Abstract
Fibroblast growth factor (FGF) 23 inhibits calcitriol production, which could exacerbate calcium deficiency or hypocalcemia unless calcium itself modulates FGF23 in this setting. In Wistar rats with normal renal function fed a diet low in both calcium and vitamin D, the resulting hypocalcemia was associated with low FGF23 despite high parathyroid hormone (PTH) and high calcitriol levels. FGF23 correlated positively with calcium and negatively with PTH. Addition of high dietary phosphorus to this diet increased FGF23 except in rats with hypocalcemia despite high PTH levels. In parathyroidectomized rats, an increase in dietary calcium for 10 days increased serum calcium, with an associated increase in FGF23, decrease in calcitriol, and no change in phosphorus. Also in parathyroidectomized rats, FGF23 increased significantly 6 hours after administration of calcium gluconate. Taken together, these results suggest that hypocalcemia reduces the circulating concentrations of FGF23. This decrease in FGF23 could be a response to avoid a subsequent reduction in calcitriol, which could exacerbate hypocalcemia.
Fibroblast growth factor (FGF) 23 production is stimulated by both calcitriol and phosphorus intake. FGF23 acts through FGFR-klotho receptors in the kidney to induce phosphaturia, a decrease in 1-α-hydroxylase activity, and an increase in 24-hydroxylase activity. The latter two effects decrease the synthesis and increase the degradation of calcitriol, respectively.1–4 Parathyroid cells also possess FGFR-klotho receptors, and experimental studies have shown that FGF23 inhibits parathyroid hormone (PTH) production and secretion.5–7 However, in uremic animals, hyperplastic parathyroid glands fail to respond to FGF23 because the expression of FGFR-klotho is downregulated.7–11
FGF23 effectively increases the output and decreases the input of phosphorus because it directly increases phosphaturia and indirectly decreases intestinal phosphorus absorption by decreasing calcitriol values. However, a conflict will arise if high FGF23 inhibits calcitriol production in a setting of calcium deficiency/hypocalcemia, where high calcitriol is needed to increase intestinal calcium absorption. We have previously observed in parathyroidectomized (PTX) rats with decreased serum levels of calcium and calcitriol that despite an increase in phosphorus levels, the concentration of FGF23 is below that in control intact rats.12,13 We also have observed in PTX rats that replacement with calcitriol sufficient to normalize serum calcitriol levels resulted in normalization of FGF23. In an experimental model of primary hyperparathyroidism, FGF23 is increased, suggesting that PTH may directly increase FGF23 and may also increase FGF23 through stimulation of calcitriol.13
Our hypothesis is that because increased calcitriol values are necessary to correct hypocalcemia in conditions of calcium deficiency, a calcitriol-induced increase in FGF23 might be prevented when serum calcium levels are low. Therefore, our primary goal was to determine whether the calcium status affects FGF23 production. We studied rats with a reduction in calcium and vitamin D in the diet, which resulted in calcium deficiency/hypocalcemia. We also investigated in PTX rats the effect of short- and long-term administration of calcium on FGF23 levels.
Results
In rats receiving low-calcium, low–vitamin D (LCa-LD) and low-calcium, high-phosphorus, low–vitamin D (LCa-HP-LD) diets, serum calcium levels were lower and serum phosphorus did not differ from levels in rats fed a normal-calcium and normal-phosphorus diet (Table 1). PTH was increased and calcitriol levels were greater than normal (i.e., in the normal-diet group). Serum FGF23 was normal in the LCa-HP-LD diet group and decreased in the LCa-LD diet group. In LCa-LD diet rats, the decrease in serum calcium was associated with a marked elevation in PTH, but serum FGF23 levels were decreased despite high calcitriol and high PTH levels. In LCa-HP-LD diet rats, FGF23 did not increase above normal despite the phosphorus load and high PTH and calcitriol levels.
Table 1.
Serum levels of creatinine, FGF23, calcium, phosphorous, 1,25 (OH)D, 25(OH)D, and PTH in the experimental groups of rats fed different diets.
| Group | Calcium (mg/dl) | Phosphorous (mg/dl) | Serum Creatinine (mg/dl) | PTH (pg/ml) | FGF23 (pg/ml) | 1,25(OH)D (pg/ml) | 25(OH)D (ng/ml) |
|---|---|---|---|---|---|---|---|
| Normal diet | 4.80±0.12 | 5.36±1.10a | 0.51±0.08a | 57±21 | 163±51 | 296±106 | 43±13 |
| LCa-LD diet | 3.24±1.16b | 5.30±1.48a | 0.52±0.06a | 1510±1072b,c | 37±56a,b,c | 427±47b | 5±1a,b |
| LCa-HP-LD diet | 3.80±0.88a,b | 4.90±1.26a | 0.57±0.07a | 825±944b | 138±109a | 452±43a,b | 4±1a,b |
| Nx-HP diet | 2.68±0.40b | 12.2±3.43 | 1.09±0.18 | 1943±685b,c | 1360±284b | 342±110 | 15±2a |
Values are expressed as the mean ± SD. Normal diet, controls with a normal (0.6% calcium, 0.6% phosphorous) diet (n=10); LCa-LD diet, low-calcium, low–vitamin D (0.15% calcium, 0.6% phosphorous, and <50 IU/kg vitamin D) diet (n=23); LCa-HP-LD diet, low-calcium, high-phosphorus, low–vitamin D (0.15% calcium, 1.2% phosphorous, and <50 IU/kg of vitamin D) diet (n=24); Nx-HP diet, rats with renal hyperparathyroidism with a high-phosphorus (1.2% phosphorous) diet (n=9).
