.1515/JPM.2002.044. [DOI] [PubMed] [Google Scholar]
- 28.Park CW, Lee SM, Park JS, Jun JK, Romero R, Yoon BH. The antenatal identification of funisitis with a rapid MMP-8 bedside test. J Perinat Med. 2008;36:497–502. doi: 10.1515/JPM.2008.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin Neonatol. 2002;7:259–274. doi: 10.1016/s1084-2756(02)90121-1. [DOI] [PubMed] [Google Scholar]
- 30.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 32.Kramer BW. Antenatal inflammation and lung injury: prenatal origin of neonatal disease. J Perinatol. 2008;28(Suppl 1):S21–S27. doi: 10.1038/jp.2008.46. [DOI] [PubMed] [Google Scholar]
- 33.Kramer BW, Kallapur S, Newnham J, Jobe AH. Prenatal inflammation and lung development. Semin Fetal Neonatal Med. 2009;14:2–7. doi: 10.1016/j.siny.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berger A, Witt A, Haiden N, Kaider A, Klebermasz K, Fuiko R, et al. Intrauterine infection with Ureaplasma species is associated with adverse neuromotor outcome at 1 and 2 years adjusted age in preterm infants. J Perinat Med. 2009;37:72–78. doi: 10.1515/JPM.2009.016. [DOI] [PubMed] [Google Scholar]
- 35.Dammann O, Leviton A. Infection remote from the brain, neonatal white matter damage, and cerebral palsy in the preterm infant. Semin Pediatr Neurol. 1998;5:190–201. doi: 10.1016/s1071-9091(98)80034-x. [DOI] [PubMed] [Google Scholar]
- 36.Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. Br J Obstet Gynaecol. 2003;110(Suppl 20):124–127. doi: 10.1016/s1470-0328(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 37.Dammann O, Leviton A. Role of the fetus in perinatal infection and neonatal brain damage. Curr Opin Pediatr. 2000;12:99–104. doi: 10.1097/00008480-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Bashiri A, Burstein E, Mazor M. Cerebral palsy and fetal inflammatory response syndrome: a review. J Perinat Med. 2006;34:5–12. doi: 10.1515/JPM.2006.001. [DOI] [PubMed] [Google Scholar]
- 39.Robertson CM, Coopersmith CM. The systemic inflammatory response syndrome. Microbes Infect. 2006;8:1382–1389. doi: 10.1016/j.micinf.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 40.Muckart DJ, Bhagwanjee S. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference definitions of the systemic inflammatory response syndrome and allied disorders in relation to critically injured patients. Crit Care Med. 1997;25:1789–1795. doi: 10.1097/00003246-199711000-00014. [DOI] [PubMed] [Google Scholar]
- 41.Romero R, Manogue KR, Mitchell MD, Wu YK, Oyarzun E, Hobbins JC, et al. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol. 1989;161:336–341. doi: 10.1016/0002-9378(89)90515-2. [DOI] [PubMed] [Google Scholar]
- 42.Romero R, Mazor M, Wu YK, Avila C, Oyarzun E, Mitchell MD. Bacterial endotoxin and tumor necrosis factor stimulate prostaglandin production by human decidua. Prostaglandins Leukot Essent Fatty Acids. 1989;37:183–186. doi: 10.1016/0952-3278(89)90083-5. [DOI] [PubMed] [Google Scholar]
- 43.Maymon E, Ghezzi F, Edwin SS, Mazor M, Yoon BH, Gomez R, et al. The tumor necrosis factor alpha and its soluble receptor profile in term and preterm parturition. Am J Obstet Gynecol. 1999;181:1142–1148. doi: 10.1016/s0002-9378(99)70097-9. [DOI] [PubMed] [Google Scholar]
- 44.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest. 1990;85:1392–1400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992;27:117–123. doi: 10.1111/j.1600-0897.1992.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 46.Pacora P, Romero R, Maymon E, Gervasi MT, Gomez R, Edwin SS, et al. Participation of the novel cytokine interleukin 18 in the host response to intra-amniotic infection. Am J Obstet Gynecol. 2000;183:1138–1143. doi: 10.1067/mob.2000.108881. [DOI] [PubMed] [Google Scholar]
- 47.Romero R, Gomez R, Galasso M, Mazor M, Berry SM, Quintero RA, et al. The natural interleukin-1 receptor antagonist in the fetal, maternal, and amniotic fluid compartments: the effect of gestational age, fetal gender, and intrauterine infection. Am J Obstet Gynecol. 1994;171:912–921. doi: 10.1016/s0002-9378(94)70058-3. [DOI] [PubMed] [Google Scholar]
- 48.Kusanovic JP, Romero R, Chaiworapongsa T, Mittal P, Mazaki-Tovi S, Vaisbuch E, et al. Amniotic fluid sTREM-1 in normal pregnancy, spontaneous parturition at term and preterm, and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2010;23:34–47. doi: 10.3109/14767050903009248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gotsch F, Romero R, Kusanovic JP, Erez O, Espinoza J, Kim CJ, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med. 2008;21:529–547. doi: 10.1080/14767050802127349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chaiworapongsa T, Romero R, Espinoza J, Kim YM, Edwin S, Bujold E, et al. Macrophage migration inhibitory factor in patients with preterm parturition and microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2005;18:405–416. doi: 10.1080/14767050500361703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dudley DJ, Hunter C, Mitchell MD, Varner MW. Elevations of amniotic fluid macrophage inflammatory protein-1 alpha concentrations in women during term and preterm labor. Obstet Gynecol. 1996;87:94–98. doi: 10.1016/0029-7844(95)00366-5. [DOI] [PubMed] [Google Scholar]
