Abstract
There have been major advances in our understanding of the cellular and molecular biology of the human malignancies collectively referred to as ovarian cancer. At a recent Helene Harris Memorial Trust meeting, an international group of researchers considered actions that should be taken to improve the outcome for women with ovarian cancer. Nine major recommendations are outlined in this Perspective.
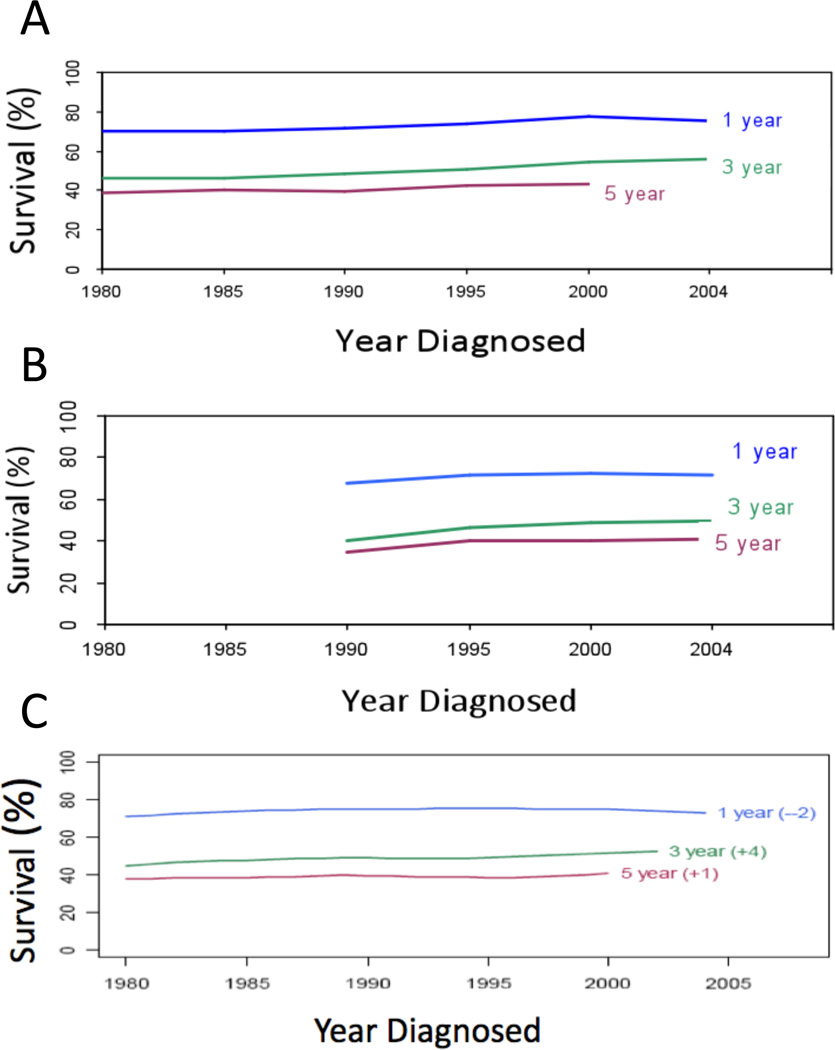
Patient prognosis has improved significantly for many solid cancer types. However, survival of women with epithelial ovarian cancer has changed little since platinum-based treatment was introduced over 30 years ago1–3 (Figure 1). Invasive epithelial ovarian cancer is widely viewed and treated as a single disease entity with little stratification of histological or molecular subtypes. At a recent Helene Harris Memorial Trust (HHMT) meeting of leading ovarian cancer researchers and clinician-scientists, sponsored and organised by Ovarian Cancer Action (Box 1), we asked the question ‘what actions can we take to improve the outcome for women with ovarian cancer?’ A consensus regarding the major barriers to success and the most pressing questions led delegates to propose nine priorities for action, which we describe in this Perspective.
Figure 1. Survival from ovarian cancer.
One-, three- and five-year survival post-diagnosis of ovarian cancer patients over the past 20 years. Data from: A) Surveillance, Epidemiology and End Results (SEER, 1980–2004); B) The Cancer Council of Victoria, Victoria, Australia (1990–2004); C) The Cheryl Brown Outcomes Unit, British Columbia, Canada (1980–2004).
Ovarian cancer is many diseases
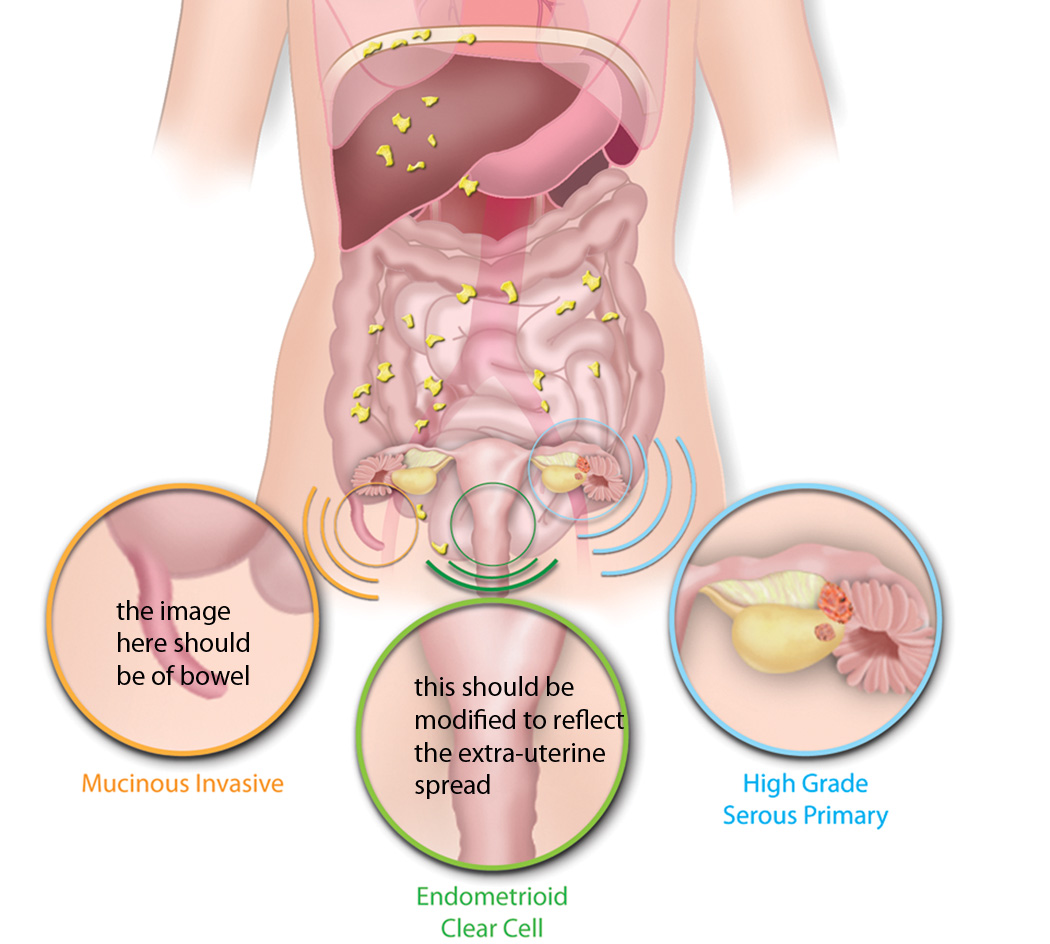
The group felt it was essential that researchers, pathologists, epidemiologists and clinicians understood that ovarian cancer is a general term for a series of molecularly and etiologically distinct diseases that simply share an anatomical location. Recent pathological and genomic findings indicate that many ovarian cancers are derived from non-ovarian tissues and that the different histotypes share few molecular similarities (Figure 2)4. For example, the distal fallopian tube has been identified as a source of high-grade serous ovarian cancers5–7. The relative importance of the fallopian tube versus the ovarian surface epithelium in the genesis of high-grade serous ovarian cancers is still debated, however, the finding has important implications for screening, prevention and understanding the molecular biology of the disease. Clear cell and endometrioid cancers have a strong epidemiological link with endometriosis. High frequency somatic mutations of the PI3K catalytic subunit PIK3CA and AT-rich interactive domain-containing protein 1A (ARID1A) in adjacent endometriotic lesions link endometriosis, clear cell and endometrioid cancers8–10. The majority of invasive mucinous cancers are metastases to the ovary11,12,13. Improved histotype classification using immunological markers and genomic studies has shown that many tumors previously designated high-grade endometrioid cancers should be classified as serous cancers14–16. Low-grade invasive tumors are still regarded as ovarian-derived, but the initiating cells are unknown and it is possible that their site of origin will be re-evaluated in the future. Nevertheless, it is clear that serous borderline cancers are not precursor lesions for the majority of high-grade serous ovarian cancer, as they have a distinct spectrum of mutational events17.
