Abstract
Background
Bipolar disorder (BPD) research has identified a number of neurocognitive deficits as potential vulnerability markers; however, very few studies have focused on patterns of performance on affective processing tasks (e.g. Affective Go/No-Go tasks) which may be more closely tied to the pathophysiology of the illness. We previously reported that stable BPD patients demonstrate a response bias toward negative affective stimuli as compared with healthy controls and schizophrenia patients. The goal of the current study was to expand upon these prior findings to investigate these patterns in the unaffected siblings of BPD patients.
Methods
An affective Go/No-Go test was used to evaluate inhibitory response to negatively-valenced, positively-valenced, and neutral stimuli in 20 unaffected siblings of bipolar I patients versus 20 healthy controls. Accuracy (d′) and response bias (beta) served as dependent variables in a series of repeated measures ANCOVAs.
Results
We found a non-significant main effect for group when comparing accuracy performance (d′) on the Affective Go/No-Go of unaffected siblings vs. healthy controls. However, very similar to the pattern that we previously reported in stable BPD patients, unaffected siblings showed a response bias (beta) toward negatively valenced stimuli vs. healthy controls [F = 3.81; p =.03].
Limitations
Small sample size.
Conclusions
The current results extend our recent work which suggested that stable bipolar patients attend more readily to negative target stimuli than do schizophrenic or healthy subjects. These data, indicating that unaffected siblings also demonstrate an affective processing bias, implicate this task as a potential endophenotype in BPD.
Keywords: affective bias, affective go/no-go; unaffected siblings; endophenotype
Background
Endophenotypes are internal and intermediate phenotypes that link a disease to its genetic diathesis. They are thought to underlie clinical phenotypes in individuals meeting criteria for a disorder, as well as those at risk for the same disorder such as unaffected relatives of probands. Gottesman and Gould (2003) posited five criteria for the identification of endophenotypes. The primary criteria are that endophenotypes are associated with the illness, heritable, and state independent. Additionally, endophenotypes should co-segregate within families and be identified in unaffected relatives of probands at a higher rate than in the general population.
Research on endophenotypes in bipolar disorder (BPD) has accelerated in the past decade, and neurocognition has been distinguished as a promising domain in which to identify these markers. Neurocognitive deficits have been consistently demonstrated in BPD, suggesting a clear association with the illness (Martinez-Aran et al., 2000; Burdick et al. 2011). Additionally, research showing neurocognitive impairments in BPD patients during periods of remission has supported independence from clinical state (Clark et al. 2002; 2005; Ferrier et al. 1999; Liu et al. 2002; Thompson et al. 2005). A large body of evidence has demonstrated the heritability of neurocognitive skills (Ando et al. 2001; Fan et al. 2001; Finkel et al. 1995; Plomin and Defries, 1998; Swan et al. 1992); however, less research has examined the co-segregation/familiality of neurocognition in BPD. Early studies have supported familial aggregation of neurocognitive traits, with performance of the unaffected siblings of BPD patients falling at an intermediate level between the patient group and unrelated healthy controls (Bora et al. 2009).
Investigations of endophenotypes in BPD have identified a number of neurocognitive deficits as potential vulnerability markers of the disorder. Attention and executive deficits have been exhibited by both euthymic BPD patients (Clark et al., 2002; Clark et al., 2005) as well as unaffected relatives of BPD probands (Clark et al., 2005; Ferrier et al., 2004; Zalla et al., 2004), specifically in set-shifting, executive control, and processing speed. Additionally, euthymic BPD patients and their unaffected relatives have also demonstrated impairments in verbal learning and memory (Altshuler et al. 2004; Gourovitch et al. 1999; Keri et al. 2001).
One neurocognitive domain that has been the subject of a substantial literature in BPD, but not widely explored as a potential endophenotype of the disorder, is affective, or emotional, processing. Adult and pediatric BPD patients have exhibited impairments and biases in facial affect recognition (George et al. 1998; Guyer et al., 2007; Lennox et al. 2004; Venn et al., 2004), and one recent study has suggested that young individuals at risk for BPD show a generalized insensitivity to facial affect (Brotman et al., 2008). Functional magnetic resonance imaging (fMRI) research has corroborated these findings, showing abnormalities in BPD within brain regions responsible for processing facial affect (Dickstein et al., 2007; Malhi et al. 2007). While much of the literature concerning facial affect recognition was conducted on patients in varying phases of the disorder (i.e., mania, depression, euthymia), research highlights a link between this fundamental skill of emotional cognition and BPD.
