Abstract
An updated review of the existing knowledge regarding uremic toxins facilitates the design of experimental studies. We performed a literature search and found 621 articles about uremic toxicity published after a 2003 review of this topic. Eighty-seven records provided serum or blood measurements of one or more solutes in patients with CKD. These records described 32 previously known uremic toxins and 56 newly reported solutes. The articles most frequently reported concentrations of β2-microglobulin, indoxyl sulfate, homocysteine, uric acid, and parathyroid hormone. We found most solutes (59%) in only one report. Compared with previous results, more recent articles reported higher uremic concentrations of many solutes, including carboxymethyllysine, cystatin C, and parathyroid hormone. However, five solutes had uremic concentrations less than 10% of the originally reported values. Furthermore, the uremic concentrations of four solutes did not exceed their respective normal concentrations, although they had been previously described as uremic retention solutes. In summary, this review extends the classification of uremic retention solutes and their normal and uremic concentrations, and it should aid the design of experiments to study the biologic effects of these solutes in CKD.
The uremic syndrome is characterized by the retention of various solutes that would normally be excreted by the kidneys. The substances that interact negatively with biologic functions are called uremic toxins. In the past years, research on uremic toxicity has been very dynamic and resulted in the identification of dozens of retention solutes, including several uremic toxins. In 2003, the European Uremic Toxin Work Group (http://www.uremic-toxins.org/) proposed a classification of 90 retention solutes providing data on normal and pathologic serum concentrations.1 In 2007, results were further discussed and expanded with the addition of 14 solutes.2,3 This collaborative work focused on the highest mean or median concentration of the solutes measured in a uremic population and the highest individual uremic concentration. These data were particularly relevant for researchers on uremic toxicity, and they became a successful tool for allowing use of standardized and biologically relevant concentrations in experimental settings. More recently, scientific and technological progress resulted in the identification of many new uremic retention solutes, particularly thanks to nontargeted approaches such as metabolomic and proteomic profiling.4,5 To maintain experimental guidelines in keeping with current knowledge, it seemed necessary to propose an update of the encyclopedic review.1 It was decided to study the publications from after the first review and compare results with previous findings. With this comparison, it became possible to identify new uremic retention solutes and provide an external validation of the original tool. We reviewed all original articles on uremic toxicity published between 2003 and April 2011 and extracted all serum concentrations of uremic retention solutes measured in uremic populations and healthy controls.
Results
Overview of Uremic Toxin Research
Between 2003 and April 2011, 621 articles dealing with uremic toxins in renal patients matched a corresponding PubMed electronic search (www.pubmed.com). Eighty-seven articles (14%) provided data on uremic retention solute concentrations in CKD patients and were also included in the analysis (Supplemental Material 1). Most studies were performed in patients undergoing dialysis (chronic hemodialysis, 80.5% of studies; peritoneal dialysis, 2% of studies), and 14% of studies were performed in nondialyzed CKD patients. The remaining 3.5% of studies included both dialyzed and nondialyzed CKD patients. According to the classification of uremic retention solutes based on size and binding properties,2 free water-soluble low molecular mass compounds (<0.5 kD) represented 46% of the 88 solutes; 28% of solutes were middle molecules (0.5–60 kD), and 24% of solutes were protein-bound solutes (Table 1). Most of the 88 solutes (59%) had only been quantified within one study; these solutes were mainly free water-soluble compounds (64%), whereas 23% of solutes were middle molecules, and the remaining 13% of solutes were protein-bound solutes. Among the 36 solutes for which several concentrations could be included, 45% were protein-bound solutes, 36% were middle molecules, and 19% were small water-soluble compounds. Uremic concentrations are presented in Tables 2–4.6–86 Molar concentrations ranged from a few picomoles per liter for ILs to micromoles per liter for phenylacetic acid.22,31 The highest mass concentration was detected for the acute phase macromolecule α1-acid glycoprotein.54 Most frequently reported concentrations concerned β2-microglobulin, indoxyl sulfate, homocysteine, uric acid, and parathyroid hormone (PTH). There were large variations in binding of protein-bound solutes. The free fraction of acrolein represented less than 1% (range=0.72–0.84) of total acrolein. On average, the free fraction of p-cresylsulfate was 9.0% (4.2–13.8), the free fraction of indoxyl sulfate was 9.4% (4.5–12.9), and the free fraction of indole-3-acetic acid was 16.3% (14.3–18.3). The free fraction was greater for hippuric acid (60%; range=49–71). Our approach failed to include 58 uremic retention solutes that had been listed along with their uremic and normal concentrations in the previous classification.1 Several isolation and detection techniques were applied to quantify toxins, including chromatography (ion exchange chromotography [IEC], gas chromatography [GC], and HPLC), spectrophotometry, fluorometry, chemilumisnescence, nephelometry, radioimmunometry, nuclear magnetic resonance, and mass spectrometry (MS). However, uremic and normal concentrations were generally, but not always, measured with similar techniques (85% of solutes) (Tables 2–4).
Table 1.
Contingency table of uremic retention solutes depending on solute classification, solute status, and number of records retrieved for each solute
| Solute Classification | Solute Status | Total (Count) | |
|---|---|---|---|
| Known Retention Solute (Count) | Newly Identified Retention Solute (Count) | ||
| Free water-soluble low molecular weight molecules | |||
| results based on one report (count) | 8 | 25 | 33 |
| results based on several reports (count) | 3 | 4 | 7 |
| total | 11 | 29 | 40 (46%) |
| Protein-bound solutes | |||
| results based on one report (count) | 3 | 4 | 7 |
| results based on several reports (count) | 8 | 8 | 16 |
| total | 11 | 12 | 23 (25%) |
| Middle molecules | |||
| results based on one report (count) | 2 | 10 | 12 |
| results based on several reports (count) | 8 | 5 | 13 |
| total | 10 | 15 | 25 (28%) |
| Total (count) | 32 (37%) | 56 (63%) | 88 (100%) |
Table 2.
Mean and highest concentrations of uremic retention solutes found in uremic populations and normal concentrations found in the general population: Free water-soluble low molecular weight molecules
| Molecule | Molecular Weight | Group | Uremic Concentrations | Normal Concentration N (SD) | Methods (U; N) | NewRetention Solutea | ||
|---|---|---|---|---|---|---|---|---|