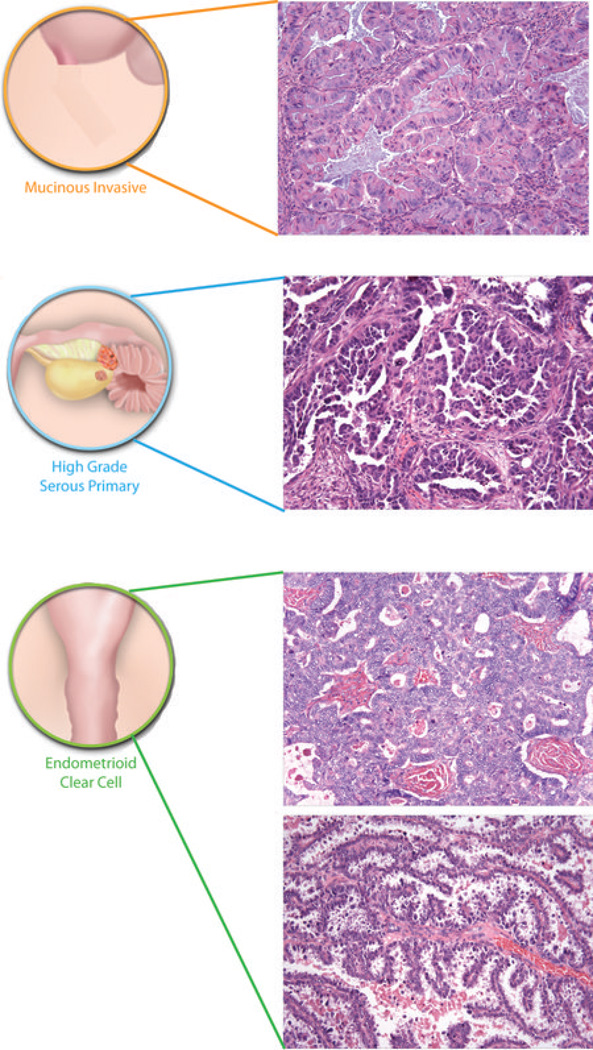
Figure 2. The origins of ‘ovarian’ cancer.
Ovarian cancer is a collective term for invasive cancers derived from different tissues. A majority of invasive mucinous ovarian cancers are metastases to the ovary, often from the gastrointestinal tract including colon (Co), appendix (Ap) or stomach (St). Endometrioid and clear cell ovarian cancers are derived from endometriosis, which in turn is associated with retrograde menstruation (blue arrow) from the endometrium (En). High grade serous ovarian cancers are derived from the surface of the ovary (Ov) and/or distal fallopian tube (FT) - the relative contribution that the two sites make to these tumours remains unclear. Benign and low malignant potential (borderline) tumours are not included in the diagram. Such tumours are thought to be of ovarian origin, however, the originating cells are not defined and their derivation may be revised in the future. Histological images courtesy of R. Drapkin, Dana-Farber Cancer Institute, USA, and C. Crum, Brigham and Women's Hospital, USA.
Further sub-classification of histotypes is based on signaling pathway activation18, genomic events or gene expression profiling19. Some ovarian cancers have more in common with certain types of renal or breast cancer than other ovarian histologies. For example, high-grade serous ovarian cancers share genomic and transcriptional features with basal-like breast cancers17, 20. Ovarian clear cell cancers have similar expression phenotypes to renal and uterine clear cell cancers21, and women with ovarian clear cell cancer may benefit from the use of drugs such as sunitinib that are active in renal cancer patients22. Taking a rigorous view, the ovarian histotypes should be regarded as distinct diseases, as their cell of origin, epidemiology, and driver mutations are quite different.
The term ‘ovarian cancer’ is therefore misleading. It is not a single disease, and a significant proportion of tumours may not arise from ovarian tissue. The unifying clinical feature is frequent loco-regional dissemination to the ovary and related pelvic organs. We considered whether ‘ovarian cancer’ should be replaced with ‘pelvic’ or ‘peritoneal’ cancer but recognised the confusion that might ensue for patients, physicians and the literature, especially during a transition period. It is appropriate to allow the changed view to become more widely understood before the term is abandoned.
Improved clinical trial design
The second action point was that there should be a shift in the emphasis from Phase III to nimble, earlier phase trials that explicitly include molecular studies. Given the differences in ovarian cancer subtypes, clinical trials should no longer aggregate the various histologies. Trial design should also consider molecular parallels with other solid cancers. Clinical reporting and scientific publications should use reproducible diagnostic methods and standardized terms for these disease entities23. The current single approach to treatment should rapidly evolve to evidence-based stratification based on molecular drivers and histotype-specific treatments.