Like facial affect recognition, other affective processing research has associated BPD with abnormal performance on Emotional Stroop paradigms, with supporting evidence for neurofunctional correlates during fMRI (Bentall and Thompson, 1990; Lagopoulos and Malhi, 2007; Lyon et al. 1999; Malhi et al. 2005). Additionally, BPD patients have demonstrated behavioral and functional abnormalities during emotional Go/No-Go tasks (Murphy and Sahakian, 2001; Wessa et al., 2007), including prior work by our group (Gopin et al. 2011).
No study to date has assessed patterns of performance on affective Go/No-Go tasks as a potential endophenotype in BPD. We have previously demonstrated that BPD patients exhibit a response bias toward negative affective stimuli as compared with healthy controls (Gopin et al. 2011). The purpose of the current study was to expand upon these prior findings and investigate the pattern of performance on this task in a group of unaffected siblings of patients with BPD as compared with unrelated healthy volunteers (with no family history of psychiatric illness). Performance profiles on these tasks may suggest affective processing abnormalities as a potential endophenotype of BPD.
Materials and Methods
Participants
Data were collected via an ongoing study (NIMH K23MH077807 to KEB), which was approved by the North Shore Long Island Jewish Health System (NSLIJHS) Institutional Review Board.
The unaffected siblings of the bipolar (BPD) patients were identified through direct contact with the affected sibling. BPD patients from the outpatient services at The Zucker Hillside Hospital (ZHH)-NSLIJHS were asked to contact their siblings to inquire about their interest in participating in the current study and the unaffected siblings were then instructed to contact study staff to set up an appointment. Details related to the recruitment and assessment of the patient sample are reported in our prior work (Gopin et al. 2011). In brief, confirmation of patients' diagnoses (Bipolar I or II) was made via the Structured Clinical Interview for DSM-IV, Patient edition (SCID – P; First et al. 1996) followed by a rigorous diagnostic consensus process.
A total of 20 unaffected siblings were recruited through direct contact by their bipolar sibling. Unaffected siblings were required to be past the modal age of onset for BPD (≥ 25 years but ≤ 65 years) and they could not be more than two years younger than their affected sibling was at the time of his/her illness onset. These criteria were set in place to reduce the likelihood that we were sampling from a group of unaffected siblings who would go on to later develop the disorder. In addition, unaffected siblings themselves could have no evidence of any major Axis I disorder. The healthy control (HC) subjects (n = 20) were recruited from community advertisement and had the same inclusion/exclusion criteria as the unaffected siblings but additionally could not have a first degree relative with any Axis I disorder. Unaffected siblings and HC participants were also administered the entire SCID to rule out Axis I disorders.
The full sample consisted of 16 men and 24 women with a mean age=40.52 +/- 11.19 years. Forty-five percent (45%) of the sample was Caucasian, 7.5% were Asian/Pacific Islander, 27.5% were African American of non-Hispanic origin, 17.5% were of Hispanic origin, and 2.5% were of mixed descent/other. The mean premorbid IQ, as measured by Wide Range Achievement Test-3rd edition-Reading subtest (WRAT-3) scores, was 101.00+/-7.51.
Measures
As part of their participation in the study, participants were administered a comprehensive assessment battery consisting of behavioral ratings, a structure diagnostic interview, an assessment of premorbid general intellectual functioning, and an affective processing task.
Diagnosis
The Structured Clinical Interview for DSM-IV (SCID-IV; First et al. 1996) was administered in full. The ZHH Research Division staff members have expertise in the use of the SCID and have demonstrable inter-rater reliability within the group (ICC > 0.8). Subjects' reports and data from all available medical records were compiled by the interviewer into a detailed narrative case summary, which was presented to senior faculty, trainees, and other trained SCID raters at a weekly diagnostic conference to establish a consensus DSM-IV diagnosis.
Current Mood Symptoms
The HAM-D (Hamilton, 1960) was used to assess the severity of participants' depressive symptoms. Current mania symptoms were assessed using the CARS-M (Altman et al. 1994), which provides a measure of manic features within the prior week, including a score for psychotic symptoms. ZHH raters are also trained to a high inter-rater reliability (ICC> 0.80) for these ratings.
Premorbid IQ and Affective Processing Assessment
Wide Range Achievement Test - Third Edition, Reading (WRAT-3; Wilkinson, 1993): The WRAT-3 Reading subtest assesses single-word reading skills. In this task, the participant is required to read 75 words of increasing difficulty. Scoring is based on correct pronunciation. Single word reading, like general knowledge and vocabulary, is believed to be particularly resistant to the effects of deterioration associated with brain disease (Nelson and O'Connell, 1978) and is considered an estimate of pre-morbid functioning in patient populations and a measure of general cognitive abilities in healthy individuals.
