Abstract
Mutations in SLC4A1 that mislocalize its product, the chloride/bicarbonate exchanger AE1, away from its normal position on the basolateral membrane of the α-intercalated cell cause autosomal dominant distal renal tubular acidosis (dRTA). We studied a family exhibiting dominant inheritance and defined a mutation (AE1-M909T) that affects the C terminus of AE1, a region rich in potential targeting motifs that are incompletely characterized. Expression of AE1-M909T in Xenopus oocytes confirmed preservation of its anion exchange function. Wild-type GFP-tagged AE1 localized to the basolateral membrane of polarized MDCK cells, but AE1-M909T localized to both the apical and basolateral membranes. Wild-type AE1 trafficked directly to the basolateral membrane without apical passage, whereas AE1-M909T trafficked to both cell surfaces, implying the gain of an apical-targeting signal. We found that AE1-M909T acquired class 1 PDZ ligand activity that the wild type did not possess. In summary, the AE1-M909T mutation illustrates the role of abnormal targeting in dRTA and provides insight into C-terminal motifs that govern normal trafficking of AE1.
Regulation of acid–base status by the kidney is essential for health. In the distal nephron, α-intercalated cells (α-ICs) are responsible for secreting protons into the tubular lumen through apical membrane H+-adenosinetriphosphatases (H+-ATPases) functionally coupled to the basolateral membrane chloride–bicarbonate transporter anion exchanger 1 (AE1).1 As with other tubular epithelial cells, α-ICs are polarized and highly sensitive to small changes in equilibrium. Disruption of α-IC function results in distal renal tubular acidosis (dRTA), with consequent hypokalemic hyperchloremic metabolic acidosis, nephrocalcinosis and nephrolithiasis, and sometimes, associated failure to thrive and rickets in infancy, osteomalacia in adults, and renal failure.2 Dominantly inherited dRTA (ddRTA) is a consequence of mutations in SLC4A1 encoding AE1.3
AE1 is a large glycosylated protein with 12–14 transmembrane spans forming the anion exchanger domain plus cytoplasmic N and C termini.4 It is predominantly expressed in two cell types, where it exists as separate isoforms; eAE1 in the erythrocyte and kAEl in the α-IC. eAE1 plays a major structural role in the red cell, anchoring cytoskeletal elements such as ankyrin, spectrin, and protein 4.2 at its N terminus. Heterozygous mutations result in membrane abnormalities, such as hereditary spherocytosis and Southeast Asian ovalocytosis,4 usually without an associated renal phenotype. In contrast, kAE1 is truncated by 65 amino acids at its N terminus (by virtue of an alternative promoter lying within intron 3), resulting in loss of its structural role.5 An entirely separate set of mutations cause ddRTA, but there is no erythroid phenotype. Rarely, a dual renal and hematologic phenotype can occur in the setting of recessive or codominant heterozygous AE1 mutations or complete absence of AE1.6–8 A recent report of kAE1 in the glomerular podocyte and interactions with nephrin and integrin-linked kinase suggests potential additional roles.9
To date, nine separate SLC4A1 mutations have been described in association with ddRTA.3,10–14 Where examined, the in vitro anion exchange function of these mutants was found to be at or near normal levels,3,6,12,15 suggesting that disease may instead occur through mistargeting of the mutant protein within the polarized α-IC.3 Expression of the C-terminal truncation mutant R901X in polarized Madin–Darby canine kidney (MDCK) cells confirmed this suggestion with mislocalization of the mutant kAE1 to the apical membrane, indicating the presence of basolateral targeting motifs within the C-terminal domain.16,17 Loss of polarized membrane expression has also been reported with the G609R mutant, whereas intracellular retention in polarized cells characterizes the R589H, S613F, and C479W mutants.12,14,17 Additional investigation confirmed the tyrosine residue at position 904 within the C terminus as a basolateral determinant; the phosphorylation states of both this determinant and an N-terminal tyrosine seem important in governing trafficking to and from the membrane.16–18 In addition, the extreme C terminus of AE1 conforms to the X-Φ-X-Φ amino acid sequence of a class II PDZ (postsynaptic density protein, Drosophila disc large tumor suppressor, Zonula occludens-1 protein) ligand; such interactions may also play a role in membrane targeting or retention.17 However, nothing else is known of targeting motifs or trafficking partners for AE1.
We report here the identification of a novel C-terminal mutation in a family with ddRTA and examination of its anion exchange function and trafficking properties compared with wild-type kAE1 (kAE1wt). This mutant kAE1 retains good anion exchange function but possesses a novel apical targeting motif, unusually without concomitant loss of basolateral determinants. The mutation introduces a C-terminal class I PDZ ligand motif, which we have confirmed in vitro. For the first time, we also show direct postsynthetic traffic of kAE1wt to the basolateral membrane.
Results
M909T Is a Novel AE1 Mutation Identified in a Family with ddRTA
A five-generation pedigree with renal stone disease and/or overt dRTA was investigated for the presence of an SLC4A1 mutation (Figure 1A). The index case was a boy of 4 years at the time of diagnosis after investigation for recurrent vomiting, muscle weakness, and back pain. Serum biochemistry showed a metabolic acidosis, and urine biochemistry revealed hypocitraturia (Supplemental Table 1). Hypocitraturia was also shown in his father, who had a history of severe recurrent nephrolithiasis and mild CKD.
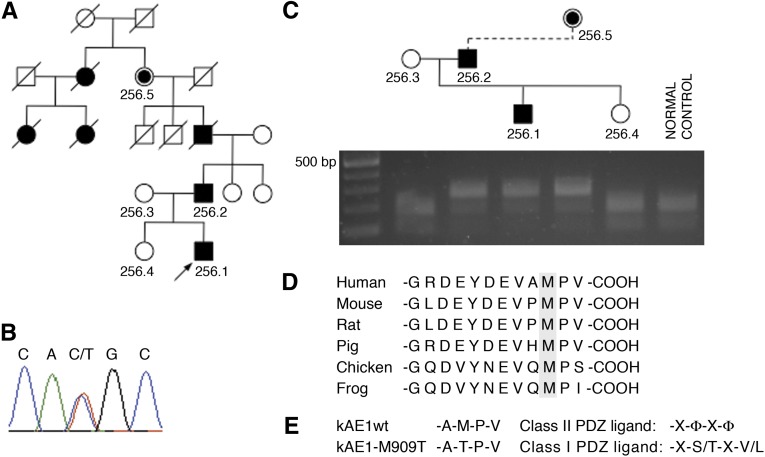
Figure 1.
Mutation identification. (A) Pedigree with index case arrowed. Filled symbols, family members affected by renal stone disease or diagnosed with dRTA; slashes, deceased; central filled symbol, obligate carrier. (B) Genomic DNA sequencing in the index case shows heterozygous substitution of T for C at nucleotide 2,840, resulting in the replacement of methionine by threonine at codon 909 (M909T). (C) This finding leads to loss of the restriction site for NlaIII amplification and NlaIII digestion of exon 20 from five family members, and a normal control shows cosegregation of the mutation with disease. (D) Multiple species sequence alignment of the C terminus of AE1 shows that Met909 is highly conserved. (E) The M909T alteration results in loss of a class II PDZ ligand and generates a potential class I PDZ ligand motif.
