Abstract
Eculizumab might benefit C3 glomerulopathies mediated by dysregulation of the alternative complement pathway. Here, we report renal biopsy findings before and after eculizumab therapy in three patients with dense deposit disease and two with C3 GN. All pretreatment biopsies had glomerular and tubular basement membrane deposits that stained exclusively for C3 without significant Ig. After 1 year of therapy, there was reduction in active glomerular proliferation and neutrophil infiltration in three of five patients, consistent with effective C5 blockade, which prevents production of chemotactin C5a. One individual with mild mesangial disease had no significant change in activity or chronicity. One patient exhibited persistent activity and worsening chronicity despite therapy. Immunofluorescence showed no significant reduction in C3 or C5b-9, and electron microscopy revealed persistent deposits in all cases, suggesting a long t1/2 of C5b-9 in extracellular matrix. Normal renal biopsies stained positive for C5b-9 in glomeruli, tubular basement membranes, and vessel walls, albeit at lower intensity than in C3 glomerulopathy. This indication of physiologic levels of C5b-9 activation in normal kidney potentially explains the localization of deposits in patients with dysregulation of the alternative complement pathway. All post-treatment biopsies showed de novo monoclonal staining for IgG-κ in the same distribution as C3 and C5b-9, mimicking monoclonal Ig deposition disease (MIDD). Staining of the γ heavy chain was restricted to the IgG2 and IgG4 subclasses, suggesting the binding of monoclonal eculizumab to C5 in renal tissues. The long-term effects of this apparent drug-tissue interaction are unknown.
Disorders of alternative complement pathway activation are increasingly recognized as causes of GN. The term C3 glomerulopathy has been proposed for the group of glomerular disorders mediated by dysregulation of the alternative complement pathway.1 By definition, C3 glomerulopathies are characterized by prominent glomerular C3 deposition in the absence of significant Ig deposits. Among these conditions, dense deposit disease (DDD) (formerly called membranoproliferative GN type II) and C3 GN (C3GN) are the best characterized. Both entities exhibit a range of histologic appearances from mild mesangial proliferative to endocapillary, membranoproliferative, and even extracapillary proliferative GN. Classically, DDD shows distinctive highly electron dense deposits within glomerular basement membranes, mesangial matrix, Bowman’s capsule, and tubular basement membranes, forming sausage-shaped or ring forms.2 C3GN characteristically shows moderately electron dense deposits in subendothelial, intramembranous, and mesangial locations. C3GN often displays the histologic pattern of type 1 membranoproliferative GN but stains only for C3.3 Other examples have features of membranoproliferative GN type 3 of Strife and Anders.4
There is currently no proven effective therapy for the C3 glomerulopathies, but targeted inhibition of complement is a rational approach based on the common underlying pathomechanisms of alternative complement pathway dysregulation, the identification of C5, C6, C7, C8, and C9 by mass spectrometry in the glomerular deposits,5 and the demonstrated amelioration of experimental models of C3GN by prevention of C5 activation.6 Identified causes of human C3 glomerulopathy include mutations in inhibitors of the alternative pathway, including factor H, factor I, CD46 (membrane cofactor protein), and the factor-H–related protein family (CFHR1, 2, 3, 4, and 5) or the development of antibodies that activate the alternative pathway either by stabilizing C3 convertase (such as C3 nephritic factor) or blocking the action of pathway inhibitors (such as anti-factor H autoantibody).3,7,8
From a therapeutic standpoint, inhibition of complement at the level of C3 convertase is predicted to have significant potential adverse clinical effects because of the critical role of C3b in innate immunity and clearance of circulating immune complexes.9 Targeting downstream complement component C5 has the advantage of preserving C3b generation and reducing infectious complications, with the exception of a reportedly increased risk of Neisserial infection.10
Monoclonal murine antibodies directed at human C5 were developed and screened for their ability to block activity of terminal complement components in a standard hemolytic assay. Murine antibody 5G1.1 was found to effectively prevent generation of chemotactin C5a and the formation of the membrane attack complex (C5b-9) and was successfully humanized, laying the groundwork for development of a clinically useful terminal complement pathway inhibitor.11 The US Food and Drug Administration approved eculizumab for treatment of paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS). Both PNH12 and aHUS13 are disorders of complement regulation. Based on their similar pathogenesis, C3GN and DDD were proposed as conditions that might benefit from eculizumab therapy. Theoretically, successful blockade of C5 in these glomerulopathies should both prevent C5a formation, resulting in less neutrophil and leukocyte infiltration, and inhibit formation of the membrane attack complex.
