Abstract
Obesity increases oxidative stress, endothelial dysfunction, and inflammation, but the effect of obesity on postoperative AKI is not known. We examined the relationship between body mass index (BMI) and AKI in 445 patients undergoing cardiac surgery and whether oxidative stress (F2-isoprostanes), inflammation (IL-6), or antifibrinolysis (plasminogen activator inhibitor-1 [PAI-1]) contribute to any identified relationship. Overall, 112 (25%) of the 445 patients developed AKI. Higher BMI was independently associated with increased odds of AKI (26.5% increase per 5 kg/m2 [95% confidence interval, 4.3%–53.4%]; P=0.02). Baseline F2-isoprostane (P=0.04), intraoperative F2-isoprostane (P=0.003), and intraoperative PAI-1 (P=0.04) concentrations also independently predicted AKI. BMI no longer predicted AKI after adjustment for the effect of F2-isoprostanes, suggesting that obesity may affect AKI via effects on oxidative stress. In contrast, adjustment for IL-6 or PAI-1 did not substantially alter the association between BMI and AKI. Further, deconstruction of the obesity-AKI relationship into direct (i.e., independent of candidate pathways) and indirect (i.e., effect of BMI on AKI via each candidate pathway) effects indicated that F2-isoprostanes, but not IL-6 or PAI-1, partially mediate the relationship between obesity and AKI (P=0.001). In conclusion, obesity independently predicts AKI after cardiac surgery, and oxidative stress may partially mediate this association.
AKI complicates the recovery of up to 30% of patients undergoing cardiac surgery, promotes systemic and sternal wound infection, is associated with myocardial injury and postoperative arrhythmias, and independently predicts an eight-fold increase in the odds of death at 30 days.1–4 The incidence and associated mortality of AKI after cardiac surgery have increased despite the advances in surgical, anesthetic, and intensive care unit care that have led to reduced perioperative mortality and shorter duration of hospitalization.5 These findings could be explained in part by the growing prevalence of obesity in the developed world because obesity and high body mass index (BMI) have recently been associated with AKI in other intensive care unit and postsurgical populations.6–9 Obesity increases oxidative stress, endothelial dysfunction, and inflammation,10,11 and systemic markers of these processes, including F2-isoprostanes, IL-6, and plasminogen activator inhibitor (PAI)-1, have been associated with BMI.12,13 These markers also increase rapidly during cardiac surgery,14–16 but their relation to AKI is not clear. If obesity is a risk factor for AKI, the identification of mechanisms by which obesity may affect AKI provides the opportunity to reduce AKI after cardiac surgery.
We conducted this study to address three questions: (1) Does BMI predict AKI after cardiac surgery? (2) Do baseline and intraoperative markers of oxidative stress (F2-isoprostanes), inflammation (IL-6), and antifibrinolysis (PAI-1) predict postoperative AKI? (3) Do these candidate pathways (i.e., oxidative stress, inflammation, and antifibrinolysis) mediate an association between BMI and AKI after cardiac surgery?
Results
AKI
One hundred twelve patients (25.2%) developed stage I AKI after cardiac surgery, defined using Acute Kidney Injury Network (AKIN) consensus criteria for AKI diagnosis: at least a 0.3-mg/dl (26.5-μmol/L) or 50% increase in serum creatinine within 72 hours of surgery.17 Patients who developed this criterion for AKI stayed in the hospital longer (8.1±9.7 days versus 5.5±2.7 days; P<0.001), were more likely to develop postoperative atrial fibrillation (37.7% versus 23.5%; P=0.003), were more likely to develop pneumonia (7.3% versus 1.2%; P=0.001), and had an increased risk for death at 30 days (4.5% versus 0.0%; P<0.001) than patients who did not develop AKI. Sixteen of these 112 patients developed stage II AKI, defined as a 100% increase in serum creatinine within 72 hours of surgery, and two patients developed stage III AKI, defined as a 200% increase in serum creatinine within 72 hours of surgery.
Baseline and intraoperative patient characteristics are shown in Table 1. The median BMI was 28.1 kg/m2, and 41.3% of all patients were obese (BMI≥30 kg/m2). After adjustment for baseline risk factors for AKI that had been determined a priori, 5-kg/m2 increases in BMI independently predicted 26.5% (95% confidence interval [CI], 4.3%–53.4%; P=0.02) increases in the odds of AKI. After adjustment for additional intraoperative risk factors for AKI, 5-kg/m2 increases in BMI independently predicted 28.7% (95% CI, 5.9%–56.4%; P=0.01) increases in the odds of AKI (Table 2, Model 1). Baseline creatinine, male sex, and duration of cardiopulmonary bypass also independently predicted AKI after cardiac surgery.
Table 1.