| Number of Original Papers | Mean Uremic Concentration M (SD) | Highest Uremic Concentration H (SD or Range) | ||||||
| 2-Heptenal (µg/L) | 112 | RCC | 1 | — | 54.7 (16.3)6 | 17.7 (5.33)6 | GC/MS | ✓ |
| 2-Hexenal (µg/L) | 98 | RCC | 1 | — | 61.7 (20.5)6 | 22.1 (6.6)6 | GC/MS | ✓ |
| 2-Nonenal (µg/L) | 140 | RCC | 1 | — | 101.8 (58.4)6 | 18.5 (5.2)6 | GC/MS | ✓ |
| 2-Octenal (µg/L) | 126 | RCC | 1 | — | 32.5 (21.2)6 | 26.1 (16.4)6 | GC/MS | ✓ |
| 4-Decenal (µg/L) | 154 | RCC | 1 | — | 100.1 (26.8)6 | 15.9 (5.3)6 | GC/MS | ✓ |
| 4-HO-decenal (µg/L) | 170 | RCC | 1 | — | 36.6 (22.3)6 | 10.3 (7.1)6 | GC/MS | ✓ |
| 4-HO-hexenal (µg/L) | 114 | RCC | 1 | — | 63.8 (25.3)6 | 25.1 (9.9)6 | GC/MS | ✓ |
| 4-HO-nonenal (µg/L) | 156 | RCC | 1 | — | 117.3 (47.7)6 | 16.4 (9.0)6 | GC/MS | ✓ |
| 4-HO-octenal (µg/L) | 142 | RCC | 1 | — | 27.8 (13.8)6 | 10.7 (3.6)6 | GC/MS | ✓ |
| 4-Pyridone-3-carboxamide-1-β-d-ribonucleoside (µg/L) | 272 | Nicotinamide | 1 | — | 156.1 (169.2)7 | 3.54 (1.63)7 | HPLC | ✓ |
| 8-Hydroxy-2'-deoxyguanosine (µg/L) | 283 | Purine | 1 | — | 0.82 (0.25)8 | 0.64 (0.23)9 | HPLC; ELISA | ✓ |
| α-Keto-δ-guanidinovaleric acid (µg/L) | 173 | Guanidine | 1 | — | 39.8 (31.1–60.6)10 | 8.23 (0.66)11 | IEC | ✓ |
| Anthranilic acid (µg/L) | 137 | 1 | — | 16.7 (6.5)12 | 4.23 (1.62)12 | HPLC | ✓ | |
| Argininic acid (µg/L) | 175 | Guanidine | 1 | — | 57.8 (40.3–78.8)10 | 21.5 (3.5)11 | IEC | |
| Asymmetric dimethylarginine (µg/L)a | 202 | Guanidine | 5 | 385.0 (288.4) | 878.7b (38.4)14 | <60.615 | HPLC; ELISA | |
| Cysteine (µg/L) | 121 | Aminoacid | 1 | — | 67.8 (3.6)16 | 43.6 (2.4)16 | HPLC | ✓ |
| Decanal (µg/L) | 156 | RCC | 1 | — | 23.4 (8.81)6 | 17.2 (5.0)6 | GC/MS | ✓ |
| Dimethylamine (mg/L) | 45 | Amine | 1 | — | 10.3 (1.6)5 | 2.18 (0.33)5 | HPLC | ✓ |
| Ethylamine (µg/L) | 45 | Amine | 1 | — | 69.0 (10.2)17 | 25.8 (5.8)17 | HPLC | ✓ |
| Guanidine (µg/L) | 59 | Guanidine | 1 | — | 96.2 (90.9–112.1)10 | <11.818 | IEC | |
| Guanidinoacetic acid (µg/L) | 117 | Guanidine | 1 | — | 220.0 (168.5–251.6)10 | 222.3 (79.6)19 | IEC | |
| Guanidino succinic acid (mg/L) | 175 | Guanidine | 1 | — | 1.43 (0.99–1.72)10 | 0.03 (0.01)18 | IEC | |
| Heptanal (µg/L) | 114 | RCC | 1 | — | 61.1 (44.1)6 | 51.1 (11.2)6 | GC/MS | ✓ |
| Hexanal (µg/L) | 100 | RCC | 1 | — | 51.7 (33.0)6 | 21.7 (10.8)6 | GC/MS | ✓ |
| Hypoxanthine (mg/L) | 136 | Purine | 1 | — | 2.57 (1.13)20 | 1.5 (0.5)21 | HPLC | |
| Malondialdehyde (µg/L) | 72 | RCC | 3 | 217.9 (148.4) | 388.8 (21.6)22 | 257.7 (81.7)23 | Spectrophotometry | |
| Methylguanidine (µg/L) | 73 | Guanidine | 1 | — | 139.4 (72.3–218.3)10 | <7.318 | IEC; HPLC | ✓ |
| Monomethylamine (µg/L) | 31 | Amine | 2 | 332 (351) | 580 (100)5 | 320 (40)5 | HPLC | ✓ |
| Neopterin (µg/L) | 253 | Purine | 1 | — | 83.3 (10.8)24 | 1.38 (0.47)24 | ELISA | ✓ |
| Nicotinamide (µg/L) | 122 | Nicotinamide | 1 | — | 35.4 (29.3)25 | 3.17 (1.22)25 | HPLC | ✓ |
| N-methyl-2-pyridone-5-carboxamide (mg/L) | 152 | Nicotinamide | 3 | 4.02 (3.28) | 7.80 (3.59)26 | 1.37 (0.68)26 | HPLC | ✓ |
| N-methyl-4-pyridone-3-carboxamide (µg/L) | 152 | Nicotinamide | 3 | 498.6 (162.8) | 636.9 (471.2)22 | 39.5 (13.7)25 | HPLC | ✓ |
| Nonanal (µg/L) | 142 | RCC | 1 | — | 68.9 (26.8)6 | 37.4 (23.3)6 | GC/MS | ✓ |
| Noradrenalin (µg/L) | 382 | Catecholamine | 1 | — | 2.02 (0.88)27 | 0.25 (0.07)28 | RIA | |
| Oxalate (mg/L) | 90 | 1 | — | 3.9 (0.6)29 | 0.3 (0.1)30 | Spectrophotometry; IEC | ✓ | |
| Phenylacetic acid (mg/L) | 136 | 2 | 467.2 (10.6) | 474.6 (44.9)31 | <1.431 | NMR | ||
| Symmetric dimethylarginine (µg/L) | 202 | Guanidine | 1 | — | 646.4 (606.0)32 | 76.1 (21.0)13 | HPLC; IEC | ✓ |
| Trimethylamine (µg/L) | 59 | Amine | 1 | — | 82.0 (28.5)33 | 24.7 (7.3)33 | GC/MS | ✓ |
| Trimethylamine-N-oxide (mg/L) | 75 | Amine | 1 | — | 7.49 (2.39)33 | 2.84 (1.53)33 | GC/MS | |
| Uric acid (mg/L) | 168 | Purine | 12 | 64.4 (20.4) | 83 (13)34 | 40.5 (13.9)35 | Spectrophotometry | |
GC/MS, gas chromatography–mass spectrometry; HPLC, high-performance liquid chromatography; IEC, ion exchange chromatography; NMR, nuclear magnetic resonance; RCC, reactive carbonyl compound; RIA, radioimmunoassay.
Highest uremic concentrations found after suppression of outliers: asymmetric dimethylarginine=364±81 µg/L.15
Table 4.
Mean and highest concentrations of uremic retention solutes found in uremic populations and normal concentrations found in the general population: Middle molecules
| Molecule | Molecular Weight | Group | Uremic Concentrations | Normal Concentration N (SD) | Methods (U; N) | New Retention Solutea | ||
|---|---|---|---|---|---|---|---|---|
| Number of Original Papers | Mean Uremic Concentration M (SD) | Highest Uremic Concentration H (SD or Range) | ||||||
| α1-Acid glycoprotein (g/L) | 43,000 | Protein | 2 | 1.24 (0.09) | 1.3 (0.1)54 | 0.63 (0.19)13 | Nephelometry | ✓ |
| α1-Microglobulin (mg/L) | 33,000 | Protein | 1 | — | 332 (45)54 | <54.055 | Nephelometry; spectrophotometry | ✓ |
| β-Trace protein (mg/L) | 26,000 | Protein | 1 | — | 12.3 (6.0–19.8)56 | <0.7457 | Nephelometry | ✓ |
| β2-Microglobulin (mg/L) | 11,818 | Protein | 24 | 30.2 (7.8) | 43.1 (18)27 | 1.9 (1.6)45 | ELISA | |
| Adiponectin (mg/L) | 30,000 | Protein | 1 | — | 16.6 (14.4–18.6)58 | <11.159 | Fluorometry; ELISA | |
| Angiogenin (µg/L) | 14,400 | Protein | 1 | — | 803 (74)60 | <150.061 | ELISA | |
| Calcitonin (ng/L) | 3450 | Protein | 1 | — | 21.8 (21.0)54 | <20.062 | RIA | ✓ |
| Complement factor D (mg/L) | 23,750 | Protein | 2 | 20.6 (13.0) | 29.8 (8.6)27 | 1.9 (0.5)63 | ELISA; RIA | |
| Cystatin C (mg/L) | 13,300 | Protein | 4 | 10.4 (8.3) | 22.9 (9.0)64 | <1.665 | Nephelometry; RIA | |
| Fibroblast growth factor-23 (ng/L) | 32,000 | Protein | 1 | — | 149.6 (102.8)66 | 26.3 (0.8)66 | ELISA | ✓ |
| Glutathion, oxidized (mg/L) | 613 | Tripeptide | 2 | 73.2 (57.6) | 114.0 (73.1–160.2)67 | 20.8 (7.3)35 | Spectrophotometry | ✓ |
| IGF-1 (µg/L) | 7650 | Protein | 1 | — | 220 (93)27 | 145.068 | RIA; chemiluminescence | ✓ |
| IL-6 (ng/L) | 24,500 | Cytokine | 7 | 5.91 (1.98) | 8.6 (3.7)22 | 4.069 | ELISA | |
| IL-8 (ng/L) | 8000 | Cytokine | 2 | 20.2 (25.1) | 38 (0.42–659)70 | 1.64 (1.85)71 | ELISA | ✓ |
| IL-10 (ng/L) | 18,000 | Cytokine | 1 | — | 10.6 (6)46 | 7.1 (1.5)72 | ELISA | ✓ |
| Leptin (µg/L) | 16,000 | Protein | 6 | 37.6 (25.1) | 70.1 (50.4)73 | 8.4 (6.7)74 | RIA | |
| Myoglobin (µg/L) | 17,000 | Protein | 1 | — | 163.6 (67.8)54 | 39.0 (2)75 | RIA | ✓ |
| Osteocalcin (µg/L) | 5800 | Protein | 2 | 189.7 (235.7) | 356.4 (607.4)54 | <18.076 | RIA | ✓ |
| PTH (ng/L) | 9500 | Protein | 12 | 673 (660) | 2195b (370)77 | <60.078 | N/A; RIA | |
| Prolactin (µg/L) | 22,000 | Protein | 2 | 25.8 (8.4) | 31.7 (6.5)54 | <19.379 | Chemiluminescence; spectrophotometry | ✓ |
| Resistin (µg/L) | 12,500 | Cytokine | 1 | — | 47.3 (35.3–62.2)80 | 15.1 (0.7)80 | ELISA | ✓ |
| Retinol binding protein (mg/L) | 21,200 | Protein | 2 | 169.1 (53.5) | 206.9 (20.5)81 | <80.065 | ELISA; RIA | |
| Soluble intracellular adhesion molecule-1 (µg/L) | 4270 | Protein | 1 | — | 80 (40–157)70 | 48 (5)82 | ELISA | ✓ |
| TNF-α (ng/L) | 26,000 | Cytokine | 4 | 21.6 (24.5) | 57.8 (10.8)83 | 7.069 | ELISA | |
| Vascular endothelial growth factor (ng/L) | 34,250 | Protein | 1 | — | 346 (887)45 | 60.0 (9)45 | ELISA | ✓ |
Table 3.
