Abstract
Angiotensin II content in the kidney is much higher than in the plasma, and it increases more in kidney diseases through an uncertain mechanism. Because the kidney abundantly expresses angiotensinogen mRNA, transcriptional dysregulation of angiotensinogen within the kidney is one potential cause of increased renal angiotensin II in the setting of disease. Here, we observed that kidney-specific angiotensinogen knockout mice had levels of renal angiotensinogen protein and angiotensin II that were similar to those levels of control mice. In contrast, liver-specific knockout of angiotensinogen nearly abolished plasma and renal angiotensinogen protein and renal tissue angiotensin II. Immunohistochemical analysis in mosaic proximal tubules of megalin knockout mice revealed that angiotensinogen protein was incorporated selectively in megalin-intact cells of the proximal tubule, indicating that the proximal tubule reabsorbs filtered angiotensinogen through megalin. Disruption of the filtration barrier in a transgenic mouse model of podocyte-selective injury increased renal angiotensin II content and markedly increased both tubular and urinary angiotensinogen protein without an increase in renal renin activity, supporting the dependency of renal angiotensin II generation on filtered angiotensinogen. Taken together, these data suggest that liver-derived angiotensinogen is the primary source of renal angiotensinogen protein and angiotensin II. Furthermore, an abnormal increase in the permeability of the glomerular capillary wall to angiotensinogen, which characterizes proteinuric kidney diseases, enhances the synthesis of renal angiotensin II.
CKDs are often progressive and involve cardiovascular diseases. Pharmacological intervention of angiotensin II (AII) is the only currently available therapeutic clinical measure with proven effectiveness in protecting the kidney from progressive loss of renal function in CKD. However, in these conditions, circulating AII is typically not elevated. Of note, AII content in renal tissues is markedly higher than content in the circulation, and it is regulated in a manner distinct from circulating AII.1–3 These findings have formed the currently prevailing notion that the kidney in and of itself is furnished with a full set of components necessary for de novo AII synthesis. In support of this notion, studies have shown that renal AII is elevated in hypertension and kidney diseases in parallel to their severity.4,5 Renal AII is also increased in animal models of glomerular diseases, including adriamycin and puromycin aminonucleoside nephropathies,6 albumin overload,7,8 and immune complex nephritis.9
Several mechanisms have been proposed to explain the local upregulation of AII in glomerular diseases, including local increase of renin,10–13 angiotensin I converting enzyme (ACE),7–9,14 or angiotensinogen (Agt),7,8,15,16 activation of prorenin by prorenin receptor,17 type 1 AII receptor–mediated uptake and endosomal accumulation of AII,18 generation of AII through alternative enzyme pathway,19,20 and decrease in ACE2.8,21 Among these mechanisms, local upregulation of Agt is thought to play a key role for progressively aggravating kidney diseases, because the Agt gene is expressed in the kidney; additionally, the intensity of renal Agt mRNA, confined to the proximal straight tubule, when expressed per cell, is equal to or more than the intensity in hepatocytes, which is the primary source of circulating Agt.22,23
To investigate the mechanism of intrarenal AII generation, we generated liver (hepatocyte) -specific and kidney (proximal tubule cell) -specific Agt knockout (KO) mice. Unexpectedly, we observed that kidney-specific Agt KO mice have renal Agt protein that is similar in quantity to the protein of Agt gene intact controls, whereas the liver-specific Agt KO showed undetectably low amounts of renal Agt protein.
Results
Kidney Agt KO Had No Effect on Renal Agt Protein and AII
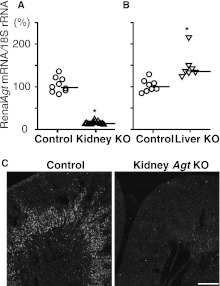
To disrupt the Agt gene in the kidney, we crossed Agt-loxP mice (Supplemental Figure 1) and KAP-Cre mice. The latter line expresses Cre recombinase only in the kidney, primarily in proximal straight tubules (Supplemental Figure 2). We quantified Agt mRNA by real-time RT-PCR performed on the RNA extracted from the whole kidney of KAP-Cre/AgtloxP/loxP mice. As expected, relative Agt/18S rRNA ratio was dramatically decreased to 14.4% of the ratio of control mice (Figure 1A and Supplemental Figure 3). In control mice, in situ hybridization showed that Agt mRNA was expressed in proximal tubule cells of the S3 segment as reported previously.22,23 In contrast, almost no Agt mRNA signal was detected in KAP-Cre/AgtloxP/loxP, confirming successful disruption of the Agt gene in the kidney (Figure 1C). Although data are not shown, Agt mRNA in the liver was not different in this line compared with control mice. This line is hereafter designated as kidney Agt KO mice.
Figure 1.
Renal Agt mRNA in kidney Agt KO mice and liver Agt KO mice versus control mice. (A and B) Real-time RT-PCR analyses for Agt mRNA/18S rRNA. *P<0.05 compared with the control group. Bars represent median values. In kidney Agt KO mice, Agt mRNA is markedly low compared with control mice. In liver Agt KO mice, renal Agt mRNA is significantly increased. (C) In situ hybridization for Agt mRNA. In control kidneys, Agt mRNA is expressed in the outer stripe of the outer medulla. In kidney Agt KO mice, the signal is almost absent. Scale bar, 0.5 mm.