Sequencing SLC4A1 identified a novel mutation: c.2840T>C in exon 20 (Figure 1B). This finding results in the substitution of methionine by threonine at position 909 within the C-terminal tail. The altered sequence leads to loss of an NlaIII restriction site; restriction digestion confirmed that the mutation segregated with disease through the family (Figure 1C). The remaining coding exons were normal. The site and nature of the M909T mutation in the C terminus of kAE1, a region known to contain targeting information, were suggestive of a functional consequence. Ala-Met909-Pro-Val constitutes a possible but to date, poorly characterized C-terminal PDZ binding motif, with methionine highly conserved across species (Figure 1D). The potential for the C terminus of AE1 to act as a class II PDZ ligand has previously only been shown in principle through a yeast two-hybrid interaction with PICK-1 that was not further characterized.19 The M909T substitution could lead either to loss of a class II motif or its conversion to a class I motif (Figure 1E).
kAE1-M909T Reaches the Cell Surface and Retains Anion Exchange Function When Expressed in Xenopus Oocytes
X. laevis oocytes, which lack endogenous AE1 and have been widely used to assess anion exchange function of transfected AE1,3,12,20 were microinjected with in vitro-transcribed cRNA encoding kAE1wt or kAE1-M909T. Biotinylation of surface proteins confirmed expression of each. No intracellular trafficking defect was evident: kAE1wt and mutant kAE1 reached the oocyte surface in approximately equal quantities (Figure 2A and Supplemental Figure 1). The minor mobility shift of the mutant may reflect less extensive glycosylation.
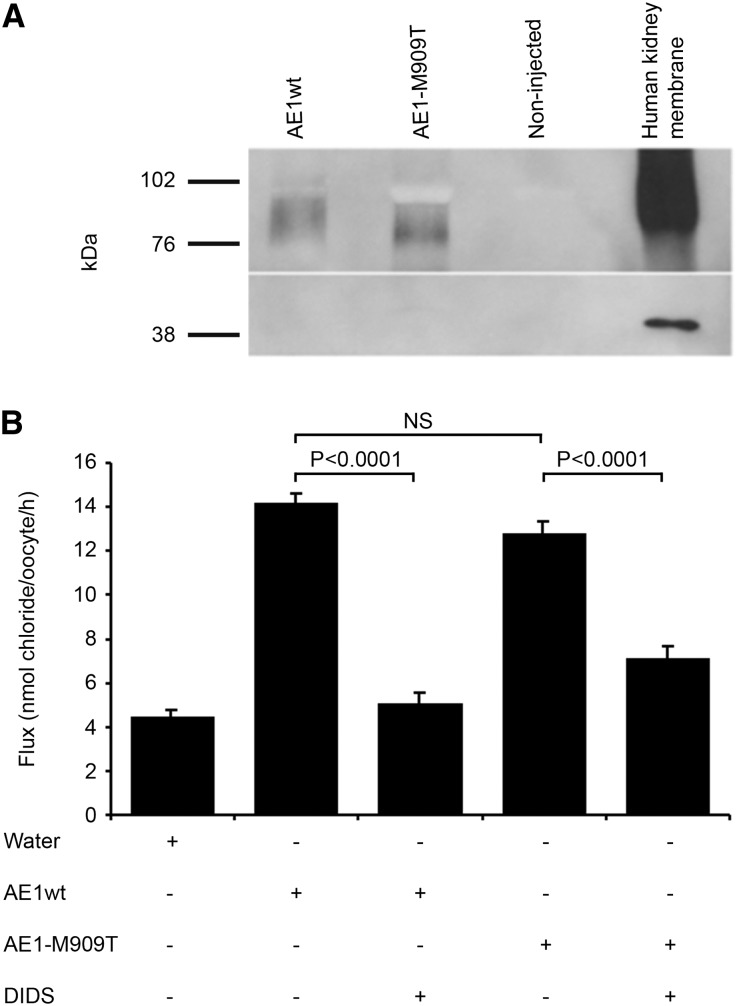
Figure 2.
Expression of kAE1 in Xenopus oocytes. (A) Western blot analysis of microinjected Xenopus oocytes confirms similar levels of surface expression of kAE1wt and kAE1-M909T (approximately 95 kDa biotinylated surface fraction) using the anti-AE1 monoclonal antibody Bric-170. Actin control confirms biotinylation of surface fraction only. (B) 36Cl uptake by oocytes injected with wild-type (WT) or mutant (M909T) kAE1 cRNA in the presence or absence of DIDS. Control oocytes were injected with water. Values are means ± SEM of influx pooled from five separate experiments with 9–33 oocytes/group. NS = not significant.
Anion exchange function was assessed through measurement of 36Cl− uptake in the same oocyte system (Figure 2B). Chloride influx was similar in oocytes expressing kAE1wt (14.22±0.42 nmol 36Cl−/oocyte per hour) or M909T (12.80±0.51 nmol 36Cl−/oocyte per hour; P=0.06); both were significantly higher than controls (P<0.0001). Although the addition of 4,4′-diisothiocyano-2,2′-stilbenedisulfonic acid (DIDS) to wild-type cells resulted in a reduction of chloride flux to water-injected levels (P=0.54; wt versus water), the effect of DIDS was less marked in mutant-expressing oocytes (P=0.002; M909T versus water). DIDS-sensitive chloride flux, a measure of AE1-specific chloride transport, was 9.17 and 5.67 nmol36Cl−/oocyte per hour for AE1wt and M909T, respectively (i.e., kAE1-M909T has 62% of the DIDS-sensitive anion exchange activity of kAE1wt). This finding is comparable with the spectrum of other kAE1 mutations, with chloride influx activity as a percentage of wild type (calculated from DIDS- or 4,4′-dinitro-2,2′-stilbene disulfonate–, a similar inhibitor, specific flux) ranging from 28% to 180%; most mutants are within the 50%–110% range.3,6,12,15,21
kAE1-M909T activity, thus, remains substantial; the additional presence of kAE1wt in the in vivo kidney predicts near-normal levels of α-IC anion transport activity, which was previously shown when kAE1wt and mutant kAE1 have been coexpressed in Xenopus oocytes.3,21 Thus, a defect in anion exchange function is unlikely to account for ddRTA in this pedigree.
Green Fluorescent Protein–Tagged kAE1-M909T Is Resident at Both Apical and Basolateral Surfaces in Polarized MDCK Cells
With this in mind, the cellular behavior of kAE1-M909T was investigated. Normal membrane expression and anion exchange function of N-terminal green fluorescent protein (GFP)-tagged kAE1wt has been shown in both K562 erythroleukemia cells and kidney epithelial cell models.22,23 Expression of GFP-kAE1 in HEK293 and nonpolarized MDCK cells showed membrane localization of kAE1wt and mutant kAE1 in both transiently and stably transfected cells (Supplemental Figure 2). Neither cell type was permeabalized before staining with Bric-6 antibody, which only recognizes an exofacial loop of properly conformed AE1.24
Transient and stable transfection of MDCK cells grown to polarity confirmed normal basolateral localization of both GFP-kAE1wt and GFP-kAE1-M909T but showed additional abnormal apical distribution of GFP-kAE1-M909T with preservation of tight junctions and normal polarity (Figure 3 and Supplemental Figure 3). Unlike kAE1wt, there was, in addition, considerable intracellular accumulation of the mutant protein. Membrane localization at both surface domains was again confirmed by Bric-6 staining (Figure 3). Expression of kAE1-M909T with an N-terminal hemagglutinin tag gave similar results (Supplemental Figure 4).