Mean and highest concentrations of uremic retention solutes found in uremic populations and normal concentrations found in the general population: Protein-bound molecules
| Molecule | Molecular Weight | Group | Uremic Concentrations | Normal Concentration N (SD) | Methods (U; N) | New Retention Solutea | ||
|---|---|---|---|---|---|---|---|---|
| Number of Original Papers | Mean Uremic Concentration M (SD) | Highest Uremic Concentration H (SD or Range) | ||||||
| 3-Carboxy-4-methyl-5-propyl-2-furan-propanoic acid (mg/L) | 240 | 3 | 6.1 (2.4) | 8.8 (5.0)36 | 3.6 (0.2)37 | HPLC; GC/MS | ||
| Acrolein, total (mg/L) | 56 | RCC | 2 | 9.8 (0.4) | 10.138 | 1.7 (0.5)38 | ELISA | ✓ |
| Acrolein, free (µg/L) | 56 | RCC | 2 | 76.2 (4.8) | 79.5 (47.0)38 | 28 (10.1)38 | HPLC | ✓ |
| Carboxymethyllysine (mg/L) | 204 | AGE | 6 | 5.4 (7.4) | 18.5 (5.0)39 | 0.35 (0.13)40 | ELISA | ✓ |
| Dihydroxyphenylalanine (mg/L) | 197 | Catecholamine | 1 | — | 11.4 (3.2)41 | 6.6 (0.73)41 | Fluorometry | ✓ |
| Hippuric acid, total (mg/L) | 179 | Hippurate | 5 | 71.3 (13.7) | 87.2 (61.7)42 | 3.0 (2)5 | HPLC | |
| Hippuric acid, free (mg/L) | 179 | Hippurate | 3 | 41.3 (14.3) | 51.1 (40.1)42 | 1.05,43 | HPLC; fluorometry | ✓ |
| Homocysteine (mg/L) | 135 | Aminoacid | 12 | 4.90 (2.15) | 9.37b (2.05)44 | 1.35 (0.14)44 | HPLC | |
| Indican (mg/L) | 295 | Indole | 1 | — | 27.3 (13.3)17 | 10.0 (0.4)17 | HPLC | ✓ |
| Indole-3-acetic acid, total (mg/L) | 175 | Indole | 4 | 2.03 (0.38) | 2.4 (2.2)36 | 0.5 (0.3)45 | HPLC | |
| Indole-3-acetic acid, free (mg/L) | 175 | Indole | 2 | 0.37 (0.10) | 0.44 (0.51)36 | — | HPLC | ✓ |
| Indoxyl sulfate, total (mg/L) | 212 | Indole | 18 | 23.1 (13.0) | 44.5 (15.3)46 | 0.53 (0.29)47 | HPLC | |
| Indoxyl sulfate, free (mg/L) | 213 | Indole | 6 | 3.22 (1.21) | 4.49 (2.67)46 | ND48 | HPLC | ✓ |
| Indoxyl-β-d-glucoronide (mg/L) | 408 | Indole | 1 | — | 3.87 (3.88)47 | 1.26 (0.52)47 | HPLC | ✓ |
| Kynurenic acid (µg/L) | 189 | Indole | 1 | — | 151.0 (76.4)5 | 5.48 (1.32)5 | HPLC | |
| p-Cresylsulfate, total (mg/L) | 31 | Phenol | 6 | 20.9 (12.2) | 41 (13.3)5 | 1.9 (1.3)5 | HPLC | ✓ |
| p-Cresylsulfate, free (mg/L) | 31 | Phenol | 2 | 1.75 (1.20) | 2.6 (5.1)49 | 0.08 (0.09)49 | HPLC | ✓ |
| Pentosidine (µg/L) | 342 | AGE | 2 | 509.7 (98.6) | 579.5 (299.3)36 | 51.6 (18.8)50 | HPLC; ELISA | |
| Phenol (mg/L) | 94 | Phenol | 2 | 2.79 (3.83) | 5.5 (3.7)51 | 0.6 (0.2)52 | HPLC; GC/MS | |
| Putrescine (µg/L) | 88 | Polyamine | 2 | 9.11 (0.44) | 9.42 (7.59)53 | 4.36 (2.75)53 | HPLC | |
| Spermidine (µg/L) | 145 | Polyamine | 1 | — | 9.99 (7.74)53 | 10.4 (5.1)53 | HPLC | |
| Spermine (µg/L) | 202 | Polyamine | 1 | — | 1.86 (1.53)53 | 6.20 (7.98)53 | HPLC | |
| Thiocyanate (mg/L) | 58 | 1 | — | 1.86 (0.17)16 | 0.29 (0.06)16 | HPLC | ✓ | |
AGE, advanced glycation end-product; ELISA, Enzyme-linked immunosorbant assay; GC/MS, Gas chromatography – mass spectrometry; HPLC, High performance liquid chromatography; RCC, reactive carbonyl compound.
Highest uremic concentrations found after suppression of outliers: homocysteine=7.8±1.3 mg/L.85
Comparison with Normal Concentrations
To evaluate the relative solute retention in uremia, we calculated the ratio of the mean of all reported uremic concentrations (M) to the normal concentration (N) measured in healthy controls (M/N). The ratio M/N ranged from 333 for phenylacetic acid to 0.3 for spermine (Tables 5–7). There were 21 solutes for which uremic concentrations were more than 10 times higher than normal (Table 5). There was a limited degree of retention in the case of 18 solutes for which the ratio M/N ranged between one and two (Table 6). For four compounds from the original uremic retention list, the ratio M/N was below one, which suggests that they might not be retention solutes (spermine, spermidine, guanidinoacetic acid, and malondialdehyde) (Table 7).
Table 5.
Comparison of the average uremic concentration (M) with normal concentrations (N): 21 solutes scoring above 10
| Molecule | M/N |
|---|---|
| Phenylacetic acid | 334 |
| Neopterin | 60.3 |
| Guanidino succinic acid | 47.7 |
| 4-Pyridone-3-carboxamide-1-β-d-ribonucleoside | 44.2 |
| Indoxyl sulfate, total | 43.2 |
| Hippuric acid, free | 41.3 |
| Kynurenic acid | 27.6 |
| Hippuric acid, total | 23.8 |
| p-Cresylsulfate, free | 21.9 |
| Methylguanidine | 19.1 |
| β-Trace protein | 16.6 |
| β2-Microglobulin | 15.9 |
| Carboxymethyllysine | 15.4 |
| Oxalate | 13.0 |
| N-Methyl-4-pyridone-3-carboxamide | 12.5 |
| IL-8 | 12.3 |
| PTH | 11.2 |
| Nicotinamide | 11.2 |
| p-Cresylsulfate, total | 11.0 |
| Complement factor D | 10.8 |
| Osteocalcin | 10.5 |
Table 7.
Comparison of the average uremic concentration (M) with normal concentrations (N): four solutes scoring below one
| Molecules | M/N |
|---|---|
| Guanidinoacetic acid | 0.99 |
| Spermidine | 0.96 |
| Malondialdehyde | 0.85 |
| Spermine | 0.30 |
Table 6.
Comparison of the average uremic concentration (M) with normal concentrations (N): 18 solutes scoring between one and two
| Molecule | M/N |
|---|---|
| α1-Acid glycoprotein | 1.96 |
| Nonanal | 1.84 |
| Dihydroxyphenylalanine | 1.72 |
| Hypoxanthine | 1.72 |
| 3-Carboxy-4-methyl-5-propyl-2-furan-propanoic acid | 1.70 |
| Soluble intracellular adhesion molecule-1 | 1.67 |
| Uric acid | 1.59 |
| Cysteine | 1.56 |
| IGF-1 | 1.52 |
| Adiponectin | 1.50 |
| IL-10 | 1.49 |
| IL-6 | 1.48 |
| Decanal | 1.36 |
| Prolactin | 1.33 |
| 8-Hydroxy-2′-deoxyguanosine | 1.28 |
| 2-Octenal | 1.25 |
| Heptanal | 1.20 |
| Calcitonin | 1.09 |
Comparison with Previously Reported Concentrations
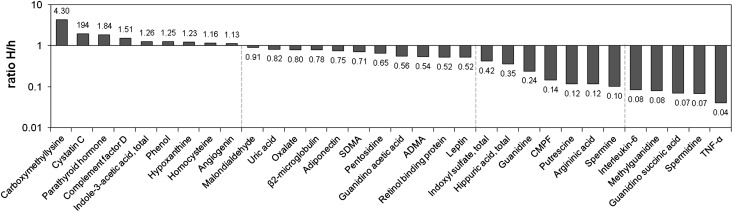
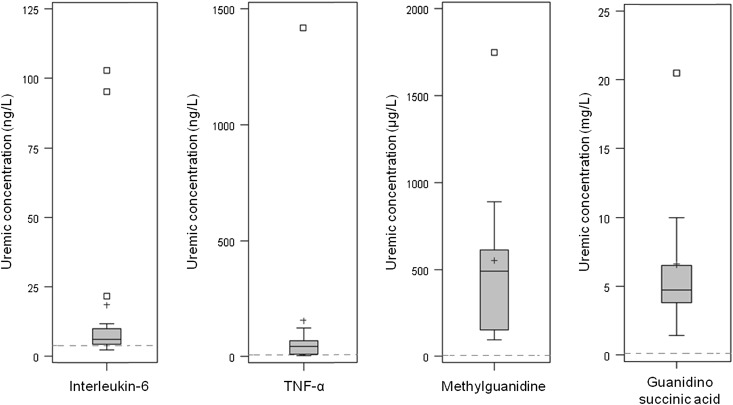
Of the 88 retention solutes, 56 solutes had not been presented in the list of uremic toxins published in 2003 and 2007 (Table 1).1,2 Because their uremic concentrations exceeded those concentrations of healthy controls, they were subsequently added to the list. However, there were 32 solutes that had already been included in the list of known uremic retention solutes. These solutes are presented in Figure 1 along with the ratio of the highest uremic concentrations found in the present analysis (H) to the concentrations found in the original review (h). This index ranged from 0.04 for TNF-α to 4.30 for carboxymethyllysine. Interestingly, the ratio H/h exceeded one for nine solutes, signifying that recent publications provided a more elevated highest concentration and justifying the need for updating the list of uremic concentrations. There were 12 solutes for which the highest concentration was less than one-half of the original highest report (ratio H/h below 0.5). For five of the solutes, it was even below 10% of the original report (H/h<0.1). For these compounds, we evaluated the representativeness of highest concentrations by graphical analyses. Data from both the present work and the previous review1 were pooled, and possible outliers were sought using box plots. Results concerning solutes with four concentrations or more are displayed in Figure 2. There were, indeed, outlying maximal values for all four solutes that had all been published before 2003. After exclusion of suspected outliers, the corrected H/h ratios substantially increased, reaching scores above 10% (H/h: IL-6, 0.73; TNF-α, 0.47; methylguanidine, 0.16; guanidino succinic acid, 0.14).