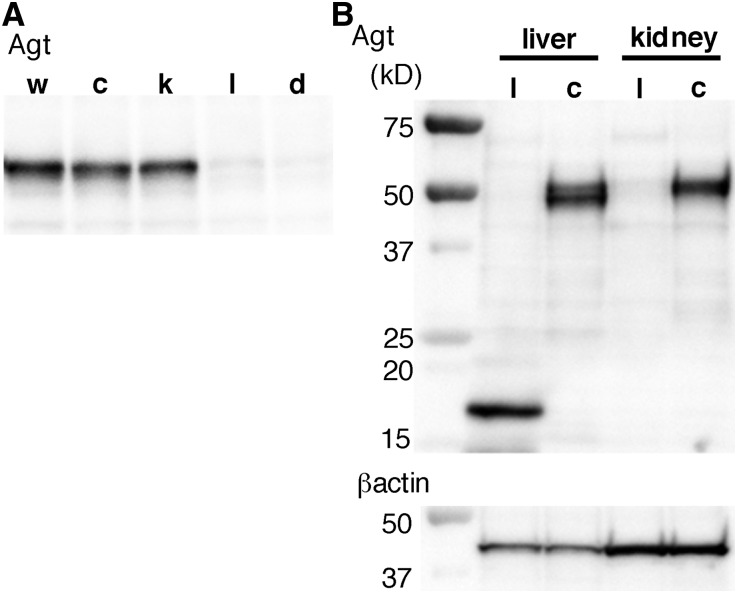
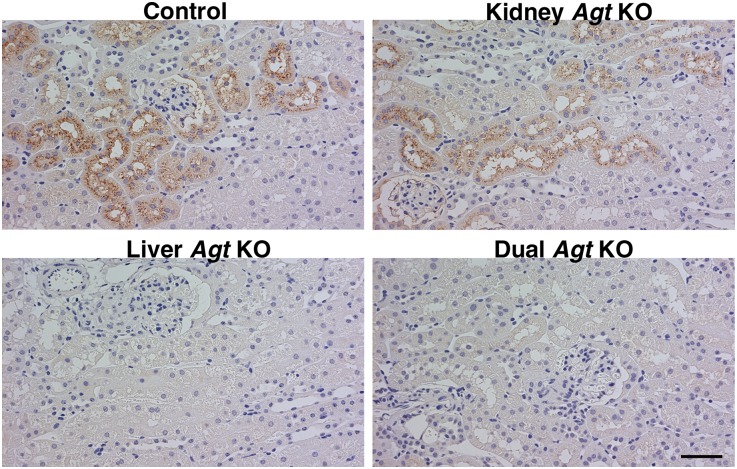
To our surprise, unlike mRNA, renal Agt protein assessed by Western analysis in the kidney Agt KO mice remained unaffected compared with the protein in control mice (Figure 2A, lane k versus lane c). Immunostaining for Agt protein confirmed that there was no difference between kidney Agt KO and control mice in both intensity and pattern (Figure 3). Of note, in both kidney Agt KO and control mice, Agt protein was stained in proximal tubule cells of S1 and S2 segments but not in the S3 segment, where renal Agt mRNA is predominantly synthesized. Specificity of this immunostaining was verified by negative staining in the kidney of whole-body Agt KO mice.
Figure 2.
Western blot analyses for Agt protein. (A) In control (c) and kidney Agt KO mice (k), renal Agt protein content was not different from the content in wild-type mice (w). In contrast, both in liver (l) and dual (d) Agt KO mice, renal Agt protein is essentially absent; 25 μg protein were loaded in each lane. Control mice carry AgtloxP/loxP but not Cre gene. (B) In the liver of liver Agt KO mice, the ∼50-kD band is absent; instead, a smaller ∼16-kD band is present. Right two lanes again show near-complete absence of Agt protein in the kidney of liver Agt KO but not control mice; 10 μg hepatic protein or 15 μg renal protein was loaded.
Figure 3.
Agt immunostaining in the kidney. In control mice, Agt protein is localized in proximal tubule cells. Unlike Agt mRNA, which is predominantly expressed in the S3 segment (Figure 1C), Agt protein is mainly localized in the S1 and S2 segments. The staining is in a granular pattern and distributes preferentially to the apical side of proximal tubule cells. Intensity of Agt staining is variable among nephrons (i.e., some nephrons contain more Agt protein than others). Within a given nephron, proximal tubule cells closer to Bowman’s space contain more Agt protein. These findings suggest that Agt protein in the proximal tubule cell represents reabsorption of Agt protein from the tubule fluid in a manner similar to the manner of albumin and many other proteins. In kidney Agt KO mice, Agt protein staining is similar to control mice in both intensity and pattern. In liver Agt KO mice, there is no Agt staining. Dual Agt KO mice are similar to liver Agt KO mice in this regard. Scale bar, 50 μm.
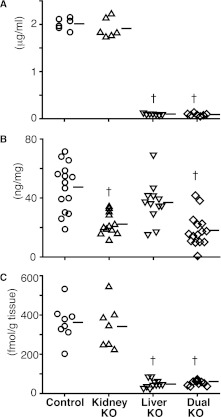
We next measured Agt protein concentration in the plasma and urine by ELISA. Plasma Agt was, on average, 1.9±0.2 μg/ml in kidney Agt KO mice, which was not different from the value in control mice of 2.0±0.1 μg/ml (Figure 4A).
Figure 4.