Results
Pretreatment and post-treatment biopsies were available for five of the six patients enrolled in the trial. Detailed clinical data are presented in a separate manuscript.14 All of the patients were Caucasian males ranging from 20 to 42 years of age. Three patients (DDD3, C3GN2, and C3GN3) had undergone renal transplantation for ESRD and developed recurrent disease in the allograft. Baseline serum creatinines ranged from 1.2 to 2.0 mg/dl. Three of the patients (DDD3, C3GN1, and C3GN2) were enrolled for proteinuria with urine protein/creatinine ratios (UPCRs) ranging from 2.6 to 4.5 g/g. Patients DDD1 and C3GN3 were enrolled for ARF with creatinines of 2.0 and 1.7 mg/dl, respectively, both with a UPCR <1. After commencing treatment with eculizumab, there was evidence of effective and sustained terminal complement inhibition in all patients. Total complement (CH50) levels declined to 0–1 CAE units by week 4 for all but one patient (DDD3), whose level declined to 4 CAE units by week 8.14
Patient DDD1 experienced improvement in serum creatinine after a year of eculizumab treatment from baseline of 1.8–2.0 mg/dl to final creatinine of 1.3–1.4 mg/dl, with low levels of proteinuria throughout. His pretreatment biopsy showed that he had moderate mesangial proliferation, segmentally prominent endocapillary proliferation with leukocyte infiltration, and focal membranoproliferative features. Biopsy after eculizumab treatment showed persistent mesangial proliferation but resolution of the endocapillary proliferation and leukocyte infiltration (Figure 1). The degree of glomerulosclerosis and tubulointerstitial scarring remained constant with approximately 20% global glomerulosclerosis and 15% tubulointerstitial scarring (Table 1). Immunofluorescence staining patterns and intensity for C3 and C5b-9 were similar in the pretreatment and post-treatment biopsies, showing no significant diminution in staining. Although there was no evidence of Ig or light chain staining in the pretreatment biopsy, after treatment with eculizumab there was strong (2–3+) staining for IgG and κ light chain within glomeruli, tubular basement membranes, and vessel walls (similar in distribution to C3 and C5b-9), with no corresponding staining for λ light chain (representative staining shown in Figure 2). IgG subtype staining revealed 2–3+ positivity for IgG4, 1+ positivity for IgG2, and no staining for IgG1 or IgG3 (Figure 3). This new and unexpected staining pattern for IgG-κ with restricted IgG subtypes was identified in all of the post-treatment biopsies (Table 2). Electron microscopy showed electron dense deposits in glomerular mesangial, subendothelial, and intramembranous distributions, as well as tubular basement membranes (TBMs) and Bowman’s capsule. The post-treatment biopsy showed a mild decrease in the number and electron density of deposits in the mesangial and intramembranous regions (Figure 1D), whereas TBMs showed an increase in electron dense deposits (Table 3 and Supplemental Figure 1). After cessation of therapy, the patient experienced a progressive rise in serum creatinine to 1.7 mg/dl and was restarted on eculizumab 8 weeks after finishing the course of therapy.
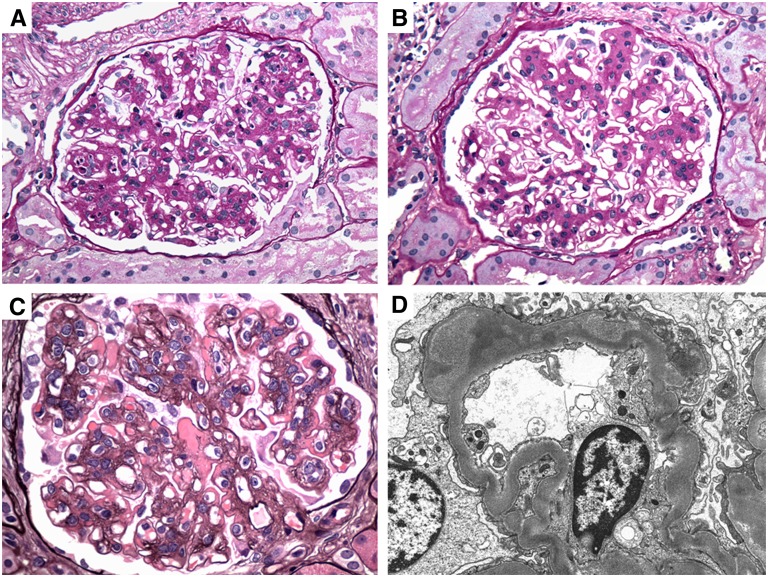
Figure 1.
Representative pretreatment and post-treatment histologic and ultrastructural findings in C3 glomerulpathy. (A) Renal biopsies from patient DDD1 show segmental endocapillary proliferation and leukocyte infiltration on a base of mesangial proliferation and sclerosis in the pretreatment biopsy (periodic acid–Schiff). (B) After treatment, there is resolution of the active features of endocapillary proliferation and leukocyte exudation, but with persistent mesangial proliferation (periodic acid–Schiff). (C) Patient C3GN1, by contrast, has persistent membranoproliferative features with large subendothelial deposits post-treatment (Jones methenamine silver). (D) Patient DDD1 exhibits a mild decrease in the electron density of the intramembranous deposits post-treatment (electron micrograph). Original magnification, ×600 in A through C; ×10,000 in D.
Table 1.
Light microscopy findings before and after eculizumab treatment
| DDD1 | DDD3 | C3GN1 | C3GN2 | C3GN3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | Before | After | |
| Predominant pattern | M/ECP | MP | MP | MP | MPGN | MPGN | MPGN | MPGN | MP | MP |
| Glomeruli (n) | 9 | 27 | 10 | 11 | 12 | 27 | 26 | 28 | 14 | 10 |
| Global sclerosis (%) | 22 | 19 | 30 | 27 | 50 | 85 | 0 | 9 | 0 | 0 |
| Segmental sclerosis (%) | 0 | 0 | 0 | 18 | 8.3 | 3.7 | 0 | 0 | 0 | 0 |
| Mesangial proliferation (0–3) | 2 | 2 | 2 | 1 | 3 | 3 | 3 | 2 | 1 | 1 |
| Membranoproliferative features (0–3) | 1 | 0 | 0 | 1 | 3 | 3 | 1 | 1 | 0 | 0 |
| Leukocyte infiltration (0–3) | 2 | 0 | 1 | 0 | 1 | 1 | 2 | 1 | 0 | 0 |
| Endocapillary proliferation (0–3) | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 |
| Crescents (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TBM deposits (0–3) | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 1 |
| Tubulointerstitial scarring (%) | 15 | 15 | 5 | 5 | 40 | 65 | 5 | 30 | 0 | 5 |
M, mesangial; M/ECP, mesangial and endocapillary proliferative GN; MP, mesangial proliferative GN; MPGN, membranoproliferative GN.