- 52.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, et al. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med. 2005;17:365–373. doi: 10.1080/14767050500141329. [DOI] [PubMed] [Google Scholar]
- 53.Hamill N, Romero R, Gotsch F, Kusanovic JP, Edwin S, Erez O, et al. Exodus-1 (CCL20): evidence for the participation of this chemokine in spontaneous labor at term, preterm labor, and intrauterine infection. J Perinat Med. 2008;36:217–227. doi: 10.1515/JPM.2008.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mittal P, Romero R, Kusanovic JP, Edwin SS, Gotsch F, Mazaki-Tovi S, et al. CXCL6 (granulocyte chemotactic protein-2): a novel chemokine involved in the innate immune response of the amniotic cavity. Am J Reprod Immunol. 2008;60:246–257. doi: 10.1111/j.1600-0897.2008.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nhan-Chang CL, Romero R, Kusanovic JP, Gotsch F, Edwin SS, Erez O, et al. A role for CXCL13 (BCA-1) in pregnancy and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2008;21:763–775. doi: 10.1080/14767050802244946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Romero R, Gomez R, Galasso M, Munoz H, Acosta L, Yoon BH, et al. Macrophage inflammatory protein-1 alpha in term and preterm parturition: effect of microbial invasion of the amniotic cavity. Am J Reprod Immunol. 1994;32:108–113. doi: 10.1111/j.1600-0897.1994.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 57.Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med. 2008;21:605–616. doi: 10.1080/14767050802212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med. 2003;13:2–21. doi: 10.1080/jmf.13.1.2.21. [DOI] [PubMed] [Google Scholar]
- 59.Erez O, Romero R, Tarca AL, Chaiworapongsa T, Kim YM, Than NG, et al. Differential expression pattern of genes encoding for anti-microbial peptides in the fetal membranes of patients with spontaneous preterm labor and intact membranes and those with preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med. 2009;22:1103–1115. doi: 10.3109/14767050902994796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soto E, Espinoza J, Nien JK, Kusanovic JP, Erez O, Richani K, et al. Human beta-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2007;20:15–22. doi: 10.1080/14767050601036212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Erez O, Romer R, Vaisbuch E, Chaiworapongsa T, Kusanovic JP, Mazaki-Tovi S, et al. Changes in amniotic fluid concentration of thrombin-antithrombin III complexes in patients with preterm labor: evidence of an increased thrombin generation. J Matern Fetal Neonatal Med. 2009;22:971–982. doi: 10.3109/14767050902994762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Erez O, Romero R, Vaisbuch E, Kusanovic JP, Mazaki-Tovi S, Chaiworapongsa T, et al. High tissue factor activity and low tissue factor pathway inhibitor concentrations in patients with preterm labor. J Matern Fetal Neonatal Med. 2010;23:23–33. doi: 10.3109/14767050902994770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soto E, Romero R, Richani K, Yoon BH, Chaiworapongsa T, Vaisbuch E, et al. Evidence for complement activation in the amniotic fluid of women with spontaneous preterm labor and intra-amniotic infection. J Matern Fetal Neonatal Med. 2009;22:983–992. doi: 10.3109/14767050902994747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vaisbuch E, Romero R, Erez O, Mazaki-Tovi S, Kusanovic JP, Soto E, et al. Fragment Bb in amniotic fluid: evidence for complement activation by the alternative pathway in women with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2009;22:905–916. doi: 10.1080/14767050902994663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cruciani L, Romero R, Vaisbuch E, Kusanovic JP, Chaiworapongsa T, Mazaki-Tovi S, et al. Pentraxin 3 in amniotic fluid: a novel association with intra-amniotic infection and inflammation. J Perinat Med. 2010;38:161–171. doi: 10.1515/JPM.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friel LA, Romero R, Edwin S, Nien JK, Gomez R, Chaiworapongsa T, et al. The calcium binding protein, S100B, is increased in the amniotic fluid of women with intra-amniotic infection/inflammation and preterm labor with intact or ruptured membranes. J Perinat Med. 2007;35:385–393. doi: 10.1515/JPM.2007.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chaiworapongsa T, Erez O, Kusanovic JP, Vaisbuch E, Mazaki-Tovi S, Gotsch F, et al. Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J Matern Fetal Neonatal Med. 2008;21:449–461. doi: 10.1080/14767050802054550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Romero R, Espinoza J, Hassan S, Gotsch F, Kusanovic JP, Avila C, et al. Soluble receptor for advanced glycation end products (sRAGE) and endogenous secretory RAGE (esRAGE) in amniotic fluid: modulation by infection and inflammation. J Perinat Med. 2008;36:388–398. doi: 10.1515/JPM.2008.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Romero R, Chaiworapongsa T, Alpay S, Madan I, Dong Z, Kusanovic J, Kim C, et al. HMGB1, A Late Mediator of Sepsis and Death, is Involved in the Host Response to Intra-amniotic Infection/Inflammation in Preterm Gestation. Reproductive Science. 2011;18:245A. [Google Scholar]
- 70.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 71.Helmig BR, Romero R, Espinoza J, Chaiworapongsa T, Bujold E, Gomez R, et al. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J Matern Fetal Neonatal Med. 2002;12:237–246. doi: 10.1080/jmf.12.4.237.246. [DOI] [PubMed] [Google Scholar]