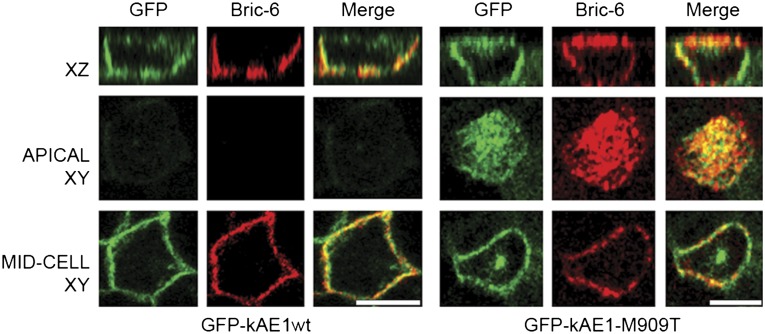
Figure 3.
Apical mistargeting of mutant GFP-tagged kAE1-M909T in polarized MDCK cells. N-terminal GFP-tagged kAE1wt or kAE1-M909T (green) expressed in stably transfected MDCK-II cells grown to polarity on Transwells shows normal basolateral localization of kAE1wt and both apical and basolateral localization of kAE1-M909T confirmed by immunostaining in these nonpermeabalized cells with Bric-6 (antibody directed against an exofacial loop). Scale bars, 10 µm.
Thus, kAE1-M909T is able to traffic to the cell surface in both nonpolarized and polarized renal epithelial cell models. The presence at both cell surface domains is intriguing, because the R901X mutant lost basolateral membrane residency when examined in stably transfected cells either as the full-length protein or a C-terminal construct.16,17
kAE1wt Traffics Directly to the Basolateral Membrane Postsynthesis
The pathway by which kAE1 reaches its final basolateral residency has not previously been elucidated.25 Direct postsynthetic targeting of various proteins to the apical or basolateral membrane, initial nonpolarized delivery followed by retrieval from the incorrect surface, and targeting to one surface with endocytosis and transport to the final membrane domain have all been described in polarized MDCK cells.26–31 Thus, AE1 could undergo transient postsynthetic traffic to the apical domain with abnormal retention of the mutant, or the mutant may be exhibiting new apical targeting. Domain-specific biotinylation remains a standard method to investigate cell surface delivery, but the work by Toye et al.17 found that expression of kAE1wt in MDCK cells increased paracellular permeability, including to biotin, thus potentially invalidating this technique. Cell surface biotinylation of filter-grown MDCK cells expressing kAE1wt in our system confirmed increased paracellular permeability to biotin in monolayers expressing GFP-AE1wt (Figure 4). Transepithelial resistance was low (∼90 Ω/cm2, with no difference between untransfected and GFP-kAE1wt-expressing cells under identical conditions), which is consistent with the known properties of MDCK-II cells.32
Figure 4.
Expression of kAE1wt increases paracellular permeability of filter-grown MDCK cells. Cells grown to polarity on Transwells were biotinylated at either the apical (AP) or basolateral (BL) surface and then lysed. Western blotting of the initial cell lysate (total lysate lanes are the loading control) and the isolated biotinylated fraction (surface fraction) shows that in untransfected cells, only trace amounts of the basolateral proteins E-Cadherin and Na+/K+-ATPase were detected at the apical surface, whereas proportions at both surfaces seemed equal in kAE1-expressing cells. A significant proportion of kAE1wt was also apparently localized to the apical surface. These biotinylation data are at odds with immunofluorescence images for all three markers in the same kAE1wt-expressing MDCK cells (Figures 3 and 6 show kAE1wt), which localize all three proteins to the basolateral membrane only, indicating that kAE1wt increases paracellular permeability to biotin. Representative of two separate experiments.
To examine the postsynthetic route to the cell surface, a domain-specific antibody-labeling assay was, therefore, designed using Bric-6. Apical or basolateral appearance of gp135 or the β1-subunit of the Na+/K+-ATPase, respectively, was consistent with known trafficking patterns, and it confirmed the integrity of the assay (Supplemental Figure 5).33,34 Apical incubation with Bric-6 in MDCK cells expressing GFP-kAE1wt failed to label the protein, the pattern of expression being normal (basolateral) when the GFP tag was examined (Figure 5A). In contrast, basolateral incubation with Bric-6 resulted in protein labeling at the membrane plus additional intracellular accumulation of Bric-6/GFP-kAE1wt complexes (Figure 5A). The proportion of cells with intracellular accumulation increased with longer incubation, whereas the total number of expressing cells seemed to decline, suggesting that degradation of the intracellular antibody–AE1 complex may occur. Taken together, these results are consistent, first, with the direct basolateral trafficking of GFP-kAE1wt without any transient apical passage and second, with GFP-kAE1wt internalization from the basolateral membrane.
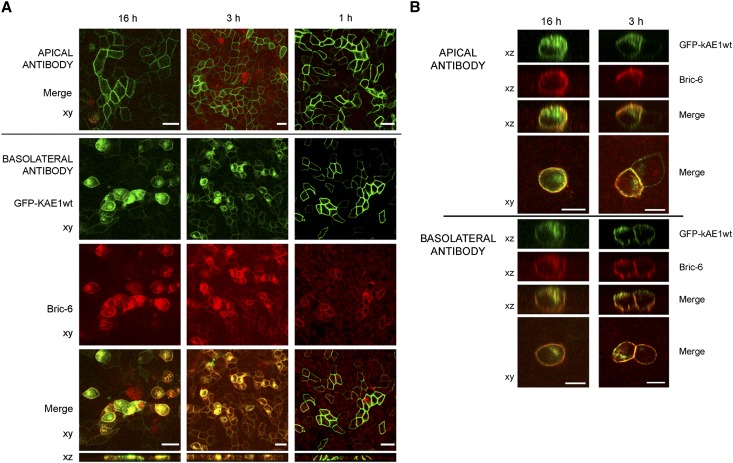
Figure 5.
Antibody uptake assay showing that kAE1wt traffics directly to the basolateral membrane. After induction of GFP-kAE1 expression, Bric-6 antibody was added to either the apical or basolateral compartment immediately (16 h) or at a later time (3 or 1 h) before fixing. (A) In cells expressing GFP-kAE1wt, no staining was evident with apical application of antibody (top panel), but basolateral application led to labeling of GFP-kAE1wt and internalization of the antibody–AE1 complex, with intracellular accumulation most evident in the 16-h assay (left column). (B) In cells expressing GFP-kAE1-M909T, application of Bric-6 to either compartment labeled both apical and basolateral surface kAE1-M909T, with increased staining at the opposing surface evident with longer incubation times (16 versus 3 h). Representative of (A) seven or (B) two separate experiments. Scale bars, 20 µm in A; 10 µm in B.