Affective Go-No Go. The test was comprised of three conditions: discrimination of positively-valenced words, discrimination of negatively-valenced words, and discrimination of animacy in words of neutral valence. Each condition was comprised of five blocks of 18 words each. Stimulus duration was 500 ms and interstimulus interval (ISI) was 1000 ms for all conditions. The ratio of targets to non-targets was 50:50 in all conditions to prevent development of an overt response-bias. Words in the emotional conditions had valences of greater than 1.93 (as based on Affective Norms of English Words; see Garolera et al. 2007), while words in the neutral condition had valences less than 1.90. For the emotional conditions nouns, verbs, and adjectives were utilized; only nouns were used for the animacy condition. Across all conditions, words were matched on frequency of use, word length, and imageability (i.e., how concrete versus abstract) using the MRC Psycholinguistic Database. In the positive affective condition, participants were instructed to press the spacebar on the keyboard whenever they viewed a word that they deemed to be positive (“happy” e.g sunshine) in the presence of negatively-valenced distracters. Similarly, in the negative condition, participants were requested to respond to words that they deemed to be negative (“sad, scary” e.g. gun) in the context of positive non-targets. In the neutral, animacy decision condition participants were told to respond to words representing a living thing (e.g. cat) versus a non-living object (e.g. house). This measure is conceptualized as a target detection task in which an individual's mood state may result in shifts in response bias, discriminability, and/or reaction time (RT) due to over- or under-processing of the various classes of stimuli (positive, negative, neutral).
Data Analysis
For each Affective Go/No-Go condition (negative, positive, neutral), the percent of correct responses was calculated, as was the percent of false alarms. Using these computations, d′ and beta were calculated (see Schultz et al. 2007 and Rosier et al. 2005, respectively) so that the accuracy (d′) and response bias (β) of the participants could be analyzed.
A series of repeated measures ANCOVAs was then conducted to analyze group differences in performance during the negative, positive, and neutral conditions of the Affective Go/No-Go; estimated premorbid IQ, HAM-D, and CARS-M scores served as covariates in the analyses. D′-accuracy and β served as the dependent variables. A series of follow-up one-way ANOVAs were then conducted to assess the nature of any significant interactions, with condition serving as the dependent variables.
Results
Subject groups were well matched demographically. Despite mood ratings that were well within the euthymic range for both groups, the unaffected siblings had significantly higher depression (HAM-D) ratings than the unrelated healthy controls and a trend-level elevation on mania (CARS-M) scores; therefore, these variables were included as covariates for all analyses. In addition, there was a trend toward higher premorbid IQ estimates in the healthy subjects and this was also included as a covariate in analyses. Participant characteristics are summarized in Table 1.
Table 1. Demographic and Clinical Features by Group.
| Unaffected Siblings Mean (SD) | Healthy Controls Mean (SD) | Statistic (p-value) | |
|---|---|---|---|
| Age | 39.15(10.54) | 41.90(11.91) | F = 0.60 (0.44) |
| Sex (% Female) | 70 | 50 | Chi2=4.20(0.12) |
| Race (% Caucasian) | 45 | 45 | Chi2=0.00(1.00) |
| WRAT-3 | 98.70 (9.39) | 103.30(4.08) | F = 4.04 (0.05) |
| HAM-D | 4.50(2.91) | 2.45(1.67) | F = 7.46 (0.01)* |
| CARS-M | 2.00 (2.66) | 0.78 (0.95) | F = 3.71 (0.06) |
p<.05
D′-accuracy
To examine the effect of group on d′-accuracy, a repeated measures ANCOVA (covarying for estimated premorbid IQ, HAM-D, and CARS-M scores) was conducted in which d′-accuracy during each condition (positive, negative, neutral) served as the dependent variable. There was a significant main effect of premorbid IQ [F=5.07; (1, 35); p=0.03] but there were no significant main effects for Subject type [F = 0.06 (1, 35); p =.81], HAM-D scores [F = 0.03 (1, 35), p =.88], or CARS-M scores [F=0.38 (1, 35); p=.54]. There was no significant main effect of Condition [F=0.10 (2, 34); p=.91] and no interaction effects were noted (data not shown).