Figure 1.
Relative change in highest uremic concentrations of known retention solutes. The H/h index is the ratio of the highest uremic concentration found in the present analysis to the highest concentration presented in the previous reviews.1,2
Figure 2.
Distribution of the concentrations of IL-6, TNF-α, methylguanidine, and guanidino succinic acid found in uremic populations. Data from the present analysis and the previous review1 were pooled and displayed. Dashed lines represent concentrations found in the general population. Values out of Tukey’s inner fence were identified as suspected outliers.
Variability in Concentrations
To analyze variability in reported concentrations, the ratio H/L of the H to the lowest concentrations (L) found in uremic populations was used as an index of the range of the observed uremic concentrations. Substantial variability, as previously defined by a ratio exceeding 8.5,2 was found for several protein-bound solutes (carboxymethyllysine, free indoxyl sulfate, and phenol) and middle molecules (PTH, TNF-α, leptin, osteocalcin, and IL-8) (Supplemental Material 2). Additional graphical analyses of dispersion for compounds including four values or more concluded with suspected outliers for asymmetric dimethyl arginine (ADMA), homocysteine, and PTH (Supplemental Material 3). Second highest uremic concentrations could be more representative of highest uremic concentrations (ADMA=364±81 µg/L15; homocysteine=7.8 mg/L [range=3.3–16.0]85; PTH=1676±69 ng/L86). Asymmetries were present in several box plots, where mean values largely exceeded the medians. For these solutes, median concentrations could be more representative of uremic populations (median: ADMA=323 µg/L; carboxymethyllysine=1.8 mg/L; cystatin C=6.3 mg/L; PTH=329 ng/L; TNF-α=12.3 ng/L; homocysteine=4.3 mg/L). Opposite asymmetries were also present for several compounds, which suggested possible underestimated mean uremic concentrations (indole-3-acetic acid, free and total indoxyl sulfate, total p-cresylsulfate, and uric acid).
Sensitivity Analyses
Sensitivity analyses were performed to evaluate the influence of methodological choices on results. Total indoxyl sulfate concentrations were increased in patients undergoing dialysis (P=0.02). Receiving dialysis or not did not affect other compound concentrations (Supplemental Material 4). There was no sign of a general over- or underestimation of normal concentrations N compared with other available control concentrations (P=0.10), and using these control concentrations as normal concentrations did not substantially influence M/N ratios (r2=0.68).
Discussion
With the appearance of extrarenal blood purification techniques, survival of patients suffering from ESRD greatly improved, thus confirming the major influence of uremic toxins and fluid control on outcome. Subsequently, many uremic solutes have been shown to be associated with mortality or morbidity in epidemiologic studies,40,49,87–89 and direct adverse effects have been proven in experimental models.31,45,90 Still, the uremic milieu is complex, and solutes may affect various biologic systems, possibly in a synergetic manner. This effect requires caution when extrapolating results from in vitro systems, and only clinical trials can allow for drawing conclusions on the clinical significance of biologic effects. Randomized clinical trials of retention solute removal on hard outcomes such as mortality are scarce, and results are not always supportive of improved survival.91,92 Nonetheless, there is a substantial number of studies which quantified the circulating levels of various compounds in uremic patients to identify retention solutes or to evaluate solute removal. The present report is the continuation of the first encyclopedic work1 published in 2003, which aimed to identify and classify uremic retention solutes. The goal of the present update was to reflect the practical definition of uremic retention solutes in recent clinical studies, extend the list of uremic retention solutes with the new compounds that have been reported in the literature since 2003, and compare the values reported in both analyses.
The review of the literature resulted in the inclusion of 88 published articles, with data on 32 known uremic toxins and 56 newly identified retention solutes, which were consequently added to the list of uremic toxins. A time limit was necessary to perform this analysis, but in such a dynamic field, new toxins are continuously identified. Recently, the strong vasoconstrictor uridine adenosine tetraphosphate (Up4A) was described as a uremic toxin. CKD patients had, on average, a 5.2-fold higher Up4A plasma concentration compared with healthy subjects, and this increased Up4A concentration may influence blood pressure, proliferation rate of vascular smooth muscle cells, and calcification processes in CKD patients.93,94 A molecular mass threshold was also applied, and molecules above 60 kD were excluded from the analysis, because these solutes cannot be filtered by the glomeruli. However, although their concentrations are not directly associated with glomerular function, several large acute phase proteins (α2-macroglobulin, fibrinogen, myeloperoxidase, and IL-12)56,95–97 and endothelium-related proteins (vascular cell adhesion molecule 1, vascular endothelial growth factor 1, and soluble vascular endothelial growth factor receptor)45,70 were increased in CKD, and they could have a significant diagnostic and pathophysiologic value.98,99 However, reviewing all solutes that were evaluated in a sample of publications on uremic patients, we identified molecules that were reduced in uremia (bilirubin, reduced glutathione, α1-antitrypsine, arginine, and homoarginine).10,35,81,100 Because these solutes are associated with antioxidant, anti-inflammatory, and vasodilating properties, their reduced concentration could be involved in additional adverse effects of uremia.
Performing this analysis, we faced several limitations related to data reporting. For instance, it is still frequent for -omics studies to report only strengths of associations without reporting concentrations.4,64 We were also limited by the use relative units, particularly concerning enzymes and advanced glycation end products (AGEs).24,39,101,102 Furthermore, we chose not to include results on κ-light chains, because the concentration found in uremic patients was less than 1% of the values previously found in healthy or CKD populations (30–80 mg/L).60,103 Finally, because it was shown in 2005 that the reported serum p-cresol concentrations were actually caused by an artifact linked to deproteinization of p-cresylsulfate and p-cresylglucuronide,104,105 results on p-cresol were also excluded. Still, we found eight reports on p-cresol published after 2005. Warnings concerning the study of p-cresol have recently been discussed elsewhere.106
Our research identified 32 solutes that had been included in the previous report in 2003 and observed considerable differences, with a general tendency to lower concentrations. Several reasons could explain these discrepancies. A general improvement in dialysis techniques could result in a more efficient toxin removal with, eventually, better blood profiles of dialyzed patients. This finding was shown with hemodiafiltration over hemodialysis for middle molecules and small water-soluble molecules such as urea.27,32,56,107 Methodological choices, in terms of quantification techniques, could also influence reported concentrations. A cross-sectional comparison of HPLC, MS, and ELISA determinations of ADMA showed large variability across and within techniques, and it found that MS was the most reliable measurement method.108 A similar technical issue could explain the lower concentrations in guanidino compounds found in our analysis. Indeed, recent results were measured using IEC, whereas the previously reported values had been quantified by HPLC.
There were four compounds that did not clearly show uremic retention, although they had been presented as uremic solutes in previous classifications.1,2 Results on guanidinoacetic acid confirmed that it is not a uremic toxin, because concentrations consistently decreased with CKD severity.13,18,19,64 Concerning spermine and spermidine, normal concentrations were not presented in the original review.1 Results from age-matched normal and uremic patients showed that both concentrations and particularly, spermine were reduced in uremia.53 Furthermore, there was a clear increase in both acrolein, a toxic spermine oxidation product, and in the activity of the responsible oxidase.53 Interestingly, concentrations in malondialdehyde actually increased with CKD severity, which supports its classification as a uremic retention solute.22,84
Whether normal and uremic concentrations of retention solutes were comparable is a difficult aspect of this study, especially when results are drawn from different sources. When available, we used internal controls to estimate normal concentrations. Furthermore, to minimize the impact of the methodological choices, efforts were made to select studies using the same techniques. When reference ranges were given by age strata, we used age-matched populations. Comfortingly, sensitivity analyses suggested that the choice of normal concentrations did not substantially modify results.