In GFP-kAE1-M909T–expressing cells, the same antibody-labeling assay confirmed trafficking of kAE1-M909T to both apical and basolateral domains (Figure 5B). When Bric-6 was applied to the apical surface, there was also staining at the basolateral membrane and vice versa; this finding was more apparent for the longer incubations, indicating that kAE1-M909T internalizes from both apical and basolateral membranes. From the recycling compartment, it can again reach either domain. Interestingly, the striking intracellular accumulation of AE1–antibody complex seen with the AE1wt cells was less apparent with the mutant protein; intracellular GFP-AE1-M909T was still present but only clearly counterstained with Bric-6 in the 16-h basolateral images and not when Bric-6 was applied to the apical surface. This finding implies that the intracellular GFP-AE1-M909T accumulation observed previously (Figure 3 and Supplemental Figures 2–4) could represent protein that reaches the membrane much more slowly (or never at all) and that the M909T substitution could alter the normal recycling pathway of kAE1.
kAE1 Mutants Lacking the C-Terminal PDZ Ligand Motif Are Retained Intracellularly
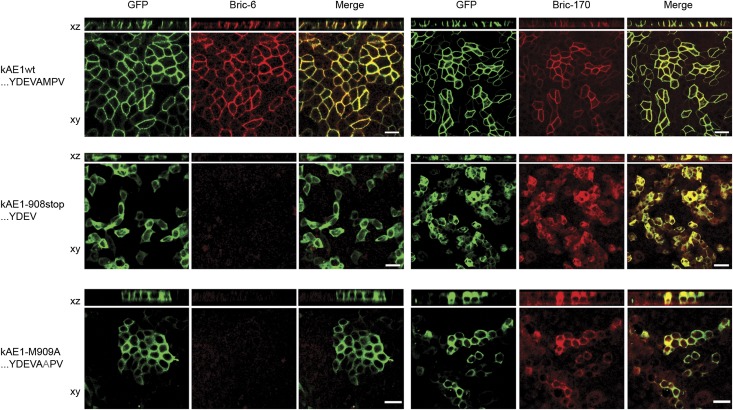
To further investigate motifs governing kAE1 traffic to the cell membrane, two other C-terminal mutants (kAE1-908X and kAE1-M909A) that mimic loss of PDZ binding capacity were created and expressed. Normal monolayer integrity was confirmed by staining for ZO-1 (Supplemental Figure 6). In both cases, GFP fluorescence seemed intracellular, and Bric-6 surface staining was negative. After permeabilization, the fluorescence colocalized with Bric-170 (directed against the cytoplasmic N terminus of AE1), confirming successful expression but intracellular retention of both mutants (Figure 6).
Figure 6.
GFP-kAE1-M909A and -908stop mutants are intracellularly retarded. Both Bric-6 (columns 1–3, nonpermeabilized) and Bric-170 (columns 4–6, permeabilized) staining failed to show membrane localization of either mutant in mixed stable lines in contrast to normal localization of kAE1wt (top row). Scale bars, 20 µm.
The antibody uptake assay was repeated for both these mutants. In neither case was there any positive staining with Bric-6, indicating that the mutant proteins never reached the cell surface (i.e., intracellular accumulation was a primary postsynthetic event did not result from a failure of membrane retention). These findings indicate that a C-terminal domain containing residues corresponding to a PDZ ligand is essential for biosynthetic traffic of kAE1 to the cell surface.
kAE1-M909T Is a Class I PDZ Ligand
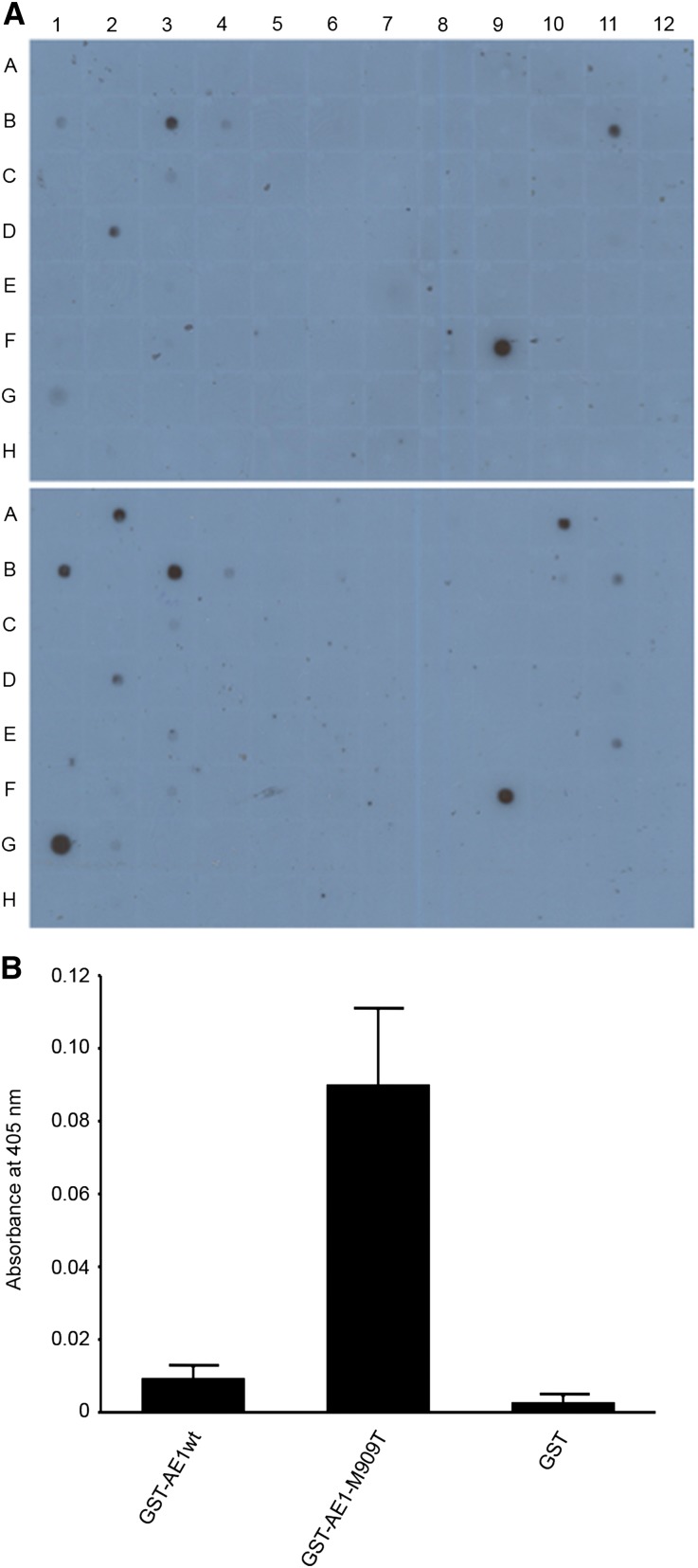
To assess whether kAE1-M909T could act as a class I PDZ ligand, we screened glutathione S-transferase (GST) fusion constructs of the C termini of AE1wt and mutant AE1 against a proteomic array of 96 PDZ proteins/peptides,35 comprising known or potential class I domains. Clear binding was found between kAE1C-M909T but not kAE1C-wt and PDZK1, membrane-associated guanylate kinase, WW, and PDZ domain-containing protein (MAGI)-1, and MAGI-3 (Figure 7A). In contrast, none of the 96 class I domains showed wild-type but not mutant binding. PDZK1 was selected for additional analysis as a candidate class I domain-containing protein. ELISA clearly showed binding of mutant but not kAE1wt to purified PDZK1, confirming acquisition of a class I motif by the mutant. Binding was optimal at 2 μg/ml concentrations of PDZK1 at 10 minutes incubation (Figure 7B) and also significant at 1 and 5 μg/ml (data not shown).
Figure 7.
The C-terminal tail of kAE1-M909T is a class I PDZ ligand. (A) A proteomic array containing 96 putative class I PDZ domains (38) showed binding of GST-kAE1-M909T (lower panel) but not its wild-type equivalent (upper panel) to bins A2, A10, and G1 (MAGI-3, MAGI-1, and PDZK1). (B) The interaction with purified PDZK1 was confirmed by ELISA. Data are means and SEM of three experiments.
Discussion
In characterizing M909T, a novel disease-causing mutation in SLC4A1, we have added new information concerning not only the functional activity and cellular phenotype of the mutant protein but also the normal behavior of kAE1. The M909T mutation had clear potential significance given its location within the C-terminal domain of kAE1 because of the known contribution of the C terminus to targeting and the important role of mistargeting in other ddRTA-causing AE1 mutations. In addition, anion transport by kAE1-M909T expressed in oocytes was reasonably preserved at approximately two-thirds of wild type, making loss of transport function unlikely to be the cause of α-IC failure in vivo.