Beta (β)
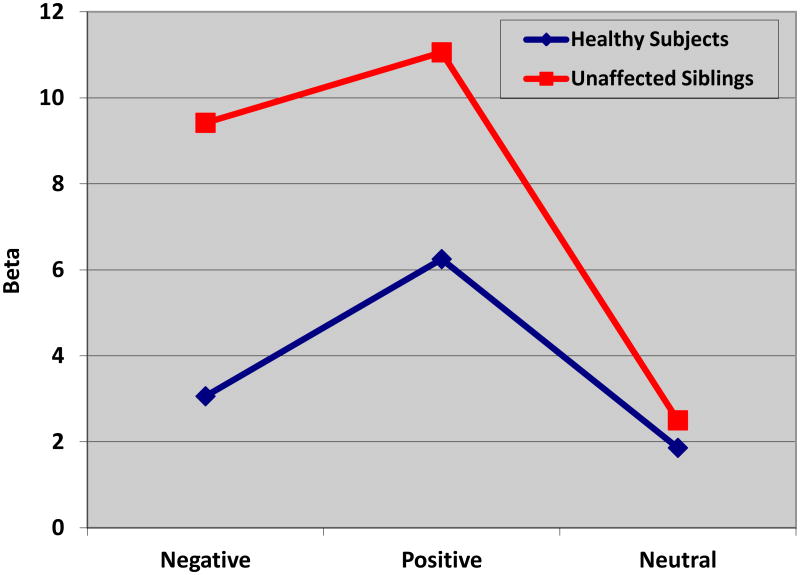
Similar analyses were then conducted to examine the group difference in response bias (β) across the three conditions. A repeated measures ANCOVA (covarying for estimated premorbid IQ, HAM-D scores, and CARS-M scores) was conducted in which β during each condition (positive, negative, neutral) served as the dependent variable. No significant main effects for HAM-D scores or CARS-M scores were noted; however, the main effect of premorbid IQ was significant [F=6.16 (1, 35); p=.02]. A main effect of Subject type was noted [F=4.68 (1, 35); p=.04] but there was no significant effect of Condition [F=1.99 (2, 34); p=.15]. Importantly, we detected an interaction effect for Subject type x Condition [F=3.81 (2, 34), p=.03].
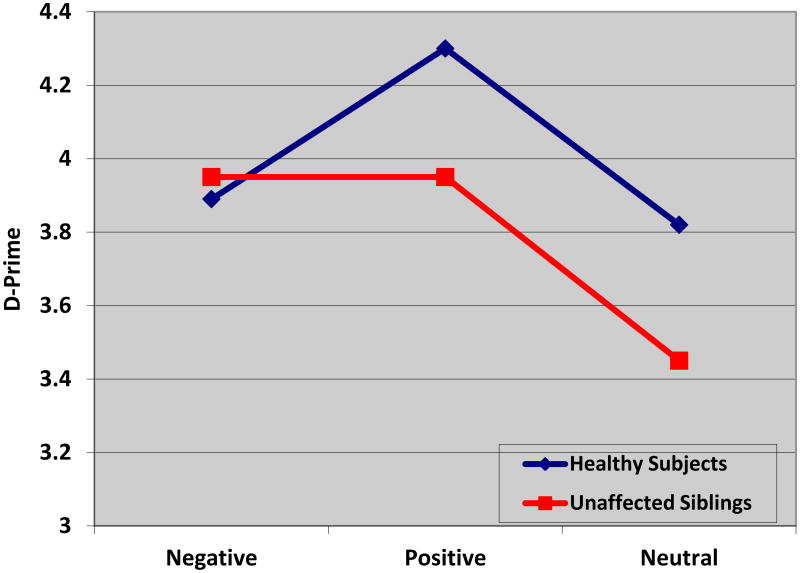
To further examine the interaction effect on β, a multivariate ANOVA was conducted in which β during each condition (positive, negative, neutral) served as the dependent variable. A significant difference between subject groups was revealed during the negative condition only [F = 5.01 (1, 39), p=.03], indicating that unaffected siblings were more biased during this condition than the healthy subjects. Non-significant differences by subject type were revealed for positive [F=1.34 (1, 39); p=.26] and neutral [F=0.10 (1,39); p=.76] conditions (Figure 1).
Figure 1. Group Performance on Affective Go/No-Go Accuracy (D′) × Condition.
The X-axis indicates the three conditions of the Affective Go/No-Go task and the Y-axis depicts the value of D-prime. The blue line represents unrelated healthy controls (n=20) and the red line represents the unaffected siblings (n=20). The results indicate no significant differences in accuracy of performance on any of the conditions.
Discussion
While other cognitive skills such as response inhibition, sustained attention, and verbal memory have been identified as endophenotypes of BPD (Bora et al. 2009), no study to date has implicated biased affective processing as a potential biomarker. Our results revealed a significant response bias towards negative stimuli in the unaffected siblings of bipolar patients when compared with unrelated healthy controls. The current results extend our recent work which suggested that bipolar patients–as opposed to individuals with schizophrenia and healthy controls–are more biased toward negative target stimuli (Gopin et al. 2011). Thus, the effect noted in stable probands has now been observed in their unaffected siblings to a greater degree than in individuals with no first degree psychiatric family history.