We believe that this analysis provided relevant information on uremic retention solutes, which should be taken into account when testing uremic retention solute toxicity. With the addition of 56 uremic retention solutes, this extended classification of uremic retention solutes proved that it was timely and useful. It should be used jointly with the previous publications1,2 as a complementary tool, giving additional insight into concentration values and variability of retention solutes found in uremic populations.
Concise Methods
Search and Selection Criteria
The PubMed database was searched for articles published from January 1, 2003 to April 1, 2011 dealing with uremic toxins in renal patients using the following keywords: (uremi* OR uraemi*) AND (toxin* OR toxic*) AND patients AND (renal failure chronic OR kidney disease OR CKD). The aim was to retrieve uremic and normal serum or plasma concentrations of uremic retention solutes and exclude common solutes such as creatinine and urea, inorganic compounds, and large molecules over 60 kD that are not filtered by the glomerulus. To be included in our study, plasma concentrations had to be measured in adult patients with CKD stages 3–5. All solutes that had been listed in the previous encyclopedic reviews were included. For other solutes, only those solutes in which uremic concentrations exceeded normal concentrations were included. For populations receiving renal replacement therapy by peritoneal or hemodialysis, only pretreatment concentrations were included. To avoid the effect of metabolic differences, reactive carbonyl compounds (RCCs), AGEs, and advanced lipoperoxidation end products were measured in nondiabetic patients. When necessary, blood concentrations were estimated using a blood protein concentration of 70 g/L and serum albumin concentration of 35 g/L.1,109
All concentrations are presented in grams per liter. Data that were originally reported in moles per liter were transformed into mass concentrations by multiplying with the molecular weight. Suspected unit errors were recorded and studied separately. All original concentrations were given as mean (SD) or median (range). For each solute, we calculated M and identified H and L reported concentrations. In contrast with our previous study, the maximal individual concentrations were not recorded or estimated. However, under the hypothesis of a normal distribution, it can be approximated using the formula Cindividual max=M+2SD.1
For each solute, we reported N measured in healthy controls. N values were preferably extracted from the same publication reporting the highest concentration H or if not possible, another included study. Otherwise, normal concentration was taken from the 2003 encyclopedic review or external sources. N levels were preferably recorded as means (SD). If reported as a range or confidence interval, the upper bound or maximal value was extracted and presented using a less than symbol. Finally, for solutes of which data were presented in the original review, the 2003 h value was also extracted.
Calculations and Analyses
The relative solute retention in uremia was studied using M/N. Solutes scoring more than 10 were arbitrarily defined as largely increased, whereas scorings below 2 were interpreted as showing limited evidence of uremic retention. For solutes that had been included in the 2003 review, highest reported values were compared using the H/h ratio. Between-study variability was assessed using H/L, which is an index of the width of the concentration range. Low evidence of variability has been previously defined as a ratio below 3, whereas a ratio exceeding 8.5 is evidence of substantial variability.5 Additionally, the coefficient of variability was calculated for results based on four data or more using the formula coefficient of variability= SD/M. Finally, graphical analyses of data dispersion using box plots were undertaken for solutes that had four values or more. Values outside Tukey’s inner fence were considered to be suspected outliers.2,110 Tukey’s inner fence is defined as the range between 1.5 times the interquartile range below the first quartile to 1.5 times the interquartile range above the third quartile. Sensitivity analyses were performed to evaluate the influence of methodological choices. The effect of CKD stage (predialytic versus dialytic stages) on concentrations was assessed by exact Wilcoxon tests for two independent samples. The difference between N and other available control concentrations was assessed by a paired t test. The correlation between M/N ratios using N or other control concentrations was evaluated using Pearson’s product moment correlation coefficient. Statistical tests were performed with a 5% type I error. Calculations and analyses were performed with Excel 2007 (Microsoft Corp., Redmond, WA) and SAS version 9.2 (SAS Institute, Cary, NC).
Disclosures
None.
Acknowledgments
The European Uremic Toxin Work Group (EUTox) is a working group from the European Society for Artificial Organs (ESAO) endorsed by the European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) that aims to analyze and discuss matters related to the identification, characterization, analytic determination, and evaluation of biological activity of uremic retention solutes. EUTox is constituted of 24 academic research groups and 7 corporate members (Abbott, Bayer Schering Pharma, BBraun Avitum, Fresenius Medical Care, Gambro, Membrana, and Vifor Pharma). Academic members are O. Abou Deif, A. Argiles, T. Drueke, U. Baurmeister, J. Beige, P. Brunet, G. Cohen, D. Fliser, G. Glorieux, S. Herget-Rosenthal, V. Jankowski, J. Jankowski, Z. Massy, H. Mischak, A. Ortiz, A. Perna, J.M. Rodriguez Portillo, G. Spasovski, B. Stegmayr, P. Stenvinkel, P. Thornalley, R. Vanholder, C. Wanner, and A. Wiecek. Member affiliations and other information about EUTox can be obtained at the website http://uremic-toxins.org. We also would like to thank Prof. J.P. Daurès for guidance and support.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011121175/-/DCSupplemental.
References
- 1.Vanholder R, De Smet R, Glorieux G, Argilés A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jörres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W, European Uremic Toxin Work Group (EUTox) : Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int 63: 1934–1943, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Meert N, Schepers E, De Smet R, Argiles A, Cohen G, Deppisch R, Drüeke T, Massy Z, Spasovski G, Stegmayr B, Zidek W, Jankowski J, Vanholder R: Inconsistency of reported uremic toxin concentrations. Artif Organs 31: 600–611, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Vanholder R, Meert N, Schepers E, Glorieux G, Argiles A, Brunet P, Cohen G, Drüeke T, Mischak H, Spasovski G, Massy Z, Jankowski J, European Uremic Toxin Work Group (EUTox) : Review on uraemic solutes II—variability in reported concentrations: causes and consequences. Nephrol Dial Transplant 22: 3115–3121, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Rhee EP, Souza A, Farrell L, Pollak MR, Lewis GD, Steele DJ, Thadhani R, Clish CB, Greka A, Gerszten RE: Metabolite profiling identifies markers of uremia. J Am Soc Nephrol 21: 1041–1051, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aronov PA, Luo FJ-G, Plummer NS, Quan Z, Holmes S, Hostetter TH, Meyer TW: Colonic contribution to uremic solutes. J Am Soc Nephrol 22: 1769–1776, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alhamdani M-SS, Al-Kassir A-HAM, Jaleel NA, Hmood AM, Ali HM: Elevated levels of alkanals, alkenals and 4-HO-alkenals in plasma of hemodialysis patients. Am J Nephrol 26: 299–303, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Slominska EM, Carrey EA, Foks H, Orlewska C, Wieczerzak E, Sowinski P, Yacoub MH, Marinaki AM, Simmonds HA, Smolenski RT: A novel nucleotide found in human erythrocytes, 4-pyridone-3-carboxamide-1-beta-D-ribonucleoside triphosphate. J Biol Chem 281: 32057–32064, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Goto S, Fujii H, Hamada Y, Yoshiya K, Fukagawa M: Association between indoxyl sulfate and skeletal resistance in hemodialysis patients. Ther Apher Dial 14: 417–423, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Forlenza MJ, Miller GE: Increased serum levels of 8-hydroxy-2′-deoxyguanosine in clinical depression. Psychosom Med 68: 1–7, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Taes YEC, Marescau B, De Vriese A, De Deyn PP, Schepers E, Vanholder