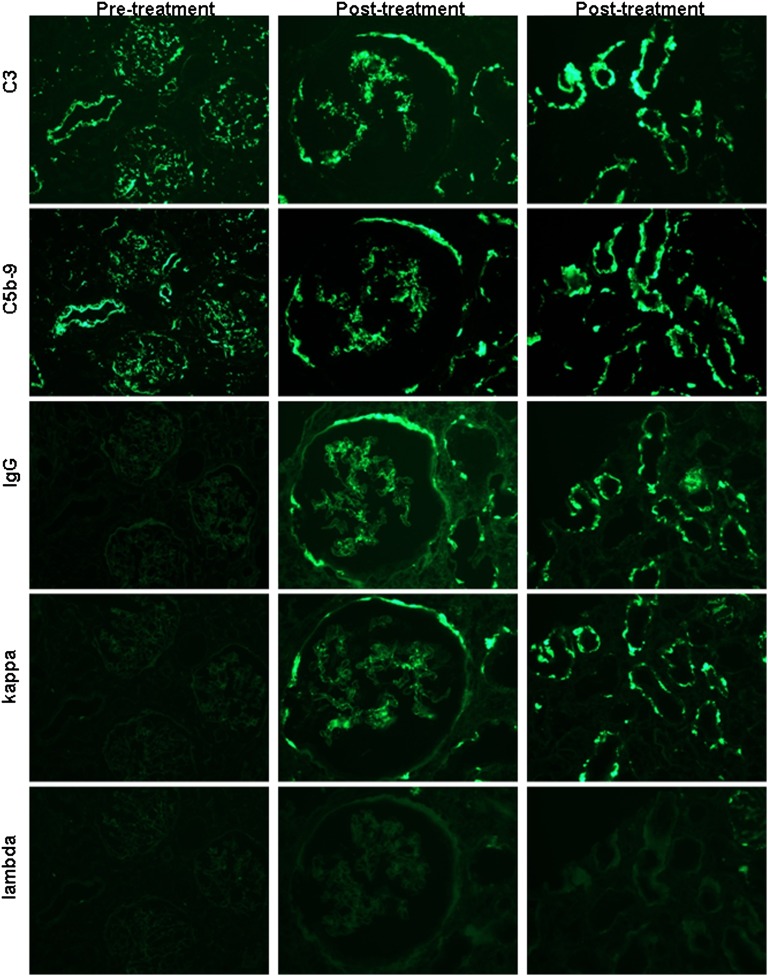
Figure 2.
A representative panel of serial sections from patient C3GN3 illustrates the evolution of staining for C3, C5b-9, IgG, κ light chain, and λ light chain in pretreatment and post-treatment biopsies. Column 1 shows pretreatment findings in a low-power (×200) view of three glomeruli and an interlobular artery, which demonstrate positivity for C3 and C5b-9, but no staining for IgG or either light chain. Columns 2 (×400; glomerulus) and 3 (×600; tubules) illustrate post-treatment biopsy findings, with the persistence of C3 and C5b-9 in glomerular tufts, Bowman’s capsules, and TBMs, as well as the new appearance of positivity for IgG and κ (with negative λ) in the same distribution.
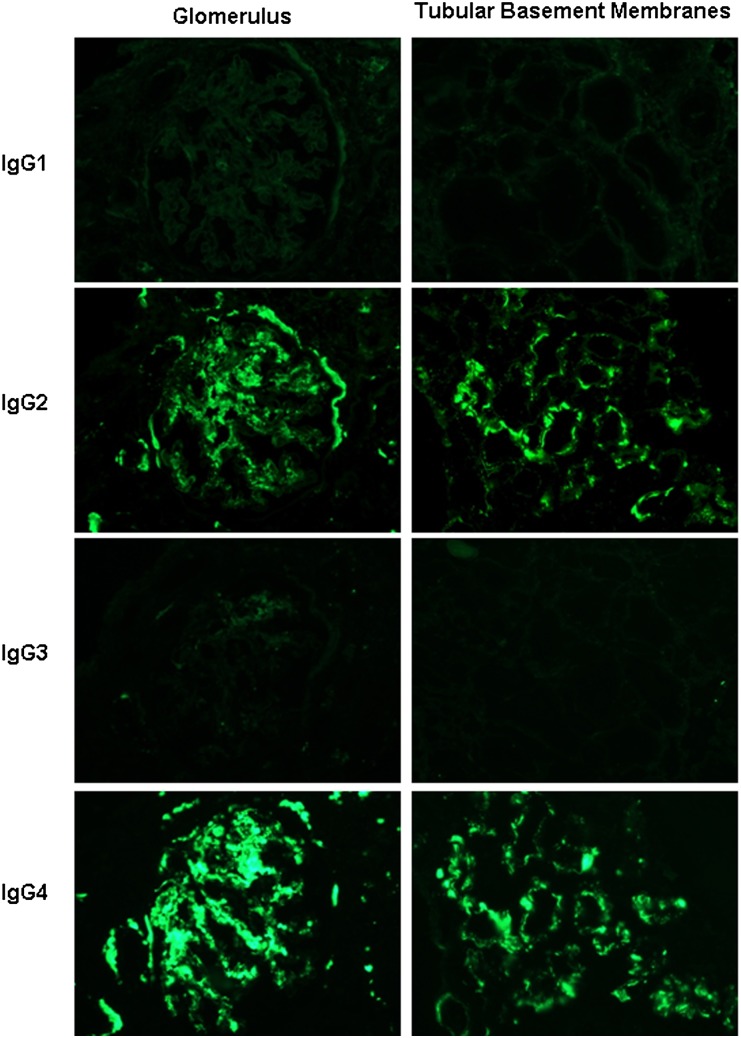
Figure 3.
Immunofluorescence staining in serial sections for the IgG subtypes in the post-treatment biopsy of patient DDD1 shown for glomeruli (×600; column 1) and tubules (×400; column 2) reveals positivity for IgG2 and IgG4, with negativity for IgG1 and IgG3 in the distribution of the IgG-κ deposits. Similar results were seen in all of the post-treatment biopsies examined.
Table 2.