As the present results were significant in unaffected individuals, even after controlling for subclinical depression and mania ratings, they should not be attributed to emotional state. In other words, the negative bias exhibited by unaffected siblings in the present study was not due to subclinical depressive or manic symptomatology, but rather to some other underlying factor. This further supports the notion of negatively-biased affective processing as a genetically-influenced trait that may represent a good candidate endophenotype for BPD.
The idea that unaffected siblings of bipolar patients are more attentive to negative affective information highlights several potential implications, including the increased risk of developing an affective disorder. It has long been suggested that viewing one's environment through a negatively biased lens may lead to depression, which in turn fosters a negative view of the world, creating a cycle of biased affective experiences (Coyne, 1976). Thus a predisposition towards negative information–which appears to be common to both individuals with BPD (Gopin et al. 2011) and their unaffected siblings–may play a role in the development of depression. Of note, the unaffected siblings in this study have not in fact developed a mood disorder, despite this attentional bias towards negative information and an increased genetic loading. These unaffected siblings were specifically selected as a group of 1st degree relatives that is highly unlikely to go on to develop the disorder, (e.g. age above modal onset; more than two years older than their affected sibling was when he/she developed the disorder), making this a particularly valuable sample in which to evaluate resilience. There are likely to be important protective factors that are unique to the unaffected siblings' genetic makeup and/or environment that have served as preventative against threshold-level affective disturbances. These, as yet undefined, protective factors or compensatory mechanisms are of great interest in our efforts to better understand risk and resilience to psychiatric illnesses and will be the subject of future research.
The link between genetic risk and biased affective processing in BPD is apt to be mediated by neural abnormalities. As aforementioned, a wealth of neuroimaging research utilizing facial affect recognition (e.g., Dickstein et al., 2007; Malhi et al., 2007), emotional Stroop (Bentall and Thompson, 1990; Lyon et al., 1999; Lagopoulos and Malhi, 2007; Malhi et al., 2005), and go/no-go paradigms (Wessa et al. 2007) have revealed functional abnormalities in the affective processing of bipolar patients. Considering research showing that bipolar patients and their unaffected relatives evidence similar grey and white matter abnormalities (Chaddock et al., 2009; McDonald et al., 2004; van der Schot et al., 2009), the functional abnormalities observed during bipolar patients' affective and cognitive processing are likely present to some degree in unaffected siblings. While some previous studies have, in fact, illustrated these shared functional abnormalities within other cognitive domains (Drapier et al., 2008; Thermenos et al., 2009), little work has been done within the area of affective or emotion-based processing. A recent study revealed regional cerebral hyperactivation in both bipolar patients and their unaffected first-degree relatives in response to facial emotional expressions of fear and happiness (Surguladze et al., 2010). Thus, given the results of the current study, it would be expected that bipolar patients and their unaffected siblings demonstrate similar functional abnormalities in response to other related affective processing tasks, such as emotional go/no-go.
There are several limitations of the current study. First, the small sample size reduced our power and thus our ability to identify small effects. Nonetheless, our significant results are notable given the relatively limited power. In addition, our sample size is comparable to that of previous research in the area (e.g., Kulkarni et al. 2010) and our data are highly consistent with our recent findings in a much larger bipolar sample (Gopin et al. 2011). However, future research should seek to utilize larger samples when further investigating affective processing bias in this population and, where possible, include additional measures such as functional magnetic resonance imaging and/or electroencephalography to further elucidate the underlying neural networks involved. Second, while the unaffected sibling group was free of current Axis I disorders, some individuals in this group had a history of other clinical presentations such as substance abuse. As described above, however, none met criteria for current Axis I disorder and no subject had a current diagnosis of substance use disorder. In addition, our sample of unaffected siblings was free of psychotropic medications, which represents a strength of the current study.
In conclusion, the current study identified a response bias toward negative affective information in the unaffected siblings of bipolar patients as compared to unrelated healthy controls. In light of previous work done by our research group showing a similar effect for stable bipolar patients, this finding suggests affective processing bias as a potential endophenotype in BPD, and supports future efforts to replicate and expand upon these findings.
Figure 2. Group Performance on Affective Go/No-Go Response Bias (β) × Condition.
The X-axis indicates the three conditions of the Affective Go/No-Go task and the Y-axis depicts the value of β. The blue line represents unrelated healthy controls (n=20) and the red line represents the unaffected siblings (n=20). The results indicate a significantly increased negative bias in the unaffected siblings as compared with healthy controls. No significant differences were noted on positive or neutral conditions.