The ovarian cancer field lags in incorporating targeted therapies into standard treatment (Figure 3). With the possible exception of poly(ADP-ribose) polymerase (PARP) and angiogenesis inhibitors (see below), single agent molecularly targeted therapies have only yielded small increments in progression-free survival in ovarian cancers. A major shift in the way clinical trials are designed is therefore urgently required. The ceiling in efficacy witnessed with all-inclusive Phase III trials means that the priority should now be subtype specific (randomised) Phase II clinical trials, based on sound scientific rationale. In addition, ‘window trials’24 with clear readouts of pathway inhibition that correlate with clinical outcome, will expedite clinical investigation of novel therapies.
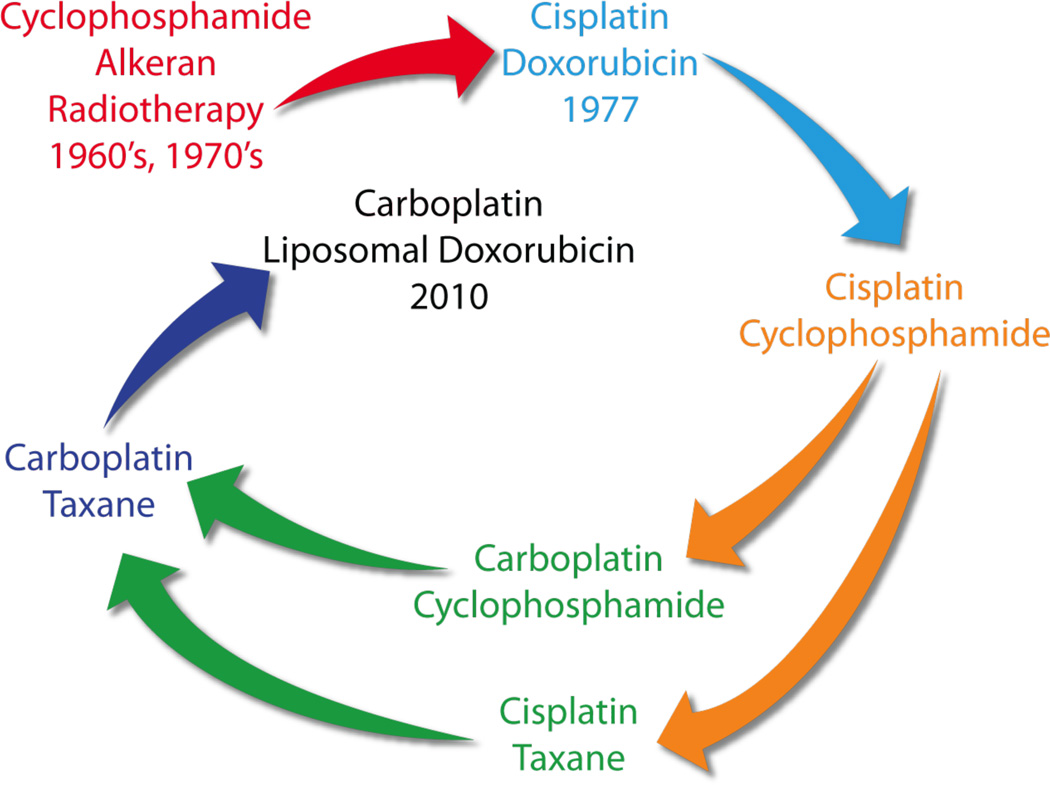
Figure 3. Evolution of chemotherapy for ovarian cancer over the last 50 years.
It has proved difficult to progress beyond platinum-based therapy, which was introduced in the late 1970s and remains standard of care. Cisplatin and subsequently carboplatin, which has a lower toxicity profile, have been combined with other agents, including taxanes. Most recently, liposomal doxorubicin has become commonly used with carboplatin, especially in a relapse setting. It is notable that the combination of carboplatin and liposomal doxorubicin involve similar drugs to those used in the mid-70s, albeit with reduced side-effects. It is likely that ovarian cancer treatment will evolve significantly in the coming years with the introduction of molecularly targeted agents, such as the poly(ADP-ribose) polymerase PARP inhibitors, histotype-specific treatments, and dose dense regimes, including use of weekly taxane.
Improved blood and imaging biomarkers that accurately measure response and residual disease are also needed; the current criteria are an impediment to better evaluation of response. Contrast-enhanced computed tomography (CT) imaging is the standard nonsurgical method for staging and assessing response, however, it is difficult to detect small peritoneal deposits. For example when peritoneal disease is <1cm, the sensitivity of CT is only 7–28%, and this is further dependent upon anatomical location25. Measurement of circulating levels of the ovarian tumour antigen CA125 (also known as Mucin-16) is routinely used to monitor disease recurrence, however, markers that are molecularly-based and sensitive for low volume residual disease are needed. Preliminary results from detection of cellular by-products of ovarian cancer cells in blood are promising but need wider evaluation26.
Prevention and early detection
The third action point was to recognize that identification of patients at increased genetic risk currently offers the most effective measure for prevention and early detection of disease. High-grade serous ovarian cancer is diagnosed at an advanced stage in approximately 70% of patients, and these women have a significantly worse outcome than those with early stage disease. It is important to recognise that the poor prognosis in advanced stage disease is a function of at least two factors – the extent of disease and therefore the ability to remove the tumour surgically, and differences in the biology of tumours that remain confined to the pelvic space versus those that disseminate widely27. Early detection tests should focus on identifying the precursors of advanced stage high-grade serous ovarian tumours, rather than the molecularly distinct stage I/II low-grade tumours with Ras pathway activation, which are typically not precursors of more lethal tumours.
Traditional concepts of metastasis are difficult to apply to ovarian cancer. In high-grade serous carcinoma originating from the ovarian surface or fallopian tubes, or clear cell cancer arising in endometriotic deposits, there is no anatomical barrier to seeding throughout the peritoneal cavity. Emerging insights into disease progression of high-grade serous ovarian cancers now suggest that early detection of low-volume advanced stage, rather than early-stage, ovarian cancer may be a more realistic goal of screening studies28 although the clinical value of this strategy remains to be determined. More data about the natural history of precursor lesions in the fallopian tube epithelium and their rate of transformation to invasive carcinoma is needed to plan future screening methods and technologies29. Recent data from the Prostate, Lung, Colorectal and Ovarian (PLCO) trial indicate that current serological diagnostics and imaging tests are insufficiently sensitive to alter outcome in screened populations30 although results from the large UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) trial remains to be reported. Based on current data, widespread screening is not yet justified and its value in the management of high-risk women is unknown31. Screening for endometrioid and clear cell ovarian cancer will be particularly challenging since endometriosis is a common, often subclinical, disease and risk factors for transformation have not yet been identified. Identification of women carrying germline mutations in BRCA1, BRCA2, RAD51C and other DNA repair proteins provides the most effective preventative strategy for high-grade serous ovarian cancers, as removal of the ovaries and fallopian tubes can reduce risk of disease in carriers by 80%32, 33. Given the newly appreciated importance of the fallopian tube in the genesis of high-grade serous ovarian cancer, it is recommended that complete removal of the fallopian tube should become standard of care in any woman undergoing hysterectomy and/or oophorectomy. Removal of the ovaries (oophorectomy) in pre-menopausal women induces early menopause. As a consequence, and with the changed view of the role of the fallopian tube in ovarian cancer, some have recommended that only the fallopian tubes be removed (salpingectomy) in women with germline BRCA1 or BRCA2 mutations, or a strong family history of breast and/or ovarian cancer34. However, until comprehensive comparative data are available, the group felt it is premature to recommend that only the fallopian tubes are removed.