R, Delanghe JR: Guanidino compounds after creatine supplementation in renal failure patients and their relation to inflammatory status. Nephrol Dial Transplant 23: 1330–1335, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Derave W, Marescau B, Vanden Eede E, Eijnde BO, De Deyn PP, Hespel P: Plasma guanidino compounds are altered by oral creatine supplementation in healthy humans. J Appl Physiol 97: 852–857, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Tankiewicz A, Pawlak D, Pawlak K, Szewc D, Myśliwiec M, Buczko W: Anthranilic acid-uraemic toxin damaged red cell’s membrane. Int Urol Nephrol 37: 621–627, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Pere P, Salonen M, Jokinen M, Rosenberg PH, Neuvonen PJ, Haasio J: Pharmacokinetics of ropivacaine in uremic and nonuremic patients after axillary brachial plexus block. Anesth Analg 96: 563–569, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Kielstein JT, Böger RH, Bode-Böger SM, Martens-Lobenhoffer J, Lonnemann G, Frölich JC, Haller H, Fliser D: Low dialysance of asymmetric dimethylarginine (ADMA)—in vivo and in vitro evidence of significant protein binding. Clin Nephrol 62: 295–300, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Zhang D-L, Liu J, Liu S, Zhang Y, Liu W-H: The differences of asymmetric dimethylarginine removal by different dialysis treatments. Ren Fail 32: 935–940, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Hasuike Y, Nakanishi T, Moriguchi R, Otaki Y, Nanami M, Hama Y, Naka M, Miyagawa K, Izumi M, Takamitsu Y: Accumulation of cyanide and thiocyanate in haemodialysis patients. Nephrol Dial Transplant 19: 1474–1479, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Raff AC, Lieu S, Melamed ML, Quan Z, Ponda M, Meyer TW, Hostetter TH: Relationship of impaired olfactory function in ESRD to malnutrition and retained uremic molecules. Am J Kidney Dis 52: 102–110, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka A, Takahashi Y, Mizokuchi M, Shimada N, Koide H: Plasma, urinary, and erythrocyte concentrations of guanidino compounds in patients with chronic renal failure. Ren Fail 21: 499–514, 1999 [DOI] [PubMed] [Google Scholar]
- 19.De Deyn P, Marescau B, Lornoy W, Becaus I, Lowenthal A: Guanidino compounds in uraemic dialysed patients. Clin Chim Acta 157: 143–150, 1986 [DOI] [PubMed] [Google Scholar]
- 20.Shahbazian H, Zand Moghadam A, Ehsanpour A, Khazaali M: Changes in plasma concentrations of hypoxanthine and uric acid before and after hemodialysis. Iran J Kidney Dis 3: 151–155, 2009 [PubMed] [Google Scholar]
- 21.Kock R, Delvoux B, Sigmund M, Greiling H: A comparative study of the concentrations of hypoxanthine, xanthine, uric acid and allantoin in the peripheral blood of normals and patients with acute myocardial infarction and other ischaemic diseases. Eur J Clin Chem Clin Biochem 32: 837–842, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Rutkowski P, Słominska EM, Szołkiewicz M, Aleksandrowicz E, Smolenski RT, Wołyniec W, Renke M, Wisterowicz K, Swierczynski J, Rutkowski B: Relationship between uremic toxins and oxidative stress in patients with chronic renal failure. Scand J Urol Nephrol 41: 243–248, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Bayés B, Pastor MC, Bonal J, Juncà J, Romero R: Homocysteine and lipid peroxidation in haemodialysis: Role of folinic acid and vitamin E. Nephrol Dial Transplant 16: 2172–2175, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Fragedaki E, Nebel M, Schupp N, Sebekova K, Völkel W, Klassen A, Pischetsrieder M, Frischmann M, Niwa T, Vienken J, Heidland A, Stopper H: Genomic damage and circulating AGE levels in patients undergoing daily versus standard haemodialysis. Nephrol Dial Transplant 20: 1936–1943, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Rutkowski B, Slominska E, Szolkiewicz M, Smolenski RT, Striley C, Rutkowski P, Swierczynski J: N-methyl-2-pyridone-5-carboxamide: a novel uremic toxin? Kidney Int Suppl 84: S19–S21, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Carrey EA, Smolenski RT, Edbury SM, Laurence A, Marinaki AM, Duley JA, Zhu L, Goldsmith DJ, Simmonds HA: Origin and characteristics of an unusual pyridine nucleotide accumulating in erythrocytes: Positive correlation with degree of renal failure. Clin Chim Acta 335: 117–129, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Beerenhout CH, Luik AJ, Jeuken-Mertens SGJ, Bekers O, Menheere P, Hover L, Klaassen L, van der Sande FM, Cheriex EC, Meert N, Leunissen KM, Kooman JP: Pre-dilution on-line haemofiltration vs low-flux haemodialysis: a randomized prospective study. Nephrol Dial Transplant 20: 1155–1163, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Abd-Allah NM, Hassan FH, Esmat AY, Hammad SA: Age dependence of the levels of plasma norepinephrine, aldosterone, renin activity and urinary vanillylmandelic acid in normal and essential hypertensives. Biol Res 37: 95–106, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Mydlík M, Derzsiová K: Oxalic Acid as a uremic toxin. J Ren Nutr 18: 33–39, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Marangella M, Petrarulo M, Mandolfo S, Vitale C, Cosseddu D, Linari F: Plasma profiles and dialysis kinetics of oxalate in patients receiving hemodialysis. Nephron 60: 74–80, 1992 [DOI] [PubMed] [Google Scholar]
- 31.Jankowski J, van der Giet M, Jankowski V, Schmidt S, Hemeier M, Mahn B, Giebing G, Tolle M, Luftmann H, Schluter H, Zidek W, Tepel M: Increased plasma phenylacetic acid in patients with end-stage renal failure inhibits iNOS expression. J Clin Invest 112: 256–264, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalousová M, Kielstein JT, Hodková M, Zima T, Dusilová-Sulková S, Martens-Lobenhoffer J, Bode-Boger SM: No benefit of hemodiafiltration over hemodialysis in lowering elevated levels of asymmetric dimethylarginine in ESRD patients. Blood Purif 24: 439–444, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Bain MA, Faull R, Fornasini G, Milne RW, Evans AM: Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol Dial Transplant 21: 1300–1304, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Taki K, Tsuruta Y, Niwa T: Indoxyl sulfate and atherosclerotic risk factors in hemodialysis patients. Am J Nephrol 27: 30–35, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Müller C, Eisenbrand G, Gradinger M, Rath T, Albert FW, Vienken J, Singh R, Farmer PB, Stockis JP, Janzowski C: Effects of hemodialysis, dialyser type and iron infusion on oxidative stress in uremic patients. Free Radic Res 38: 1093–1100, 2004 [DOI] [PubMed] [Google Scholar]
- 36.De Smet R, Dhondt A, Eloot S, Galli F, Waterloos MA, Vanholder R: Effect of the super-flux cellulose triacetate dialyser membrane on the removal of non-protein-bound and protein-bound uraemic solutes. Nephrol Dial Transplant 22: 2006–2012, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Sassa T, Matsuno H, Niwa M, Kozawa O, Takeda N, Niwa T, Kumada T, Uematsu T: Measurement of furancarboxylic acid, a candidate for uremic toxin, in human serum, hair, and sweat, and analysis of pharmacological actions in vitro. Arch Toxicol 73: 649–654, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Sakata K, Kashiwagi K, Sharmin S, Ueda S, Irie Y, Murotani N, Igarashi K: Increase in putrescine, amine oxidase, and acrolein in plasma of renal failure patients. Biochem Biophys Res Commun 305: 143–149, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Ueda S, Yamagishi S, Takeuchi M, Kohno K, Shibata R, Matsumoto Y, Kaneyuki U, Fujimura T, Hayashida A, Okuda S: Oral adsorbent AST-120 decreases serum levels of AGEs in patients with chronic renal failure. Mol Med 12: 180–184, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner Z, Molnár M, Molnár GA, Tamaskó M, Laczy B, Wagner L, Csiky B, Heidland A, Nagy J, Wittmann I: Serum carboxymethyllysine predicts mortality in hemodialysis patients. Am J Kidney Dis 47: 294–300, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Sutherland WHF, Gieseg SP, Walker RJ, de Jong SA, Firth CA, Scott N: Serum protein-bound 3,4-dihydroxyphenylalanine and related products of protein oxidation and chronic hemodialysis. Ren Fail 25: 997–1009, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Dhondt AW, Vanholder RC, De Smet RV, Claus SA, Waterloos MA, Glorieux GL, Delanghe JR, Lameire NH: Studies on dialysate mixing in the Genius single-pass batch system for hemodialysis therapy. Kidney Int 63: 1540–1547, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Farrell PC, Gotch FA, Peters JH, Berridge BJ, Jr, Lam M: Binding of hippurate in normal plasma and in uremic plasma pre- and postdialysis. Nephron 20: 40–46, 1978 [DOI] [PubMed] [Google Scholar]