Acknowledgments
The authors would like to thank the patients who participated in the study and to acknowledge Denise Coscia, Gail Reiter, James Riley, Brady Berman, Nisha Chitkara, and Anu Tyagi, all of whom played a critical role in the data collection.
Financial support for this work was received from the National Institute of Mental Health [K23MH077807 (to KEB)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman EG, Hedeker DR, Janicak PG, Peterson JL, Davis JM. The clinician-administered rating scale for mania (CARS-M): Development, reliability, and validity. Biological Psychiatry. 1994;36:24–134. doi: 10.1016/0006-3223(94)91193-2. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Ventura J, Van Gorp WG, Green MF, Theberge DC, Mintz J. Neurocognitive function in clinically stable men with bipolar disorder or schizophrenia and normal control subjects. Biological Psychiatry. 2004;56:560–569. doi: 10.1016/j.biopsych.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Ando J, Ono Y, Wright MJ. Genetic structure of spatial and verbal working memory. Behavioral Genetics. 2001;31:615–624. doi: 10.1023/a:1013353613591. [DOI] [PubMed] [Google Scholar]
- Bentall RP, Thompson M. Emotional Stroop performance and the manic defence. British Journal of Clinical Psychology. 1990;29:235–237. doi: 10.1111/j.2044-8260.1990.tb00877.x. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first degree relatives. Journal of Affective Disorders. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Skup M, Rich BA, Blair KS, Pine DS, Blair JR, et al. Risk for bipolar disorder is associated with face-processing deficits across emotions. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:1455–1461. doi: 10.1097/CHI.0b013e318188832e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick KE, Goldberg TE, Cornblatt BA, Keefe RS, Gopin CB, Derosse P, Braga RJ, Malhotra AK. The MATRICS Consensus Cognitive Battery in Patients with Bipolar I Disorder. Neuropsychopharmacology. 2011;36(8):1587–1592. doi: 10.1038/npp.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock CA, Barker GJ, Marshall N, Schulze K, Hall MH, Fern A, et al. White matter microstructural impairments and genetic liability to familial bipolar I disorder. British Journal of Psychiatry. 2009;194:527–534. doi: 10.1192/bjp.bp.107.047498. [DOI] [PubMed] [Google Scholar]
- Clark L, Iversen SD, Goodwin GM. Sustained attention deficit in bipolar disorder. British Journal of Psychiatry. 2002;180:313–319. doi: 10.1192/bjp.180.4.313. [DOI] [PubMed] [Google Scholar]
- Clark L, Kempton MJ, Scarna A, Grasby PM, Goodwin GM. Sustained attention deficit confirmed in euthymic bipolar disorder but not in first degree relatives of bipolar patients or euthymic unipolar depression. Biological Psychiatry. 2005a;57:183–187. doi: 10.1016/j.biopsych.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Clark L, Sarna A, Goodwin GM. Impairment of executive function but not memory in first-degree relatives of patients with bipolar I disorder and in euthymic patients with unipolar depression. American Journal of Psychiatry. 2005b;162:1980–1982. doi: 10.1176/appi.ajp.162.10.1980. [DOI] [PubMed] [Google Scholar]
- Coyne JC. Toward an interactional description of depression. Psychiatry: Interpersonal and Biological Processes. 1976;39:28–40. doi: 10.1080/00332747.1976.11023874. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Rich BA, Roberson-Nay R, Berghorst L, Vinton D, Pine DS, et al. Neural activation during encoding of emotional faces in pediatric bipolar disorder. Bipolar Disorders. 2007;9:679–692. doi: 10.1111/j.1399-5618.2007.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapier D, Surguladze S, Marshall N, Schulze K, Fern A, Hall H, et al. Genetic liability for bipolar disorder is characterized by excess frontal activation in response to a working memory task. Biological Psychiatry. 2008;115:513–520. doi: 10.1016/j.biopsych.2008.04.038. [DOI] [PubMed] [Google Scholar]
- Fan J, Wu Y, Fossella JA, Posner MI. Assessing the heritability of attentional networks. BioMedCentral Neuroscience. 2001;2:14. doi: 10.1186/1471-2202-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier IN, Chowdury R, Thompson JM, Watson S, Young AH. Neurocognitive function in unaffected first-degree relatives of patients with bipolar disorder: a preliminary report. Bipolar Disorders. 2004;6:319–322. doi: 10.1111/j.1399-5618.2004.00122.x. [DOI] [PubMed] [Google Scholar]