Immunofluorescence findings before and after eculizumab treatment
| DDD1 | DDD3 | C3GN1 | C3GN2 | C3GN3 | aHUS | Normal Controls | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | ||
| C3 | |||||||||||||
| glomeruli | 3+ G M/CW/BC | 3+ G M/S CW | 2+ G M/BC | 1–2+ S M/CW/BC | 3+ G M/CW/BC | 3+ G M/CW | 3+ G M | 3+ G M | 3+ G M/CW | 3+ G M/CW/BC | 2–3+ G M/CW | 3+ G M/CW | ± to 1+ S M |
| TBMs | 3+ in 80% | 3+ in 50% | 1+ in 15% | 2+ in 50% | 2+ in 30% | 2+ in 30% | neg | neg | 3+ in 30% | 3+ in 50% | 2–3+ patchy | 3+ diffuse | 1+ rare |
| vessels | 1+ | 2+ | 3+ | 2+ | 1+ | 1+ | neg | neg | 3+ | 1+ | 3+ | 3+ | 1+ |
| C5b-9 | |||||||||||||
| glomeruli | 3+ G M/CW/BC | 3+ G M/S CW | 3+ G M/BC | 2–3+ S M/CW/BC | 3+ G M/CW/BC | 2+ G M/CW/BC | N/A | N/A | 3+ G M/CW | 3+ G M/CW/BC | 3+ G M/CW | 3+ G M/CW | 1–2+ G M |
| TBMs | 3+ in 90% | 3+ in 60% | 2–3+ in 70% | 2–3+ in 50% | 3+ in 100% | 2+ in 80% | N/A | N/A | 3+ in 70% | 3+ in 80% | 3+ diffuse | 3+diffuse | 2+ patchy to diffuse |
| vessels | 2+ | 1+ | 3+ | 3+ | 2+ | 2+ | N/A | N/A | 3+ | 3+ | 3+ | 3+ | 3+ |
| IgG | |||||||||||||
| glomeruli | neg | 2+ G M/S CW | ± sparse CW | 1–2+ S M/CW | neg | 2+ G M/CW/BC | neg | 2+ G M/CW | neg | 2+ S M/CW | neg | 1–2+G M/CW | neg |
| TBMs | neg | 3+ in 25% | neg | 2+ in 10% | neg | 2+ in 30% | neg | 1+ patchy | neg | 2+ patchy | neg | 2+ patchy | neg |
| vessels | neg | 3+ | neg | 3+ | neg | neg | neg | neg | neg | 1+ | neg | 2+ | neg |
| κ | |||||||||||||
| glomeruli | neg | 2+ G M/S CW | ± sparse CW | 1–2+ S M/CW | neg | 2+ G M/CW/BC | neg | 2+ G M/CW | neg | 2+ S M/CW/BC | neg | 1–2+ G M/CW | neg |
| TBMs | neg | 3+ in 25% | neg | 2+ in 10% | neg | 2+ in 40% | neg | 1+ patchy | neg | 2+ patchy | neg | 2+ patchy | neg |
| vessels | neg | 3+ | neg | 3+ | neg | neg | neg | neg | neg | 1+ | neg | 2+ | neg |
| λ | |||||||||||||
| glomeruli | neg | neg | ± sparse CW | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg |
| TBMs | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg |
| vessels | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg |
| Glomeruli sampled (n) | 2 | 7 | 2 | 5 | 3 | 3 | 11 | 5 | 11 | 4 | 7 | 9 | 10 |
The staining intensity was graded on a scale from 0 to 3+. G, global; M, mesangial; CW, glomerular capillary wall; BC, Bowman's capsule; S, segmental; neg, negative; N/A, not applicable.
Table 3.
Electron microscopy findings before and after eculizumab treatment
| Deposits (S/G, 0–3+) | DDD1 | DDD3 | C3GN1 | C3GN2 | C3GN3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | Before | After | |
| Mesangial | G 3+ | G 2+ | G 2+ | S 1+ | G 2+ | G 2+ | G 3+ | G 3+a | G 2+ | G 2+ |
| Subendothelial | 0 | 0 | 0 | 0 | S 2+ | G 3+a | G 3+ | G 3+a | S 1+ | S 1+ |
| Subepithelial | 0 | 0 | 0 | 0 | S 1+ | 0 | 0 | 0 | S 1+ | S 1+ |
| Intramembranous | G 3+ | G 2+ | S 1+ | S 1+ | S 2+ | S 1+ | 0 | S 1+a | S 1+ | S 1+ |
| TBM | S 2+ | S 3+ | S 2+ | S 2+ | S 1+ | S 1+ | S 2+ | S 2+ | S 1+ | S 2+ |
| Bowman's capsule | S 2+ | S 2+ | S 1+ | S 1+ | 0 | 0 | 0 | 0 | 0 | 0 |
| Vessel wall | 0 | 0 | 0 | 0 | 0 | S 1+a | 0 | 0 | 0 | 0 |
| Podocyte effacement (%) | 80 | 50 | 20 | 30 | 80 | 80 | 20 | 75 | 20 | 10 |
| Glomeruli (n) | 4 | 5 | 3 | 3 | 6 | 4 | 3 | 4 | 5 | 6 |
S, segmental; G, global.
Some of these deposits had a distinctly punctate, finely granular appearance, different from the deposits seen in the pretreatment biopsies. These granular deposits may represent deposition of the mAb, eculizumab.
Patient DDD3 had recurrent disease diagnosed in his renal allograft 20 months after living related renal transplant. He experienced a decrease in proteinuria from a peak of 10.58 g/g to <0.3 g/g after 1 year of therapy. Light microscopy showed a mild decrease in mesangial proliferation and resolution of neutrophil infiltration. Although mild segmental sclerosing features and focal membranoproliferative features had developed in the post-treatment biopsy, tubulointerstitial scarring remained stable at 5% (Table 1). The pattern and intensity of immunofluorescence staining for C3 and C5b-9 were comparable in the pretreatment and post-treatment biopsies. The post-treatment biopsy was notable for similar deposition of IgG, κ light chain, and IgG2 and IgG4 subtypes as described above for DDD1. Electron microscopy showed a decrease in mesangial dense deposits. Laboratory values 4 weeks after cessation of eculizumab therapy remain unchanged.