Baseline and intraoperative patient characteristics separated by AKI with unadjusted analyses
| Characteristic | No AKI (n=333) | AKI (n=112) | P Value |
|---|---|---|---|
| Age (yr) | 60 (51–68) | 62 (53–68) | 0.30 |
| Women | 118 (35.4) | 31 (27.7) | 0.13 |
| African American | 19 (5.7) | 7 (6.2) | 0.83 |
| Medical history | |||
| diabetes | 68 (20.4) | 28 (25.0) | 0.31 |
| hypertension | 203 (61.0) | 76 (67.9) | 0.19 |
| smoking | 53 (15.9) | 21 (18.8) | 0.49 |
| BMI (kg/m2) | 28.0 (25.0–32.0) | 29.1 (26.0–34.0) | 0.02 |
| Obese (BMI>30 kg/m2) | 130 (39.0) | 54 (48.2) | 0.09 |
| Systolic BP (mmHg) | 128 (118–139) | 134 (122–149) | 0.001 |
| Diastolic BP (mmHg) | 75 (66–82) | 76 (68–82) | 0.62 |
| Heart rate (beats/min) | 62 (53–73) | 64 (56–74) | 0.30 |
| Central venous pressure (mmHg) | 12 (9–15) | 14 (10–17) | 0.002 |
| Left ventricular ejection fraction (%) | 55 (55–65) | 55 (55–61) | 0.19 |
| Baseline creatinine (mg/dl) | 1.00 (0.85–1.10) | 1.00 (0.80–1.14) | 0.37 |
| eGFR (ml/min per 1.73 m2) | 77.2 (65.5–89.7) | 80.3 (66.0–96.0) | |
| Preoperative medications | |||
| statin | 184 (55.3) | 58 (51.8) | 0.52 |
| diuretic | 117 (34.7) | 40 (37.0) | 0.66 |
| Treatment group | 0.77 | ||
| placebo | 110 (33.0) | 36 (32.1) | |
| ramipril | 116 (34.8) | 36 (32.1) | |
| spironolactone | 107 (32.1) | 40 (35.7) | |
| CABG surgery | 132 (39.6) | 52 (46.4) | 0.21 |
| Valve surgery | 210 (63.1) | 74 (66.1) | 0.57 |
| On-pump surgery | 279 (83.8) | 96 (85.7) | 0.63 |
| Cardiopulmonary bypass time (min)a | 114 (91–148) | 117 (91–162) | 0.35 |
| Aortic cross-clamp time (min)b | 90 (68–113) | 91 (64–125) | 0.62 |
| Perioperative radiocontrast exposurec | 18 (5.4) | 7 (6.3) | 0.74 |
| Red blood cell transfusion (intraoperative units) | 0 (0–1) | 0 (0–2) | 0.39 |
| Postoperative vasopressor used | 116 (35.2) | 63 (56.3) | <0.001 |
Data are represented as median (interquartile range) or number of patients (percentage). eGFR, estimated GFR; CABG, coronary artery bypass grafting.
Among on-pump surgery patients.
Among patients receiving aortic cross-clamp.
“Perioperative” includes 48 hours before, during, and 48 hours after surgery.
Norepinephrine, epinephrine, dopamine, vasopressin, or phenylephrine infusions on postoperative days 1 or 2.
Table 2.
Effects of BMI and baseline or intraoperative F2-isoprostane, IL-6, or PAI-1 concentrations on risk for AKI
| Time | Model | Independent Variable | No AKI (n=333)a | AKI (n=112)a | Adjusted Odds Ratio (95% CI) for AKI | P Value |
|---|---|---|---|---|---|---|
| Baseline | 1 | BMI | 28.0 kg/m2 (25.0–32.0) | 29.1 kg/m2 (26.0–34.0) | 1.265 (1.043–1.534) | 0.02 |
| 2 | BMI | 1.165 (0.946–1.436) | 0.15 | |||
| F2-isoprostanes | 36.0 pg/ml (25.6–49.1) | 40.3 pg/ml (30.5–52.4) | 1.234 (1.009–1.508) | 0.04 | ||
| BMI | 1.232 (1.011–1.502) | 0.04 | ||||
| IL-6 | 3.2 pg/ml (0.0–5.7) | 3.0 pg/ml (0.0–8.2) | 0.996 (0.981–1.012) | 0.66 | ||
| BMI | 1.225 (1.001–1.499) | 0.05 | ||||
| PAI-1 | 14.0 ng/ml (8.7–23.3) | 13.1 ng/ml (8.2–24.9) | 0.988 (0.883–1.106) | 0.84 | ||
| Intraoperative | 1 | BMI | 28.0 kg/m2 (25.0–32.0) | 29.1 kg/m2 (26.0–34.0) | 1.287 (1.059–1.564) | 0.01 |
| 2 | BMI | 1.182 (0.954–1.463) | 0.13 | |||
| F2-isoprostanes | 37.4 pg/ml (27.1–52.7) | 43.7 pg/ml (32.0–62.3) | 1.381 (1.117–1.707) | 0.003 | ||
| BMI | 1.278 (1.045–1.564) | 0.01 | ||||
| IL-6 | 46.9 pg/ml (20.8–101.2) | 56.3 pg/ml (33.3–172.4) | 1.073 (0.995–1.156) | 0.07 | ||
| BMI | 1.222 (0.995–1.501) | 0.06 | ||||
| PAI-1 | 28.4 ng/ml (18.5–45.8) | 37.5 ng/ml (20.8–50.4) | 1.181 (1.008–1.383) | 0.04 |
Model 1 represents the total adjusted association between BMI and AKI (β1,BMI). Model 2 represents the direct associations between BMI (β2,BMI) and each pathway biomarker measured at baseline or intraoperatively (β2,Biomarker) on AKI. Odds ratios represent 5 kg/m2 BMI or 50% biomarker increases adjusted for age, sex, African-American race, diabetes, log-transformed baseline creatinine, use of cardiopulmonary bypass, valvular heart surgery, study drug, and duration of cardiopulmonary bypass (intraoperative models only).