- Ferrier IN, Stanton BR, Kelly TP, Scott J. Neuropsychological function in euthymic patients with bipolar disorder. British Journal of Psychiatry. 1999;175:246–251. doi: 10.1192/bjp.175.3.246. [DOI] [PubMed] [Google Scholar]
- Finkel D, Pedersen NL, McGue M, McClearn GE. Heritability of cognitive abilities in adult twins: Comparison of Minnesota and Swedish data. Behavioral Genetics. 1995;25:421–431. doi: 10.1007/BF02253371. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version. Washington, D.C.: American Psychiatric Press, Inc.; 1996. [Google Scholar]
- Garolera M, Coppola R, Muñoz KE, Elvevåg B, Carver FW, Weinberger DR, et al. Amygdala activation in affective priming: a magnetoencephalogram study. Neuroreport. 2007;18:1449–53. doi: 10.1097/WNR.0b013e3282efa253. [DOI] [PubMed] [Google Scholar]
- George MS, Huggins T, McDermut W, Parekh PI, Rubinow D, Post RM. Abnormal facial emotion recognition in depression: Serial testing in an ultra-rapid-cycling patient. Behavior Modification. 1998;22:192–204. doi: 10.1177/01454455980222007. [DOI] [PubMed] [Google Scholar]
- Gopin CB, Burdick KE, DeRosse P, Goldberg TE, Malhotra AK. Emotional modulation of response inhibition in stable patients with bipolar I disorder: A comparison with healthy and schizophrenia subjects. Bipolar Disorders. 2011;13(2):164–172. doi: 10.1111/j.1399-5618.2011.00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gourovitch ML, Torrey EF, Gold JM, Randolph C, Weinberger DR, Goldberg TE. Neuropsychological performance of monozygotic twins discordant for bipolar disorder. Biological Psychiatry. 1999;45:639–646. doi: 10.1016/s0006-3223(98)00148-6. [DOI] [PubMed] [Google Scholar]
- Guyer AE, McClure EB, Adler AD, Brotman MA, Rich BA, Kimes AS, et al. Specificity of facial expression labeling deficits in childhood psychopathology. Journal of Child Psychology and Psychiatry. 2007;48:863–871. doi: 10.1111/j.1469-7610.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keri S, Kelemen O, Benedek G, Janka Z. Different trait markers for schizophrenia and bipolar disorder: a neurocognitive approach. Psychological Medicine. 2001;31:915–922. doi: 10.1017/s0033291701004068. [DOI] [PubMed] [Google Scholar]
- Kulkarni S, Jain S, Reddy YC, Kumar KJ, Kandavel T. Impairment of verbal learning and memory and executive function in unaffected siblings of probands with bipolar disorder. Bipolar Disorders. 2010;12:647–656. doi: 10.1111/j.1399-5618.2010.00857.x. [DOI] [PubMed] [Google Scholar]
- Lagopoulos J, Malhi GS. A functional magnetic resonance imaging study of emotional Stroop in euthymic bipolar disorder. Brain Imaging. 2007;18:1583–1587. doi: 10.1097/WNR.0b013e3282efa07a. [DOI] [PubMed] [Google Scholar]
- Lennox BR, Jacob R, Calder AJ, Lupson V, Bullmore ET. Behavioural and neurocognitive responses to sad facial affect are attenuated in patients with mania. Psychological Medicine. 2004;34:795–802. doi: 10.1017/s0033291704002557. [DOI] [PubMed] [Google Scholar]
- Liu SK, Chiu CH, Chang CJ, Hwang TJ, Hwu HG, Chen WJ. Deficits in sustained attention in schizophrenia and affective disorders: Stable versus state-dependent markers. American Journal of Psychiatry. 2002;159:975–982. doi: 10.1176/appi.ajp.159.6.975. [DOI] [PubMed] [Google Scholar]
- Lyon HM, Startup M, Bentall RP. Social cognition and the manic defense: Attributions, selective attention, and self-schema in bipolar affective disorder. Journal of Abnormal Psychology. 1999;108:273–282. doi: 10.1037//0021-843x.108.2.273. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Lagopoulos J, Sachdev PS, Ivanovski B, Shnier R. An emotional Stroop functional MRI study of euthymic bipolar disorder. Bipolar Disorders. 2005;7:58–69. doi: 10.1111/j.1399-5618.2005.00255.x. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Lagopoulos J, Sachdev PS, Ivanovski B, Shnier R, Ketter T. Is a lack of disgust something to fear? A functional magnetic resonance imaging facial emotion recognition study in euthymic bipolar disorder patients. Bipolar Disorders. 2007;9:345–357. doi: 10.1111/j.1399-5618.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Arán A, Vieta E, Colom F, Reinares M, Benabarre A, Gastó C, et al. Cognitive dysfunctions in bipolar disorder: evidence of neuropsychological disturbances. Psychotherapy and Psychosomatics. 2000;69:2–18. doi: 10.1159/000012361. [DOI] [PubMed] [Google Scholar]