Patient C3GN1 did not show clinical evidence of a response to eculizumab therapy. The pretreatment biopsy was notable for significant disease chronicity with 50% global glomerulosclerosis and 40% tubulointerstitial scarring. In both the pretreatment and post-treatment biopsies, there was prominent mesangial proliferation with membranoproliferative features (Figure 1C). Despite treatment, fibrosis increased to 65% and glomerulosclerosis increased to 85% (Table 1). Immunofluorescence showed similar levels and patterns of C3 and C5b-9 staining. Again the development of IgG-κ staining with restricted γ subtypes was noted in the post-treatment biopsy (Table 2). Electron microscopy (Table 3) showed an apparent increase in subendothelial deposits, some of which had a punctate powdery texture similar to that seen in monoclonal Ig deposition disease (Figure 4). These powdery deposits were also noted in the intimal and medial basement membranes of small arteries in the post-treatment biopsy (Supplemental Figure 2).
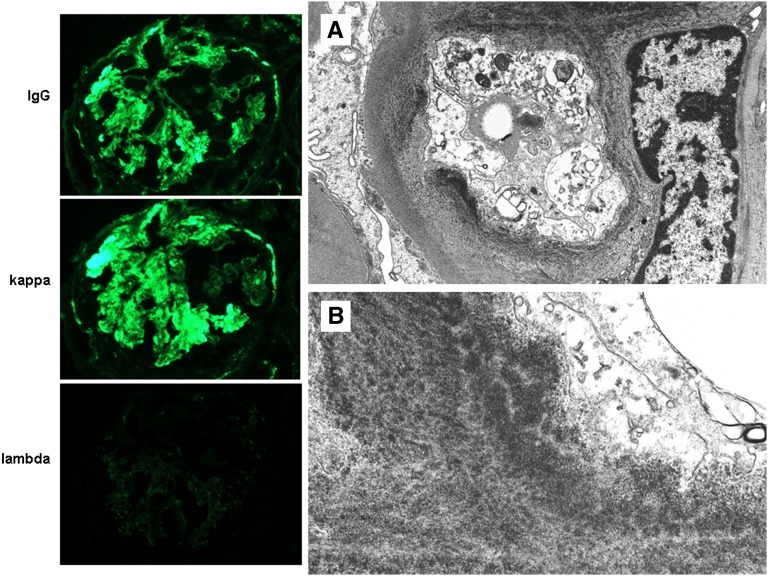
Figure 4.
Representative images of the post-treatment biopsy from patient C3GN1 illustrate strong glomerular staining for IgG and κ light chain, with negativity for λ light chain (column 1; ×600). By electron microscopy, the electron dense subendothelial deposits exhibited an unusual punctate highly electron dense powdery texture, resembling those seen in MIDD. This transformation in the appearance of the deposits post-therapy may represent binding of eculizumab to existing deposits. Original magnification, ×15,000 in A; 40,000 in B.
Patient C3GN2 had recurrent C3GN diagnosed in the renal allograft at 4 months post-transplant. He began eculizumab therapy 8 months later with elevated creatinine (1.7–1.9 mg/dl) and a UPCR of 4.4 g/g. His creatinine and proteinuria remained stable over the 1 year of therapy. The pretreatment biopsy showed prominent mesangial and endocapillary proliferation with abundant leukocyte infiltration. Post-treatment biopsy showed a decrease in both the endocapillary proliferation and leukocyte infiltration with a mild increase in chronic glomerular scarring and interstitial fibrosis (Table 1). Immunofluorescence revealed similar C3 staining in the pretreatment and post-treatment biopsies and again showed the development of IgG-κ staining with γ subtype restriction after eculizumab treatment. Electron microscopy showed a similar degree of deposit formation with the added finding of powdery subendothelial and mesangial deposits reminiscent of monoclonal Ig deposition disease, similar to those seen in patient C3GN1. Seven weeks after his last dose of eculizumab, the patient’s creatinine had increased to 8.4 mg/dl, requiring initiation of dialysis. Repeat biopsy after recrudescence showed a marked increase in glomerular endocapillary proliferation and neutrophil infiltration and the new development of crescents, consistent with reactivation. Notably, this is the only biopsy in the series that demonstrated crescent formation. He was restarted on eculizumab, and is showing ongoing improvement with the latest creatinine value of 3.3 mg/dl.
Patient C3GN3 had recurrent disease diagnosed in his allograft 1 month after transplantation. He began eculizumab 3 months post-transplant with a creatinine of 1.8 mg/dl and no proteinuria. Creatinine decreased to 1.4 mg/dl by the end of therapy. Both the pretreatment and post-treatment biopsies showed mild mesangial proliferation with no evidence of endocapillary proliferation or exudative features, and minimal chronic scarring (Table 1). Immunofluorescence and electron microscopy findings (Tables 2 and 3) were similar between the two biopsies, with the exception of the development of IgG-κ staining with restricted subtypes in the post-treatment biopsy. Follow-up laboratory parameters 4 weeks after completing therapy remain stable.
Discussion
We present a detailed account of the pathologic findings in patients with DDD and C3GN before and after treatment with C5 inhibitor eculizumab. The clinical responses of the patients are provided in a companion manuscript.14 Clinical responses were variable with three patients showing evidence of clinical improvement either in the form of reduced serum creatinine (patients DDD1 and C3GN3) or remission of nephrotic syndrome (patient DDD3). Corresponding improvement in histology was observed in two of these patients, with repeat biopsy in patient DDD1 showing a decrease in leukocyte infiltration, endocapillary proliferation and membranoproliferative features, and patient DDD3 showing decreased leukocyte infiltration and mesangial proliferation. Histologic findings in patient C3GN3 were very mild on the pretreatment biopsy and remained unchanged, although he showed clinical improvement. Patient C3GN2 showed neither clinical improvement nor deterioration while taking eculizumab, but histology revealed a significant reduction in proliferative activity within glomeruli. Notably, 1 month after discontinuing eculizumab, this patient developed a severe reactivation of C3GN, which appeared to respond to reinstitution of eculizumab therapy. Patient C3GN1 had a slow clinical deterioration despite therapy, and this was paralleled by persistent histologic activity and a marked increase in chronic scarring on post-treatment biopsy.