While there is no evidence that current screening strategies reduce mortality in high risk women, it seems inevitable that effective early detection screening must be coupled with the identification of those women at increased genetic risk, to reduce the number of false positives30. Advances in high throughput sequencing and mutation scanning offer the potential to greatly reduce the cost of mutation testing, and therefore extend testing to more women, especially women with high-grade serous ovarian cancers. Recent genome wide association studies have identified a number of new ovarian cancer risk loci35, 36, enabled by large cancer cohorts assembled by research consortia (see below). Identification of novel genetic risk loci is improved by histotype stratification35, 36, and therefore genetic and epidemiological studies of tumours should embrace the appropriate classification of ovarian cancer.
Identification of new targets
The fourth action was to recognize the need for novel approaches to identify therapeutic targets. Over the last decade, increasingly comprehensive genomic analyses of ovarian cancer have failed to reveal new oncogenic drivers in the more common histotypes. Large scale gene expression19, DNA copy number37, 38 and mutational screens39 have not identified new high frequency drugable targets in high grade serous ovarian cancers, perhaps with the possible exception of CyclinE1 gene (CCNE1). CCNE1 amplification is associated with poor outcome40 and oncogene addiction in vitro41. Further studies are needed to validate the prognostic significance of other amplified genes in high grade serous ovarian cancers and develop therapeutic approaches to targeting them. Mutations in the RAS pathway are found in more than 70% of low-grade serous ovarian cancers and specific clinical trials for this unique serous neoplasm are urgently needed. Activating mutations in PIK3CA8, and amplification of receptor tyrosine kinases including MET22 and ERBB242, provide novel therapeutic approaches in clear cell cancers. Although high frequency novel somatic mutations have been found in granulosa43 and clear cell10 ovarian cancer, these uncommon subtypes only account for a small fraction of the disease mortality.
Genomic studies have reinforced the centrality of mutations in the tumour suppressor TP5320, BRCA pathway disruption44 and homologous recombination repair (HRR) deficiency in high-grade serous ovarian cancers. The notion that many high-grade serous ovarian cancers are BRCA-like through alterations in other proteins in the HRR pathway45 has resulted in exploration of PARP inhibitors46. Preliminary findings suggest efficacy of PARP inhibitors in a number of settings, including the use of olaparib as maintenance treatment after chemotherapy in women with platinum sensitive recurrent high-grade serous ovarian cancers47. About half of all high-grade serous ovarian cancers show disruption of the BRCA pathway either by germline or somatic mutation, or epigenic silencing of pathway members39. Consistent with mutational studies39, functional assays show that roughly half of all high-grade serous ovarian cancers have defective formation of HRR foci following DNA damage48. If the repair-defective tumours correspond to those patients with relatively good responses to PARP inhibitors, this may provide an important predictive test of response. These data suggest that mutator phenotypes in high-grade serous ovarian cancer offer a valuable approach to novel therapies49, 50. Synthetic lethality as a strategy for targeting DNA repair and p53 pathways in high-grade serous ovarian cancer should be further explored with RNA interference and small molecule screens.
One of the most challenging aspects of high-grade serous ovarian cancer is the recognition that widespread gene expression and DNA copy number changes provide extensive opportunities for adaptation and the development of resistance17. Ongoing genomic instability may drive intra-tumoral genetic heterogeneity and increase the probability of treatment-resistant clone selection51, 52. Genetic alterations in tumour cells are not the only contributor to therapy resistance. Cell-non-autonomous effects are mediated by the tumour microenvironment, which is recruited by the tumour cells (see also below), providing a cytokine milieu on which the tumour thrives, becomes resistant to current therapies, and becomes more invasive. Single agent treatment is likely to be ineffective when cancerous cells are capable of utilising other non-targeted pathways. Therefore, it is crucial that we develop combination therapeutic approaches that anticipate tumour adaptive responses to individual targeted agents. The exploration of novel combinations of targeted therapeutics will only be possible if we can overcome the current reluctance of the pharmaceutical industry to be involved in clinical trials that combine drugs from different companies. Combination studies must be underpinned by preclinical investigation of dosing schedules in appropriate model systems.
The tumor microenvironment
The fifth point was to emphasize the importance of targeting the tumour microenvironment as an adjunct to other molecular therapeutics and chemotherapy. The inherent instability of the genome of high-grade serous ovarian cancer has shifted attention to targeting the tumor microenvironment, which comprises a large proportion of the cell mass of many ovarian cancers. To date, encouraging results have been obtained with trials in ovarian cancer that target the angiogenic factor VEGF using the therapeutic antibody bevacizumab. The addition of bevacizumab to conventional chemotherapy improves progression-free survival in relapsed, platinum-resistant ovarian cancer53. In addition, large randomised phase III trials investigating the role of maintenance bevacizumab in the first-line setting (GOG218, ICON7) and in platinum-sensitive relapsed disease (OCEANS) have been completed. The results of all three trials, presented in abstract form only, suggest improvements in progression-free survival when given as concurrent or maintenance therapy. However, despite the presumed stability of the tumor endothelium, resistance to anti-VEGF agents has emerged. Understanding resistance pathways and developing predictors of patient response are crucial to better exploiting anti-angiogenic therapies54, 55.
A spontaneous anti-tumour immune response in the form of tumour-reactive T cells and/or antibodies has been demonstrated in some patients with ovarian cancer56, 57. The increased infiltration of lymphocytes in tumour islets, predicts significantly longer survival in ovarian cancer58. Vice versa, the detection of high numbers of T regulatory cells, which mediate immune suppression, predicts poor patient survival59, 60. Additional immunosuppressive cell subtypes, such as B7-H4 expressing tumour macrophages,61 have also been correlated with poor outcome.