- 44.Perna AF, Satta E, Acanfora F, Lombardi C, Ingrosso D, De Santo NG: Increased plasma protein homocysteinylation in hemodialysis patients. Kidney Int 69: 869–876, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Jourde-Chiche N, Dou L, Sabatier F, Calaf R, Cerini C, Robert S, Camoin-Jau L, Charpiot P, Argiles A, Dignat-George F, Brunet P: Levels of circulating endothelial progenitor cells are related to uremic toxins and vascular injury in hemodialysis patients. J Thromb Haemost 7: 1576–1584, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Lee C-T, Kuo C-C, Chen Y-M, Hsu CY, Lee WC, Tsai YC, Ng HY, Kuo LC, Chiou TT, Yang YK, Cheng BC, Chen JB: Factors associated with blood concentrations of indoxyl sulfate and p-cresol in patients undergoing peritoneal dialysis. Perit Dial Int 30: 456–463, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Ujhelyi L, Balla G, Jeney V, Varga Z, Nagy E, Vercellotti GM, Agarwal A, Eaton JW, Balla J: Hemodialysis reduces inhibitory effect of plasma ultrafiltrate on LDL oxidation and subsequent endothelial reactions. Kidney Int 69: 144–151, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Niwa T, Takeda N, Tatematsu A, Maeda K: Accumulation of indoxyl sulfate, an inhibitor of drug-binding, in uremic serum as demonstrated by internal-surface reversed-phase liquid chromatography. Clin Chem 34: 2264–2267, 1988 [PubMed] [Google Scholar]
- 49.Liabeuf S, Barreto DV, Barreto FC, Meert N, Glorieux G, Schepers E, Temmar M, Choukroun G, Vanholder R, Massy ZA, European Uraemic Toxin Work Group (EUTox) : Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant 25: 1183–1191, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Taneda S, Monnier VM: ELISA of pentosidine, an advanced glycation end product, in biological specimens. Clin Chem 40: 1766–1773, 1994 [PubMed] [Google Scholar]
- 51.Nakabayashi I, Nakamura M, Kawakami K, Ohta T, Kato I, Uchida K, Yoshida M: Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: a preliminary study. Nephrol Dial Transplant 26: 1094–1098, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Hida M, Aiba Y, Sawamura S, Suzuki N, Satoh T, Koga Y: Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron 74: 349–355, 1996 [DOI] [PubMed] [Google Scholar]
- 53.Sakata K, Kashiwagi K, Sharmin S, Ueda S, Igarashi K: Acrolein produced from polyamines as one of the uraemic toxins. Biochem Soc Trans 31: 371–374, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Testa A, Gentilhomme H, Le Carrer D, Orsonneau JL: In vivo removal of high- and low-molecular-weight compounds in hemodiafiltration with on-line regeneration of ultrafiltrate. Nephron Clin Pract 104: c55–c60, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Ekström B, Berggård I: Human alpha1-microglobulin. Purification procedure, chemical and physiochemical properties. J Biol Chem 252: 8048–8057, 1977 [PubMed] [Google Scholar]
- 56.Lindström V, Grubb A, Alquist Hegbrant M, Christensson A: Different elimination patterns of beta-trace protein, beta2-microglobulin and cystatin C in haemodialysis, haemodiafiltration and haemofiltration. Scand J Clin Lab Invest 68: 685–691, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Pöge U, Gerhardt TM, Stoffel-Wagner B, Palmedo H, Klehr HU, Sauerbruch T, Woitas RP: beta-Trace protein is an alternative marker for glomerular filtration rate in renal transplantation patients. Clin Chem 51: 1531–1533, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Kim S, Oh KH, Chin HJ, Na KY, Kim YS, Chae DW, Ahn C, Han JS, Kim S, Joo KW: Effective removal of leptin via hemodiafiltration with on-line endogenous reinfusion therapy. Clin Nephrol 72: 442–448, 2009 [DOI] [PubMed] [Google Scholar]
- 59.Guebre-Egziabher F, Bernhard J, Funahashi T, Hadj-Aissa A, Fouque D: Adiponectin in chronic kidney disease is related more to metabolic disturbances than to decline in renal function. Nephrol Dial Transplant 20: 129–134, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Ouseph R, Hutchison CA, Ward RA: Differences in solute removal by two high-flux membranes of nominally similar synthetic polymers. Nephrol Dial Transplant 23: 1704–1712, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Shapiro R, Strydom DJ, Olson KA, Vallee BL: Isolation of angiogenin from normal human plasma. Biochemistry 26: 5141–5146, 1987 [DOI] [PubMed] [Google Scholar]
- 62.d’Herbomez M, Caron P, Bauters C, Do Cao C, Schlienger JL, Sapin R, Baldet L, Carnaille B, Wémeau JL, French Group GTE (Groupe des Tumeurs Endocrines) : Reference range of serum calcitonin levels in humans: Influence of calcitonin assays, sex, age, and cigarette smoking. Eur J Endocrinol 157: 749–755, 2007 [DOI] [PubMed] [Google Scholar]
- 63.Pascual M, Steiger G, Estreicher J, Macon K, Volanakis JE, Schifferli JA: Metabolism of complement factor D in renal failure. Kidney Int 34: 529–536, 1988 [DOI] [PubMed] [Google Scholar]
- 64.Toyohara T, Akiyama Y, Suzuki T, Takeuchi Y, Mishima E, Tanemoto M, Momose A, Toki N, Sato H, Nakayama M, Hozawa A, Tsuji I, Ito S, Soga T, Abe T: Metabolomic profiling of uremic solutes in CKD patients. Hypertens Res 33: 944–952, 2010 [DOI] [PubMed] [Google Scholar]
- 65.Kabanda A, Jadoul M, Pochet JM, Lauwerys R, van Ypersele de Strihou C, Bernard A: Determinants of the serum concentrations of low molecular weight proteins in patients on maintenance hemodialysis. Kidney Int 45: 1689–1696, 1994 [DOI] [PubMed] [Google Scholar]
- 66.Shigematsu T, Kazama JJ, Yamashita T, Fukumoto S, Hosoya T, Gejyo F, Fukagawa M: Possible involvement of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism associated with renal insufficiency. Am J Kidney Dis 44: 250–256, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Yu M, Kim YJ, Kang D-H: Indoxyl sulfate-induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin J Am Soc Nephrol 6: 30–39, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Friedrich N, Alte D, Völzke H, Spilcke-Liss E, Lüdemann J, Lerch MM, Kohlmann T, Nauck M, Wallaschofski H: Reference ranges of serum IGF-1 and IGFBP-3 levels in a general adult population: Results of the Study of Health in Pomerania (SHIP). Growth Horm IGF Res 18: 228–237, 2008 [DOI] [PubMed] [Google Scholar]
- 69.Rysz J, Banach M, Cialkowska-Rysz A, Stolarek R, Barylski M, Drozdz J, Okonski P: Blood serum levels of IL-2, IL-6, IL-8, TNF-alpha and IL-1beta in patients on maintenance hemodialysis. Cell Mol Immunol 3: 151–154, 2006 [PubMed] [Google Scholar]
- 70.Stinghen AE, Gonçalves SM, Martines EG, Nakao LS, Riella MC, Aita CA, Pecoits-Filho R: Increased plasma and endothelial cell expression of chemokines and adhesion molecules in chronic kidney disease. Nephron Clin Pract 111: c117–c126, 2009 [DOI] [PubMed] [Google Scholar]
- 71.Berrahmoune H, Lamont JV, Herbeth B, FitzGerald PS, Visvikis-Siest S: Biological determinants of and reference values for plasma interleukin-8, monocyte chemoattractant protein-1, epidermal growth factor, and vascular endothelial growth factor: Results from the STANISLAS cohort. Clin Chem 52: 504–510, 2006 [DOI] [PubMed] [Google Scholar]
- 72.Sarris AH, Kliche KO, Pethambaram P, Preti A, Tucker S, Jackow C, Messina O, Pugh W, Hagemeister FB, McLaughlin P, Rodriguez MA, Romaguera J, Fritsche H, Witzig T, Duvic M, Andreeff M, Cabanillas F: Interleukin-10 levels are often elevated in serum of adults with Hodgkin’s disease and are associated with inferior failure-free survival. Ann Oncol 10: 433–440, 1999 [DOI] [PubMed] [Google Scholar]
- 73.Ottonello L, Gnerre P, Bertolotto M, Mancini M, Dapino P, Russo R, Garibotto G, Barreca T, Dallegri F: Leptin as a uremic toxin interferes with neutrophil chemotaxis. J Am Soc Nephrol 15: 2366–2372, 2004 [DOI] [PubMed] [Google Scholar]
- 74.Dagogo-Jack S, Ovalle F, Landt M, Gearing B, Coyne DW: Hyperleptinemia in patients with end-stage renal disease undergoing continuous ambulatory peritoneal dialysis. Perit Dial Int 18: 34–40, 1998 [PubMed] [Google Scholar]
- 75.Chen IW, David R, Maxon HR, Sperling M, Stein EA: Age-, sex-, and race-related differences in myoglobin concentrations in the serum of healthy persons. Clin Chem 26: 1864–1868, 1980 [PubMed] [Google Scholar]
- 76.Kusec V, Lukić M, Stavljenić-Rukavina A: Osteocalcin reference range in a Croatian population sample. Acta Med Croatica 48: 59–62, 1994 [PubMed] [Google Scholar]