P<0.05 versus Nx-HP diet.
P<0.05 versus normal diet.
P<0.05 versus LCa-HP-LD diet.
Compared with controls (normal-diet group), rats in an additional group with uremic hyperparathyroidism (Nx-HP diet group) had higher serum creatinine levels (Table 1). Serum calcium and phosphorus levels were, respectively, decreased and increased compared with those in the normal-diet group. Serum PTH was elevated, as was FGF23. Calcitriol levels did not differ from those in the normal-diet rats. As expected, the serum concentrations of 25-hydroxyvitamin D (25[OH]D) were markedly decreased in rats receiving a vitamin D–deficient diet; they were also reduced in the rats receiving the Nx-HP diet.
The wide distribution of serum calcium levels in the LCa-LD and LCa-HP-LD diet groups allowed for a more detailed study of the relationship between FGF23 and the other variables.
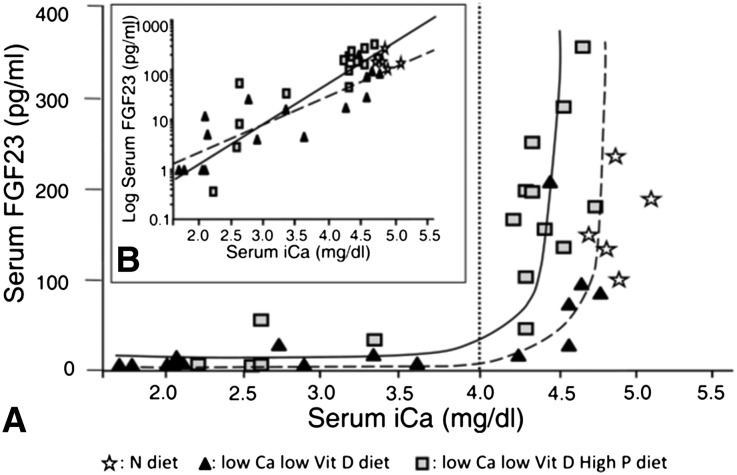
The relationship between serum concentrations of FGF23 and calcium is shown in Figure 1. FGF23 levels significantly correlated with the serum ionized calcium concentration. In rats fed the LCa-LD and LCa-HP-LD diets, a low serum calcium concentration was associated with reduced FGF23 levels. In fact, FGF23 was reduced in all rats with serum calcium <4.0 mg/dl. Only rats with serum calcium >4.0 mg/dl had serum FGF23 close to normal. Of note, in rats with increased dietary phosphorus (LCa-HP-LD), FGF23 started to increase at lower calcium concentrations than in LCa-LD rats with no phosphorus added to the diet (left shift of the FGF23-Ca curve). At serum calcium concentrations >4.0 mg/dl (which matches PTH values <700 pg/ml), serum FGF23 levels were greater in rats fed a high-phosphorus than in those fed a normal-phosphorus diet (85.7±68.4 versus 192.3±86.7 pg/ml; P=0.02) (Figure 1B). However, when the serum calcium concentration was <4.0 mg/dl, FGF23 values were similarly decreased in rats fed a high- or normal-phosphorus diet (8.0±9.3 versus 21.3±25.0 pg/ml; P=NS). In the normal-diet rats, FGF23 levels were elevated independently of the serum calcium concentration (Table 1).
Figure 1.
PTH does not appear to stimulate FGF23 in the presence of hypocalcemia. FGF23 remains low when calcium is <4.0 mg/dl and increases steeply when calcium rises through the physiologic range. (A) The relationship between serum FGF23 concentration and serum ionized calcium is shown in three groups of rats on different diets: (1) controls fed a normal diet; (2) low calcium, low vitamin D (LCa-LD diet); and (3) low calcium, high phosphorus, low vitamin D (LCa-HP-LD diet). Rats were maintained on this diet for 6 weeks. The vertical dotted line at ionized calcium (iCa) = 4 mg/dl represents the approximate threshold concentration of calcium that allows an increase in FGF23. (B) Inset shows the relationship between log-transformed serum FGF23 concentration and serum ionized calcium in the same groups of rats shown in A. Normal diet: r2=0.07, P=0.66; LCa-LD diet (dotted line): r2=0.73, P<0.001; LCa-HP-LD diet (continuous line): r2=0.77, P<0.001.