Complex networks of inflammatory cytokines and chemokines62 regulate communication between malignant cells and supporting stroma in ovarian cancer. These cytokines and their intracellular signalling pathways can make malignant cells resistant to apoptosis, facilitate evasion of tumour immunity and promote angiogenesis. Tumour cells typically trigger inflammatory cytokine networks as a means to escape immune recognition in spite of surrounding inflammation63, 64. In experimental animal models, targeting these key inflammatory cytokines and chemokines has been shown to abrogate these processes that are key to the progression of ovarian cancer62, 65.
The association of intra-tumoral T cells with increased survival, and T regulatory cells with worse survival, indicates that ovarian cancers could respond to immune therapy. Pilot studies indicate that therapies capitalizing on pre-existing antitumour immune responses can be successful in ovarian cancer. For example, objective responses and/or prolonged survival have been seen with immune checkpoint blockade using an antibody against cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4)66, 67 and Phase II studies are currently underway. The understanding that select conventional chemotherapy drugs also have immunomodulatory activity offers new opportunities to design combinatorial approaches for women with ovarian cancer whose tumours exhibit pre-existing antitumor immunity in terms of high levels of intraepithelial tumour-infiltrating lymphocytes. In addition, trials targeting inflammatory cytokines68, 69 are currently in early stages, but they can offer important biological lessons for clinicians.
There needs to be a rapid development of further Phase II/III studies focusing on agents targeting key pathways in the tumour microenvironment that not only assess their efficacy alongside established cytotoxic regimes, but also aim to establish the optimal use of these agents for maintenance therapy.
Tumor adaptation and resistance
The sixth action point was that understanding clonal diversity, tumor adaptation and acquisition of resistance is essential if more durable responses to therapy are to be achieved. Many women respond well to first line treatment, but frequently relapse with chemotherapy-resistant disease. Evolutionary models of clonal selection may explain drug resistance in cancer. In acute lymphocytic leukaemia and chronic myeloid leukaemia, point mutations conferring resistance to imatinib exist at low prevalence prior to treatment and can become highly enriched during relapse70, 71. Autopsy studies on advanced pancreatic cancer have shown profound genetic heterogeneity, with 52% of mutations being present in sub-clonal populations72, 73. Whether this paradigm can explain resistance in ovarian cancer, and in particular whether heterogeneity is associated with primary platinum resistance, needs further investigation. With advances in nextgeneration sequencing we now have the tools to investigate clonal diversity and to start inferring the evolutionary changes that may contribute to resistance.
Despite the plethora of research dedicated to the mechanisms of platinum resistance, these are yet to translate to clinical practise. Undoubtedly, deficiencies in our knowledge of the fundamental changes occurring within the tumor – both the epithelial fraction and the microenvironment - throughout treatment have impeded progress. More recently, studies with paired tumour samples collected prior to treatment and following disease relapse have provided some of the first insights into clonal variation and mechanisms of resistance in vivo52, 74. Serially obtained biopsies of disease sites should be a central component in clinical trial design.
Importance of international consortia
The seventh action point was that international consortia involving large biological datasets must be encouraged, but they require high fidelity and transparency in analytical approaches. Ovarian cancer is a relatively uncommon disease and, together with its histologic diversity, this makes it difficult to collect substantial numbers of samples of specific subtypes. With the increased recognition of the importance of stratification by subtype, global collaborations and consortia such as Ovarian Cancer Association Consortium (OCAC), The Cancer Genome Atlas (TCGA), The Australian Ovarian Cancer Study (AOCS), OCTIPS (Ovarian Cancer Therapy – Innovative Models Prolong Survival) and Ovarian Tumor Tissue Analysis Consortium (OTTA) are crucial to furthering our understanding of the molecular biology of ovarian tumors and genetic risk. Such collaborative endeavours require highly ordered, standardised and quality-controlled strategies for the collection and management of biological specimens. The combination of high-throughput technologies with large sample sets allows detailed biological and translational studies. However, the challenges of managing large molecular datasets, each identifying a multitude of putative abnormalities — many of which may prove to be artefacts — are significant. Moreover, the opportunity to introduce human operator error remains unacceptably high. Methodologies used in the analysis of such data sets should be wholly transparent, reproducible, and biologically validated in the laboratory before any such findings can be put into practice. Errors made in the development of the OvaCheck early detection test underscore the importance of rigorous biostatistical support, especially when dealing with complex molecular datasets75.
Better experimental models
The eighth action is to speed the development of more appropriate experimental models, which should occur as a result of improved understanding of ovarian cancer. New model systems are needed that reflect the various originating cells, as well as the underlying genomic events driving each disease. Although mouse models of ovarian cancer have been developed76–79, these have not for the most part recapitulated human disease. A model that recapitulates the oncogenic drivers and biology of high-grade serous ovarian cancer has remained elusive. Mouse transgenic and knockout studies are likely to benefit substantially from improved understanding of the key driver mutations, such as loss of ARID1A in clear cell cancer and the importance of secretory cells of the fallopian tube in high-grade serous ovarian cancer. Recent reports of fallopian tube-based model systems will help define the physiology and susceptibility of this epithelium to transformation7, 80.
Now that it is appreciated that clear cell, mucinous, low-grade endometrioid and high-grade serous ovarian cancers are as molecularly distinct as, for example, breast and renal cancer, researchers must pay strict attention to the human cell line models they use to explore different aspects of the disease. Comparative molecular studies that fail to recognise the histopathological origins of the ovarian cancer cell lines used are no longer appropriate. Currently, the origin of many of the ovarian cancer cell lines are poorly defined - the field would benefit enormously from the creation of new lines that reflect the different histotypes and molecular subtypes.
It is also timely to consider in vitro models that are more appropriate than 2-dimensional growth of cells on plastic. Three-dimensional culture systems that mimic the peritoneal microenvironment have provided important insights into ovarian cancer biology81,82.
Quality of life and symptom benefit
The ninth action was that quality of life and symptom benefit should be included with response and survival rates as a primary endpoint in clinical trials investigating palliative treatment. The primary endpoint of most clinical trials has traditionally been objective response and survival. Most patients relapse after first line treatment; response rates and time to progression typically diminish with each recurrence. Although there are numerous studies of palliative chemotherapy regimes in platinum resistant ovarian cancer, there is scant evidence to confirm that these treatments are truly palliative and improve symptom control83. Attempts to address this important question with the currently available quality of life questionnaires have not been successful, and toxicity data completed by physicians may give a false impression of the patient’s experience. Robust instruments are needed that measure the impact of palliative chemotherapy on symptom control given that this should be the major aim of treatment. Treatment should be stratified in accordance to prognosis, with more emphasis being placed on minimizing side effects and avoiding inappropriate therapy in patients with a low likelihood of benefit. Prognostic indices are required to better categorize patients with recurrent ovarian cancer into poor, intermediate and good subsets rather than the current approach of grouping patients together.