- 72.Kusanovic JP, Romero R, Mazaki-Tovi S, Chaiworapongsa T, Mittal P, Gotsch F, et al. Resistin in amniotic fluid and its association with intra-amniotic infection and inflammation. J Matern Fetal Neonatal Med. 2008;21:902–916. doi: 10.1080/14767050802320357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, Mittal P, et al. Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J Perinat Med. 2008;36:485–496. doi: 10.1515/JPM.2008.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mazaki-Tovi S, Romero R, Vaisbuch E, Kusanovic JP, Erez O, Mittal P, et al. Adiponectin in amniotic fluid in normal pregnancy, spontaneous labor at term, and preterm labor: a novel association with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2010;23:120–130. doi: 10.3109/14767050903026481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vaisbuch E, Mazaki-Tovi S, Kusanovic JP, Erez O, Than NG, Kim SK, et al. Retinol binding protein 4: an adipokine associated with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2010;23:111–119. doi: 10.3109/14767050902994739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chaiworapongsa T, Hong JS, Hull WM, Romero R, Whitsett JA. Amniotic fluid concentration of surfactant proteins in intra-amniotic infection. J Matern Fetal Neonatal Med. 2008;21:663–670. doi: 10.1080/14767050802215664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pacora P, Romero R, Chaiworapongsa T, Kusanovic JP, Erez O, Vaisbuch E, et al. Amniotic fluid angiopoietin-2 in term and preterm parturition, and intra-amniotic infection/inflammation. J Perinat Med. 2009;37:503–511. doi: 10.1515/JPM.2009.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alpay Savasan Z, Romero R, Chaiworapongsa T, Kusanovic JP, Kim SK, Mazaki-Tovi S, et al. Evidence in support of a role for anti-angiogenic factors in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2010;23:828–841. doi: 10.3109/14767050903440471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maymon E, Romero R, Pacora P, Gomez R, Athayde N, Edwin S, et al. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;183:94–99. doi: 10.1067/mob.2000.105344. [DOI] [PubMed] [Google Scholar]
- 80.Maymon E, Romero R, Pacora P, Gervasi MT, Gomez R, Edwin SS, et al. Evidence of in vivo differential bioavailability of the active forms of matrix metalloproteinases 9 and 2 in parturition, spontaneous rupture of membranes, and intra-amniotic infection. Am J Obstet Gynecol. 2000;183:887–894. doi: 10.1067/mob.2000.108878. [DOI] [PubMed] [Google Scholar]
- 81.Maymon E, Romero R, Pacora P, Gervasi MT, Edwin SS, Gomez R, et al. Matrilysin (matrix metalloproteinase 7) in parturition, premature rupture of membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;182:1545–1553. doi: 10.1067/mob.2000.107652. [DOI] [PubMed] [Google Scholar]
- 82.Maymon E, Romero R, Pacora P, Gervasi MT, Bianco K, Ghezzi F, et al. Evidence for the participation of interstitial collagenase (matrix metalloproteinase 1) in preterm premature rupture of membranes. Am J Obstet Gynecol. 2000;183:914–920. doi: 10.1067/mob.2000.108879. [DOI] [PubMed] [Google Scholar]
- 83.Maymon E, Romero R, Chaiworapongsa T, Kim JC, Berman S, Gomez R, et al. Value of amniotic fluid neutrophil collagenase concentrations in preterm premature rupture of membranes. Am J Obstet Gynecol. 2001;185:1143–1148. doi: 10.1067/mob.2001.118166. [DOI] [PubMed] [Google Scholar]
- 84.Maymon E, Romero R, Pacora P, Gomez R, Mazor M, Edwin S, et al. A role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intraamniotic infection. J Perinat Med. 2001;29:308–316. doi: 10.1515/JPM.2001.044. [DOI] [PubMed] [Google Scholar]
- 85.Park KH, Chaiworapongsa T, Kim YM, Espinoza J, Yoshimatsu J, Edwin S, et al. Matrix metalloproteinase 3 in parturition, premature rupture of the membranes, and microbial invasion of the amniotic cavity. J Perinat Med. 2003;31:12–22. doi: 10.1515/JPM.2003.002. [DOI] [PubMed] [Google Scholar]
- 86.Fortunato SJ, Menon R, Lombardi SJ. MMP/TIMP imbalance in amniotic fluid during PROM: an indirect support for endogenous pathway to membrane rupture. J Perinat Med. 1999;27:362–368. doi: 10.1515/JPM.1999.049. [DOI] [PubMed] [Google Scholar]
- 87.Fortunato SJ, Menon R. Distinct molecular events suggest different pathways for preterm labor and premature rupture of membranes. Am J Obstet Gynecol. 2001;184:1399–1405. doi: 10.1067/mob.2001.115122. [DOI] [PubMed] [Google Scholar]
- 88.Jacobsson B, Hagberg G. Antenatal risk factors for cerebral palsy. Best Pract Res Clin Obstet Gynaecol. 2004;18:425–436. doi: 10.1016/j.bpobgyn.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 89.Willoughby RE, Jr, Nelson KB. Chorioamnionitis and brain injury. Clin Perinatol. 2002;29:603–621. doi: 10.1016/s0095-5108(02)00058-1. [DOI] [PubMed] [Google Scholar]
- 90.Kasper DC, Mechtler TP, Bohm J, Petricevic L, Gleiss A, Spergser J, et al. In utero exposure to Ureaplasma spp. is associated with increased rate of bronchopulmonary dysplasia and intraventricular hemorrhage in preterm infants. J Perinat Med. 2011;39:331–336. doi: 10.1515/jpm.2011.022. [DOI] [PubMed] [Google Scholar]