As shown in Figure 2, in both groups fed a low-calcium diet, the serum phosphorus values were inversely correlated with serum ionized calcium (r2=0.73, P<0.001 for LCa-LD; r2=0.51, P<0.002 for LCa-HP-LD).
Figure 2.
Serum phosphorus (P) concentration is inversely correlated with serum ionized calcium (iCa) in rats fed a low-calcium diet. The relationship between serum phosphorus concentration and serum ionized calcium is shown in three groups of rats fed different diets: (1) controls fed a normal diet; (2) low calcium, low vitamin D (LCa-LD diet); and (3) low calcium, high phosphorus, low vitamin D (LCa-HP-LD diet). Rats were maintained on this diet for 6 weeks. r2=0.41, P=0.24 for normal diet; r2=0.73, P<0.001 for LCa-LD diet (dotted line); and r2=0.51, P<0.002 for LCa-HP-LD diet (continuous line).
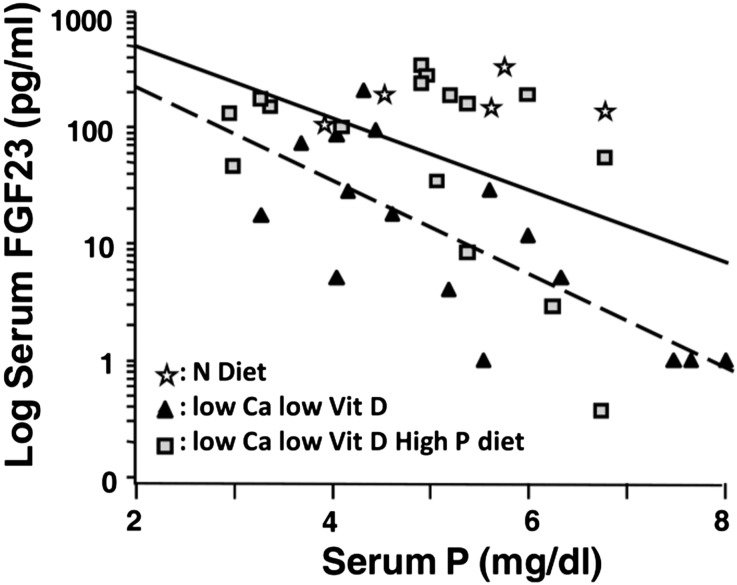
In rats receiving a calcium- and vitamin D–deficient diet (LCa-LD), FGF23 was negatively correlated with the serum phosphorus concentration (r2=0.54; P=0.001) (Figure 3). In rats fed a calcium- and vitamin D–deficient diet with high phosphorus (LCa-HP-LD), the correlation between FGF23 and serum phosphorus was r2=0.23 (P=0.06). Despite the high-phosphorus diet in the LCa-HP-LD rats, FGF23 did not reach values above those in controls fed a normal-calcium and normal-phosphorus diet (Table 1).
Figure 3.
The direct association between FGF23 and phosphorus (P) does not occur in the presence of hypocalcemia. The relationship between serum log-transformed FGF23 concentration and serum phosphorus is shown in three groups of rats fed different diets: (1) controls fed a normal (N) diet; (2) low calcium (Ca), low vitamin D (Vit D) (LCa-LD diet); and (3) low calcium, high phosphorus, low vitamin D (LCa-HP-LD diet). Rats were maintained on this diet for 6 weeks. r2=0.06, P=0.68 for normal diet; r2=0.54, P=0.001 for LCa-LD diet (dotted line); and r2=0.23, P=0.06 for LCa-HP-LD diet (continuous line).
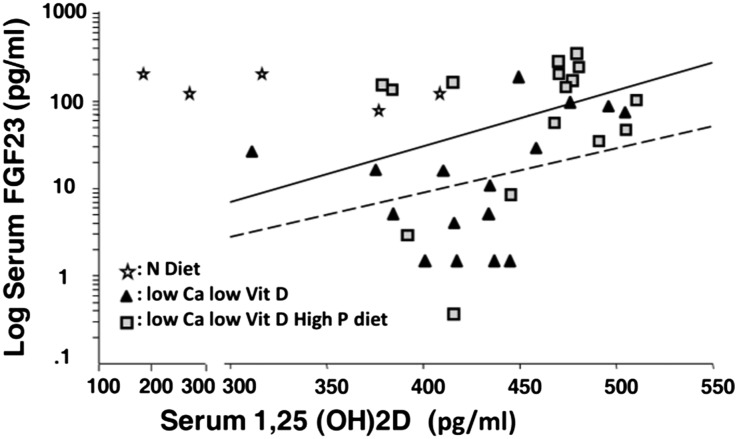
Figure 4 shows no correlation between FGF23 and calcitriol. In rats fed a LCa-HP-LD diet, high levels of FGF23 were not associated with a reduction in calcitriol levels. Rather, the opposite trend was observed: High calcitriol was associated with higher FGF23 levels (and higher calcium). Suggesting that elevated calcitriol was stimulating FGF23 only when serum calcium was not low or normal is the observation that in the LCa-LD and LCa-HP-LD diet groups with higher serum calcitriol levels (>450 pg/ml), the mean serum calcium concentration was 4.24±0.56 mg/dl, a value significantly higher than the 2.96±1.00 mg/dl (P<0.01) in rats with a calcitriol level <450 pg/ml.