Median (interquartile range).
Oxidative Stress, Inflammation, and Antifibrinolysis Pathway Markers
Median F2-isoprostane concentrations were 11.9% higher at baseline and 16.8% higher during surgery in patients who subsequently developed AKI. Baseline concentrations of IL-6 and PAI-1 did not differ between AKI and non-AKI groups, but intraoperative concentrations of IL-6 and PAI-1 were 20.0% and 32.0% higher, respectively, in patients who developed AKI.
In adjusted analyses, baseline F2-isoprostane (P=0.04) but not baseline IL-6 (P=0.66) or PAI-1 (P=0.84) concentrations predicted AKI. Intraoperative F2-isoprostane (P=0.003) and intraoperative PAI-1 (P=0.04) concentrations predicted AKI, and a trend between intraoperative IL-6 and AKI (P=0.07) was observed. The odds of AKI were 23.4% (95% CI, 0.9%–50.8%), 38.1% (95% CI, 11.7%–70.7%), and 18.1% (95% CI, 0.8%–38.3%) higher for 50% increases in baseline F2-isoprostane, intraoperative F2-isoprostane, and intraoperative PAI-1 concentrations, respectively (Table 2, Model 2, biomarker variables). Baseline IL-6 and baseline PAI-1 concentrations were not associated with AKI after cardiac surgery. These differences between the prediction of AKI by baseline versus intraoperative concentrations of F2-isoprostanes, IL-6, and PAI-1 suggest that surgical events induce systemic inflammatory and pro-oxidant changes that independently predict AKI.
BMI correlated with baseline F2-isoprostane (Spearman correlation coefficient r=0.21; P<0.001) and PAI-1 (r=0.24; P<0.001) concentrations. BMI also correlated with intraoperative F2-isoprostane (r=0.17; P=0.001) concentrations, and for any given BMI, the intraoperative concentrations of F2-isoprostanes tended to be higher in patients with AKI (Figure 1). In linear regression analyses, BMI was independently associated with baseline and intraoperative F2-isoprostane and baseline PAI-1 concentrations but not with IL-6 or intraoperative PAI-1 concentrations (Table 3, Model 3). After adjustment for AKI risk factors and randomized treatment assignment, 5-kg/m2 increases in BMI were associated with 10.9% (95% CI, 6.4%–15.5%), 9.5% (95% CI, 4.9%–14.3%), and 10.1% (95% CI, 2.6%–18.3%) median value increases in baseline F2-isoprostane, intraoperative F2-isoprostane, and baseline PAI-1 concentrations, respectively.
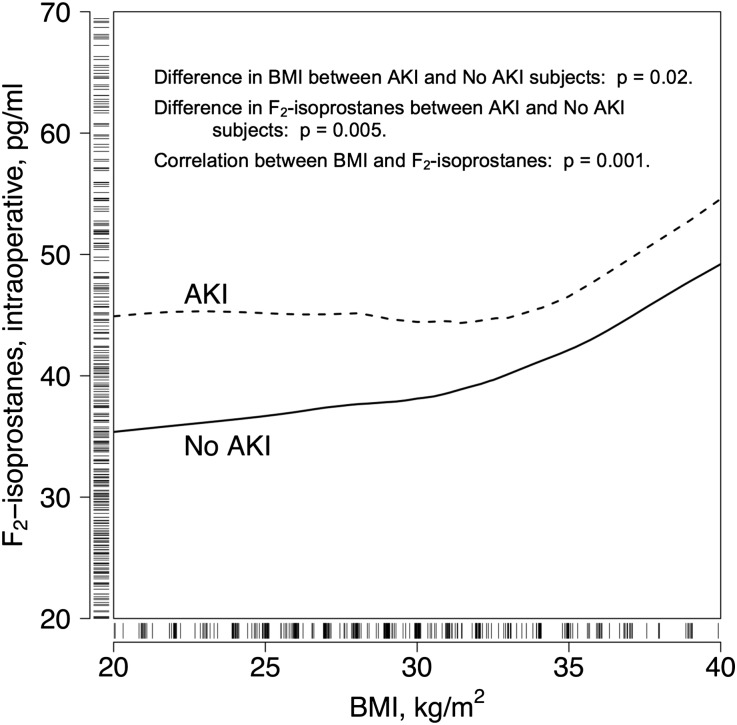
Figure 1.
For any given BMI, the intraoperative concentrations of F2-isoprostanes were higher in patients with AKI than those without AKI. Tick marks on the x- and y-axes represent patients, and the curves are smoothed estimates of the groups with and without AKI. The x-axis is truncated at the 5th and 95th patient percentiles and the y-axis at the 5th and 90th percentiles for ease of exposition.
Table 3.