All post-treatment biopsies showed unexpected immunofluorescence findings. In contrast to the improvement in histologic activity observed post-treatment in three patients, the staining for C3 and C5b-9 appeared largely unchanged in the pretreatment and post-treatment biopsies. Whereas C3 is upstream of eculizumab and therefore would not be expected to be affected by therapy, eculizumab should inhibit formation of new C5b-9. Thus, the unchanged staining for C5b-9 observed post-treatment may be due in part to its long t1/2 when bound to extracellular matrix, reflecting pretreatment levels of deposition.
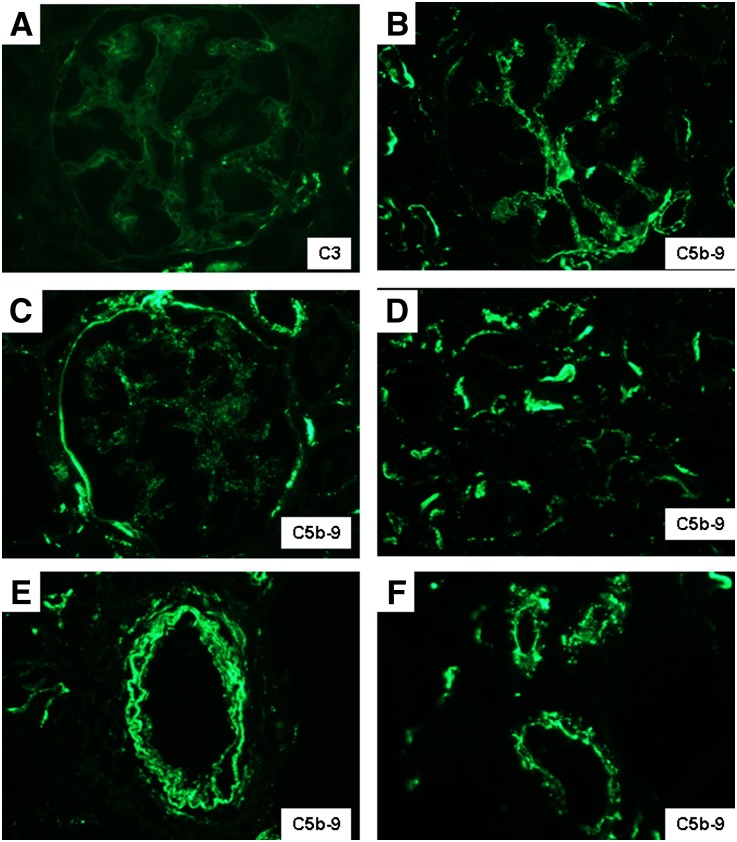
Notably, normal kidney biopsies (Table 2) showed mild to moderate (1–2+) C5b-9 staining within glomerular mesangium and some glomerular capillary walls and Bowman’s capsules, with moderate patchy to diffuse staining within TBMs and prominent arterial and arteriolar wall staining, despite the absence of any light microscopic or ultrastructural abnormalities (Figure 5). These findings suggest that C5b-9 is deposited extracellularly in normal kidney tissue, probably in the course of fluid fluxes across membranes of the glomerulus, tubules, and blood vessels, without causing histologic abnormalities or electron dense deposits. The intensity and quantity of C5b-9 exceeded the sparse staining for C3 typically observed in normal biopsies (Figure 5A), supporting the concept of low level activation of C3 in aqueous environments by the so-called “tick-over” mechanism, which leads to C5 recruitment in the fluid phase.15 These physiologic levels of C5b-9 activation in the course of normal glomerular filtration and tubular function could explain the propensity for deposits to form in glomeruli and TBMs in patients with complement regulatory disorders.
Figure 5.
Representative distribution of C5b-9 in normal human renal biopsies is illustrated. (A) In contrast to the weak and sparse mesangial staining for C3, (B) the identical field stained for C5b-9 reveals stronger mesangial positivity, in addition to staining of the preglomerular arteriolar wall. Focal staining of (C) Bowman’s capsule and (D) TBMs and diffuse staining of (E) arterial walls and (F) arteriolar walls are also observed. Original magnification, ×600 in A through C and F; 400 in D and E.
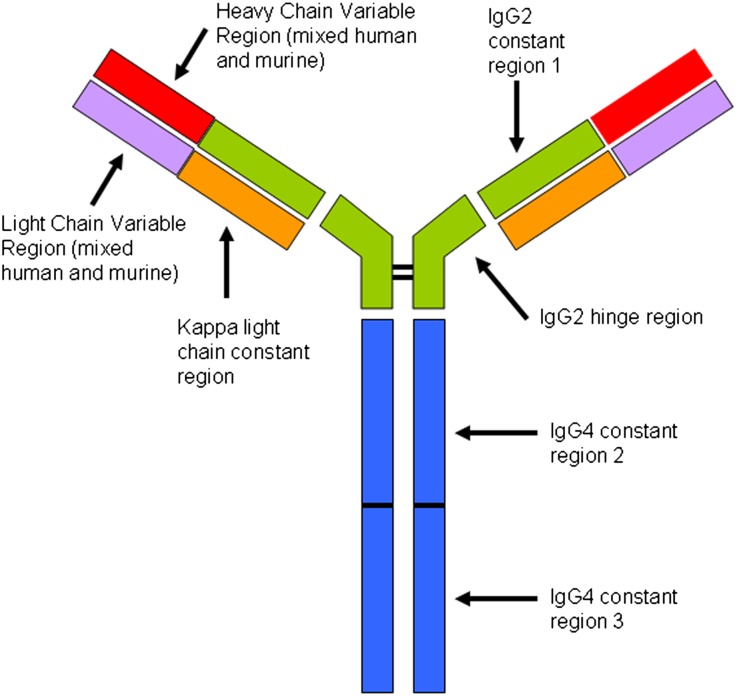
Immunofluorescence also revealed a unique pattern of staining for Ig. By definition, patients with C3GN and DDD do not have significant staining for any Ig heavy chains or light chains. Interestingly, after treatment with eculizumab, all patients showed variable degrees of glomerular, tubular basement membrane, and vessel wall staining for IgG and κ light chain, with absence of all other heavy chain classes and λ light chain. The presence of κ restriction suggested a monoclonal process. We next performed IgG subtype staining, which revealed 1+ positivity for IgG2 and 2–3+ staining for IgG4. Because this finding was restricted to post-treatment biopsies, we considered the possibility that it represented tissue binding of the humanized eculizumab mAb itself. Eculizumab has been engineered to target C5 while minimizing its own potential to elicit an inflammatory response. Accordingly, the C5 specific variable regions were fused to a κ light chain and a hybrid heavy chain created from portions of IgG2 and IgG4 (Figure 6). The IgG2 constant region 1 and hinge region were chosen because they do not bind Fc receptors or activate complement. IgG4 constant regions 2 and 3 were selected for their inability to activate complement.15 The presence of staining exclusively for IgG2, IgG4, and κ are exactly what would be detected if eculizumab were being deposited in tissues, which we propose is the explanation for our findings.