- 91.Botet F, Figueras J, Carbonell-Estrany X, Narbona E. The impact of clinical maternal chorioamnionitis on neurological and psychological sequelae in very-low-birth weight infants: a case-control study. J Perinat Med. 2011;39:203–208. doi: 10.1515/jpm.2011.005. [DOI] [PubMed] [Google Scholar]
- 92.Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995;172:960–970. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 93.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 94.Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 95.Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675–681. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 96.Mammen EF. The haematological manifestations of sepsis. J Antimicrob Chemother. 1998;41(Suppl A):17–24. doi: 10.1093/jac/41.suppl_1.17. [DOI] [PubMed] [Google Scholar]
- 97.Groselj-Grenc M, Ihan A, Pavcnik-Arnol M, Kopitar AN, Gmeiner-Stopar T, Derganc M. Neutrophil and monocyte CD64 indexes, lipopolysaccharide-binding protein, procalcitonin and C-reactive protein in sepsis of critically ill neonates and children. Intensive Care Med. 2009;35:1950–1958. doi: 10.1007/s00134-009-1637-7. [DOI] [PubMed] [Google Scholar]
- 98.Fung YL, Fraser JF, Wood P, Minchinton RM, Silliman CC. The systemic inflammatory response syndrome induces functional changes and relative hyporesponsiveness in neutrophils. J Crit Care. 2008;23:542–549. doi: 10.1016/j.jcrc.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 99.Abidi K, Khoudri I, Belayachi J, Madani N, Zekraoui A, Zeggwagh AA, et al. Eosinopenia is a reliable marker of sepsis on admission to medical intensive care units. Crit Care. 2008;12:R59. doi: 10.1186/cc6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Koussoulas V, Tzivras M, Karagianni V, Spyridaki E, Plachouras D, Giamarellou H, et al. Monocytes in systematic inflammatory response syndrome: differences between sepsis and acute pancreatitis. World J Gastroenterol. 2006;12:6711–6714. doi: 10.3748/wjg.v12.i41.6711. [DOI] [PubMed] [Google Scholar]
- 101.Cavaillon JM, dib-Conquy M. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care. 2006;10:233. doi: 10.1186/cc5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Asadollahi K, Beeching NJ, Gill GV. Leukocytosis as a predictor for non-infective mortality and morbidity. QJM. 2010;103:285–292. doi: 10.1093/qjmed/hcp182. [DOI] [PubMed] [Google Scholar]
- 103.Grimm RH, Jr, Neaton JD, Ludwig W. Prognostic importance of the white blood cell count for coronary, cancer, and all-cause mortality. JAMA. 1985;254:1932–1937. [PubMed] [Google Scholar]
- 104.Weiss ST, Segal MR, Sparrow D, Wager C. Relation of FEV1 and peripheral blood leukocyte count to total mortality. The Normative Aging Study. Am J Epidemiol. 1995;142:493–498. doi: 10.1093/oxfordjournals.aje.a117665. [DOI] [PubMed] [Google Scholar]
- 105.Brown DW, Ford ES, Giles WH, Croft JB, Balluz LS, Mokdad AH. Associations between white blood cell count and risk for cerebrovascular disease mortality: NHANES II Mortality Study, 1976–1992. Ann Epidemiol. 2004;14:425–430. doi: 10.1016/j.annepidem.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 106.Friedman GD, Klatsky AL, Siegelaub AB. The leukocyte count as a predictor of myocardial infarction. N Engl J Med. 1974;290:1275–1278. doi: 10.1056/NEJM197406062902302. [DOI] [PubMed] [Google Scholar]
- 107.Margolis KL, Manson JE, Greenland P, Rodabough RJ, Bray PF, Safford M, et al. Leukocyte count as a predictor of cardiovascular events and mortality in postmenopausal women: the Women's Health Initiative Observational Study. Arch Intern Med. 2005;165:500–508. doi: 10.1001/archinte.165.5.500. [DOI] [PubMed] [Google Scholar]
- 108.Papoff P, Christensen RD, Calhoun DA, Juul SE. Granulocyte colony-stimulating factor, granulocyte macrophage colony-stimulating factor and neutrophils in the bronchoalveolar lavage fluid of premature infants with respiratory distress syndrome. Biol Neonate. 2001;80:133–141. doi: 10.1159/000047132. [DOI] [PubMed] [Google Scholar]
- 109.Schrama AJ, de Beaufort AJ, Poorthuis BJ, Berger HM, Walther FJ. Secretory phospholipase A(2) in newborn infants with sepsis. J Perinatol. 2008;28:291–296. doi: 10.1038/sj.jp.7211929. [DOI] [PubMed] [Google Scholar]
- 110.Nupponen I, Pesonen E, Andersson S, Makela A, Turunen R, Kautiainen H, et al. Neutrophil activation in preterm infants who have respiratory distress syndrome. Pediatrics. 2002;110:36–41. doi: 10.1542/peds.110.1.36. [DOI] [PubMed] [Google Scholar]
- 111.Nupponen I, Venge P, Pohjavuori M, Lassus P, Andersson S. Phagocyte activation in preterm infants following premature rupture of the membranes or chorioamnionitis. Acta Paediatr. 2000;89:1207–1212. doi: 10.1080/080352500750027583. [DOI] [PubMed] [Google Scholar]
- 112.Manroe BL, Rosenfeld CR, Weinberg AG, Browne R. The differential leukocyte count in the assessment and outcome of early-onset neonatal group B streptococcal disease. J Pediatr. 1977;91:632–637. doi: 10.1016/s0022-3476(77)80522-2. [DOI] [PubMed] [Google Scholar]
- 113.Selimovic A, Skokic F, Selimovic Z, Bazardzanovic M. [The predictive values of total white blood count and differential count in the diagnosis of early-onset neonatal sepsis] Med Arh. 2008;62:205–210. [PubMed] [Google Scholar]
- 114.Wirbelauer J, Thomas W, Speer CP. Response of leukocytes and nucleated red blood cells in very low-birth weight preterm infants after exposure to intrauterine inflammation. J Matern Fetal Neonatal Med. 2010;24:348–353. doi: 10.3109/14767058.2010.497568. [DOI] [PubMed] [Google Scholar]