Effects of BMI on baseline or intraoperative F2-isoprostanes, IL-6, or PAI-1 concentrations (Model 3)
| Time | Model | Outcome Variable | Median Ratio (95% CI) | P Value | P Value for Indirect Effect between BMI and AKI via Biomarker |
|---|---|---|---|---|---|
| Baseline | 3 | F2-isoprostanes | 1.109 (1.064–1.155) | <0.001 | 0.03 |
| IL-6 | 0.989 (0.603–1.624) | 0.97 | 0.99 | ||
| PAI-1 | 1.101 (1.026–1.183) | 0.008 | 0.84 | ||
| Intraoperative | 3 | F2-isoprostanes | 1.095 (1.049–1.143) | <0.001 | 0.001 |
| IL-6 | 1.002 (0.872–1.151) | 0.98 | 0.93 | ||
| PAI-1 | 1.057 (0.997–1.120) | 0.06 | 0.08 |
Median ratios represent the change in log-transformed biomarker values per 5-kg/m2 changes in BMI adjusted for age, sex, African-American race, diabetes, log-transformed baseline creatinine, use of cardiopulmonary bypass, valvular heart surgery, study drug, and duration of cardiopulmonary bypass (intraoperative models only). The P values for the indirect effects (effects of BMI on AKI via effect of BMI on each biomarker) are also listed.
Mediation of the BMI-AKI Association by Candidate Pathways
The effect of BMI on AKI via candidate pathway biomarkers, represented by the product of β3,BMI and β2,Biomarker (Figure 2B), was non-zero for baseline F2-isoprostanes and for intraoperative F2-isoprostanes. Specifically, 5-kg/m2 increases in BMI were associated with 11.6% (95% CI, 1.0%–29.7%; P=0.03) increases in the odds of AKI via the effect of BMI on baseline F2-isoprostanes and 16.2% (95% CI, 5.7%–33.6%; P<0.001) increases in the odds of AKI via the effect of BMI on intraoperative F2-isoprostanes. There was no evidence of an indirect effect between BMI and AKI via PAI-1 or IL-6 (product estimate ∼0) (see indirect effect P value column in Table 3). In addition, the association between BMI and AKI was 37% weaker and no longer statistically significant when intraoperative F2-isoprostane concentrations were included in AKI prediction models (odds ratio for AKI per 5-kg/m2 change in BMI was 1.182 when intraoperative F2-isoprostanes were included versus 1.287 when intraoperative F2-isoprostanes were not included) (Model 2 versus Model 1, Table 2). The association between BMI and AKI was 38% weaker and also no longer statistically significant when baseline F2-isoprostane concentrations were included in AKI prediction models. Similar findings were not observed (IL-6) or less apparent (PAI-1) when we examined mediation between BMI and AKI via effects of BMI on IL-6 or PAI-1, respectively. Taken together, these findings are consistent with an indirect pathway from BMI to AKI via baseline and, to a larger extent, intraoperative F2-isoprostane concentrations and the hypothesis that oxidative stress partially mediates the association between BMI and AKI.
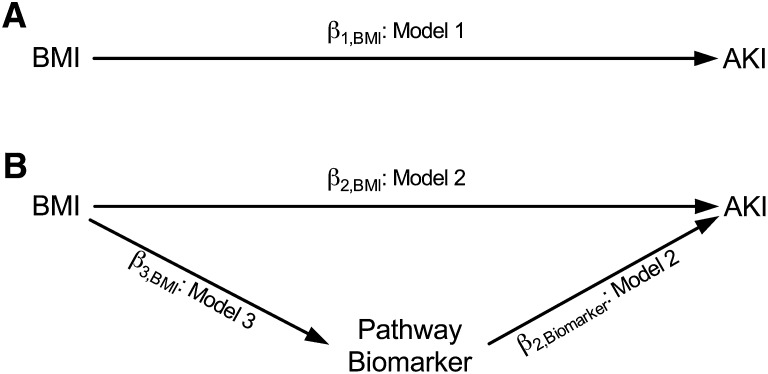
Figure 2.
Regression models for theoretical causal relationships. Adjustment covariates (AKI risk factors) are not shown for ease of exposition. (A) β1,BMI captures the total effect of BMI on AKI. (B) Total effect of BMI on AKI separated into direct (independent of candidate pathway biomarker) and indirect (via biomarker) effects. β2,BMI captures the direct effect of BMI on AKI adjusted for biomarker, β2,Biomarker captures the direct effect of biomarker on AKI, and β3,BMI captures the direct effect of BMI on candidate pathway biomarker. The product of β3,BMI and β2,Biomarker captures the indirect effect of BMI on AKI that is mediated by that candidate pathway biomarker.
Secondary Exploratory Analyses and Alternative Criteria for Defining AKI
We conducted exploratory analyses to further examine the relationship between BMI and AKI. These analyses included additional established risk factors for AKI after cardiac surgery, added individually to the regression models, or were conducted on the subset of patients who underwent surgery with cardiopulmonary bypass (84.3% of patients). Results and conclusions for the BMI-AKI analysis, the analyses for candidate pathway biomarkers and AKI, and the mediation analyses were similar when we included duration of cardiopulmonary bypass, mean arterial pressure during cardiopulmonary bypass, perioperative exposure to radiocontrast agents (defined as during or within 2 days of surgery), or intraoperative red blood cell transfusion in the regression models or when we restricted our cohort to only patients who underwent surgery with cardiopulmonary bypass. Mean arterial pressure 60 minutes into cardiopulmonary bypass (P=0.41), perioperative exposure to iodinated radiocontrast agents (P=0.64), and intraoperative red blood cell transfusion (P=0.92) were not independently associated with AKI. Vasopressor use on postoperative day 1 or 2 was highly associated with AKI (P<0.001), but inclusion of this variable in the AKI prediction model did not change the independent association between BMI and AKI.