Conclusion
The last five years have seen an explosion in our understanding of the heterogeneity of ovarian cancer. This comes with an emphatic commitment to changing in the way that clinical trials in ovarian cancer must be designed. Treatment in ovarian cancer will benefit from the careful alignment of target, drug, patient, and trial design. The greatest challenge will be to craft combinations of therapy that result in years of improved survival. There is an outstanding level of cooperation and willingness to share ideas amongst those in the ovarian cancer research field, as exemplified by the HHMT meeting. A high level of cooperation bodes well for the women whose lives we hope to improve.
Box 1: The meeting.
For twenty-five years the Helene Harris Memorial Trust has recognised the importance of communication between scientists and clinicians in improving the early detection of ovarian cancers and treatment of patients with advanced disease, bringing together international experts on a biannual basis http://www.ovarian.org.uk/. In January 2011 researchers met in Florida over 4 days to consider the latest findings in basic, translational and clinical research in ovarian cancer. With a both a sense of optimism associated with recent advances and frustration with limited improvements in outcomes in the past, we considered new ways forward based on recent findings. The delegates attending the meetings are outlined in Supplementary Table 1. The resulting review reflects the consensus of the meeting and the listed authors have all contributed to the manuscript.
Acknowledgements
With thanks to Ovarian Cancer Action, its Chair, Allyson Kaye, staff, and its many supporters, without whose drive and generosity the Helene Harris Memorial Trust meetings would not be possible.
We thank Ken Swenerton, Cheryl Brown Outcomes Unit, British Columbia Cancer Agency for providing some of the data in Figure 1 and for the concept of Figure 3.
Further acknowledgements and sources of funding for some of the authors of this Perspective can be found in the Supplementary information.
Contributor Information
Sebastian Vaughan, Ovarian Cancer Action Research Centre, Imperial College London Hammersmith Campus, Du Cane Road, London, W12 0NN.
Jermaine I. Coward, Royal Marsden Hospital, Fulham Road, London, SW3 6JJ
Robert C. Bast Jr., MD Anderson Cancer Center, Ovarian Cancer Research Lab, 1515 Holcombe Blvd, Houston, Texas 77030, USA
Andy Berchuck, Duke University Medical Center, Division of Gynecologic Oncology, DUMC 3079, Durham, NC 27710.
Jonathan S. Berek, Stanford Women’s Cancer Center, Department of Obstetrics and Gynecology, Stanford University School of Medicine, Stanford, California, 94305, USA
James D. Brenton, Cancer Research UK, Li Ka Shing Centre, Robinson Way, Cambridge, CB2 0RE
George Coukos, University of Pennsylvania, Center for Research on Women’s Health, 1315 Rm, BRB II/III, Philadelphia, Pennsylvania 19104, USA.
Christopher C. Crum, Brigham and Women’s Hospital, 75 Francis Street, Boston, Massachusetts 02445, USA
Ronny Drapkin, Dana-Farber Cancer Institute, Harvard Medical School, JFB 215D, 450 Brookline Avenue, Boston, Massachusetts 02215, USA.
Dariush Etemadmoghadam, Peter MacCallum Cancer Centre, St Andrew’s Place, East Melbourne, 3002, Australia.
Michael Friedlander, Prince of Wales Cancer Centre, Department of Medical Oncology, Barker Street, Randwick, 2031, Australia.
Hani Gabra, Ovarian Cancer Action Research Centre, Imperial College London Hammersmith Campus, Du Cane Road, London, W12 0NN.
Stan B. Kaye, Institute of Cancer Research/Royal Marsden Hospital, Section of Medicine, Downs Road, Sutton, SM2 5PT
Chris J. Lord, Institute of Cancer Research, Breakthrough Toby Robins Breast Cancer Research Institute, 237 Fulham Road, London, SW3 6JB
Ernst Lengyel, University of Chicago Medical Center, 5841 S. Maryland Avenue, MC 2050, Chicago, Illinois 60637, USA.
Douglas A. Levine, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, New York 10021, USA
Iain A. McNeish, Queen Mary University of London, Barts Cancer Institute, Charterhouse Square, London, EC1M 6BQ
Usha Menon, University College London Elizabeth Garrett Institute of Women’s Health, Euston Road, London, NW1 2BU.
Gordon B. Mills, University of Texas M.D. Anderson Cancer Center, 1515, Holcombe Boulevard, Houston, Texas 77030, USA
Kenneth P. Nephew, Indiana University School of Medicine, 1001 E. Third Street, Bloomington, Indiana 47405, USA
Amit M. Oza, Princess Margaret Hospital, 5th Floor Rm 5-717, 610 University Ave, Toronto, Ontario M5G 2M9, Canada
Anil K. Sood, University of Texas M. D. Anderson Cancer Center, Departments of Gynecologic Oncology and Cancer Biology, Unit 1362, P.O. BOX 301439, Houston, Texas 77230, USA
Euan A. Stronach, Ovarian Cancer Action Research Centre, Imperial College London Hammersmith Campus, Du Cane Road, London, W12 0NN
Henning Walczak, Division of Immunology and Inflammation, Imperial College London, South Kensington Campus, London, SW7 2AZ.