- 115.Paul DA, Leef KH, Stefano JL. Increased leukocytes in infants with intraventricular hemorrhage. Pediatr Neurol. 2000;22:194–199. doi: 10.1016/s0887-8994(99)00155-1. [DOI] [PubMed] [Google Scholar]
- 116.Baschat AA, Gungor S, Kush ML, Berg C, Gembruch U, Harman CR. Nucleated red blood cell counts in the first week of life: a critical appraisal of relationships with perinatal outcome in preterm growth-restricted neonates. Am J Obstet Gynecol. 2007;197:286–288. doi: 10.1016/j.ajog.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 117.Blackwell SC, Refuerzo JS, Hassan SS, Wolfe HM, Berry SM, Sorokin Y. Nucleated red blood cell counts in term neonates with umbilical artery pH < or = 7.00. Am J Perinatol. 2001;18:93–98. doi: 10.1055/s-2001-13634. [DOI] [PubMed] [Google Scholar]
- 118.Hanlon-Lundberg KM, Kirby RS. Nucleated red blood cells as a marker of acidemia in term neonates. Am J Obstet Gynecol. 1999;181:196–201. doi: 10.1016/s0002-9378(99)70459-x. [DOI] [PubMed] [Google Scholar]
- 119.Minior VK, Bernstein PS, Divon MY. Nucleated red blood cells in growth-restricted fetuses: associations with short-term neonatal outcome. Fetal Diagn Ther. 2000;15:165–169. doi: 10.1159/000020998. [DOI] [PubMed] [Google Scholar]
- 120.Bernstein PS, Minior VK, Divon MY. Neonatal nucleated red blood cell counts in small-for-gestational age fetuses with abnormal umbilical artery Doppler studies. Am J Obstet Gynecol. 1997;177:1079–1084. doi: 10.1016/s0002-9378(97)70018-8. [DOI] [PubMed] [Google Scholar]
- 121.Axt-Fliedner R, Wrobel M, Hendrik HJ, Ertan AK, Mink D, Konig J, et al. Nucleated red blood cell count and doppler ultrasound in low- and high-risk pregnancies. Clin Exp Obstet Gynecol. 2000;27:85–88. [PubMed] [Google Scholar]
- 122.Dulay AT, Buhimschi IA, Zhao G, Luo G, bdel-Razeq S, Cackovic M, et al. Nucleated red blood cells are a direct response to mediators of inflammation in newborns with early-onset neonatal sepsis. Am J Obstet Gynecol. 2008;198:426–429. doi: 10.1016/j.ajog.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hermansen MC. Potential for brief but severe intrapartum injury among neonates with early-onset seizures and elevated nucleated red blood cell counts. Am J Obstet Gynecol. 2001;184:782–783. doi: 10.1067/mob.2001.112108. [DOI] [PubMed] [Google Scholar]
- 124.Blackwell SC, Refuerzo JS, Wolfe HM, Hassan SS, Berry SM, Sokol RJ, et al. The relationship between nucleated red blood cell counts and early-onset neonatal seizures. Am J Obstet Gynecol. 2000;182:1452–1457. doi: 10.1067/mob.2000.106854. [DOI] [PubMed] [Google Scholar]
- 125.Silva AM, Smith RN, Lehmann CU, Johnson EA, Holcroft CJ, Graham EM. Neonatal nucleated red blood cells and the prediction of cerebral white matter injury in preterm infants. Obstet Gynecol. 2006;107:550–556. doi: 10.1097/01.AOG.0000195066.43243.56. [DOI] [PubMed] [Google Scholar]
- 126.Buonocore G, Perrone S, Gioia D, Gatti MG, Massafra C, Agosta R, et al. Nucleated red blood cell count at birth as an index of perinatal brain damage. Am J Obstet Gynecol. 1999;181:1500–1505. doi: 10.1016/s0002-9378(99)70396-0. [DOI] [PubMed] [Google Scholar]
- 127.Leikin E, Verma U, Klein S, Tejani N. Relationship between neonatal nucleated red blood cell counts and hypoxic-ischemic injury. Obstet Gynecol. 1996;87:439–443. doi: 10.1016/0029-7844(95)00436-x. [DOI] [PubMed] [Google Scholar]
- 128.Strijbis EM, Oudman I, van EP, MacLennan AH. Cerebral palsy and the application of the international criteria for acute intrapartum hypoxia. Obstet Gynecol. 2006;107:1357–1365. doi: 10.1097/01.AOG.0000220544.21316.80. [DOI] [PubMed] [Google Scholar]
- 129.Redline RW. Elevated circulating fetal nucleated red blood cells and placental pathology in term infants who develop cerebral palsy. Hum Pathol. 2008;39:1378–1384. doi: 10.1016/j.humpath.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 130.Kil TH, Han JY, Kim JB, Ko GO, Lee YH, Kim KY, et al. A study on the measurement of the nucleated red blood cell (nRBC) count based on birth weight and its correlation with perinatal prognosis in infants with very low birth weights. Korean J Pediatr. 2011;54:69–78. doi: 10.3345/kjp.2011.54.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boskabadi H, Maamouri G, Sadeghian MH, Ghayour-Mobarhan M, Heidarzade M, Shakeri MT, et al. Early diagnosis of perinatal asphyxia by nucleated red blood cell count: a case-control study. Arch Iran Med. 2010;13:275–281. [PubMed] [Google Scholar]
- 132.Wirbelauer J, Thomas W, Rieger L, Speer CP. Intrauterine growth retardation in preterm infants </=32 weeks of gestation is associated with low white blood cell counts. Am J Perinatol. 2010;27:819–824. doi: 10.1055/s-0030-1254547. [DOI] [PubMed] [Google Scholar]
- 133.Bayram F, Ozerkan K, Cengiz C, Develioglu O, Cetinkaya M. Perinatal asphyxia is associated with the umbilical cord nucleated red blood cell count in pre-eclamptic pregnancies. J Obstet Gynaecol. 2010;30:383–386. doi: 10.3109/01443611003706928. [DOI] [PubMed] [Google Scholar]
- 134.Martinelli S, Francisco RP, Bittar RE, Zugaib M. Hematological indices at birth in relation to arterial and venous Doppler in small-for-gestational-age fetuses. Acta Obstet Gynecol Scand. 2009;88:888–893. doi: 10.1080/00016340903090985. [DOI] [PubMed] [Google Scholar]