Plasma and urinary Agt protein and renal AII content in control, kidney Agt KO, liver Agt KO, and dual Agt KO mice. (A) Plasma Agt concentration is unaffected in kidney Agt KO mice and almost null in liver and dual Agt KO mice. (B) Urinary Agt to creatinine (Cr) ratio is significantly lower in kidney and dual Agt KO than control mice, but the ratio in liver Agt KO mice is not different from the ratio in control mice. (C) In kidney Agt KO mice, renal AII content is not different from the content in control. Contrastingly, it is markedly low in liver and dual Agt KO mice. Horizontal bars represent mean values in A and B and median in C. †P<0.01 versus control mice.
Urinary Agt/creatinine ratio was, on average, 22.2±7.4 ng/mg in kidney Agt KO mice, which was significantly lower than the value in control mice of 47.4±16.2 ng/mg (Figure 4B).
We next quantified AII content in the kidney homogenate by RIA. The median and 0.25–0.75 quantiles of renal AII in kidney Agt KO mice were 344 and 241–388 fmol/g tissue, respectively, which were not significantly different from those values in control mice of 362 and 317–402, respectively (Figure 4C).
These findings collectively indicate that Agt protein translated from renal Agt mRNA is immediately secreted into the urine, is not retained in the kidney, and contributes little to renal AII.
Liver Agt KO Markedly Decreases Renal Agt Protein and Renal AII
In liver Agt KO mice, liver Agt mRNA was only 0.17% of the value of control mice (Supplemental Figure 4), confirming near-complete inactivation of the Agt gene in the liver. Western blotting failed to detect the ∼50-kD band of full-length Agt protein in the liver of liver Agt KO mice (Figure 2B). Instead, a smaller 16-kD band was observed. This band presumably reflects a truncated product, the transcription of which started from the ATG codon in exon 3.
In the kidney of liver Agt KO mice, Agt mRNA was significantly increased, on average, by 45% compared with the value in control mice (Figure 1B), suggesting the existence of a compensatory mechanism to upregulate renal Agt transcription.
Despite this small increase in Agt mRNA in the kidney of liver Agt KO mice, Western blot analysis revealed that Agt protein almost disappeared from the kidney (Figure 2). Immunostaining confirmed that Agt protein was undetectable in the kidney of liver Agt KO mice (Figure 3).
Plasma Agt protein in liver Agt KO mice was, on average, 0.1±0.0 μg/ml, which was only 5% of the value in control mice (Figure 4A). Urinary Agt/creatinine ratio in liver Agt KO mice was, on average, 37.0±14.0 ng/mg, a value somewhat lower than but not significantly different from the value in controls (Figure 4B).
Importantly, renal AII content was markedly low in liver Agt KO with median value of 46 fmol/g tissue, a value only 13% of the value in control mice (Figure 4C).
These data (summarized in Table 1) collectively indicate that hepatic Agt is the major source not only of plasma Agt but also renal Agt and AII.
Table 1.
Summary of changes in Agt mRNA and proteins in Agt KO mice
| Control | Kidney Agt KO | Liver Agt KO | Dual Agt KO | |
|---|---|---|---|---|
| Liver | ||||
| Agt mRNA | ∼ | ∼ | ↓↓↓ | ↓↓↓ |
| Agt protein | ∼ | ∼ | ↓↓↓ | ↓↓↓ |
| Plasma | ||||
| Agt protein | ∼ | ∼ | ↓↓ | ↓↓ |
| Kidney | ||||
| Agt mRNA | ∼ | ↓ | ↑ | ↑ |
| Agt protein | ∼ | ∼ | ↓↓↓ | ↓↓↓ |
| AII content | ∼ | ∼ | ↓↓ | ↓↓ |
Dual Agt KO Mice Were Similar to Liver Agt KO Mice
We also generated liver and kidney dual Agt KO mice. Similar to liver Agt KO mice, dual Agt KO mice showed markedly low plasma Agt protein (0.1±0.0 μg/ml) (Figure 4A) and almost undetectable renal Agt protein (Figures 2A and 3). Urinary Agt/creatinine ratio was, on average, 18.1±10.8 ng/mg, which was as low as the value in kidney Agt KO mice (Figure 4B). Renal AII was again markedly low, with a median value of 41 fmol/g tissue, a value similar to the value in liver Agt KO mice (Figure 4C). These results confirm the notion derived from the above renal Agt KO studies that the Agt protein translated in the kidney does not contribute to the renal AII in the basal condition.
Liver Agt KO but Not Kidney Agt KO Causes Abnormal Morphologic Phenotypes
Most of the phenotypes seen in whole-body Agt KO mice were duplicated in liver Agt KO mice but not kidney Agt KO mice. Thus, systolic BP of kidney Agt KO mice was not different from the systolic BP in control mice. In contrast, both liver Agt KO and dual Agt KO mice were similarly and markedly hypotensive. Liver Agt KO mice and dual Agt KO mice but not kidney Agt KO mice were polyuric compared with control mice (Table 2).
Table 2.