Figure 4.
Elevated calcitriol stimulates FGF23 only when serum calcium is not low. The relationship between serum log-transformed FGF23 concentration and serum calcitriol concentration is shown in three groups of rats fed different diets: (1) controls fed a normal (N) diet; (2) low calcium (Ca), low vitamin D (Vit D) (LCa-LD diet); and (3) low calcium, high phosphorus, low vitamin D (LCa-HP-LD diet). Rats were maintained on this diet for 6 weeks. r2=0.14, P=0.53 for normal diet; r2=0.11, P=0.21 for LCa-LD diet (dotted line); and r2=0.11, P=0.21 for LCa-HP-LD diet (continuous line).
In rats fed a low-calcium, vitamin D–deficient diet with or without high phosphorus (LCa-LD and LCa-HP-LD), the increase in PTH was associated with a decrease in FGF23 levels (Figure 5). Of note, FGF23 levels were normal or increased only if the PTH concentration was <700 pg/ml. For a PTH concentration <700 pg/ml, the serum FGF23 was greater in the LCa-HP-LD than in the LCa-LD diet group (Figure 5B). Indeed, in rats with a PTH level <700 pg/ml, the respective FGF23 levels in the LCa-LD and LCa-HP-LD diet groups were 85.7±68.4 and 192.3±86.7 pg/ml (P=0.02). However, in rats with a PTH level >700 pg/ml, the respective FGF23 levels in the LCa-LD and LCa-HP-LD diet rats were 8.0±9.3 and 21.3±25.0 pg/ml (P=NS).
Figure 5.
FGF23 levels are normal or increased only if the PTH concentration is <700 pg/ml (which matches with a serum calcium [Ca] >4.0 mg/dl). (A) The relationship between serum FGF23 concentration and serum PTH concentration is shown in three groups of rats fed different diets: (1) controls fed a normal diet; (2) low calcium, low vitamin D (LCa-LD diet); and (3) low calcium, high phosphorus, low vitamin D (Vit D) (LCa-HP-LD diet). Rats were maintained on this diet for 6 weeks. (B) The relationship between log-transformed serum FGF23 concentration and serum PTH concentration in the same groups of rats shown in A. normal diet: r2=0.08, P=0.64; LCa-LD diet (dotted line): r2=0.62, P<0.001; LCa-HP-LD diet (continuous line): r2=0.42, P=0.007.
A multiple regression analysis was performed using pooled data from the LCa-LD and LCa-HP-LD diet groups. FGF23 level (log-transformed) was the dependent variable, and serum levels of calcium, calcitriol, phosphorus, and PTH were the independent variables. The results shown in Table 2 indicate that serum calcium level was the only independent variable that significantly influenced the level of FGF23.
Table 2.
Multiple regression analysis with FGF23 level (log-transformed) as the dependent variable and serum levels of calcium, calcitriol, phosphorus, and PTH as the independent variables
| Dependent Variable | Independent Variable | Regression Coefficient | SEM | T Value to Test H0≠0 | P Value | Reject H0 at 5.0%? | Power Test at 5.0% |
|---|---|---|---|---|---|---|---|
| FGF23 levels | Intercept | −2.31 | 1.20 | −1.93 | 0.06 | No | 0.46 |
| Calcium | 3.55 | 0.87 | 4.08 | 0.0004 | Yes | 0.98 | |
| Calcitriol | −0.0008 | 0.002 | −0.39 | 0.70 | No | 0.07 | |
| phosphorus | 0.19 | 0.11 | 1.79 | 0.08 | No | 0.41 | |
| PTH | 0.00 | 0.0001 | −0.06 | 0.95 | No | 0.05 |
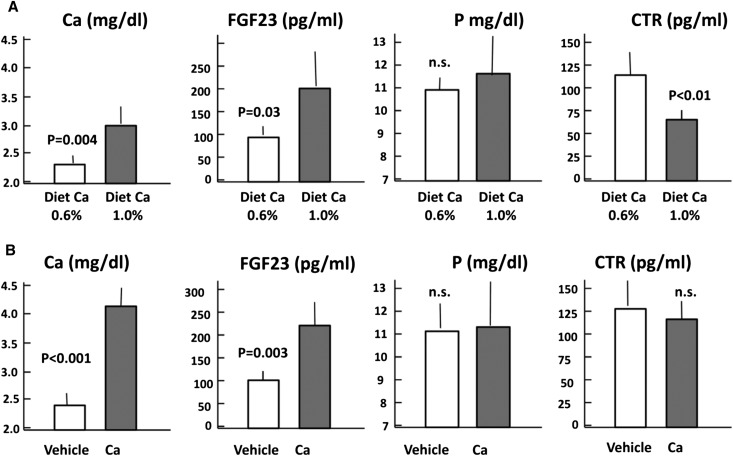
Two additional experiments were performed in PTX rats to evaluate whether the serum calcium concentration directly affects circulating levels of FGF23. In the first experiment, PTX rats were fed a diet with normal or high calcium content (0.6% versus 1.0%). After 10 days, the mean serum calcium concentration was greater in rats fed a high-calcium than those fed a normal-calcium diet (3.04±0.36 versus 2.28±0.16 mg/dl; P<0.01) (Figure 6A). Likewise, the serum concentration of FGF23 was also greater in rats ingesting more calcium (194±73 versus 94±9.6 pg/ml; P<0.05). Serum levels of phosphorus remained similarly increased for the two diets. However, the concentration of calcitriol was significantly reduced in rats fed the 1.0% calcium diet compared with those fed the normal-calcium diet (63±8 versus 114±22 pg/ml; P<0.01).