In addition, defining AKI as a 50% or 0.3-mg/dl (26.5-μmol/L) increase in serum creatinine within 48 hours of surgery, as an ordinal end point (stage 0, I, II, or III AKI at 72 hours after surgery), or as the maximum creatinine level at 48 or 72 hours after surgery (a continuous marker of AKI severity) did not change the independent prediction of AKI by BMI or candidate pathway markers or the evidence that F2-isoprostanes, but not IL-6 or PAI-1, partially mediated the association between BMI and AKI (see Supplemental Table 1).
Discussion
We report that BMI is an independent risk factor for AKI after cardiac surgery. In addition, this study is the first to report that an intraoperative marker of oxidative stress (plasma concentrations of F2-isoprostanes) predicts AKI after cardiac surgery. Our data suggest that the association between obesity and AKI is partially mediated by BMI’s effect on oxidative stress but not inflammation or antifibrinolysis.
Prior studies have not demonstrated a consistent association between obesity and AKI after cardiac surgery. Yap and coworkers found that obesity and morbid obesity were associated with an increased risk for AKI, defined as at least two of the following: postoperative creatinine > 200 μmol/L, increase in creatinine to twice the baseline value, or requirement of postoperative dialysis.18 Wigfield et al. reported that extreme obesity (BMI>40 kg/m2) but not obesity (BMI, 30–40 kg/m2) was associated with renal failure, defined as an increase in creatinine to twice the baseline value and >2.0 mmol/L.19 Reis et al. reported no significant association between BMI and renal dysfunction in older patients undergoing coronary artery bypass grafting.20 This inconsistency may reflect differences in AKI diagnostic criteria, differences in patient selection, or lack of adjustment for established AKI risk factors.
Using recent AKIN consensus guidelines for AKI diagnosis,17 as recommended by the Acute Dialysis Quality Initiative for the diagnosis of AKI after cardiac surgery,21 and adjusting for established risk factors for AKI, we found increases in BMI of 5 kg/m2 to be independently associated with 26.5% increases in the odds of postoperative AKI. Clinicians may use these data to better stratify the risk for AKI in patients undergoing elective cardiac surgery and to help inform their decision to operate.
Our results suggest that the mechanism underlying the prediction of AKI by obesity involves oxidative stress but not inflammation or antifibrinolysis, a novel finding of this study. F2-isoprostanes not only provide sensitive and specific in vivo quantification of oxidative stress but also have deleterious biologic properties. F2-isoprostanes reduce renal perfusion, reduce creatinine clearance, and cause cellular and functional renal damage in experimental models.22 Our data indicate that elevated F2-isoprostane concentrations predict clinical AKI and suggest that interventions that reduce the formation of these products of oxidative stress could potentially reduce risk for AKI, particularly in obese patients. Nonspecific antioxidants have thus far failed to reduce AKI after cardiac surgery, although these antioxidants have not been tested at sufficient doses and duration to suppress oxidative stress.23,24 Alternate therapies, including perioperative statin or mitochondrial-targeted antioxidant use, merit investigation.
Although our correlative approach prevents us from drawing conclusions about causation, our statistical methods allowed us to define relationships between BMI, candidate pathway biomarkers, and AKI by deconstructing the total effect of BMI on AKI into direct effects (independent of candidate pathways’ biomarkers) and indirect effects (the effect of BMI on AKI via the effect of BMI on biomarker and the effect of biomarker on AKI). Study inclusion criteria limit our conclusions to patients undergoing cardiac surgery who did not have severe baseline renal insufficiency (creatinine > 1.6 mg/dl [141.6 μmol/L]) and may account for the observation that the incidence of diabetes was not independently associated with AKI. The exclusion of patients with severe CKD from the parent trial may have eliminated diabetic patients with advanced CKD who may have developed AKI. Reports that plasma F2-isoprostane and C-reactive protein concentrations are associated with adiposity in patients with stage III and IV CKD, however, suggest that F2-isoprostanes and AKI may be associated with obesity in patients with severe chronic renal insufficiency as well.25 Most patients with kidney injury had stage I AKI, and it is uncertain whether BMI or oxidative stress predicts severe AKI. Nevertheless, stage I AKI independently predicts morbidity and death in critical care patient populations.26,27 In addition, we observed similar associations among BMI, AKI, and biomarkers when we defined AKI as an ordinal end point (stage 0, I, II, or III AKI) and when we defined AKI as postoperative creatinine concentrations (a continuous end point reflecting AKI severity) (see Supplemental Table 1). Obtaining consistent results when we defined AKI using these alternate criteria for AKI diagnosis and severity at both 48 and 72 hours after surgery, along with the size of our well phenotyped surgical cohort, further strengthens our conclusions.
In conclusion, increased BMI predicts an increased risk for AKI after cardiac surgery, and increased oxidative stress may partially account for the risk for AKI associated with obesity. Although intraoperative PAI-1 and, to a lesser extent, IL-6 also predict the development of AKI after cardiac surgery, it is not evident that they contribute to the relationship between BMI and AKI in these patients. Therapies that reduce intraoperative oxidative stress might reduce the incidence, severity, and associated morbidity of AKI after cardiac surgery.