Systolic BP, urine volume, and renin activity
| Genotypes | Control | Kidney Agt KO | Liver Agt KO | Dual Agt KO |
|---|---|---|---|---|
| Systolic BP (mmHg) | 120.2±9.2 (n=25) | 114.6±10.1 (n=17) | 74.9±4.9a (n=8) | 80.7±6.5a (n=10) |
| Urine volume (ml/d) | 1.15±0.54 (n=13) | 0.87±0.21 (n=9) | 2.29±0.67a (n=10) | 2.01±0.69a (n=13) |
| Plasma renin activity (μg AI/h per ml) | 1.3±0.6 (n=7) | 1.1±0.5 (n=5) | 22.3±14.1a (n=5) | 25.9±4.4a (n=5) |
| Renal renin activity (μg AI/h per mg protein) | 14.7 (14.5–17.9; n=6) | 15.8 (13.7–16.2; n=7) | 120.3 (102.5–150.4a; n=7) | 123.1 (92.5–138.3a; n=9) |
Data are represented by means ± SD. Renal renin activities are represented by the median and 0.25–0.75 quartiles.
P<0.01 for the difference compared with control and kidney Agt KO mice.
Histologically, liver Agt KO mice showed medial hyperplasia in the renal interlobular artery and the afferent arteriole, hypertrophy of juxtaglomerular cells, and mesangial matrix expansion (Figure 5). Renin-expressing cells were extended in liver and dual Agt KO mice (Supplemental Figure 5). These phenotypes were less in magnitude than those phenotypes in whole-body Agt KO mice. Hypoplastic papillae, tubular dilatation, and interstitial fibrosis, which are commonly seen in whole-body Agt KO mice, were not commonly observed in liver Agt KO mice. Kidney Agt KO mice showed normal renal morphology, and dual Agt KO mice showed similar phenotypes to those phenotypes of liver Agt KO mice with comparable severity.
Figure 5.
Renal histology of Agt KO mice. Renal morphology is unaffected in control and kidney Agt KO mice. In liver and dual Agt KO mice, juxtaglomerular cells, afferent arterioles, and interlobular arteries are hypertrophic. Periodic acid-Schiff staining. Scale bar, 50 μm.
Plasma and renal renin activities were dramatically elevated in liver Agt KO mice but not kidney Agt KO mice. Dual Agt KO mice showed elevated plasma and renal renin activities, which were similar in degree to those activities of liver Agt KO mice (Table 2).
Megalin-Dependent Distribution of Renal Agt Protein
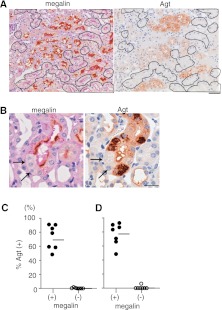
The above data indicated that most renal Agt protein originated from the liver, is filtered through the glomerulus, and is reabsorbed in a manner similar to albumin. To test the role of megalin for Agt protein reabsorption, we analyzed the kidney of megalin KO mice, in which megalin gene is null-mutated in approximately 60% of proximal tubule cells in a mosaic fashion. Analysis of serial sections revealed that Agt staining is strictly limited to proximal tubule cells expressing megalin (Figure 6, A and C). Thus, in the seven megalin KO mice analyzed, the majority of megalin-expressing cells (median=78.9%) were positive for Agt, whereas virtually no (median-0.0%) megalin KO cells were positive for Agt, clearly showing that Agt is reabsorbed from the tubule fluid by proximal tubule cells in a strictly megalin-dependent manner. Megalin dependency was also shown in nephrotic state (Figure 6, B and D).
Figure 6.
Agt protein reabsorption is dependent on megalin. (A) The left panel is megalin immunostaining in proximal tubule-specific mosaic megalin KO mouse kidney without podocyte injury. The proximal tubule cells outlined lack megalin staining. The adjacent section was stained for Agt (right panel). None of the outlined megalin KO cells are stained for Agt. Scale bar, 50 μm. (B) Serial kidney sections from NEP25/megalin KO mice 10 days after injection with LMB2 are stained for megalin and Agt. These pictures are taken from the outer stripe of outer medulla. Massive amounts of Agt protein are reabsorbed only in megalin-intact cells of the S3 segment but not megalin KO cells (arrows). Agt staining is in s granular pattern. Scale bar, 20 μm. (C and D) Agt positivity in mosaic megalin KO mice (D) with or (C) without glomerular sieving defect. The cortex that mainly contains S1 and S2 segments of the proximal tubule was photographed. Agt positivity was determined separately in megalin (+) and megalin proximal tubule cells (−). Regardless of the glomerular sieving conditions, megalin proximal tubule cells (−) did not contain Agt protein. Horizontal bars represent medians.
Sieving Defect Causes Increase in Renal Agt Protein and AII
We next analyzed the effect of impairment of glomerular sieving function on the renal content of Agt protein. For this purpose, we used Nephrin-hCD25 (NEP25) transgenic mice,24 in which selective podocyte injury can be induced by injection of anti–Tac(Fv)-PE38 (LMB2), a recombinant immunotoxin specific to hCD25. To prevent volume depletion caused by resultant nephrotic syndrome, NEP25 mice given LMB2 were supplemented with mouse serum. Eight days after the injection of LMB2, NEP25 mice developed severe proteinuria with median urinary albumin to creatinine ratio of 76.0 mg/mg, whereas the ratio in the control group was 0.1 mg/mg. At this stage, glomerulosclerosis had yet to develop, and tubule injury was unremarkable (Supplemental Figure 6).