Figure 6.
Hypocalcemia (induced by dietary calcium [Ca] restriction) decreases FGF23, whereas hypercalcemia (induced by intraperitoneal calcium administration) increases FGF23. (A) Serum concentration of ionized calcium, FGF23, phosphorus (P), and calcitriol (CTR) in PTX rats fed a 0.6% or 1.0% calcium diet for 10 days. (B) Serum concentration of ionized calcium, FGF23, phosphorus, and calcitriol in PTX rats after intraperitoneal infusion of calcium (10 mg/kg per hour) during 6 hours.
In the second experiment, calcium was administered intraperitoneally for 6 hours (Figure 6B). An increase in serum calcium concentration from 2.36±0.20 to 4.12±0.32 mg/dl (P<0.01) was accompanied by an increase in serum FGF23 from 101±5 to 218±62 pg/ml (P<0.01). Serum phosphorus and serum calcitriol were not changed by this short-term calcium increase.
Discussion
The aim of the present study was to evaluate FGF23 regulation in conditions of calcium deficiency and hypocalcemia. In rats with normal renal function fed a low-calcium and low–vitamin D diet, the resulting hypocalcemia was associated with low FGF23 despite high PTH and 1,25(OH)2 vitamin D levels. FGF23 and calcium were positively correlated. FGF23 correlated negatively with PTH levels. The addition of phosphorus to the diet produced a discrete increase in FGF23, but not in rats with hypocalcemia despite high PTH levels. In the absence of PTH (PTX rats), an increase in dietary calcium for 10 days produced an increase in serum calcium with an associated increase in FGF23 and a decrease in serum calcitriol, but no change in phosphorus levels. In addition, short-term (6 hours) intraperitoneal administration of calcium to PTX rats also produced a significant increase in FGF23 levels. These results indicate that hypocalcemia reduces the circulating levels of FGF23; this effect is reversed by the administration of calcium. The reduction of FGF23 associated with low calcium could be a physiologic response to avoid a decrease in calcitriol, which could exacerbate hypocalcemia.
Rats fed a calcium- and vitamin D–deficient diet developed hyperparathyroidism, and many of these rats were hypocalcemic with very high PTH levels. FGF23 was reduced, indicating that high PTH levels are not always associated with increased production of FGF23. In a previous study,13 we observed in an experimental model of primary hyperparathyroidism in rats that high PTH was associated with hypercalcemia and high FGF23. In the present experiment, the excess of PTH was secondary to hypocalcemia. This finding suggests that in the presence of hypocalcemia, PTH does not seem to stimulate FGF23. This notion is supported by the fact that with the diet deficient in calcium and vitamin D, only rats able to maintain ionized calcium at a level >4.0 mg/dl had “close to normal” FGF23 levels. Our results suggest a threshold phenomenon for the calcium effect on FGF23: FGF23 remains low, independent of the phosphorus status, when ionized calcium is <4.0 mg/dl and increases steeply when ionized calcium rises through the physiologic range. This observation favors the concept that a minimum concentration of ambient calcium is needed to permit FGF23 secretion. Moreover, because FGF23 increases as ionized calcium rises through the physiologic range, this type of regulation of FGF23 may have a significant physiologic role.
Because low calcium correlated with both low FGF23 and high PTH, we expected to find an association between high PTH and low FGF23. The PTH effect seems counterintuitive, but we believe that the threshold effect of calcium on FGF23 regulation provides an answer to this paradox. If, as shown by the data, FGF23 is not stimulated when calcium is very low (essentially because this would reduce calcitriol and help aggravate hypocalcemia) in situations of severe hypocalcemia in which PTH is very high, FGF23 will not be stimulated. These data also support the precedence of calcium (during hypocalcemia) over other regulatory factors of FGF23 (i.e., low calcium overrides the expected counter-regulatory effect of calcitriol and PTH). Conversely, many authors have shown a positive correlation between PTH and FGF23 in patients with renal failure. In this study we also observed that in uremic rats, high FGF23 is associated with elevated PTH levels. In uremia, FGF23 seems to be regulated by a variety of factors, including high phosphorus, high PTH, low GFR, low klotho, and possibly others.14
Serum FGF23 concentration has been shown to correlate positively with serum phosphorus levels.15 Our uremic rats had high serum phosphorus concentrations, which were associated with high FGF23 levels despite hypocalcemia. However, in normal rats receiving a calcium-deficient diet, even with phosphorus added to the diet, serum FGF23 was inversely correlated with the serum phosphorus concentration. Although in these rats serum phosphorus concentrations were not as high as in uremic rats, this was an unexpected finding. There was also a significant inverse correlation between serum calcium and phosphorus. If high FGF23 is associated with an increasing calcium concentration, it cannot be also associated with high serum phosphorus. The finding that in rats fed a low-calcium diet the increase in dietary phosphorus did not increase FGF23 above that in control rats (which were fed a normal-phosphorus diet) suggests that low calcium intake/hypocalcemia limits phosphorus-induced FGF23 production.