Concise Methods
Data were analyzed from the Atrial Fibrillation and Renin Angiotensin Aldosterone System study (ClinicalTrials.gov: NCT00141778), a randomized clinical trial that tested the hypothesis that interruption of the renin-angiotensin-aldosterone system by angiotensin-converting enzyme inhibition (ramipril) or aldosterone receptor antagonism (spironolactone) decreases the incidence of atrial fibrillation after elective cardiac surgery.28 Prespecified end points of the parent clinical trial were the occurrence of atrial fibrillation from surgery until hospital discharge, serum potassium and creatinine concentrations, length of hospital stay, and death. Predefined biomarkers included markers of oxidative stress (F2-isoprostanes), antifibrinolysis (PAI-1), and inflammation (IL and C-reactive protein). Five days before surgery, patients were randomly assigned to treatment with placebo, spironolactone (25 mg/d), or ramipril (5 mg/d). Patients were eligible for the study if they were undergoing elective cardiac surgery, including coronary artery bypass grafting or valvular surgery. Exclusion criteria included chronic or paroxysmal atrial fibrillation within 6 months, baseline serum creatinine level >1.6 mg/dl (141.6 μmol/L), hyperkalemia with potassium level >5.0 mEq/L, a left ventricular ejection fraction <30%, and emergency surgery. This study was approved by the Vanderbilt University Institutional Review Board and was conducted according to the principles of the Declaration of Helsinki. All patients provided written informed consent.
Study Population
Six hundred ten patients consented to participate. One hundred fourteen of these patients had surgery emergently, did not require surgery, or did not meet inclusion criteria after review of their chart. An additional 38 patients decided to withdraw before randomization. Four hundred fifty-eight patients were randomly assigned. Thirteen withdrew after randomization but before taking study medication. Four hundred forty-five patients completed the study and make up the data set.
Standardized Patient Treatment and AKI Diagnosis
Anesthetic and surgical management were conducted according to institutional protocols, as detailed in the Supplemental Methods and as described elsewhere.28
AKI was defined according to AKIN criteria:17 stage I, an increase in serum creatinine concentration of 50% or 0.3 mg/dl (26.5 μmol/L); stage II, 100% increase in serum creatinine; and stage III, 200% increase in serum creatinine within 72 hours of surgery. The AKIN urine output criteria for AKI diagnosis were not used because of confounding by intravascular hypovolemia and diuretic use,29 both of which are common among patients undergoing cardiac surgery. Baseline creatinine and BMI were determined during the preoperative anesthesiology assessment clinic visit for outpatients and on the morning of surgery for inpatients.
Measurement of Oxidative Stress, Antifibrinolysis, and Inflammation
Blood was sampled at anesthetic induction (baseline) and during surgery after protamine reversal of heparin at the conclusion of cardiopulmonary bypass or off-pump coronary artery bypass grafting (intraoperative) in 0.105 M sodium citrate–coated tubes, immediately placed on ice, centrifuged for 20 minutes at 3120 rpm, and plasma frozen at −80°C.
Oxidative stress was quantified by measurement of plasma F2-isoprostane concentrations. Internal standard [2H4]-15-F2T-isoprostane was added to plasma and the sample purified by sequential C-18 and silica solid-phase extraction and then derivatized to penta-fluorobenzyl ester, trimethylsilyl ether for gas chromatography/ negative ion chemical ionization/mass spectrometry analysis.30 PAI-1 antigen concentrations were determined using a two-site ELISA (Biopool AB). IL-6 concentrations were determined using the Human Inflammation Cytokine Cytometric Bead array ELISA kit (BD Biosciences Pharmingen).
Statistical Analyses
Descriptive summaries were calculated for patient characteristics. The Wilcoxon rank-sum test was used for between-group comparisons of continuous variables, and the Pearson chi-squared test was used for categorical variables. The Spearman test was used to assess correlations between continuous variables.
Logistic and linear regression analyses, adjusted for sets of potential confounders determined a priori and for the randomized study drug treatment, were used to examine the relationships between BMI and AKI (logistic), BMI and candidate pathway biomarkers (linear), candidate pathway biomarkers and AKI (logistic), and candidate pathway mediation of any association between BMI and AKI (logistic and linear). All regression models included adjustment variables or covariates: age, sex, African-American race (yes/no), history of diabetes (yes/no), baseline creatinine (log-transformed because of positive skewness), use of cardiopulmonary bypass (yes/no), valvular heart surgery (yes/no), the randomized treatment (placebo, ramipril, or spironolactone), and duration of cardiopulmonary bypass (intraoperative models only). To adjust for CKD, each of the Modified Diet in Renal Disease formula variables were included individually in all regression models.
The overall BMI effect on AKI was summarized with an adjusted odds ratio and 95% CI per 5-kg/m2 change. This model (Model 1) quantifies the association between BMI and AKI adjusted for covariates. Model 1 can be written as follows:
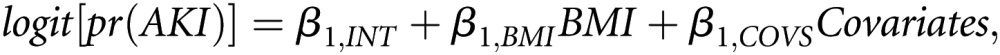 |
where logit[pr(AKI)] is the log odds of AKI, β1,INT is the model intercept, β1,BMI is the adjusted log odds ratio associated with changes in BMI, and Covariates is the set of adjustment covariates (Figure 2A).