The mistargeting hypothesis to explain ddRTA was originally proposed after functional studies of the first mutants showed near-normal AE1 anion exchange activity.3 Subsequent expression of various ddRTA-associated mutant kAE1 in polarized cells has characterized subsets of mutations resulting in either intracellular retention (R589H and S613F) or mistargeting to apical and basolateral surfaces (R901X and G609R in transiently transfected cells) or the apical surface alone (R901X in stably transfected cells).12,16,17 Overexpression in transiently transfected cells may lead to abnormal nonpolarized expression (LLC-PK1 cells are discussed in the work by Toye et al.17); ideally, stably transfected cells combined with determination of cell surface residency through Bric-6 staining should be employed for assessment of all mutants.
Using this system, expression of GFP-AE1wt or GFP-M909T in polarized MDCK cells indicated a clearly aberrant targeting phenotype for the mutant, which was confirmed with a hemagglutinin-tagged construct. Because we have here shown that the wild-type construct moves directly to the basolateral compartment, the apical membrane appearance of the mutant indicated likely gain of a new targeting motif; basolateral signals, which are known not to reside solely at the far C terminus, would be preserved. Against the theory that cell polarity was more widely disrupted by introducing the mutant, normal tight junctions and apical gp135 were observed.
It remains true that no groups have yet been able to identify the previously hypothesized class II ligand activity for AE1.19 We nevertheless considered whether simple loss of a class II motif, without gain of class I, could explain our observations. This test should have resulted in the same appearances as reported for kAE1-V911X (the lack of C-terminal valine likely leading to loss of class II ligand activity17), which resulted in basolateral residency with increased levels of intracellular protein but no apical appearance, suggesting that a class II ligand might be important in either maximizing efficiency of basolateral biosynthetic membrane traffic or mediating basolateral retention in situ. Instead, the results from the PDZ array and ELISA with PDZK1 clearly show that kAE1-M909T is capable of acting as a class I PDZ ligand. The alternative explanation that M909T disrupts the C terminus to such an extent that previously suppressed apical determinants within kAE1 (e.g., N-glycans36) can exert a targeting effect is less likely for several reasons. First, there was no apical expression of kAE1-V911stop; second, kAE1-M909T shows persistent basolateral presence, implying preservation of its other determinants, and third, the overall structural effect of a single residue substitution so close to the C terminus is unlikely to be major. It is a shame that PDZK1, although an excellent candidate to show the potential for interaction and well characterized as an apical scaffolding protein for a number of proteins within renal tubular cells, is only present in the brush border of the proximal tubular epithelium and not in α-ICs.37–39 MAGI-1 is present within glomerular podocytes,40 and an isoform colocalizes with Kir4.1 in collecting duct principle cells41; expression of MAGI-1 in α-ICs has not been reported, and renal expression of MAGI-3 is as yet undefined. We have not proceeded to try to identify the actual PDZ protein involved, because this protein is a unique pathologic mutant that would not be likely to provide generalizable information and α-ICs do not readily submit to in vitro growth.
A considerable number of protein–protein interactions have been described where PDZ domains modulate membrane retention (reviewed in ref. 42). Because we show that kAE1 does not normally traffic through the apical domain, M909 cannot form part of an apical retention signal, even transiently. The situation is more akin to the situation of the cell surface proteoglycan syndecan-1, which contains a C-terminal type II PDZ ligand controlling localization to the basolateral membrane in MDCK cells by polarized sorting within the biosynthetic pathway.43 Also, sorting of the widely expressed gp135 to the apical domain is dependent on a type I PDZ interaction with ezrin/radixin/moesin–binding phosphoprotein 50 at the level of the trans-Golgi network; knockdown of ezrin/radixin/moesin–binding phosphoprotein 50 or removal of the PDZ ligand motif results in basolateral missorting of gp135.44
The previously reported C-terminal truncation mutants that were partially or completely retained in nonpolarized cells implied the presence of sequence information essential for kAE1 traffic to the surface.45 In our system, kAE1wt and M909T mutant kAE1 each reached the cell surface in both nonpolarized HEK and MDCK-II cells, suggesting preservation of motifs involved in quality control, although there was increased intracellular retention of the mutant implying reduced efficiency of intracellular trafficking.
PDZ proteins also mediate transit from/through the endoplasmic reticulum (ER) and Golgi. For example, proTGF-α contains a type I PDZ ligand domain (-ETVV) at its C terminus, which binds the PDZ protein proTGF-α cytoplasmic domain-interacting protein 18 (TACIP18) or syntenin, an interaction necessary for exit from the ER. TACIP18/syntenin can, in fact, bind both classes I and II PDZ ligands.46 Pro-TGF mutants lacking a C-terminal valine were retained in the ER, such as with AE1-V911stop. Similar mechanisms and interactions may, therefore, also control kAE1, because presence of an intact PDZ ligand domain seems essential for normal traffic to the cell surface. Promiscuity of the kAE1 cognate PDZ protein, the identity of which remains unknown, may, as in TACIP18, allow binding of both types I and II ligands; hence, there is the persistent ability of kAE1-M909T to exit the synthetic compartment, albeit with reduced efficiency. Whether kAE1wt contains a specific ER retention signal—suppressed by the PDZ motif or its binding partners—is unclear. However, the AE1 C terminus does not contain any of the classic lysine- or arginine-based motifs previously identified as retention signals.47 Also against this idea is the ability of the truncation mutant kAE1-R901X to reach the cell surface, although it may have lost both PDZ binding domain and retention signal, be significantly misfolded at the C terminus to avoid PDZ interaction, or attract different trafficking intermediates.
Hetero-oligomerization of kAE1 explains how each type of mutation results in α-IC failure in vivo. Intracellularly retained mutants will also retain kAE1wt, thus leading to insufficiency of basolateral membrane anion exchange capability.48 In contrast, where abnormal apical expression occurs, functional hetero-oligomers will appear apically, potentially together with reduced basolateral expression of kAE1wt; the former would result in neutralization of secreted H+ and thus, failure of acid secretion by the distal nephron, whereas the latter would also reduce bicarbonate reabsorption, both leading to α-IC failure. Although coexpression of differently tagged kAE1wt and mutant kAE1 in polarized cells would permit assessment of these possibilities, our attempts and the attempts of others17 at coexpression in MDCK cells were unsuccessful in achieving significant expression levels using transient and stable transfection (of one or both constructs). Most recently, the work by Chu et al.14 has reported some success with coexpression of the kAE1 C479W and G701D mutants.
We have here also confirmed the previous, and somewhat unexpected, finding that the presence of kAE1wt increases the paracellular permeability of epithelial monolayers in culture. The validity of this finding is increased by the similar changes in both subtypes of MDCK cells, which have different compositions of the tight junction complex.49 Expression levels were not significantly high enough to ascertain whether this effect was also true in vitro for kAE1-M909T. Whether kAE1 exerts the same effect on the tubular epithelium in vivo is also unknown.
The intracellular accumulation of antibody–kAE1wt complex in our antibody-labeling assay implies that bound antibody resulted in defective recycling, an unusual phenomenon but one previously reported for antibodies against the phosphomannosyl receptor in cultured fibroblasts, the transferrin receptor in chicken erythroblasts, and gp280 in rat yolk sac epithelial cells.50–52 Unfortunately, no clear pattern emerges from these reports of a mechanism by which Bric-6 could cause intracellular accumulation of GFP-kAE1wt. Regulation of cell surface kAE1wt has recently been described to depend on the phosphorylation state of tyrosine residues at positions 359 and 904 within the N and C termini, respectively18; whether antibody binding affects phosphorylation state is also unknown.