Similar to urinary albumin, urinary Agt was markedly increased in NEP25 mice after LMB2 injection, with Agt to creatinine ratio averaging 7520±510 ng/mg compared with the control mice ratio of 18.8±4.1 ng/mg. Western blotting revealed that renal Agt protein was dramatically increased (Figure 7A). Immunostaining showed that Agt protein in the S1 and S2 segments was markedly intensified. In addition, Agt staining focally extended to the S3 segment, distributed in a lysosomal pattern, and was completely dependent on megalin (Figure 6B). Importantly, renal AII in mice with sieving defect was significantly higher than the value in control mice, with a median value of 363 versus 111 fmol/g tissue, although renal renin activity was somewhat decreased in the former group (7.4±2.8 versus 14.6±2.7 μg Ang I/h per mg protein) (Figure 7, C and D).
Figure 7.
Sieving defect increases renal Agt protein and AII. NEP25 mice were injected with LMB2, an immunotoxin specific to hCD25, to induce sieving defect. Control NEP25 mice were injected with saline. (A) Western blot analysis for renal Agt protein. Kidneys of NEP25 mice with podocyte injury (+) contain more Agt protein than the kidneys of control mice (−). (B) Immunostaining for Agt protein. In kidneys with podocyte injury, Agt staining is enhanced and extended to the S3 segment. Scale bar, 100 μm. (C) Renal AII content. NEP25 mice with podocyte injury show significant increase in renal AII content. (D) Renal renin activity. NEP25 mice with podocyte injury show downregulated renin activity, reflecting sufficient volume repletion with plasma infusion in these nephrotic mice. Thus, the increase in renal AII found in these mice is not ascribed to increased renin. In C and D, data from individual mice are shown by open or closed circles. The median and 0.25 and 0.75 quantile values are shown by box plots placed next to the individual data. †P<0.01 versus control NEP25 mice without podocyte injury.
Discussion
When the Agt gene is intact, proximal tubule cells of the S3 segment intensely express Agt mRNA. However, our liver Agt KO study revealed that the Agt protein synthesized from the renal Agt mRNA is too low to be appreciated by either immunostaining or Western blot analysis. However, kidney Ag KO led to a decrease in urinary Agt protein, suggesting that most Agt protein synthesized in the S3 segment is immediately secreted into the urine; thus, our findings support the notion previously offered in the work by Ding et al.25 Our study also showed that, whereas disruption of kidney Agt gene has no effect on renal AII content, disruption of liver Agt gene markedly reduces renal AII. In fact, although liver Agt KO led to a moderate increase in renal Agt mRNA in a compensatory fashion, the increase was not translated into an increase in renal AII content. Collectively, these findings indicate that the kidney Agt gene does not contribute to renal AII. Also, kidney Agt KO mice had no appreciable abnormal phenotype, indicating that the kidney Agt gene does not play a functionally significant role in maintaining normal phenotypes. Nevertheless, the present study does not rule out the possibility that, in some other conditions not tested in the present study, renal Agt may contribute to the renal Ang II synthesis to a significant extent.26,27
The present study unequivocally showed that Agt protein of liver origin is the primary source not only of renal Agt protein but also renal AII. The liver is the primary source of circulating Agt, a minor but significant fraction of which is filtered through the glomerulus and reabsorbed by proximal tubule cells in a megalin-dependent manner. When the liver Agt gene was not inactivated and glomerular sieving function was intact, most filtered Agt protein was reabsorbed by proximal tubule cells and did not appear in the urine. This finding is consistent with the findings in the previous study by Kobori et al.28 In that work, human Agt protein was infused into rats, which was not detectable in the urine, although this observation was taken in their study as evidence for negligible filterability of plasma Agt.
Megalin dependency of the reabsorption of Agt protein has recently been shown by Pohl et al.,23 and our findings echo their observations. Using opossum kidney cell sheet cultured on permeable filter, they showed that 5% of Agt protein added in one side of the chamber medium was transferred to the opposite side by transcytosis, suggesting the possibility that AII can be generated from the Agt reabsorbed into the renal interstitium. Alternatively, reabsorbed Agt may be transferred back to the tubular lumen and then converted to AII, or it may be processed to AII by lysosomal enzymes.29
The present study does not determine how much of the renal AII is attributed to the Agt reabsorbed by proximal tubule cells as its precursor. To address this quantitative issue, mutant mice with 100% of proximal tubular cells carrying megalin null mutation need to be generated in view of our observation that, in mosaic megalin KOs, Agt protein incorporation of megalin gene intact cells becomes upregulated in a compensatory manner (Supplemental Figure 7).
Importantly, when the molecular barrier function of the glomerular capillary wall is impaired, massive Agt protein is leaked into the tubular lumen and reabsorbed by proximal tubular cells. When the amount of leaked Agt protein exceeds the capacity of reabsorption by the S1 and S2 segments, reabsorption can also occur in the S3 segment, where ACE is abundantly expressed.23 In these nephrotic mice, renal AII was increased, although renal renin was suppressed, indicating that a fraction of the Agt that is leaked into Bowman’s space is converted to AII and that renal AII content is determined by the intactness of glomerular sieving function to restrict filtration of circulating Agt. Our observations with podocyte disruption echo the earlier findings in patients with glomerular diseases that immunoreactive Agt in proximal tubules was increased along with increased urinary Agt excretion,30 although we found that the origin of Agt is the liver and not the kidney itself.