Frances R. Balkwill, Queen Mary University of London, Centre for Cancer and Inflammation, Charterhouse Square, Barts and the London Medical School, London, EC1M 6BQ
References
- 1.McGuire WP. Maintenance therapy for ovarian cancer: of Helsinki and Hippocrates. J Clin Oncol. 2009;27:4633–4634. doi: 10.1200/JCO.2009.23.6653. [DOI] [PubMed] [Google Scholar]
- 2.Omura G, et al. A randomized trial of cyclophosphamide and doxorubicin with or without cisplatin in advanced ovarian carcinoma. A Gynecologic Oncology Group Study. Cancer. 1986;57:1725–1730. doi: 10.1002/1097-0142(19860501)57:9<1725::aid-cncr2820570903>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 3.Coleman MP, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK-1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011;377:127–138. doi: 10.1016/S0140-6736(10)62231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. The American Journal of Surgical Pathology. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piek JM, et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–456. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 6.Lee Y, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 7.Levanon K, et al. Primary ex vivo cultures of human fallopian tube epithelium as a model for serous ovarian carcinogenesis. Oncogene. 2010;29:1103–1113. doi: 10.1038/onc.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo KT, et al. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. The American Journal of Pathology. 2009;174:1597–1601. doi: 10.2353/ajpath.2009.081000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones S, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228–231. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiegand KC, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. The New England Journal of Medicine. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee KR, Young RH. The distinction between primary and metastatic mucinous carcinomas of the ovary: gross and histologic findings in 50 cases. The American Journal of Surgical Pathology. 2003;27:281–292. doi: 10.1097/00000478-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Zaino RJ, et al. Advanced stage mucinous adenocarcinoma of the ovary is both rare and highly lethal: a Gynecologic Oncology Group study. Cancer. 2011;117:554–562. doi: 10.1002/cncr.25460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelemen LE, Kobel M. Mucinous carcinomas of the ovary and colorectum: different organ, same dilemma. The Lancet Oncology. 2011 May 24; doi: 10.1016/S1470-2045(11)70058-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Kobel M, et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med. 2008;5:e232. doi: 10.1371/journal.pmed.0050232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobel M, et al. A limited panel of immunomarkers can reliably distinguish between clear cell and high-grade serous carcinoma of the ovary. The American Journal of Surgical Pathology. 2009;33:14–21. doi: 10.1097/PAS.0b013e3181788546. [DOI] [PubMed] [Google Scholar]
- 16.Madore J, et al. Characterization of the molecular differences between ovarian endometrioid carcinoma and ovarian serous carcinoma. The Journal of Pathology. 2010;220:392–400. doi: 10.1002/path.2659. [DOI] [PubMed] [Google Scholar]
- 17.Bowtell DD. The genesis and evolution of high-grade serous ovarian cancer. Nat Rev Cancer. 2010;10:803–808. doi: 10.1038/nrc2946. [DOI] [PubMed] [Google Scholar]
- 18.Ho CL, Kurman RJ, Dehari R, Wang TL, Shih Ie M. Mutations of BRAF and KRAS precede the development of ovarian serous borderline tumors. Cancer Research. 2004;64:6915–6918. doi: 10.1158/0008-5472.CAN-04-2067. [DOI] [PubMed] [Google Scholar]
- 19.Tothill RW, et al. Novel molecular subtypes of serous and endometroid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14:5198–5208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed AA, et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J Pathol. 2010;221:49–56. doi: 10.1002/path.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zorn KK, et al. Gene expression profiles of serous, endometrioid, and clear cell subtypes of ovarian and endometrial cancer. Clinical Cancer Research. 2005;11:6422–6430. doi: 10.1158/1078-0432.CCR-05-0508. [DOI] [PubMed] [Google Scholar]
- 22.Anglesio MS, et al. IL6-STAT3-HIF signaling and therapeutic response to the angiogenesis inhibitor sunitinib in ovarian clear cell cancer. Clinical Cancer Research. 2011;17:2538–2548. doi: 10.1158/1078-0432.CCR-10-3314. [DOI] [PubMed] [Google Scholar]
- 23.Kobel M, et al. Diagnosis of ovarian carcinoma cell type is highly reproducible: a transcanadian study. The American Journal of Surgical Pathology. 2010;34:984–993. doi: 10.1097/PAS.0b013e3181e1a3bb. [DOI] [PubMed] [Google Scholar]
- 24.Glimelius B, Lahn M. Window-of-opportunity trials to evaluate clinical activity of new molecular entities in oncology. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2011;22:1717–1725. doi: 10.1093/annonc/mdq622. [DOI] [PubMed] [Google Scholar]
- 25.Kyriazi S, Kaye SB, deSouza NM. Imaging ovarian cancer and peritoneal metastases--current and emerging techniques. Nat Rev Clin Oncol. 2010;7:381–393. doi: 10.1038/nrclinonc.2010.47. [DOI] [PubMed] [Google Scholar]
- 26.He W, et al. Quantitation of circulating tumor cells in blood samples from ovarian and prostate cancer patients using tumor-specific fluorescent ligands. International Journal of Cancer. 2008;123:1968–1973. doi: 10.1002/ijc.23717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shih Ie M, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–1518. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menon U, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) Lancet Oncology. 2009;10:327–340. doi: 10.1016/S1470-2045(09)70026-9. [DOI] [PubMed] [Google Scholar]
- 29.Brown PO, Palmer C. The preclinical natural history of serous ovarian cancer: defining the target for early detection. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000114. e1000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buys SS, et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011;305:2295–2303. doi: 10.1001/jama.2011.766. [DOI] [PubMed] [Google Scholar]
- 31.Hogg R, Friedlander M. Biology of epithelial ovarian cancer: implications for screening women at high genetic risk. J Clin Oncol. 2004;22:1315–1327. doi: 10.1200/JCO.2004.07.179. [DOI] [PubMed] [Google Scholar]
- 32.Domchek SM, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304:967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80–87. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dietl J, Wischhusen J. The forgotten fallopian tube. Nat Rev Cancer. 2011;11:227. doi: 10.1038/nrc2946-c1. author reply 227. [DOI] [PubMed] [Google Scholar]
- 35.Bolton KL, et al. Common variants at 19p13 are associated with susceptibility to ovarian cancer. Nature Genetics. 2010;42:880–884. doi: 10.1038/ng.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song H, et al. A genome-wide association study identifies a new ovarian cancer susceptibility locus on 9p22.2. Nature Genetics. 2009;41:996–1000. doi: 10.1038/ng.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorringe KL, et al. Copy number analysis identifies novel interactions between genomic loci in ovarian cancer. PloS One. 2010;5:e11408. doi: 10.1371/journal.pone.0011408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorringe KL, et al. High-resolution single nucleotide polymorphism array analysis of epithelial ovarian cancer reveals numerous microdeletions and amplifications. Clinical Cancer Research. 2007;13:4731–4739. doi: 10.1158/1078-0432.CCR-07-0502. [DOI] [PubMed] [Google Scholar]
- 39.The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farley J, et al. Cyclin E expression is a significant predictor of survival in advanced, suboptimally debulked ovarian epithelial cancers: a Gynecologic Oncology Group study. Cancer Research. 2003;63:1235–1241. [PubMed] [Google Scholar]
- 41.Etemadmoghadam D, et al. Amplicon-dependent CCNE1 expression is critical for clonogenic survival after cisplatin treatment and is correlated with 20q11 gain in ovarian cancer. PloS one. 2010;5:e15498. doi: 10.1371/journal.pone.0015498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan DS, et al. Genomic analysis reveals the molecular heterogeneity of ovarian clear cell carcinomas. Clinical Cancer Research. 2011;17:1521–1534. doi: 10.1158/1078-0432.CCR-10-1688. [DOI] [PubMed] [Google Scholar]