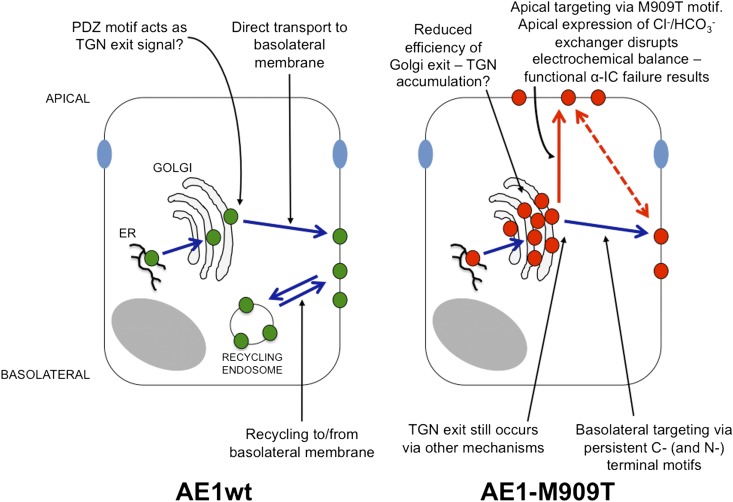
The diagnosis of ddRTA in affected families and the molecular and functional classification of novel AE1 mutants continue to provide insights into both disease mechanisms and normal trafficking behavior of kAE1. Figure 8 illustrates our proposed model for kAE1 trafficking based on these observations. Future work should focus on identification and investigation of interacting proteins—particularly those proteins containing PDZ domains—that mediate traffic of AE1.
Figure 8.
Suggested model for trafficking of kAE1wt and -M909T. For clarity, the recycling endosome has been omitted from the kAE1-M909T cell. The dotted red line indicates a possible transcytotic route.
Concise Methods
Kindred RTA256
Under the terms of Cambridge Local Research Ethics Committee approval 99/078, written informed consent was received from participants/parents before inclusion in the study.
SLC4A1 Mutation Detection
Genomic DNA was amplified using intronic primers to examine all coding exons and intron–exon boundaries of SLC4A1 and sequenced by Geneservice Medical Solutions PLC. Sequences were analyzed using Sequencher analysis software (Gene Codes Corporation).
Xenopus Oocyte Expression
Vector pXG-kAE1, available within our laboratory,12 was used as a template for site-directed mutagenesis using the QuikChange kit (Stratagene) to create pXG-kAE1-M909T. After linearization of sequence-verified plasmids with NheI, DNA transcription was performed using T7 RNA polymerase and the mMESSAGE mMACHINE kit (Ambion). Xenopus oocyte preparation, microinjection, maintenance, protein extraction, Western blot analysis, biotinylation, and chloride influx assessment were performed according to previously published techniques.12,20,53,54
Antibodies
Anti-AE1 antibodies used were Bric-170, which recognizes a cytoplasmic N-terminal epitope from residues 368–382,55 and Bric-6, which recognizes an extracellular epitope of AE1 situated within the fourth exofacial loop24 (both mouse monoclonal antibodies from IBGRL). Additional antibodies used (all mouse monoclonal antibodies unless indicated otherwise) were directed against the β1-subunit Na+/K+-ATPase (Sigma-Aldrich), hemagglutinin (rat; Covance), ZO-1 (rat; Santa Cruz), gp135 (gift of F. Buss, Cambridge, United Kingdom), E-cadherin (Transduction Labs), and β-actin (Abcam). Species-specific horseradish peroxidase-conjugated secondary antibodies (Dako) and AlexaFluor-conjugated antibodies (488, 555, and 568; Invitrogen) were used in Western blotting and immunofluorescence studies, respectively.
Mammalian Expression Constructs
kAE1wt was excised from laboratory stocks of pHM6-kAE1wt12,16 and cloned into the vector pEGFP-C2 to create an N-terminal GFP-tagged kAE1 construct for transient transfection of HEK and MDCK cells. For generation of stable cell lines, the GFP-kAE1 construct was subcloned into the ΔpMEP4 vector56 (gift of J. P. Luzio, Cambridge, United Kingdom). Site-directed mutagenesis to create M909T was again performed using the QuikChange kit and confirmed by direct sequencing.
Cell Transfection
HEK293 and MDCK-II cells were grown in DMEM supplemented with 10% FBS and 2 mM l-glutamine and maintained at 37°C in 5% CO2-balanced air. HEK and MDCK transfection used FuGENE 6 Transfection Reagent (Roche) or the Cell Line Nucleofection Kit L (Amaxa), respectively, as per manufacturer’s protocols. Selection of stable transfectants over a 2-week period was followed by induction using 2 μM CdCl2 and 100 μM ZnCl2 followed 16 h later by FACS sorting for GFP fluorescence (MoFlo Cell Sorter; Beckman Coulter).
Cell Culture and Immunofluorescence Microscopy
Nonpolarized cells were seeded at low density onto coverslips; cell polarization was achieved by seeding onto semipermeable polycarbonate filters (Transwells; Corning Life Sciences). In the case of coverslip-grown cells, those cells transfected with ΔpMEP4 were induced on day 3 using 2 μM cadmium chloride and 100 μM zinc chloride, and cells were fixed/stained 16 h later. Cells grown to polarity on Transwells were induced on day 4 and fixed/stained 16 h later (using the same concentrations of cadmium and zinc for the ΔpMEP4-inducible promoter and 5 mM sodium butyrate to enhance pHM6 vector expression).
Immunostaining (for all antibodies except Bric-6) was performed as described previously.23 In the case of Bric-6, the antibody in 1% BSA was applied directly to washed intact unpolarized cells on coverslips or both compartments of polarized cells on Transwells (previously incubated with PBS for 20 min at 4°C) for 1 h at room temperature. After washing, cells were fixed by incubation in ice-cold methanol at −20°C for 5 min, washed again, and blocked with 3% BSA for 15 min before addition of fluorescently labeled secondary antibody for 45 min at room temperature. For optimum results, we used the AlexaFluor 568 Signal-Amplification Kit for mouse primary antibodies (Invitrogen).
Cell Surface Biotinylation and Western Blot Analyses
Cell surface biotinylation was performed using the Pierce Cell Surface Protein Isolation Kit. Protein electrophoresis and Western blot analysis were performed as described previously.12
Electrical Properties of Cell Monolayers
MDCK-II cells were grown to polarity on Snapwell polycarbonate filters (Corning), and their electrical properties were assessed by the voltage clamp technique.57 Comparisons were made between untransfected and ΔpMEP4-kAE1wt transfected cell monolayers both with and without the addition of cadmium and zinc 16 h before.
Antibody-Labeling Assay in Live Cells
Bric-6 labeling of surface kAE1 at either the apical or basolateral Transwell compartment was based on the transcytosis assay in the work by Ihrke et al.58 Antibodies directed against external epitopes on gp135 or the β1-subunit of the Na+/K+-ATPase were used as controls. Using the ΔpMEP4 stably transfected cells grown to polarity on Transwells, promoter expression was induced as above on day 4, with antibody added to either apical or basolateral compartment at the same point (16 h prefixation) or for shorter time points (3 or 1 h prefixation). Cells were fixed, labeled with fluorescent secondary antibody, and imaged 16 h after induction of expression.