Figure 6.
Structural features of eculizumab. Adapted from Rother et.al.15 Constant regions CH2 and CH3 from IgG4 are fused to the IgG2 hinge region and CH1 domain and then paired with a κ light chain. The variable regions of the light and heavy chains are composed of the murine-derived sequences that have high affinity for C5, admixed with human germline framework regions. The result is a hybrid Ig with high affinity for C5 but without the ability to activate complement or bind Fc receptors. Post-treatment biopsies show evidence of eculizumab binding to glomeruli, TBMs, and vessel walls in the form of immunofluorescence staining for IgG2, IgG4, and κ light chain, with negative staining for IgG1, IgG3, and λ light chain.
We also tested pretreatment and post-treatment biopsies on a patient with aHUS who received eculizumab. Despite the absence of glomerular electron dense deposits, this patient also developed IgG-κ deposition in a distribution similar to that seen in the DDD and C3GN patients.
The distribution and characteristics of the IgG-κ binding are similar to what is seen in MIDD due to dysproteinemia.16 Two of the post-treatment biopsies (C3GN1 and C3GN2) also displayed the finely granular, powdery type of electron dense deposits seen in MIDD, again suggesting an effect of eculizumab binding to C5 deposits in glomeruli, TBMs, and vessel walls. The clinical significance of the binding of eculizumab to renal tissue is unclear. Long-term eculizumab use has been studied in patients with PNH and there is no evidence of the development of proteinuria and renal insufficiency that is typically seen in MIDD.17 Nonetheless, the same design features of eculizumab that make it anti-inflammatory could also inhibit its clearance from sites of tissue deposition by blocking the usual routes of elimination via the Fc-γ receptor and complement receptor bearing leukocytes, thereby promoting its accumulation in tissues over the course of prolonged therapy.
In conclusion, eculizumab therapy in patients with C3GN and DDD is associated with reduced glomerular endocapillary proliferation and neutrophil infiltration in the majority of patients studied, particularly those who manifest other clinical evidence of response. The decrease in neutrophil infiltration may serve as a useful marker of histologic response in future studies. Importantly, treatment does not appear to be associated with a significant diminution in staining for C3 or C5b-9 or a resolution of glomerular electron dense deposits after 1 year of therapy. Eculizumab appears to bind to and deposit within glomeruli, TBMs, and vessel walls of treated patients, and the resulting distinctive immunofluorescence findings should not be misinterpreted as a transformation to immune complex-mediated GN or MIDD. The long-term clinical significance of this drug-tissue interaction is unclear and requires further study.
Concise Methods
Six adult participants, three with DDD and three with C3GN, were enrolled in an open-label, nonblinded, proof-of-concept, efficacy, and safety study of eculizumab (Soliris; Alexion Pharmaceuticals, Cheshire, CT). Inclusion and exclusion criteria are detailed in a separate clinical manuscript.14 Chief inclusion criteria included a pretreatment renal biopsy performed within 6 months of enrollment with diagnosis of either DDD or C3GN and the presence of proteinuria (>1 g/d or 24-hour urine collection) or ARF (defined as a ≥50% rise in serum creatinine from baseline). Participants received 900 mg of eculizumab intravenously once weekly for 4 weeks, followed by 1200 mg intravenously on week 5 and then 1200 mg intravenously every other week up to week 53. This study was approved by the Institutional Review Board of Columbia University Medical Center and is registered with ClinicalTrials.gov (NCT01221181).
Laboratory measurements were performed monthly during the study period and are detailed in the companion clinical manuscript.14 Primary endpoints were a reduction in urine protein excretion for those participants enrolled for proteinuria and a reduction in serum creatinine for those enrolled for ARF. Secondary endpoints included histopathologic changes in a biopsy performed after 1 year of treatment, which are detailed in this manuscript. Data are presented descriptively, because the small number of participants and study design preclude formal statistical analysis.
Biopsies both before and after treatment with eculizumab on four of the enrolled patients (DDD1, DDD3, C3GN1, and C3GN3) were processed at the Renal Pathology Laboratory of Columbia University. One patient had his pretreatment and two post-treatment biopsies processed at Massachusetts General Hospital (C3GN2). One patient (DDD2) refused post-treatment biopsy and is not evaluated in this manuscript. Renal biopsy samples were processed using standard techniques for light microscopy, immunofluorescence, and electron microscopy. For light microscopy, multiple serial paraffin sections were stained with hematoxylin and eosin, periodic acid–Schiff, Masson trichrome, and Jones methenamine silver. Standard immunofluorescence was performed on 3-µm cryostat sections using polyclonal FITC-conjugated antibodies to IgG, IgM, IgA, C3, C1q, κ, and λ (Dako, Carpinteria, CA). Immunofluorescence staining for the IgG subtypes (IgG1, IgG2, IgG3, and IgG4) was performed using FITC-conjugated polyclonal sheep antibodies (The Binding Site, Birmingham, UK). Staining for C5b-9 was performed by indirect immunofluorescence using a monoclonal mouse anti-human antibody (clone aE11) followed by FITC-conjugated rabbit anti-mouse secondary antibody (F0261) (Dako). Ultrastructural evaluation was performed according to standard transmission electron microscopic techniques. Six normal renal biopsies were used as negative controls. Biopsies with primary membranous glomerulopathy and active lupus nephritis served as positive controls for C5b-9 staining. Findings by light microscopy, immunofluorescence, and electron microscopy were scored by group consensus of three renal pathologists (L.C.H., G.S.M., and V.D.D.) for severity, intensity, and distribution on a semiquantitative scale (0–3+). Specifically, for light microscopy, the 0–3+ scale was based on the proportion of glomeruli or percentage of cortical area displaying the described lesion with 1%–25% involvement described as 1+, 26%–50% involvement as 2+, and >50% involvement as 3+. For immunofluorescence, the 0–3+ scale refers to intensity of the staining (negative, mild, moderate, or marked), whereas distribution is separately described as segmental/patchy (involving <50% of the glomerulus or tubulointerstitial area) or global/diffuse (involving ≥50% of the glomerulus or tubulointerstitial area). For electron microscopy, the distribution (segmental or global) was specified as described above, and the prominence of the deposits was graded 0–3+. The pathologists were not blinded to whether biopsies were pretreatment or post-treatment.