Because renin is also reabsorbed by proximal tubule cells through megalin and ACE is expressed on proximal tubule cells,23 it is likely that AII is generated within the vicinity where Agt protein is accumulated. Because proximal tubule cell–specific type 1 AII receptor KO mice are reported to have 10 mmHg lower BP compared with control mice,31 a possible function of the renal AII is BP regulation. In this regard, BP change in kidney or liver Agt KO mice was in parallel with the change in renal AII content. Although BP was not measured in NEP25 mice in the present study, nephrotic NEP25 mice measured earlier were not hypertensive.24 Nevertheless, the increased renal AII may contribute to maintenance of BP and also may underlie the sodium retention seen in nephrotic syndrome. Indeed, in the present study, although our mice with podocyte-selective injury were supplemented with plasma to ensure suppression of renal renin activity, all mice remained nephrotic with ascites and edema.
Our remaining interest in the potential function of renal AII is its profibrogenic effect on the glomerulus32 and the tubulointerstitium.33 In this regard, AII can raise intraglomerular pressure independently of systemic BP,34 which in turn, causes disproportionate increase in macromolecular traffic across the glomerular capillary wall.35 This connection, which is geared to increasing filtered Agt, may constitute a positive feedback loop of AII generation.2,36 Moreover, AII and high glomerular capillary pressure can promote podocyte injury,37–39 thereby ensuring the formation of this feedback mechanism for AII synthesis. Collectively, the observed filtered Agt dependency of renal AII synthesis can be viewed as one of the key elements of the vicious nature of progressive glomerular diseases, in which pharmacological blockade of AII action is often effective.40–43
Concise Methods
Generation of Tissue-Specific Agt KO Mice
A mouse line, Agt-loxP, that carries two loxP sites in the first and second introns of the Agt gene was generated using homologous recombination in embryonic stem cells (Supplemental Figure 1). To disrupt the Agt gene in the kidney (proximal straight tubule), kidney androgen-regulated protein (KAP) -Cre transgenic line, which carries a promoter segment of KAP and Cre, was generated. These lines were characterized and backcrossed with the C57BL/6N strain for more than 10 times (Supplemental Text). To delete the Agt gene in hepatocytes, C57BL/6TgN(AlbCre)21Mgn strain (stock number JR#003574; N7+1F15N1F2; Alb-Cre), which expresses Cre in hepatocyte, was obtained from Jackson Laboratory. This line was further backcrossed with C57BL/6N three times in our facility before mating with Agt-loxP mice.
Except for initial experiments examining Agt mRNA expression, adult male mice obtained from mating between KAP-Cre/AgtloxP/loxP and Alb-Cre/AgtloxP/loxP were treated with testosterone and used for the present studies.
Measurement of Renal AII
Kidneys were cut in one-half and immediately frozen in liquid nitrogen and stored at −80°C. Renal AII content was measured as previously reported with slight modification.44 Thus, frozen kidney tissues were homogenized in 5 ml ice-cold methanol. The homogenate was centrifuged at 3000 rpm for 15 minutes. The supernatant was dried by heating at 85°C for complete and irreversible inactivation of renin. The residue was reconstituted in 1 ml Ang assay buffer (50 mM Na phosphate, 1 mM EDTA, 0.25 mM thimerosal, 2.5 mg/ml BSA, pH 7.4). Ang peptides were extracted from this solution using phenyl-bonded, solid-phase extraction columns (Bond Elut; Varian Inc., Lake Forest, CA). The efficiency of AII recovery determined by 125I-AII was about 80%. The eluates were vacuum-dried to remove methanol, reconstituted in 130 μl Ang assay buffer, and subjected to RIA for AII.
The above samples and standard solutions (0–191 fmol AII; Sigma) were incubated with diluted anti-AII antibody solution and 5000 cpm 125I-AII at 4°C for 24 hours. Free 125I-AII was reabsorbed by charcoal (C4386; Sigma) suspended in 1 ml assay buffer containing 0.1% dextran (Sigma) and 1% human serum albumin (MP Biomedicals). After removal of charcoal by centrifugation, the radioactivity of the solution was measured for 10 minutes by a γ-scintillation counter.
Percent crossreactivities are <0.01% for Ang I and <0.1% for Ang (1–7). When whole-body Agt KO mouse kidneys were measured, the AII content was always calculated to be less than 0.8 fmol/tube. Because the whole-body Agt KO mice are expected to have no AII, lower limit of reliable AII measurement by our method is approximately 10 pmol/kg (fmol/g) tissue.
Analysis of Mosaic Megalin KO Mice
Baseline characteristics of renal tubule cell-specific megalin KO mice (MegalinloxP/loxP/ApoE-Cre) were previously reported.45 Serial paraffin sections from seven MegalinloxP/loxP/ApoE-Cre mice were stained for either Agt or megalin. Agt positivity was determined separately in megalin-intact versus -deficient proximal tubule cells. Kidneys harvested from MegalinloxP/loxP/ApoE-Cre/NEP25 mice (n=7) that were injected with 1.25 ng/g body wt LMB2 and killed 10 days later were analyzed in a similar fashion.
Induction of Glomerular Sieving Defect
Male NEP25 mice on C57BL/6 genetic background at 9–12 months of age were injected with LMB2 (1.25 ng/g body wt; n=9) or saline solution (n=10).24 To prevent volume depletion caused by severe nephrotic syndrome, NEP25 mice given LMB2 were supplemented with 0.5 ml normal mouse serum intraperitoneally from day 3 to day 6, and the control NEP25 mice were injected with 0.5 ml saline. On day 7, mice were killed, and kidneys were harvested for assays for AII, renin, Agt, and histology. Before LMB2 injection and death, 24-hour urine was collected, and the concentrations of creatinine, albumin, and Agt were determined.