- McDonald C, Bullmore ET, Sham PC, Chitnis X, Wickham H, Bramon E, et al. Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Archives of General Psychiatry. 2004;61:974–984. doi: 10.1001/archpsyc.61.10.974. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Sahakian BJ. Neuropsychology of bipolar disorder. British Journal of Psychiatry. 2001;41:s120–127. [PubMed] [Google Scholar]
- Nelson H, O'Connell A. Dementia: The estimation of premorbid intelligence levels using the new adult reading test. Cortex. 1978;14:234–244. doi: 10.1016/s0010-9452(78)80049-5. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC. The genetics of cognitive abilities and disabilities. Scientific American. 1998;278:62–69. doi: 10.1038/scientificamerican0598-62. [DOI] [PubMed] [Google Scholar]
- Rosier JP, McLean A, Ogilvie AD, Blackwell AD, Bamber DJ, Goodyer I, et al. The subjective and cognitive effects of acute phenulalanine and tyrosine depletion in patients recovered from depression. Neuropsychopharmacology. 2005;30:775–785. doi: 10.1038/sj.npp.1300659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KP, Fan J, Magidina O, Marks DJ, Hahn B, Halperin JM. Does the emotional go/no-go task really measure behavioral inhibition? Convergence with measures of a non-emotional analog. Archives of Clinical Neurophysiology. 2007;22:151–160. doi: 10.1016/j.acn.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze SA, Marshall N, Schulze K, Hall MH, Walshe M, Bramon E, et al. Exaggerated neural response to emotional faces in patients with bipolar disorder and their first-degree relatives. Neuroimage. 2010;53:58–64. doi: 10.1016/j.neuroimage.2010.05.069. [DOI] [PubMed] [Google Scholar]
- Swan GE, LaRue A, Carmelli D, Reed TE, Fabsitz RR. Decline in cognitive performance in aging twins. Heritability and biobehavioral predictors from the National Heart, Lung, and Blood Institute Twin Study. Archives of Neurology. 1992;49:476–481. doi: 10.1001/archneur.1992.00530290058012. [DOI] [PubMed] [Google Scholar]
- Thermenos HW, Goldstein JM, Milanovic SM, Whitfield-Gabrieli S, Makris N, Laviolette P, et al. An fMRI study of working memory in persons with bipolar disorder or at genetic risk for bipolar disorder. American Journal of Medical Genetics. Part B, Neuropsychiatry genetics. 2010;153B:120–131. doi: 10.1002/ajmg.b.30964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JM, Gallagher P, Hughes JH, Watson S, Gray JM, Ferrier IN, et al. Neurocognitive impairment in euthymic patients with bipolar disorder. British Journal of Psychiatry. 2005;186:32–40. doi: 10.1192/bjp.186.1.32. [DOI] [PubMed] [Google Scholar]
- van der Schot AC, Vonk R, Brans RGH, van Haren NEM, Koolschijn PC, Nuboer V, et al. Influence of genes and environment on brain volumes in twin pairs concordant and discordant for bipolar disorder. Archives of General Psychiatry. 2009;66:142–151. doi: 10.1001/archgenpsychiatry.2008.541. [DOI] [PubMed] [Google Scholar]
- Venn HR, Gray JM, Montagne B, Murray LK, Burt DM, Frigerio E, et al. Perception of facial expressions of emotion in bipolar disorder. Bipolar Disorders. 2004;6:286–293. doi: 10.1111/j.1399-5618.2004.00121.x. [DOI] [PubMed] [Google Scholar]
- Wessa M, Houenou J, Paillere-Martinot M, Berthoz S, Artiges E, Leboyer M, et al. Fronto-striatal overactivation in euthymic bipolar patients during an emotional Go/NoGo task. The American Journal of Psychiatry. 2007;164:638–646. doi: 10.1176/ajp.2007.164.4.638. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. Wide-Range Achievement Test 3: Administration Manual. Wilmington, Del.: Wide Range; 1993. [Google Scholar]
- Zalla T, Joyce C, Szoke A, Schurhoff F, Pillon B, Komano O, et al. Executive dysfunctions as potential markers of familial vulnerability to bipolar disorder and schizophrenia. Psychiatry Ressearch. 2004;121:207–217. doi: 10.1016/s0165-1781(03)00252-x. [DOI] [PubMed] [Google Scholar]