Disclosures
R.B.C. and G.B.A. have served as consultants to Alexion.
Acknowledgments
The clinical study was supported by Alexion Pharmaceuticals, manufacturer of Soliris (eculizumab); however, the pathology specimens were processed independently, without any financial support from Alexion.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011121186/-/DCSupplemental.
References
- 1.Fakhouri F, Frémeaux-Bacchi V, Noël LH, Cook HT, Pickering MC: C3 glomerulopathy: A new classification. Nat Rev Nephrol 6: 494–499, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Nasr SH, Valeri AM, Appel GB, Sherwinter J, Stokes MB, Said SM, Markowitz GS, D’Agati VD: Dense deposit disease: Clinicopathologic study of 32 pediatric and adult patients. Clin J Am Soc Nephrol 4: 22–32, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Servais A, Frémeaux-Bacchi V, Lequintrec M, Salomon R, Blouin J, Knebelmann B, Grünfeld JP, Lesavre P, Noël LH, Fakhouri F: Primary glomerulonephritis with isolated C3 deposits: A new entity which shares common genetic risk factors with haemolytic uraemic syndrome. J Med Genet 44: 193–199, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Agati VD, Jennette JC, Silva FG: Non-Neoplastic Kidney Diseases, Washington, DC, American Registry of Pathology, 2005, pp 250–255 [Google Scholar]
- 5.Sethi S, Gamez JD, Vrana JA, Theis JD, Bergen HR, 3rd, Zipfel PF, Dogan A, Smith RJ: Glomeruli of dense deposit disease contain components of the alternative and terminal complement pathway. Kidney Int 75: 952–960, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pickering MC, Warren J, Rose KL, Carlucci F, Wang Y, Walport MJ, Cook HT, Botto M: Prevention of C5 activation ameliorates spontaneous and experimental glomerulonephritis in factor H-deficient mice. Proc Natl Acad Sci USA 103: 9649–9654, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Appel GB, Cook HT, Hageman G, Jennette JC, Kashgarian M, Kirschfink M, Lambris JD, Lanning L, Lutz HU, Meri S, Rose NR, Salant DJ, Sethi S, Smith RJ, Smoyer W, Tully HF, Tully SP, Walker P, Welsh M, Würzner R, Zipfel PF: Membranoproliferative glomerulonephritis type II (dense deposit disease): An update. J Am Soc Nephrol 16: 1392–1403, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Sethi S, Fervenza FC, Zhang Y, Nasr SH, Leung N, Vrana J, Cramer C, Nester CM, Smith RJ: Proliferative glomerulonephritis secondary to dysfunction of the alternative pathway of complement. Clin J Am Soc Nephrol 6: 1009–1017, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daha MR: Role of complement in innate immunity and infections. Crit Rev Immunol 30: 47–52, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Ross SC, Densen P: Complement deficiency states and infection: Epidemiology, pathogenesis and consequences of Neisserial and other infections in an immune deficiency. Medicine (Baltimore) 63: 243–273, 1984 [PubMed] [Google Scholar]
- 11.Thomas TC, Rollins SA, Rother RP, Giannoni MA, Hartman SL, Elliott EA, Nye SH, Matis LA, Squinto SP, Evans MJ: Inhibition of complement activity by humanized anti-C5 antibody and single-chain Fv. Mol Immunol 33: 1389–1401, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Hillmen P, Young NS, Schubert J, Brodsky RA, Socié G, Muus P, Röth A, Szer J, Elebute MO, Nakamura R, Browne P, Risitano AM, Hill A, Schrezenmeier H, Fu CL, Maciejewski J, Rollins SA, Mojcik CF, Rother RP, Luzzatto L: The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med 355: 1233–1243, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Maga TK, Nishimura CJ, Weaver AE, Frees KL, Smith RJ: Mutations in alternative pathway complement proteins in American patients with atypical hemolytic uremic syndrome. Hum Mutat 31: E1445–E1460, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Bomback AS, Smith RJ, Barile GR, Zhang Y, Heher EC, Herlitz LC, Stokes MB, Markowitz GS, D’Agati VD, Canetta PA, Radhakrishnan J, Appel GB: Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin J Am Soc Nephrol 7: 748–756, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L: Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol 25: 1256–1264, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Lin J, Markowitz GS, Valeri AM, Kambham N, Sherman WH, Appel GB, D’Agati VD: Renal monoclonal immunoglobulin deposition disease: The disease spectrum. J Am Soc Nephrol 12: 1482–1492, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Hillmen P, Elebute M, Kelly R, Urbano-Ispizua A, Hill A, Rother RP, Khursigara G, Fu CL, Omine M, Browne P, Rosse W: Long-term effect of the complement inhibitor eculizumab on kidney function in patients with paroxysmal nocturnal hemoglobinuria. Am J Hematol 85: 553–559, 2010 [DOI] [PubMed] [Google Scholar]