In rats receiving LCa-LD and LCa-HP-LD diets, the increase in calcitriol was associated with an increase in FGF23, but only when the calcium concentration approached normal. Again, it seems that the calcium concentration modulates FGF23 levels. Thus, it is reasonable to hypothesize that calcitriol will stimulate FGF23 only when it is high enough to prevent hypocalcemia. High levels of FGF23 were not associated with a reduction in calcitriol; rather, high calcitriol was associated with higher FGF23 levels and, importantly, higher serum calcium. The low levels of serum 25(OH)D may have limited the ability to increase calcitriol in the presence of hypocalcemia and high PTH. Multiple regression analysis would suggest that hypocalcemia, rather than any decrease in calcitriol, is the cause of a reduction in FGF23.
The effect of serum calcium concentration on FGF23 production was also tested in PTX rats. The serum calcium concentration was increased by augmenting the calcium content of the diet or by administration of calcium intraperitoneally. The increase in calcium produced a concomitant increase in FGF23. Serum phosphorus did not change and serum calcitriol decreased in rats after a prolonged increase in serum calcium. Calcitriol levels did not change in rats after a short period of calcium infusion, probably because changes in calcitriol induced by calcium may require a more prolonged stimulus.
It is noteworthy that the concept of calcium as a modulator of FGF23 production is not completely new. In vitamin D–receptor null mice, calcium supplementation increases FGF23 mRNA levels.16 Serum calcium has also been reported to be independently associated with FGF23 in patients with primary and secondary (renal) hyperparthyroidism.17,18
The increase in FGF23 in the PTX rats during a calcium infusion and increased dietary calcium may provide potential insights into unexplained clinical observations from many years ago. In studies from the 1930s and 1940s, vitamin D or dihydrotachysterol was given to patients with hypoparathyroidism19,20 and pseudohypoparathyroidism;21 not only did vitamin D and dihydrotachysterol normalize the serum calcium, but they also decreased serum phosphorus by increasing urine phosphorus excretion. Failure to increase serum calcium because of low dietary calcium during vitamin D treatment of hypoparathyroidism also failed to decrease the serum phosphorus.19 But in these studies, it could be argued that an increase in serum calcium was not the cause of the decrease in serum phosphorus; rather, vitamin D or dihydrotachysterol may have resulted in stimulation of FGF23 production. However, in 1965 Eisenberg demonstrated in hypoparathyroid patients that the phosphaturia was independent of vitamin D by showing that a 48-hour infusion of calcium sufficient to normalize the serum calcium concentration also was phosphaturic and decreased the serum phosphorus.22 In the present study, short-term calcium administration (6 hours) and 10 days of high dietary calcium were associated with an increase in FGF23, but no changes in serum phosphorus were observed. However, in the high-calcium diet group the rats remained hypocalcemic at the end of the study; in contrast, with intraperitoneal administration of calcium, higher calcium levels were achieved but the duration was only 6 hours. Thus, additional studies will be needed to link the calcium-induced increase in FGF23 with phosphaturia and decreases in serum phosphorus.
The combination of calcium and vitamin D deficiency results in hypocalcemia and hyperparathyroidism and, at least early in the process, elevated calcitriol values. Calcitriol stimulates the phosphaturic hormone, FGF23, as does PTH. In turn, FGF23 suppresses calcitriol and PTH. From a teleologic standpoint, in hypocalcemia the stimulation of FGF23 would be counterproductive because FGF23-induced suppression of calcitriol and PTH would decrease their calcemic actions during hypocalcemia. Moreover, in this setting the high PTH values would provide the needed phosphaturic action even in the absence of FGF23. Results in patients with primary hyperparathyroidism after parathyroidectomy have suggested that hypocalcemia may blunt FGF23 production.23 The results of our current study in rats with calcium and vitamin D deficiency and in those with parathyroidectomy provide compelling evidence that hypocalcemia prevents stimulation of FGF23 by calcitriol and PTH. The mechanism by which hypocalcemia modifies FGF23 stimulation by calcitriol and PTH remains to be determined.
In conclusion, the results of this study show that calcium modulates FGF23 production. This putatively regulatory mechanism may be a response to counteract FGF23-induced calcitriol inhibition during hypocalcemia.