- 135.Shah V, Beyene J, Shah P, Perlman M. Association between hematologic findings and brain injury due to neonatal hypoxic-ischemic encephalopathy. Am J Perinatol. 2009;26:295–302. doi: 10.1055/s-0028-1103512. [DOI] [PubMed] [Google Scholar]
- 136.Steurer MA, Berger TM. Massively elevated nucleated red blood cells and cerebral or pulmonary hemorrhage in severely growth-restricted infants--is there more than coincidence? Neonatology. 2008;94:314–319. doi: 10.1159/000151654. [DOI] [PubMed] [Google Scholar]
- 137.Haiju Z, Suyuan H, Xiufang F, Lu Y, Sun R. The combined detection of umbilical cord nucleated red blood cells and lactate: early prediction of neonatal hypoxic ischemic encephalopathy. J Perinat Med. 2008;36:240–247. doi: 10.1515/JPM.2008.035. [DOI] [PubMed] [Google Scholar]
- 138.Redline RW. Cerebral palsy in term infants: a clinicopathologic analysis of 158 medicolegal case reviews. Pediatr Dev Pathol. 2008;11:456–464. doi: 10.2350/08-05-0468.1. [DOI] [PubMed] [Google Scholar]
- 139.Akercan F, Cirpan T, Saydam G. Nucleated red blood cells in infants of women with preterm labor and pre-eclampsia. Int J Gynaecol Obstet. 2005;90:138–139. doi: 10.1016/j.ijgo.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 140.Shivhare K, Chawla K, Khan MA, Mathur PS. Effect of maternal toxaemia on total haemoglobin, foetal haemoglobin and nucleated red blood cells in cord blood. Indian J Pediatr. 1976;43:349–356. doi: 10.1007/BF03177154. [DOI] [PubMed] [Google Scholar]
- 141.Sinha HB, Mukherjee AK, Bala D. Cord blood haemoglobin (including foetal haemoglobin), and nucleated red cells in normal and toxaemic pregnancies. Indian Pediatr. 1972;9:540–543. [PubMed] [Google Scholar]
- 142.Dollberg S, Marom R, Mimouni FB, Yeruchimovich M. Normoblasts in large for gestational age infants. Arch Dis Child Fetal Neonatal Ed. 2000;83:F148–F149. doi: 10.1136/fn.83.2.F148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yeruchimovich M, Mimouni FB, Green DW, Dollberg S. Nucleated red blood cells in healthy infants of women with gestational diabetes. Obstet Gynecol. 2000;95:84–86. doi: 10.1016/s0029-7844(99)00511-6. [DOI] [PubMed] [Google Scholar]
- 144.Green DW, Mimouni F. Nucleated erythrocytes in healthy infants and in infants of diabetic mothers. J Pediatr. 1990;116:129–131. doi: 10.1016/s0022-3476(05)81662-2. [DOI] [PubMed] [Google Scholar]
- 145.Ulich TR, del Castillo J, Guo KZ. In vivo hematologic effects of recombinant interleukin-6 on hematopoiesis and circulating numbers of RBCs and WBCs. Blood. 1989;73:108–110. [PubMed] [Google Scholar]
- 146.Salafia CM, Ghidini A, Pezzullo JC, Rosenkrantz TS. Early neonatal nucleated erythrocyte counts in preterm deliveries: clinical and pathologic correlations. J Soc Gynecol Investig. 1997;4:138–143. doi: 10.1016/s1071-5576(97)00022-1. [DOI] [PubMed] [Google Scholar]
- 147.Leikin E, Garry D, Visintainer P, Verma U, Tejani N. Correlation of neonatal nucleated red blood cell counts in preterm infants with histologic chorioamnionitis. Am J Obstet Gynecol. 1997;177:27–30. doi: 10.1016/s0002-9378(97)70433-2. [DOI] [PubMed] [Google Scholar]
- 148.Nicolaides KH, Soothill PW, Clewell WH, Rodeck CH, Mibashan RS, Campbell S. Fetal haemoglobin measurement in the assessment of red cell isoimmunisation. Lancet. 1988;1:1073–1075. doi: 10.1016/s0140-6736(88)91896-x. [DOI] [PubMed] [Google Scholar]
- 149.Nicolaides KH, Thilaganathan B, Mibashan RS. Cordocentesis in the investigation of fetal erythropoiesis. Am J Obstet Gynecol. 1989;161:1197–1200. doi: 10.1016/0002-9378(89)90664-9. [DOI] [PubMed] [Google Scholar]
- 150.Davies NP, Buggins AG, Snijders RJ, Jenkins E, Layton DM, Nicolaides KH. Blood leucocyte count in the human fetus. Arch Dis Child. 1992;67:399–403. doi: 10.1136/adc.67.4_spec_no.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Van den Hof MC, Nicolaides KH. Platelet count in normal, small, and anemic fetuses. Am J Obstet Gynecol. 1990;162:735–739. doi: 10.1016/0002-9378(90)90997-l. [DOI] [PubMed] [Google Scholar]
- 152.Nicolaides KH, Soothill PW, Rodeck CH, Campbell S. Ultrasound-guided sampling of umbilical cord and placental blood to assess fetal wellbeing. Lancet. 1986;1:1065–1067. doi: 10.1016/s0140-6736(86)91333-4. [DOI] [PubMed] [Google Scholar]
- 153.Slayton WB, Li Y, Calhoun DA, Juul SE, Iturraspe J, Braylan RC, et al. The first-appearance of neutrophils in the human fetal bone marrow cavity. Early Hum Dev. 1998;53:129–144. doi: 10.1016/s0378-3782(98)00049-8. [DOI] [PubMed] [Google Scholar]
- 154.De Waele M, Foulon W, Renmans W, Segers E, Smet L, Jochmans K, et al. Hematologic values and lymphocyte subsets in fetal blood. Am J Clin Pathol. 1988;89:742–746. doi: 10.1093/ajcp/89.6.742. [DOI] [PubMed] [Google Scholar]
- 155.Carroll SG, Nicolaides KH. Fetal haematological response to intra-uterine infection in preterm prelabour amniorrhexis. Fetal Diagn Ther. 1995;10:279–285. doi: 10.1159/000264244. [DOI] [PubMed] [Google Scholar]
- 156.Berry SM, Romero R, Gomez R, Puder KS, Ghezzi F, Cotton DB, et al. Premature parturition is characterized by in utero activation of the fetal immune system. Am J Obstet Gynecol. 1995;173:1315–1320. doi: 10.1016/0002-9378(95)91378-5. [DOI] [PubMed] [Google Scholar]