Expression and Purification of GST-Tagged Fusion Proteins
GST-tagged AE1C-WT (containing the final 36 residues of AE1) or mutant fusion protein (GST_AE1C-M909T) were separately expressed in Escherichia coli BL21 cells and purified using glutathione sepharose beads (Amersham Biosciences).
PDZ Membrane Array and ELISA
Nylon Super Charge membranes (pore size = 0.45 μM; Schleicher and Schuell) spotted with His- and S-tagged PDZ domain fusion proteins (each at 1 μg) were supplied by Randy Hall (Atlanta, GA). They were exposed to GST fusion proteins (GST_AE1C-WT, GST_AE1C_M909T or GST) as previously described.35
ELISA Analysis
GST_AE1C-WT, GST_AE1C-M909T, or an equivalent amount of GST was diluted in 0.05 M Na2CO3/NaHCO3 buffer (pH 9.6) to 20 μM and immobilized onto 96-well plates at 37°C for 1 h. After nonspecific site blockade, PDZK1 protein (Abnova) in the range of 1–5 μg/ml was applied and incubated at 37°C for 1 h. Mouse monoclonal α-PDZK1 antibody (Abcam) and horseradish peroxidase-conjugated α-mouse IgG antibody (DAKO) were used to detect and quantify bound protein according to standard methods.
Statistical Analyses
Statistical analyses were performed using a t test.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dr. Folma Buss (Cambridge, United Kingdom), Prof. Paul Luzio (Cambridge, United Kingdom), and Prof. Randy Hall (Atlanta, GA) for reagents and Dr. Gudrun Ihrke (Bethesda, MD) for advice and expertise.
This work was funded by a Kidney Research United Kingdom Training Fellowship (to A.C.F.), Wellcome Trust Senior Fellowship 088489 (to F.E.K.F.), and Award 079895 (to Cambridge Institute for Medical Research), and the Cambridge National Institute for Health Research Biomedical Research Centre.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012020112/-/DCSupplemental.
References
- 1.Brown D, Breton S: Mitochondria-rich, proton-secreting epithelial cells. J Exp Biol 199: 2345–2358, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Karet FE: Inherited distal renal tubular acidosis. J Am Soc Nephrol 13: 2178–2184, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Bruce LJ, Cope DL, Jones GK, Schofield AE, Burley M, Povey S, Unwin RJ, Wrong O, Tanner MJ: Familial distal renal tubular acidosis is associated with mutations in the red cell anion exchanger (Band 3, AE1) gene. J Clin Invest 100: 1693–1707, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alper SL: Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. J Exp Biol 212: 1672–1683, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kollert-Jöns A, Wagner S, Hübner S, Appelhans H, Drenckhahn D: Anion exchanger 1 in human kidney and oncocytoma differs from erythroid AE1 in its NH2 terminus. Am J Physiol 265: F813–F821, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Bruce LJ, Wrong O, Toye AM, Young MT, Ogle G, Ismail Z, Sinha AK, McMaster P, Hwaihwanje I, Nash GB, Hart S, Lavu E, Palmer R, Othman A, Unwin RJ, Tanner MJ: Band 3 mutations, renal tubular acidosis and South-East Asian ovalocytosis in Malaysia and Papua New Guinea: Loss of up to 95% band 3 transport in red cells. Biochem J 350: 41–51, 2000 [PMC free article] [PubMed] [Google Scholar]
- 7.Inaba M, Yawata A, Koshino I, Sato K, Takeuchi M, Takakuwa Y, Manno S, Yawata Y, Kanzaki A, Sakai J, Ban A, Ono K, Maede Y: Defective anion transport and marked spherocytosis with membrane instability caused by hereditary total deficiency of red cell band 3 in cattle due to a nonsense mutation. J Clin Invest 97: 1804–1817, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ribeiro ML, Alloisio N, Almeida H, Gomes C, Texier P, Lemos C, Mimoso G, Morlé L, Bey-Cabet F, Rudigoz RC, Delaunay J, Tamagnini G: Severe hereditary spherocytosis and distal renal tubular acidosis associated with the total absence of band 3. Blood 96: 1602–1604, 2000 [PubMed] [Google Scholar]
- 9.Wu F, Saleem MA, Kampik NB, Satchwell TJ, Williamson RC, Blattner SM, Ni L, Toth T, White G, Young MT, Parker MD, Alper SL, Wagner CA, Toye AM: Anion exchanger 1 interacts with nephrin in podocytes. J Am Soc Nephrol 21: 1456–1467, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheidde L, Vieira TC, Lima PR, Saad ST, Heilberg IP: A novel mutation in the anion exchanger 1 gene is associated with familial distal renal tubular acidosis and nephrocalcinosis. Pediatrics 112: 1361–1367, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Karet FE, Gainza FJ, Györy AZ, Unwin RJ, Wrong O, Tanner MJ, Nayir A, Alpay H, Santos F, Hulton SA, Bakkaloglu A, Ozen S, Cunningham MJ, di Pietro A, Walker WG, Lifton RP: Mutations in the chloride-bicarbonate exchanger gene AE1 cause autosomal dominant but not autosomal recessive distal renal tubular acidosis. Proc Natl Acad Sci USA 95: 6337–6342, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rungroj N, Devonald MA, Cuthbert AW, Reimann F, Akkarapatumwong V, Yenchitsomanus PT, Bennett WM, Karet FE: A novel missense mutation in AE1 causing autosomal dominant distal renal tubular acidosis retains normal transport function but is mistargeted in polarized epithelial cells. J Biol Chem 279: 13833–13838, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Wrong O, Unwin R, Cohen E, Tanner MJ, Thakker R: Unravelling of the molecular mechanisms of kidney stones. Report of a Meeting of Physicians and Scientists. Lancet 348: 1561–1565, 1996 [PubMed] [Google Scholar]
- 14.Chu C, Woods N, Sawasdee N, Guizouarn H, Pellissier B, Borgese F, Yenchitsomanus PT, Gowrishankar M, Cordat E: Band 3 Edmonton I, a novel mutant of the anion exchanger 1 causing spherocytosis and distal renal tubular acidosis. Biochem J 426: 379–388, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Toye AM, Bruce LJ, Unwin RJ, Wrong O, Tanner MJ: Band 3 Walton, a C-terminal deletion associated with distal renal tubular acidosis, is expressed in the red cell membrane but retained internally in kidney cells. Blood 99: 342–347, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Devonald MA, Smith AN, Poon JP, Ihrke G, Karet FE: Non-polarized targeting of AE1 causes autosomal dominant distal renal tubular acidosis. Nat Genet 33: 125–127, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Toye AM, Banting G, Tanner MJ: Regions of human kidney anion exchanger 1 (kAE1) required for basolateral targeting of kAE1 in polarised kidney cells: Mis-targeting explains dominant renal tubular acidosis (dRTA). J Cell Sci 117: 1399–1410, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Williamson RC, Brown AC, Mawby WJ, Toye AM: Human kidney anion exchanger 1 localisation in MDCK cells is controlled by the phosphorylation status of two critical tyrosines. J Cell Sci 121: 3422–3432, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Cowan CA, Yokoyama N, Bianchi LM, Henkemeyer M, Fritzsch B: EphB2 guides axons at the midline and is necessary for normal vestibular function. Neuron 26: 417–430, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Garcia AM, Lodish HF: Lysine 539 of human band 3 is not essential for ion transport or inhibition by stilbene disulfonates. J Biol Chem 264: 19607–19613, 1989 [PubMed] [Google Scholar]