Concise Methods
Male Wistar rats, 9–10 weeks old and weighing 250–300 g, were housed using a 12-hour/12-hour light/dark cycle and given ad libitum access to a standard diet (calcium 0.9%, phosphorus 0.6%). All experimental protocols were reviewed and approved by the Ethics Committee for Animal Research of the University of Cordoba, and all rats received humane care in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research.
Rats were randomly assigned to the following experimental groups to determine the effect of calcium deficiency/hypocalcemia on FGF23:
Controls with a normal diet (0.6% calcium, 0.6% phosphorous); n=10.
Low-calcium, low–vitamin D diet (LCa-LD diet) (0.15% calcium, 0.6% phosphorous, and <50 IU/kg vitamin D); n=23.
Low-calcium, high-phosphorous, low–vitamin D diet (LCa- HP-LD diet) (0.15% calcium, 1.2% phosphorous, and <50 IU/kg vitamin D). n=24.
Rats were maintained on this diet for 6 weeks.
Because in groups 2 and 3 an increase in PTH is expected to occur after calcium deficiency, an additional group of rats with uremic hyperparathyroidism (the Nx-HP diet group) was included for comparison (n=9). Uremia was induced by 5∕6 nephrectomy, a two-step procedure. In the first step, animals were anesthetized using xylazine (5 mg/kg intraperitoneally) and ketamine (80 mg/kg intraperitoneally), and a 5- to 8-mm incision was made on the left mediolateral surface of the abdomen. The left kidney was exposed, and the two poles (two thirds of renal mass) were ablated. After 1 week, the animals were reanesthetized and the right kidney was removed. Adrenal glands were left in place. One day after the second surgery, the diet was switched from 0.6% calcium and 0.6% phosphorus to 0.6% calcium and 1.2% phosphorus. Rats were maintained on this diet for 15 days. All diets were obtained from Altromin, Lage, Germany.
A second set of experiments was performed in PTX rats to better define the effect of hypocalcemia on FGF23 values during short-term (6 hours) and long-term (10 days) increases in serum calcium. For the short-term study, PTX rats fed a 0.6% calcium and 0.6% phosphorus diet were randomly separated into two groups that received either vehicle (n=9) or calcium gluconate (10 mg/kg per hour) intraperitoneally for 6 hours (n=9), after which rats were euthanized. To determine the effect of long-term increases in serum calcium, PTX rats were randomly separated into two groups fed diets of 0.6% phosphorus and 0.6% (n=7) or 1.0% (n=10) calcium for 10 days, after which they were euthanized.
Selective PTX was performed as described elsewhere with the aid of a dissecting microscope.24 Briefly, with the rat under general anesthesia (Sevofluorane, Abbott, Madrid, Spain), the skin on the ventral part of the neck was incised; the thyroid was exposed; and the parathyroid glands were identified, dissected, and ablated. Hemorrhage was prevented by electrocautery. One week after PTX (day 0), blood was drawn to measure serum calcium levels. Parathyroidectomy was considered successful in rats when ionized calcium levels decreased to <2.8 mg/dl.
Rats were euthanized by aortic puncture and exsanguination under general anesthesia (thiopental sodium intraperitoneally, 50 mg/kg). The collected blood was used to measure the levels of calcium, phosphorus, creatinine, calcitriol, 25(OH)D, PTH, and FGF23.
Blood Determinations
Serum ionized calcium levels were determined using a Spotlyte Ca2/pH Analyzer (Menarini Diagnostics, Barcelona, Spain). Intact rat PTH levels were quantified using ELISA (Immutopics, San Clemente, CA). Serum creatinine and phosphorus were measured by spectrophotometry (Sigma Diagnostics, St. Louis, MO). Serum calcitriol (1,25[OH]2D) and 25(OH)D concentrations were quantified by ELISA (Immunodiagnostic Systems, Boldon, United Kingdom). FGF23 levels were determined using a rat FGF23 kit (Kainos Laboratories, Tokyo, Japan).
Statistical Analyses
Values are expressed as the mean ± SD. The difference between means for two different groups was determined by t test. The difference between means for three or more groups was assessed by ANOVA, followed by post hoc Duncan analysis. A linear regression test was used to assess the correlation between two variables. Multiple regression analysis was used to analyze the effect of multiple independent variables on serum FGF23 levels. A P value < 0.05 was considered to represent a statistically significant difference.
Disclosure
M.R. has received research grants from Amgen and Fresenius and lecture fees from the following companies: Amgen, Abbott, Shire, and Fresenius.