Statistical Analyses
Plasma Agt concentration, urinary Agt/creatinine ratio, systolic BP, urine volume, and plasma renin activity were compared by ANOVA with Turley–Kramer method, and they are presented as means ± SD. These parameters were also compared by the Steel–Dwass procedure, which revealed the same results.
Percentage of Agt positivity in megalin mosaic KO mice was compared by Wilcoxon signed rank test. For Agt mRNA/18S rRNA ratio, renal AII content, and renal renin activity, Mann–Whitney U test (for two groups) or Steel–Dwass procedure (for multigroups) was used as data from some groups that did not display normal distribution. For these groups, data are represented by median and 0.25 and 0.75 quantiles.
All analyses were performed using KyPlot software. Values were regarded as significant at two-sided P<0.05.
Disclosures
None.
Acknowledgments
We acknowledge Dr. Thomas Willnow at Max Delbruck Center for providing megalin KO mice, Dr. Shinichi Uchida at Tokyo Medical and Dental University for providing the KAP promoter fragment, Dr. Tadashi Inagami at Vanderbilt University for helpful discussion, and Ms. Shiho Imai and Ms. Chie Sakurai for excellent technical assistance.
This study was supported by a Grant-in Aid for Scientific Research of Japan Society for the Promotion of Science, the Ministry of Education, Culture, Sports, Science and Technology in Japan, High-Tech Research Center Project of the Ministry of Education, Culture, Sports, Science and Technology in Japan, Tokai University School of Medicine, Project Research (2010-2011), the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Disease, and National Institutes of Health Grants DK037868 and DK44757 and in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and Center for Cancer Research.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “The Increasing Complexity of the Intratubular Renin-Angiotensin System,” on pages 1130–1132.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011121159/-/DCSupplemental.
References
- 1.Navar LG, Nishiyama A: Why are angiotensin concentrations so high in the kidney? Curr Opin Nephrol Hypertens 13: 107–115, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Kobori H, Nangaku M, Navar LG, Nishiyama A: The intrarenal renin-angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC: Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2. Lewis rat. Am J Physiol Renal Physiol 290: F1497–F1506, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Siragy HM, Carey RM: Role of the intrarenal renin-angiotensin-aldosterone system in chronic kidney disease. Am J Nephrol 31: 541–550, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bader M, Ganten D: Update on tissue renin-angiotensin systems. J Mol Med (Berl) 86: 615–621, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Li J, Zhou C, Yu L, Wang H: Renal protective effects of blocking the intrarenal renin-angiotensin system. Hypertens Res 22: 223–228, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Largo R, Gómez-Garre D, Soto K, Marrón B, Blanco J, Gazapo RM, Plaza JJ, Egido J: Angiotensin-converting enzyme is upregulated in the proximal tubules of rats with intense proteinuria. Hypertension 33: 732–739, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Takase O, Marumo T, Imai N, Hirahashi J, Takayanagi A, Hishikawa K, Hayashi M, Shimizu N, Fujita T, Saruta T: NF-kappaB-dependent increase in intrarenal angiotensin II induced by proteinuria. Kidney Int 68: 464–473, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Ruiz-Ortega M, González S, Serón D, Condom E, Bustos C, Largo R, González E, Ortiz A, Egido J: ACE inhibition reduces proteinuria, glomerular lesions and extracellular matrix production in a normotensive rat model of immune complex nephritis. Kidney Int 48: 1778–1791, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Ibrahim HN, Hostetter TH: The renin-aldosterone axis in two models of reduced renal mass in the rat. J Am Soc Nephrol 9: 72–76, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Anderson S, Jung FF, Ingelfinger JR: Renal renin-angiotensin system in diabetes: Functional, immunohistochemical, and molecular biological correlations. Am J Physiol 265: F477–F486, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Hsieh TJ, Zhang SL, Filep JG, Tang SS, Ingelfinger JR, Chan JS: High glucose stimulates angiotensinogen gene expression via reactive oxygen species generation in rat kidney proximal tubular cells. Endocrinology 143: 2975–2985, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Singh R, Singh AK, Leehey DJ: A novel mechanism for angiotensin II formation in streptozotocin-diabetic rat glomeruli. Am J Physiol Renal Physiol 288: F1183–F1190, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi M, Kim S, Zhan Y, Iwao H: Role of intrarenal angiotensin-converting enzyme in nephropathy of type II diabetic rats. Hypertens Res 25: 287–294, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Kobori H, Nishiyama A, Abe Y, Navar LG: Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension 41: 592–597, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobori H, Ozawa Y, Suzaki Y, Nishiyama A: Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol 16: 2073–2080, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ichihara A, Hayashi M, Kaneshiro Y, Suzuki F, Nakagawa T, Tada Y, Koura Y, Nishiyama A, Okada H, Uddin MN, Nabi AH, Ishida Y, Inagami T, Saruta T: Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest 114: 1128–1135, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhuo JL, Li XC: Novel roles of intracrine angiotensin II and signalling mechanisms in kidney cells. J Renin Angiotensin Aldosterone Syst 8: 23–33, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urata H: Pathological involvement of chymase-dependent angiotensin II formation in the development of cardiovascular disease. J Renin