The effect of baseline and intraoperative F2-isoprostanes, IL-6, and PAI-1 on AKI was summarized with adjusted odds ratios associated with percentage changes in candidate pathway biomarker values. Percentage changes were used because the markers were log-transformed (because of positive skewness) in the regression analyses. For convenience, we discuss effects of 50% changes in biomarker values. Independent variables in these models included the candidate pathway biomarker, BMI, and adjustment covariates. This model (Model 2) simultaneously quantifies the associations of a biomarker on AKI and BMI on AKI, adjusted for that biomarker. Model 2 can be written as follows:
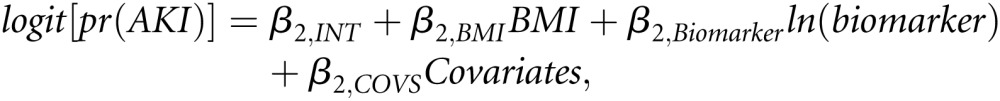 |
where ln(biomarker) is a log-transformed biomarker value. β2,BMI and β2,Biomarker capture the strengths of the relationships between BMI and AKI and between biomarker and AKI, respectively (Figure 2B).
To address the hypothesis that risk for AKI associated with BMI is mediated by oxidative stress, inflammation, or antifibrinolysis, we used a strategy based on the original approach of Baron and Kenny.31 We sought to deconstruct the total effect of BMI on AKI shown in Figure 2A and captured by β1,BMI from Model 1 into the direct (BMI to AKI independent of candidate pathway) and indirect (BMI to AKI through candidate pathway biomarker) effects shown in Figure 2B. The direct effect is captured by β2,BMI from Model 2. Assessment of the hypothesized indirect effect required a third regression model (Model 3) in which the log-transformed biomarker values were regressed on BMI and the adjustment covariates. Model 3 can be written as follows:
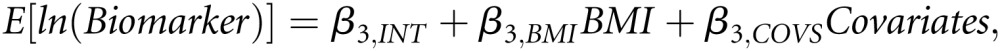 |
where E[ln(Biomarker)] is the expected value of the log-transformed biomarker value and β3,BMI captures the strength of the relationship between BMI and each candidate pathway biomarker. The indirect effect is captured by the product of β3,BMI from Model 3 and β2,Biomarker from Model 2 (Figure 2B). A positive or negative (i.e., non-zero) effect of BMI on the candidate pathway biomarker (β3,BMI from Model 3) and a positive or negative effect of the candidate pathway biomarker on AKI (β2,Biomarker from Model 2) would be consistent with the existence of an indirect pathway between BMI and AKI that was mediated by that biomarker.31,32 The presence of an indirect effect was examined by testing the null hypothesis that the product β3,BMI × β2,Biomarker was equal to zero, and this test was conducted using a bootstrap based extension of the Sobel test with 5000 bootstrap replicates.33 For interpretation, the exponentiated product estimate approximates the odds ratio for AKI associated with 1-kg/m2 increases in BMI that are secondary to the effect of BMI on AKI via the candidate pathway biomarker.
To further evaluate the relationships among BMI, candidate pathways (oxidative stress, inflammation, and antifibrinolysis), and postoperative renal dysfunction, we also conducted the same analyses while defining AKI as the outcome of a proportional odds model (ordinal regression using AKI stage [0, I, II, or III at 72 hours after surgery] as the dependent variable), as a 50% or 0.3-mg/dl (26.5-μmol/L) serum creatinine increase within 48 hours of surgery, and as the maximum creatinine concentration 48 or 72 hours after surgery as continuous dependent variables (see Supplemental Methods). All data were analyzed using the R programming language (www.R-project.org).
Disclosures
None.
Acknowledgments
We thank Carol Meisch for nursing assistance, Anthony DeMatteo and Jeff Petro for technical assistance, Stephanie Sanchez and Ginger Milne for measurement of F2-isoprostanes, and Jessica Moore for advice on manuscript preparation.
This study was funded by the National Institutes of Health (R01HL77389, R01HL65193, and UL1RR024975). Clinical Trial Registration: NCT00141778.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011090940/-/DCSupplemental.