Acknowledgments
This study was supported by Instituto Carlos III (FIS 07/0287, FIS 07/0315, 010/1311), Consejeria de Salud (JA 0127/2008), a UE Grant from Framework Programme 7 Syskid (FP7-241544), and Consejeria de Innovacion, Ciencia y Empresa (CTS-5205 and CTS-170) of Junta de Andalucia. Y.A. is a senior researcher supported by the Fundacion Progreso y Salud, Consejeria de Salud (Junta de Andalucia).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T: FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19: 429–435, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T: Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113: 561–568, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD: Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol 17: 1305–1315, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Zisman AL, Wolf M: Recent advances in the rapidly evolving field of fibroblast growth factor 23 in chronic kidney disease. Curr Opin Nephrol Hypertens 19: 335–342, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J: The parathyroid is a target organ for FGF23 in rats. J Clin Invest 117: 4003–4008, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krajisnik T, Björklund P, Marsell R, Ljunggren O, Akerström G, Jonsson KB, Westin G, Larsson TE: Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol 195: 125–131, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Canalejo R, Canalejo A, Martinez-Moreno JM, Rodriguez-Ortiz ME, Estepa JC, Mendoza FJ, Munoz-Castaneda JR, Shalhoub V, Almaden Y, Rodriguez M: FGF23 fails to inhibit uremic parathyroid glands. J Am Soc Nephrol 21: 1125–1135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galitzer H, Ben-Dov IZ, Silver J, Naveh-Many T: Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int 77: 211–218, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Komaba H, Goto S, Fujii H, Hamada Y, Kobayashi A, Shibuya K, Tominaga Y, Otsuki N, Nibu K, Nakagawa K, Tsugawa N, Okano T, Kitazawa R, Fukagawa M, Kita T: Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int 77: 232–238, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Krajisnik T, Olauson H, Mirza MA, Hellman P, Akerström G, Westin G, Larsson TE, Björklund P: Parathyroid Klotho and FGF-receptor 1 expression decline with renal function in hyperparathyroid patients with chronic kidney disease and kidney transplant recipients. Kidney Int 78: 1024–1032, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Kumata C, Mizobuchi M, Ogata H, Koiwa F, Nakazawa A, Kondo F, Kadokura Y, Kinugasa E, Akizawa T: Involvement of alpha-klotho and fibroblast growth factor receptor in the development of secondary hyperparathyroidism. Am J Nephrol 31: 230–238, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T: PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: A bone parathyroid feedback loop. Am J Physiol Renal Physiol 299: F882–F889, 2010 [DOI] [PubMed] [Google Scholar]
- 13.López I, Rodríguez-Ortiz ME, Almadén Y, Guerrero F, de Oca AM, Pineda C, Shalhoub V, Rodríguez M, Aguilera-Tejero E: Direct and indirect effects of parathyroid hormone on circulating levels of fibroblast growth factor 23 in vivo. Kidney Int 80: 475–482, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Komaba H, Fukagawa M: FGF23-parathyroid interaction: Implications in chronic kidney disease. Kidney Int 77: 292–298, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Imanishi Y, Inaba M, Nakatsuka K, Nagasue K, Okuno S, Yoshihara A, Miura M, Miyauchi A, Kobayashi K, Miki T, Shoji T, Ishimura E, Nishizawa Y: FGF-23 in patients with end-stage renal disease on hemodialysis. Kidney Int 65: 1943–1946, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Shimada T, Yamazaki Y, Takahashi M, Hasegawa H, Urakawa I, Oshima T, Ono K, Kakitani M, Tomizuka K, Fujita T, Fukumoto S, Yamashita T: Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol 289: F1088–F1095, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Evenepoel P, Naesens M, Claes K, Kuypers D, Vanrenterghem Y: Tertiary ‘hyperphosphatoninism’ accentuates hypophosphatemia and suppresses calcitriol levels in renal transplant recipients. Am J Transplant 7: 1193–1200, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Evenepoel P, Viaene L, Meijers BKI: Targeting FGF23 and phosphorus in CKD, do not forget calcium. Nephrol Dial Transplant 26: 1749–1750, author reply 1750, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Bauer W, Marble A, Claflin D: Studies on the mode of action of irradiated ergosterol: IV. In hypoparathyroidism. J Clin Invest 11: 47–62, 1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albright F, Bloomberg E, Drake T, Sulkowitch HW: A comparison of the effects of A.T. 10 (dihydrotachysterol) and vitamin D on calcium and phosphorus metabolism in hypoparathyroidism. J Clin Invest 17: 317–329, 1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albright F, Burnett CH, Smith PH, Parson W: Pseudo-hypoparathyroidism-example of 'Seabright-Bantam syndrome'; report of three cases. Endocrinology 30: 922–932, 1942 [Google Scholar]
- 22.Eisenberg E: Effects of serum calcium level and parathyroid extracts on phosphate and calcium excretion in hypoparathyroid patients. J Clin Invest 44: 942–946, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi K, Imanishi Y, Miyauchi A, Onoda N, Kawata T, Tahara H, Goto H, Miki T, Ishimura E, Sugimoto T, Ishikawa T, Inaba M, Nishizawa Y: Regulation of plasma fibroblast growth factor 23 by calcium in primary hyperparathyroidism. Eur J Endocrinol 154: 93–99, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez M, Felsenfeld AJ, Llach F: Aluminum administration in the rat separately affects the osteoblast and bone mineralization. J Bone Miner Res 5: 59–67, 1990 [DOI] [PubMed] [Google Scholar]