Angiotensin Aldosterone Syst 1[Suppl]: S35–S37, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Graciano ML, Cavaglieri RC, Dellê H, Dominguez WV, Casarini DE, Malheiros DM, Noronha IL: Intrarenal Renin-Angiotensin system is upregulated in experimental model of progressive renal disease induced by chronic inhibition of nitric oxide synthesis. J Am Soc Nephrol 15: 1805–1815, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM: Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 417: 822–828, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Niimura F, Okubo S, Fogo A, Ichikawa I: Temporal and spatial expression pattern of the angiotensinogen gene in mice and rats. Am J Physiol 272: R142–R147, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Pohl M, Kaminski H, Castrop H, Bader M, Himmerkus N, Bleich M, Bachmann S, Theilig F: Intrarenal renin angiotensin system revisited: Role of megalin-dependent endocytosis along the proximal nephron. J Biol Chem 285: 41935–41946, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsusaka T, Xin J, Niwa S, Kobayashi K, Akatsuka A, Hashizume H, Wang QC, Pastan I, Fogo AB, Ichikawa I: Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol 16: 1013–1023, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Ding Y, Davisson RL, Hardy DO, Zhu LJ, Merrill DC, Catterall JF, Sigmund CD: The kidney androgen-regulated protein promoter confers renal proximal tubule cell-specific and highly androgen-responsive expression on the human angiotensinogen gene in transgenic mice. J Biol Chem 272: 28142–28148, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Kang N, Walther T, Tian XL, Bohlender J, Fukamizu A, Ganten D, Bader M: Reduced hypertension-induced end-organ damage in mice lacking cardiac and renal angiotensinogen synthesis. J Mol Med (Berl) 80: 359–366, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Kobori H, Ozawa Y, Satou R, Katsurada A, Miyata K, Ohashi N, Hase N, Suzaki Y, Sigmund CD, Navar LG: Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol Renal Physiol 293: F938–F945, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG: Urinary angiotensinogen as an indicator of intrarenal Angiotensin status in hypertension. Hypertension 41: 42–49, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rykl J, Thiemann J, Kurzawski S, Pohl T, Gobom J, Zidek W, Schlüter H: Renal cathepsin G and angiotensin II generation. J Hypertens 24: 1797–1807, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Kobori H, Katsurada A, Ozawa Y, Satou R, Miyata K, Hase N, Suzaki Y, Shoji T: Enhanced intrarenal oxidative stress and angiotensinogen in IgA nephropathy patients. Biochem Biophys Res Commun 358: 156–163, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH, Coffman TM: AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab 13: 469–475, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson S, Rennke HG, Brenner BM: Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in the rat. J Clin Invest 77: 1993–2000, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishidoya S, Morrissey J, McCracken R, Reyes A, Klahr S: Angiotensin II receptor antagonist ameliorates renal tubulointerstitial fibrosis caused by unilateral ureteral obstruction. Kidney Int 47: 1285–1294, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Ichikawa I, Miele JF, Brenner BM: Reversal of renal cortical actions of angiotensin II by verapamil and manganese. Kidney Int 16: 137–147, 1979 [DOI] [PubMed] [Google Scholar]
- 35.Yoshioka T, Mitarai T, Kon V, Deen WM, Rennke HG, Ichikawa I: Role for angiotensin II in an overt functional proteinuria. Kidney Int 30: 538–545, 1986 [DOI] [PubMed] [Google Scholar]
- 36.Kobori H, Harrison-Bernard LM, Navar LG: Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol 12: 431–439, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benigni A, Tomasoni S, Gagliardini E, Zoja C, Grunkemeyer JA, Kalluri R, Remuzzi G: Blocking angiotensin II synthesis/activity preserves glomerular nephrin in rats with severe nephrosis. J Am Soc Nephrol 12: 941–948, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Matsusaka T, Asano T, Niimura F, Kinomura M, Shimizu A, Shintani A, Pastan I, Fogo AB, Ichikawa I: Angiotensin receptor blocker protection against podocyte-induced sclerosis is podocyte angiotensin II type 1 receptor-independent. Hypertension 55: 967–973, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsusaka T, Kobayashi K, Kon V, Pastan I, Fogo AB, Ichikawa I: Glomerular sclerosis is prevented during urinary tract obstruction due to podocyte protection. Am J Physiol Renal Physiol 300: F792–F800, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, Ponticelli C, Ritz E, Zucchelli P, The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group : Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. N Engl J Med 334: 939–945, 1996 [DOI] [PubMed] [Google Scholar]
- 41.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I, Collaborative Study Group : Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, RENAAL Study Investigators : Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P, Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group : The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 345: 870–878, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Nishiyama A, Yoshizumi M, Rahman M, Kobori H, Seth DM, Miyatake A, Zhang GX, Yao L, Hitomi H, Shokoji T, Kiyomoto H, Kimura S, Tamaki T, Kohno M, Abe Y: Effects of AT1 receptor blockade on renal injury and mitogen-activated protein activity in Dahl salt-sensitive rats. Kidney Int 65: 972–981, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Motoyoshi Y, Matsusaka T, Saito A, Pastan I, Willnow TE, Mizutani S, Ichikawa I: Megalin contributes to the early injury of proximal tubule cells during nonselective proteinuria. Kidney Int 74: 1262–1269, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]