- 43.Shah SP, et al. Mutation of FOXL2 in granulosa-cell tumors of the ovary. The New England Journal of Medicine. 2009;360:2719–2729. doi: 10.1056/NEJMoa0902542. [DOI] [PubMed] [Google Scholar]
- 44.Press JZ, et al. Ovarian carcinomas with genetic and epigenetic BRCA1 loss have distinct molecular abnormalities. BMC Cancer. 2008;8:17. doi: 10.1186/1471-2407-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turner N, Tutt A, Ashworth A. Hallmarks of 'BRCAness' in sporadic cancers. Nature Reviews Cancer. 2004;4:814–819. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 46.Banerjee S, Kaye SB, Ashworth A. Making the best of PARP inhibitors in ovarian cancer. Nature Reviews Clinical oncology. 2010;7:508–519. doi: 10.1038/nrclinonc.2010.116. [DOI] [PubMed] [Google Scholar]
- 47.Ledermann JA, et al. Phase II randomized placebo-controlled study of olaparib (AZD2281) in patients with platinum-sensitive relapsed serous ovarian cancer (PSR SOC) J Clin Oncol. 2011:29. [Google Scholar]
- 48.Mukhopadhyay A, et al. Development of a functional assay for homologous recombination status in primary cultures of epithelial ovarian tumor and correlation with sensitivity to poly(ADP-ribose) polymerase inhibitors. Clinical Cancer Research. 2010;16:2344–2351. doi: 10.1158/1078-0432.CCR-09-2758. [DOI] [PubMed] [Google Scholar]
- 49.Martin SA, et al. Methotrexate induces oxidative DNA damage and is selectively lethal to tumour cells with defects in the DNA mismatch repair gene MSH2. EMBO Molecular Medicine. 2009;1:323–337. doi: 10.1002/emmm.200900040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin SA, et al. DNA polymerases as potential therapeutic targets for cancers deficient in the DNA mismatch repair proteins MSH2 or MLH1. Cancer Cell. 2010;17:235–248. doi: 10.1016/j.ccr.2009.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cooke SL, et al. Intra-tumour genetic heterogeneity and poor chemoradiotherapy response in cervical cancer. British Journal of Cancer. 2011;104:361–368. doi: 10.1038/sj.bjc.6605971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cooke SL, et al. Genomic analysis of genetic heterogeneity and evolution in high-grade serous ovarian carcinoma. Oncogene. 2010;29:4905–4913. doi: 10.1038/onc.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O'Malley DM, et al. Addition of bevacizumab to weekly paclitaxel significantly improves progression-free survival in heavily pretreated recurrent epithelial ovarian cancer. Gynecologic oncology. 2011;121:269–272. doi: 10.1016/j.ygyno.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 54.Ebos JM, Kerbel RS. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nature reviews. Clinical oncology. 2011;8:210–221. doi: 10.1038/nrclinonc.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jubb AM, Harris AL. Biomarkers to predict the clinical efficacy of bevacizumab in cancer. The lancet oncology. 2010;11:1172–1183. doi: 10.1016/S1470-2045(10)70232-1. [DOI] [PubMed] [Google Scholar]
- 56.Goodell V, et al. Antibody immunity to the p53 oncogenic protein is a prognostic indicator in ovarian cancer. J Clin Oncol. 2006;24:762–768. doi: 10.1200/JCO.2005.03.2813. [DOI] [PubMed] [Google Scholar]
- 57.Schlienger K, et al. TRANCE- and CD40 ligand-matured dendritic cells reveal MHC class I-restricted T cells specific for autologous tumor in late-stage ovarian cancer patients. Clinical Cancer Research. 2003;9:1517–1527. [PubMed] [Google Scholar]
- 58.Zhang L, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 59.Curiel TJ, Coukos G, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature Medicine. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 60.Sato E, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. PNAS. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kryczek I, et al. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Research. 2007;67:8900–8905. doi: 10.1158/0008-5472.CAN-07-1866. [DOI] [PubMed] [Google Scholar]
- 62.Kulbe H, et al. The inflammatory cytokine TNF-a generates an autocrine tumourpromoting network in epithelial ovarian cancer cells. Cancer Res. 2007;67:585–592. doi: 10.1158/0008-5472.CAN-06-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 64.Facciabene X, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 65.Charles KA, et al. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest. 2009;119:3011–3023. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hodi FS, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. PNAS. 2003;100:4712–4714. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hodi FS, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. PNAS. 2008;105:3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coward J, et al. Interleukin-6 as a therapeutic target in human ovarian cancer. Clinical Cancer Research. 2011 Jul 27; doi: 10.1158/1078-0432.CCR-11-0945. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Balkwill F, Mantovani A. Cancer and inflammation: implications for pharmacology and therapeutics. Clin Pharmacol Ther. 2010;87:401–406. doi: 10.1038/clpt.2009.312. [DOI] [PubMed] [Google Scholar]
- 70.Choi S, et al. Relapse in children with acute lymphoblastic leukemia involving selection of a preexisting drug-resistant subclone. Blood. 2007;110:632–639. doi: 10.1182/blood-2007-01-067785. [DOI] [PubMed] [Google Scholar]
- 71.Roche-Lestienne C, et al. Several types of mutations of the Abl gene can be found in chronic myeloid leukemia patients resistant to STI571, and they can pre-exist to the onset of treatment. Blood. 2002;100:1014–1018. doi: 10.1182/blood.v100.3.1014. [DOI] [PubMed] [Google Scholar]
- 72.Yachida S, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Campbell PJ, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–1113. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stronach EA, et al. HDAC4-regulated STAT1 activation mediates platinum resistance in ovarian cancer. Cancer Research. 2011;71:4412–4422. doi: 10.1158/0008-5472.CAN-10-4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baggerly KA, Morris JS, Edmonson SR, Coombes KR. Signal in noise: evaluating reported reproducibility of serum proteomic tests for ovarian cancer. Journal of the National Cancer Institute. 2005;97:307–309. doi: 10.1093/jnci/dji008. [DOI] [PubMed] [Google Scholar]
- 76.Connolly DC, et al. Female mice chimeric for expression of the simian virus 40 TAg under control of the MISIIR promoter develop epithelial ovarian cancer. Cancer Res. 2003;63:1389–1397. [PubMed] [Google Scholar]
- 77.Wu R, et al. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/B-catenin and P13K/Pten signaling pathways. Cancer Cell. 2007;11:321–333. doi: 10.1016/j.ccr.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 78.Xing D, Orsulic S. A genetically defined mouse ovarian carcinoma model for the molecular characterization of pathway-targeted therapy and tumor resistance. PNAS. 2005;102:6936–6941. doi: 10.1073/pnas.0502256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Orsulic S, et al. Induction of ovarian cancer by defined multiple genetic changes in a mouse model system. Cancer Cell. 2002;1:53–62. doi: 10.1016/s1535-6108(01)00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karst AM, Levanon K, Drapkin R. Modeling high-grade serous ovarian carcinogenesis from the fallopian tube. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7547–7552. doi: 10.1073/pnas.1017300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kenny HA, Kaur S, Coussens LM, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest. 2008:1–13. doi: 10.1172/JCI33775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iwanicki MP, et al. Ovarian cancer spheroids use myosin-generated force to clear the mesothelium. Cancer Discovery. 2011;1:144–157. doi: 10.1158/2159-8274.CD-11-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Al-Barrak J, et al. Exploring palliative treatment outcomes in women with advanced or recurrent ovarian clear cell carcinoma. Gynecologic Oncology. 2011;122:107–110. doi: 10.1016/j.ygyno.2011.03.011. [DOI] [PubMed] [Google Scholar]