- 157.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 158.Kim SK, Romero R, Chaiworapongsa T, Kusanovic JP, Mazaki-Tovi S, Mittal P, et al. Evidence of changes in the immunophenotype and metabolic characteristics (intracellular reactive oxygen radicals) of fetal, but not maternal, monocytes and granulocytes in the fetal inflammatory response syndrome. J Perinat Med. 2009;37:543–552. doi: 10.1515/JPM.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Calhoun DA, Chegini N, Polliotti BM, Gersting JA, Miller RK, Christensen RD. Granulocyte colony-stimulating factor in preterm and term pregnancy, parturition, and intra-amniotic infection. Obstet Gynecol. 2001;97:229–234. doi: 10.1016/s0029-7844(00)01120-0. [DOI] [PubMed] [Google Scholar]
- 160.Nicola NA, Vadas MA, Lopez AF. Down-modulation of receptors for granulocyte colony-stimulating factor on human neutrophils by granulocyte-activating agents. J Cell Physiol. 1986;128:501–509. doi: 10.1002/jcp.1041280320. [DOI] [PubMed] [Google Scholar]
- 161.Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991;78:2791–2808. [PubMed] [Google Scholar]
- 162.Berry SM, Gomez R, Athayde N, Ghezzi F, Mazor M, Yoon BH, Edwin SS, et al. The Role of Granulocyte Colony Stimulating Factor in the Neutrophilia Observed in the Fetal Inflammatory Response Syndrome. Am J Obstet Gynecol. 1998;178:202. [Google Scholar]
- 163.Calhoun DA, Christensen RD. A randomized pilot trial of administration of granulocyte colony-stimulating factor to women before preterm delivery. Am J Obstet Gynecol. 1998;179:766–771. doi: 10.1016/s0002-9378(98)70080-8. [DOI] [PubMed] [Google Scholar]
- 164.Romero R. Prenatal medicine: the child is the father of the man. 1996. J Matern Fetal Neonatal Med. 2009;22:636–639. doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- 165.Nguyen DP, Gerber S, Hohlfeld P, Sandrine G, Witkin SS. Mycoplasma hominis in mid-trimester amniotic fluid: relation to pregnancy outcome. J Perinat Med. 2004;32:323–326. doi: 10.1515/JPM.2004.060. [DOI] [PubMed] [Google Scholar]
- 166.Pressman EK, Thornburg LL, Glantz JC, Earhart A, Wall PD, Ashraf M, et al. Inflammatory cytokines and antioxidants in midtrimester amniotic fluid: correlation with pregnancy outcome. Am J Obstet Gynecol. 2011;204:155–157. doi: 10.1016/j.ajog.2010.08.064. [DOI] [PubMed] [Google Scholar]
- 167.Thomakos N, Daskalakis G, Papapanagiotou A, Papantoniou N, Mesogitis S, Antsaklis A. Amniotic fluid interleukin-6 and tumor necrosis factor-alpha at mid-trimester genetic amniocentesis: relationship to intra-amniotic microbial invasion and preterm delivery. Eur J Obstet Gynecol Reprod Biol. 2010;148:147–151. doi: 10.1016/j.ejogrb.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 168.Malamitsi-Puchner A, Vrachnis N, Samoli E, Baka S, Iliodromiti Z, Puchner KP, et al. Possible early prediction of preterm birth by determination of novel proinflammatory factors in midtrimester amniotic fluid. Ann N Y Acad Sci. 2006;1092:440–449. doi: 10.1196/annals.1365.043. [DOI] [PubMed] [Google Scholar]
- 169.Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006;34:13–19. doi: 10.1515/JPM.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Biggio JR, Jr, Ramsey PS, Cliver SP, Lyon MD, Goldenberg RL, Wenstrom KD. Midtrimester amniotic fluid matrix metalloproteinase-8 (MMP-8) levels above the 90th percentile are a marker for subsequent preterm premature rupture of membranes. Am J Obstet Gynecol. 2005;192:109–113. doi: 10.1016/j.ajog.2004.06.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gerber S, Vial Y, Hohlfeld P, Witkin SS. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J Infect Dis. 2003;187:518–521. doi: 10.1086/368205. [DOI] [PubMed] [Google Scholar]
- 172.Wenstrom KD, Andrews WW, Hauth JC, Goldenberg RL, DuBard MB, Cliver SP. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol. 1998;178:546–550. doi: 10.1016/s0002-9378(98)70436-3. [DOI] [PubMed] [Google Scholar]
- 173.Chaiworapongsa T, Romero R, Tolosa JE, Yoshimatsu J, Espinoza J, Kim YM, et al. Elevated monocyte chemotactic protein-1 in amniotic fluid is a risk factor for pregnancy loss. J Matern Fetal Neonatal Med. 2002;12:159–164. doi: 10.1080/jmf.12.3.159.164. [DOI] [PubMed] [Google Scholar]
- 174.Yoon BH, Oh SY, Romero R, Shim SS, Han SY, Park JS, et al. An elevated amniotic fluid matrix metalloproteinase-8 level at the time of mid-trimester genetic amniocentesis is a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol. 2001;185:1162–1167. doi: 10.1067/mob.2001.117678. [DOI] [PubMed] [Google Scholar]
- 175.Dame C, Juul SE. The switch from fetal to adult erythropoiesis. Clin Perinatol. 2000;27:507–526. doi: 10.1016/s0095-5108(05)70036-1. [DOI] [PubMed] [Google Scholar]
- 176.Palis J, Segel GB. Developmental biology of erythropoiesis. Blood Rev. 1998;12:106–114. doi: 10.1016/s0268-960x(98)90022-4. [DOI] [PubMed] [Google Scholar]
- 177.Loukovaara M, Teramo K, Alfthan H, Hamalainen E, Stefanovic V, Andersson S. Amniotic fluid S100B protein and erythropoietin in pregnancies at risk for fetal hypoxia. Eur J Obstet Gynecol Reprod Biol. 2009;142:115–118. doi: 10.1016/j.ejogrb.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 178.Ferber A, Minior VK, Bornstein E, Divon MY. Fetal "nonreassuring status" is associated with elevation of nucleated red blood cell counts and interleukin-6. Am J Obstet Gynecol. 2005;192:1427–1429. doi: 10.1016/j.ajog.2004.12.076. [DOI] [PubMed] [Google Scholar]