References
- 1.Rosner MH, Okusa MD: Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 1: 19–32, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J: Independent association between acute renal failure and mortality following cardiac surgery. Am J Med 104: 343–348, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Mehta RH, Grab JD, O’Brien SM, Bridges CR, Gammie JS, Haan CK, Ferguson TB, Peterson ED, Society of Thoracic Surgeons National Cardiac Surgery Database Investigators : Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation 114: 2208–2216, quiz 2208, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Thakar CV, Yared J-P, Worley S, Cotman K, Paganini EP: Renal dysfunction and serious infections after open-heart surgery. Kidney Int 64: 239–246, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Swaminathan M, Shaw AD, Phillips-Bute BG, McGugan-Clark PL, Archer LE, Talbert S, Milano CA, Patel UD, Stafford-Smith M: Trends in acute renal failure associated with coronary artery bypass graft surgery in the United States. Crit Care Med 35: 2286–2291, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Thakar CV, Kharat V, Blanck S, Leonard AC: Acute kidney injury after gastric bypass surgery. Clin J Am Soc Nephrol 2: 426–430, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Praga M, Hernández E, Herrero JC, Morales E, Revilla Y, Díaz-González R, Rodicio JL: Influence of obesity on the appearance of proteinuria and renal insufficiency after unilateral nephrectomy. Kidney Int 58: 2111–2118, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Glance LG, Wissler R, Mukamel DB, Li Y, Diachun CAB, Salloum R, Fleming FJ, Dick AW: Perioperative outcomes among patients with the modified metabolic syndrome who are undergoing noncardiac surgery. Anesthesiology 113: 859–872, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Druml W, Metnitz B, Schaden E, Bauer P, Metnitz PGH: Impact of body mass on incidence and prognosis of acute kidney injury requiring renal replacement therapy. Intensive Care Med 36: 1221–1228, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Silver AE, Beske SD, Christou DD, Donato AJ, Moreau KL, Eskurza I, Gates PE, Seals DR: Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47(phox) expression and evidence of endothelial oxidative stress. Circulation 115: 627–637, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Greenberg AS, Obin MS: Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr 83: 461S–465S, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney JF, Jr, Meigs JB, Lipinska I, Kathiresan S, Murabito JM, O’Donnell CJ, Benjamin EJ, Fox CS: Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation 116: 1234–1241, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Skurk T, Hauner H: Obesity and impaired fibrinolysis: Role of adipose production of plasminogen activator inhibitor-1. Int J Obes Relat Metab Disord 28: 1357–1364, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Ulus AT, Aksoyek A, Ozkan M, Katircioglu SF, Basu S: Cardiopulmonary bypass as a cause of free radical-induced oxidative stress and enhanced blood-borne isoprostanes in humans. Free Radic Biol Med 34: 911–917, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Czerny M, Baumer H, Kilo J, Lassnigg A, Hamwi A, Vukovich T, Wolner E, Grimm M: Inflammatory response and myocardial injury following coronary artery bypass grafting with or without cardiopulmonary bypass. Eur J Cardiothorac Surg 17: 737–742, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Pretorius M, Donahue BS, Yu C, Greelish JP, Roden DM, Brown NJ: Plasminogen activator inhibitor-1 as a predictor of postoperative atrial fibrillation after cardiopulmonary bypass. Circulation 116[Suppl]: I1–I7, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury Network : Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yap CH, Mohajeri M, Yii M: Obesity and early complications after cardiac surgery. Med J Aust 186: 350–354, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Wigfield CH, Lindsey JD, Muñoz A, Chopra PS, Edwards NM, Love RB: Is extreme obesity a risk factor for cardiac surgery? An analysis of patients with a BMI > or = 40. Eur J Cardiothorac Surg 29: 434–440, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Reis C, Barbiero SM, Ribas L: The effect of the body mass index on postoperative complications of coronary artery bypass grafting in elderly. Rev Bras Cir Cardiovasc 23: 524–529, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Hoste EA, Cruz DN, Davenport A, Mehta RL, Piccinni P, Tetta C, Viscovo G, Ronco C: The epidemiology of cardiac surgery-associated acute kidney injury. Int J Artif Organs 31: 158–165, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Takahashi K, Nammour TM, Fukunaga M, Ebert J, Morrow JD, Roberts LJ, 2nd, Hoover RL, Badr KF: Glomerular actions of a free radical-generated novel prostaglandin, 8-epi-prostaglandin F2 alpha, in the rat. Evidence for interaction with thromboxane A2 receptors. J Clin Invest 90: 136–141, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haase M, Haase-Fielitz A, Bagshaw SM, Reade MC, Morgera S, Seevenayagam S, Matalanis G, Buxton B, Doolan L, Bellomo R: Phase II, randomized, controlled trial of high-dose N-acetylcysteine in high-risk cardiac surgery patients. Crit Care Med 35: 1324–1331, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Roberts LJ, 2nd, Oates JA, Linton MF, Fazio S, Meador BP, Gross MD, Shyr Y, Morrow JD: The relationship between dose of vitamin E and suppression of oxidative stress in humans. Free Radic Biol Med 43: 1388–1393, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramos LF, Shintani A, Ikizler TA, Himmelfarb J: Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J Am Soc Nephrol 19: 593–599, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M: Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: A prospective cohort study. J Am Soc Nephrol 15: 1597–1605, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Billings FT, 4th, Pretorius M, Siew ED, Yu C, Brown NJ: Early postoperative statin therapy is associated with a lower incidence of acute kidney injury after cardiac surgery. J Cardiothorac Vasc Anesth 24: 913–920, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macedo E, Malhotra R, Claure-Del Granado R, Fedullo P, Mehta RL: Defining urine output criterion for acute kidney injury in critically ill patients. Nephrol Dial Transplant 26: 509–515, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milne GL, Sanchez SC, Musiek ES, Morrow JD: Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat Protoc 2: 221–226, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Baron RM, Kenny DA: The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol 51: 1173–1182, 1986 [DOI] [PubMed] [Google Scholar]
- 32.MacKinnon D, Fairchild A, Fritz M: Mediation analysis. Ann Rev Psychol 58: 593–614, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sobel M: Asymptomatic confidence intervals for indirect effects in structural equations models. In: Sociological Methodology, Washington, DC, 1982, pp. 290–312 [Google Scholar]