- 77.Scholze A, Jankowski V, Henning L, Haass W, Wittstock A, Suvd-Erdene S, Zidek W, Tepel M, Jankowski J: Phenylacetic acid and arterial vascular properties in patients with chronic kidney disease stage 5 on hemodialysis therapy. Nephron Clin Pract 107: c1–c6, 2007 [DOI] [PubMed] [Google Scholar]
- 78.Tsukamoto Y, Hanaoka M, Matsuo T, Saruta T, Nomura M, Takahashi Y: Effect of 22-oxacalcitriol on bone histology of hemodialyzed patients with severe secondary hyperparathyroidism. Am J Kidney Dis 35: 458–464, 2000 [DOI] [PubMed] [Google Scholar]
- 79.Beltran L, Fahie-Wilson MN, McKenna TJ, Kavanagh L, Smith TP: Serum total prolactin and monomeric prolactin reference intervals determined by precipitation with polyethylene glycol: Evaluation and validation on common immunoassay platforms. Clin Chem 54: 1673–1681, 2008 [DOI] [PubMed] [Google Scholar]
- 80.Hung C-Y, Chen Y-L, Chen C-S, Yang C-S, Peng S-J: Association of leptin with hemodialysis-related muscle cramps: A cross-sectional study. Blood Purif 27: 159–164, 2009 [DOI] [PubMed] [Google Scholar]
- 81.Ficheux A, Gayrard N, Szwarc I, Andress D, Soullier S, Duny Y, Goubert G, Thomas M, Bismuth-Mondolfo J, Daurès JP, Brunet P, Servel MF, Argilés A: The use of SDS-PAGE scanning of spent dialysate to assess uraemic toxin removal by dialysis. Nephrol Dial Transplant 26: 2281–2289, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hackman A, Abe Y, Insull W, Jr, Pownall H, Smith L, Dunn K, Gotto AM, Jr, Ballantyne CM: Levels of soluble cell adhesion molecules in patients with dyslipidemia. Circulation 93: 1334–1338, 1996 [DOI] [PubMed] [Google Scholar]
- 83.Nolin TD, Appiah K, Kendrick SA, Le P, McMonagle E, Himmelfarb J: Hemodialysis acutely improves hepatic CYP3A4 metabolic activity. J Am Soc Nephrol 17: 2363–2367, 2006 [DOI] [PubMed] [Google Scholar]
- 84.Terrier-Lenglet A, Nollet A, Liabeuf S, Barreto DV, Brazier M, Lemke HD, Vanholder R, Choukroun G, Massy ZA, Groupe EUTox (European Uremic toxin) : Plasma malondialdehyde may not predict mortality in patient with chronic kidney disease. Nephrol Ther 7: 219–224, 2011 [DOI] [PubMed] [Google Scholar]
- 85.Splendiani G, De Angelis S, Tullio T, Ferranini M, Dessì MR, Pastore A, Casciani S, Liberatoscioli L, Federici G, Cortese C: Selective adsorption of homocysteine using an HFR-ON LINE technique. Artif Organs 28: 592–595, 2004 [DOI] [PubMed] [Google Scholar]
- 86.Chen ZJ, Cao G, Tang WX, Lv XY, Huang SM, Qin W, Ping F, Ye T: A randomized controlled trial of high-permeability haemodialysis against conventional haemodialysis in the treatment of uraemic pruritus. Clin Exp Dermatol 34: 679–683, 2009 [DOI] [PubMed] [Google Scholar]
- 87.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 88.Bammens B, Evenepoel P, Keuleers H, Verbeke K, Vanrenterghem Y: Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int 69: 1081–1087, 2006 [DOI] [PubMed] [Google Scholar]
- 89.Cheung AK, Rocco MV, Yan G, Leypoldt JK, Levin NW, Greene T, Agodoa L, Bailey J, Beck GJ, Clark W, Levey AS, Ornt DB, Schulman G, Schwab S, Teehan B, Eknoyan G: Serum beta-2 microglobulin levels predict mortality in dialysis patients: Results of the HEMO study. J Am Soc Nephrol 17: 546–555, 2006 [DOI] [PubMed] [Google Scholar]
- 90.Schepers E, Glorieux G, Dou L, Cerini C, Gayrard N, Louvet L, Maugard C, Preus P, Rodriguez-Ortiz M, Argiles A, Brunet P, Cohen G, Jankowski J, Jankowski V, Massy Z, Rodriguez M, Vanholder R, European Uremic Toxin Work Group (EUTox) : Guanidino compounds as cause of cardiovascular damage in chronic kidney disease: an in vitro evaluation. Blood Purif 30: 277–287, 2010 [DOI] [PubMed] [Google Scholar]
- 91.Zoungas S, McGrath BP, Branley P, Kerr PG, Muske C, Wolfe R, Atkins RC, Nicholls K, Fraenkel M, Hutchison BG, Walker R, McNeil JJ: Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: a multicenter, randomized, controlled trial. J Am Coll Cardiol 47: 1108–1116, 2006 [DOI] [PubMed] [Google Scholar]
- 92.Locatelli F, Martin-Malo A, Hannedouche T, Loureiro A, Papadimitriou M, Wizemann V, Jacobson SH, Czekalski S, Ronco C, Vanholder R, Membrane Permeability Outcome (MPO) Study Group : Effect of membrane permeability on survival of hemodialysis patients. J Am Soc Nephrol 20: 645–654, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jankowski V, Tölle M, Vanholder R, Schönfelder G, van der Giet M, Henning L, Schlüter H, Paul M, Zidek W, Jankowski J: Uridine adenosine tetraphosphate: A novel endothelium-derived vasoconstrictive factor. Nat Med 11: 223–227, 2005 [DOI] [PubMed] [Google Scholar]
- 94.Schuchardt M, Tölle M, Prüfer J, Prüfer N, Huang T, Jankowski V, Jankowski J, Zidek W, van der Giet M: Uridine adenosine tetraphosphate activation of the purinergic receptor P2Y enhances in vitro vascular calcification. Kidney Int 81: 256–265, 2012 [DOI] [PubMed] [Google Scholar]
- 95.Maduell F, Navarro V, Torregrosa E, Rius A, Dicenta F, Cruz MC, Ferrero JA: Change from three times a week on-line hemodiafiltration to short daily on-line hemodiafiltration. Kidney Int 64: 305–313, 2003 [DOI] [PubMed] [Google Scholar]
- 96.Capeillère-Blandin C, Gausson V, Nguyen AT, Descamps-Latscha B, Drüeke T, Witko-Sarsat V: Respective role of uraemic toxins and myeloperoxidase in the uraemic state. Nephrol Dial Transplant 21: 1555–1563, 2006 [DOI] [PubMed] [Google Scholar]
- 97.Lim WH, Kireta S, Leedham E, Russ GR, Coates PT: Uremia impairs monocyte and monocyte-derived dendritic cell function in hemodialysis patients. Kidney Int 72: 1138–1148, 2007 [DOI] [PubMed] [Google Scholar]
- 98.Schrijvers BF, Flyvbjerg A, De Vriese AS: The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int 65: 2003–2017, 2004 [DOI] [PubMed] [Google Scholar]
- 99.Suliman ME, Qureshi AR, Heimbürger O, Lindholm B, Stenvinkel P: Soluble adhesion molecules in end-stage renal disease: A predictor of outcome. Nephrol Dial Transplant 21: 1603–1610, 2006 [DOI] [PubMed] [Google Scholar]
- 100.Moon SJ, Kim DK, Chang JH, Kim CH, Kim HW, Park SY, Han SH, Lee JE, Yoo TH, Han DS, Kang SW: The impact of dialysis modality on skin hyperpigmentation in haemodialysis patients. Nephrol Dial Transplant 24: 2803–2809, 2009 [DOI] [PubMed] [Google Scholar]
- 101.Brandenburg VM, Schlieper G, Heussen N, Holzmann S, Busch B, Evenepoel P, Vanholder R, Meijers B, Meert N, Fassbender WJ, Floege J, Jahnen-Dechent W, Ketteler M: Serological cardiovascular and mortality risk predictors in dialysis patients receiving sevelamer: A prospective study. Nephrol Dial Transplant 25: 2672–2679, 2010 [DOI] [PubMed] [Google Scholar]
- 102.Campo A, Lanfranco G, Gramaglia L, Goia F, Cottino R, Giusto V: Could plasma cystatin C be useful as a marker of hemodialysis low molecular weight proteins removal? Nephron Clin Pract 98: c79–c82, 2004 [DOI] [PubMed] [Google Scholar]
- 103.Cohen G, Rudnicki M, Schmaldienst S, Hörl WH: Effect of dialysis on serum/plasma levels of free immunoglobulin light chains in end-stage renal disease patients. Nephrol Dial Transplant 17: 879–883, 2002 [DOI] [PubMed] [Google Scholar]
- 104.de Loor H, Bammens B, Evenepoel P, De Preter V, Verbeke K: Gas chromatographic-mass spectrometric analysis for measurement of p-cresol and its conjugated metabolites in uremic and normal serum. Clin Chem 51: 1535–1538, 2005 [DOI] [PubMed] [Google Scholar]
- 105.Martinez AW, Recht NS, Hostetter TH, Meyer TW: Removal of P-cresol sulfate by hemodialysis. J Am Soc Nephrol 16: 3430–3436, 2005 [DOI] [PubMed] [Google Scholar]
- 106.Vanholder R, Bammens B, de Loor H, Glorieux G, Meijers B, Schepers E, Massy Z, Evenepoel P: Warning: The unfortunate end of p-cresol as a uraemic toxin. Nephrol Dial Transplant 26: 1464–1467, 2011 [DOI] [PubMed] [Google Scholar]
- 107.Kerr PB, Argilés A, Flavier JL, Canaud B, Mion CM: Comparison of hemodialysis and hemodiafiltration: A long-term longitudinal study. Kidney Int 41: 1035–1040, 1992 [DOI] [PubMed] [Google Scholar]
- 108.Horowitz JD, Heresztyn T: An overview of plasma concentrations of asymmetric dimethylarginine (ADMA) in health and disease and in clinical studies: Methodological considerations. J Chromatogr B Analyt Technol Biomed Life Sci 851: 42–50, 2007 [DOI] [PubMed] [Google Scholar]
- 109.Meert N, Waterloos M-A, Van Landschoot M, Dhondt A, Ledebo I, Glorieux G, Goeman J, Van der Eycken J, Vanholder R: Prospective evaluation of the change of predialysis protein-bound uremic solute concentration with postdilution online hemodiafiltration. Artif Organs 34: 580–585, 2010 [DOI] [PubMed] [Google Scholar]
- 110.Tukey JW: Exploratory Data Analysis, Reading, MA, Addison-Wesley, 1977 [Google Scholar]