- 179.Prandota J. Possible pathomechanisms of sudden infant death syndrome: key role of chronic hypoxia, infection/inflammation states, cytokine irregularities, and metabolic trauma in genetically predisposed infants. Am J Ther. 2004;11:517–546. doi: 10.1097/01.mjt.0000140648.30948.bd. [DOI] [PubMed] [Google Scholar]
- 180.Wolf RB, Moise KJ, Jr, Brace RA. Antibody-induced anemia in fetal sheep: model for hemolytic disease of the fetus and newborn. J Soc Gynecol Investig. 2001;8:224–232. doi: 10.1016/s1071-5576(01)00107-1. [DOI] [PubMed] [Google Scholar]
- 181.Thomas RM, Canning CE, Cotes PM, Linch DC, Rodeck CH, Rossiter CE, et al. Erythropoietin and cord blood haemoglobin in the regulation of human fetal erythropoiesis. Br J Obstet Gynaecol. 1983;90:795–800. doi: 10.1111/j.1471-0528.1983.tb09318.x. [DOI] [PubMed] [Google Scholar]
- 182.Jazayeri A, Tsibris JC, Spellacy WN. Fetal erythropoietin levels in growth-restricted and appropriately grown neonates with and without abnormal fetal heart rate tracings: a comparison with cord blood gases and Apgar scores. J Perinatol. 1999;19:255–259. doi: 10.1038/sj.jp.7200158. [DOI] [PubMed] [Google Scholar]
- 183.Widness JA, Susa JB, Garcia JF, Singer DB, Sehgal P, Oh W, et al. Increased erythropoiesis and elevated erythropoietin in infants born to diabetic mothers and in hyperinsulinemic rhesus fetuses. J Clin Invest. 1981;67:637–642. doi: 10.1172/JCI110078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Stonestreet BS, Goldstein M, Oh W, Widness JA. Effects of prolonged hyperinsulinemia on erythropoiesis in fetal sheep. Am J Physiol. 1989;257:R1199–R1204. doi: 10.1152/ajpregu.1989.257.5.R1199. [DOI] [PubMed] [Google Scholar]
- 185.Ferber A, Fridel Z, Weissmann-Brenner A, Minior VK, Divon MY. Are elevated fetal nucleated red blood cell counts an indirect reflection of enhanced erythropoietin activity? Am J Obstet Gynecol. 2004;190:1473–1475. doi: 10.1016/j.ajog.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 186.Maier RF, Gunther A, Vogel M, Dudenhausen JW, Obladen M. Umbilical venous erythropoietin and umbilical arterial pH in relation to morphologic placental abnormalities. Obstet Gynecol. 1994;84:81–87. [PubMed] [Google Scholar]
- 187.Dijkstra F, Jozwiak M, De MR, Duncan J, Hale N, Harding R, et al. Erythropoietin ameliorates damage to the placenta and fetal liver induced by exposure to lipopolysaccharide. Placenta. 2010;31:282–288. doi: 10.1016/j.placenta.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 188.Kumral A, Baskin H, Yesilirmak DC, Ergur BU, Aykan S, Genc S, et al. Erythropoietin attenuates lipopolysaccharide-induced white matter injury in the neonatal rat brain. Neonatology. 2007;92:269–278. doi: 10.1159/000105493. [DOI] [PubMed] [Google Scholar]
- 189.Mandel D, Oron T, Mimouni GS, Littner Y, Dollberg S, Mimouni FB. The effect of prolonged rupture of membranes on circulating neonatal nucleated red blood cells. J Perinatol. 2005;25:690–693. doi: 10.1038/sj.jp.7211389. [DOI] [PubMed] [Google Scholar]
- 190.Leikin E, Garry D, Visintainer P, Verma U, Tejani N. Correlation of neonatal nucleated red blood cell counts in preterm infants with histologic chorioamnionitis. Am J Obstet Gynecol. 1997;177:27–30. doi: 10.1016/s0002-9378(97)70433-2. [DOI] [PubMed] [Google Scholar]
- 191.Gomez R, Romero R, Ghezzi F, David SF, Berry SM. Are fetal hypoxia and acidemia causes of preterm labor and delivery? Am J Obstet Gynecol. 1997;176:S115. [Google Scholar]
- 192.Regulation of the acute phase and immune responses: interleukin-6. Ann N Y Acad Sci. 1989;557:1–583. [PubMed] [Google Scholar]
- 193.Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175:3463–3468. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- 194.Spiropoulos A, Goussetis E, Margeli A, Premetis E, Skenderi K, Graphakos S, et al. Effect of inflammation induced by prolonged exercise on circulating erythroid progenitors and markers of erythropoiesis. Clin Chem Lab Med. 2010;48:199–203. doi: 10.1515/CCLM.2010.034. [DOI] [PubMed] [Google Scholar]
- 195.Ernst LM, Gonzalez J, Ofori E, Elovitz M. Inflammation-Induced Preterm Birth in a Murine Model is Associated with Increases in Fetal Macrophages and Circulating Erythroid Precursors. Pediatr Dev Pathol. 2009;13:273–281. doi: 10.2350/09-05-0649-OA.1. [DOI] [PubMed] [Google Scholar]
- 196.Spellacy WN, McCarthy JM, Tsibris JC, Gilbert-Barness E, Downes KL. Fetal umbilical cord blood erythropoietin, interleukin-6, pH, pO(2), pCO(2), and base excess levels in histologic and/or clinical chorioamnionitis: is the response only inflammatory? Fetal Pediatr Pathol. 2009;28:239–246. doi: 10.3109/15513810903139699. [DOI] [PubMed] [Google Scholar]
- 197.Stachon A, Bolulu O, Holland-Letz T, Krieg M. Association between nucleated red blood cells in blood and the levels of erythropoietin, interleukin 3, interleukin 6, and interleukin 12p70. Shock. 2005;24:34–39. doi: 10.1097/01.shk.0000164693.11649.91. [DOI] [PubMed] [Google Scholar]
- 198.Hermansen MC. Nucleated red blood cells in the fetus and newborn. Arch Dis Child Fetal Neonatal Ed. 2001;84:F211–F215. doi: 10.1136/fn.84.3.F211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.