- 21.Jarolim P, Shayakul C, Prabakaran D, Jiang L, Stuart-Tilley A, Rubin HL, Simova S, Zavadil J, Herrin JT, Brouillette J, Somers MJ, Seemanova E, Brugnara C, Guay-Woodford LM, Alper SL: Autosomal dominant distal renal tubular acidosis is associated in three families with heterozygosity for the R589H mutation in the AE1 (band 3) Cl-/HCO3- exchanger. J Biol Chem 273: 6380–6388, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Beckmann R, Toye AM, Smythe JS, Anstee DJ, Tanner MJ: An N-terminal GFP tag does not alter the functional expression to the plasma membrane of red cell and kidney anion exchanger (AE1) in mammalian cells. Mol Membr Biol 19: 187–200, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Su Y, Blake-Palmer KG, Fry AC, Best A, Brown AC, Hiemstra TF, Horita S, Zhou A, Toye AM, Karet FE: Glyceraldehyde 3-phosphate dehydrogenase is required for band 3 (anion exchanger 1) membrane residency in the mammalian kidney. Am J Physiol Renal Physiol 300: F157–F166, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smythe JS, Spring FA, Gardner B, Parsons SF, Judson PA, Anstee DJ: Monoclonal antibodies recognizing epitopes on the extracellular face and intracellular N-terminus of the human erythrocyte anion transporter (band 3) and their application to the analysis of South East Asian ovalocytes. Blood 85: 2929–2936, 1995 [PubMed] [Google Scholar]
- 25.Cordat E: Unraveling trafficking of the kidney anion exchanger 1 in polarized MDCK epithelial cells. Biochem Cell Biol 84: 949–959, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Anderson E, Maday S, Sfakianos J, Hull M, Winckler B, Sheff D, Fölsch H, Mellman I: Transcytosis of NgCAM in epithelial cells reflects differential signal recognition on the endocytic and secretory pathways. J Cell Biol 170: 595–605, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campo C, Mason A, Maouyo D, Olsen O, Yoo D, Welling PA: Molecular mechanisms of membrane polarity in renal epithelial cells. Rev Physiol Biochem Pharmacol 153: 47–99, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Casanova JE, Mishumi Y, Ikehara Y, Hubbard AL, Mostov KE: Direct apical sorting of rat liver dipeptidylpeptidase IV expressed in Madin-Darby canine kidney cells. J Biol Chem 266: 24428–24432, 1991 [PubMed] [Google Scholar]
- 29.Keller P, Simons K: Post-Golgi biosynthetic trafficking. J Cell Sci 110: 3001–3009, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Mostov KE, Verges M, Altschuler Y: Membrane traffic in polarized epithelial cells. Curr Opin Cell Biol 12: 483–490, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Praetor A, Ellinger I, Hunziker W: Intracellular traffic of the MHC class I-like IgG Fc receptor, FcRn, expressed in epithelial MDCK cells. J Cell Sci 112: 2291–2299, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Richardson JC, Scalera V, Simmons NL: Identification of two strains of MDCK cells which resemble separate nephron tubule segments. Biochim Biophys Acta 673: 26–36, 1981 [PubMed] [Google Scholar]
- 33.Farr GA, Hull M, Mellman I, Caplan MJ: Membrane proteins follow multiple pathways to the basolateral cell surface in polarized epithelial cells. J Cell Biol 186: 269–282, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grindstaff KK, Bacallao RL, Nelson WJ: Apiconuclear organization of microtubules does not specify protein delivery from the trans-Golgi network to different membrane domains in polarized epithelial cells. Mol Biol Cell 9: 685–699, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fam SR, Paquet M, Castleberry AM, Oller H, Lee CJ, Traynelis SF, Smith Y, Yun CC, Hall RA: P2Y1 receptor signaling is controlled by interaction with the PDZ scaffold NHERF-2. Proc Natl Acad Sci USA 102: 8042–8047, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Boulan E, Kreitzer G, Müsch A: Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol 6: 233–247, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Gisler SM, Pribanic S, Bacic D, Forrer P, Gantenbein A, Sabourin LA, Tsuji A, Zhao ZS, Manser E, Biber J, Murer H: PDZK1: I. A major scaffolder in brush borders of proximal tubular cells. Kidney Int 64: 1733–1745, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Kocher O, Comella N, Tognazzi K, Brown LF: Identification and partial characterization of PDZK1: A novel protein containing PDZ interaction domains. Lab Invest 78: 117–125, 1998 [PubMed] [Google Scholar]
- 39.Thomson RB, Wang T, Thomson BR, Tarrats L, Girardi A, Mentone S, Soleimani M, Kocher O, Aronson PS: Role of PDZK1 in membrane expression of renal brush border ion exchangers. Proc Natl Acad Sci USA 102: 13331–13336, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaufman L, Potla U, Coleman S, Dikiy S, Hata Y, Kurihara H, He JC, D’Agati VD, Klotman PE: Up-regulation of the homophilic adhesion molecule sidekick-1 in podocytes contributes to glomerulosclerosis. J Biol Chem 285: 25677–25685, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanemoto M, Toyohara T, Abe T, Ito S: MAGI-1a functions as a scaffolding protein for the distal renal tubular basolateral K+ channels. J Biol Chem 283: 12241–12247, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Brône B, Eggermont J: PDZ proteins retain and regulate membrane transporters in polarized epithelial cell membranes. Am J Physiol Cell Physiol 288: C20–C29, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Maday S, Anderson E, Chang HC, Shorter J, Satoh A, Sfakianos J, Fölsch H, Anderson JM, Walther Z, Mellman I: A PDZ-binding motif controls basolateral targeting of syndecan-1 along the biosynthetic pathway in polarized epithelial cells. Traffic 9: 1915–1924, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu CY, Chen JY, Lin YY, Shen KF, Lin WL, Chien CL, ter Beest MB, Jou TS: A bipartite signal regulates the faithful delivery of apical domain marker podocalyxin/Gp135. Mol Biol Cell 18: 1710–1722, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cordat E, Li J, Reithmeier RA: Carboxyl-terminal truncations of human anion exchanger impair its trafficking to the plasma membrane. Traffic 4: 642–651, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Fernández-Larrea J, Merlos-Suárez A, Ureña JM, Baselga J, Arribas J: A role for a PDZ protein in the early secretory pathway for the targeting of proTGF-alpha to the cell surface. Mol Cell 3: 423–433, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Ellgaard L, Helenius A: Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol 4: 181–191, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Quilty JA, Li J, Reithmeier RA: Impaired trafficking of distal renal tubular acidosis mutants of the human kidney anion exchanger kAE1. Am J Physiol Renal Physiol 282: F810–F820, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Balkovetz DF: Claudins at the gate: Determinants of renal epithelial tight junction paracellular permeability. Am J Physiol Renal Physiol 290: F572–F579, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Killisch I, Steinlein P, Römisch K, Hollinshead R, Beug H, Griffiths G: Characterization of early and late endocytic compartments of the transferrin cycle. Transferrin receptor antibody blocks erythroid differentiation by trapping the receptor in the early endosome. J Cell Sci 103: 211–232, 1992 [DOI] [PubMed] [Google Scholar]
- 51.Le Panse S, Ayani E, Mulliez N, Chatelet F, Cywiner-Golenzer C, Galceran M, Citadelle D, Roux C, Ronco P, Verroust P: Antibodies to the 280-kd coated pit protein, target of teratogenic antibodies, produce alterations in the traffic of internalized proteins. Am J Pathol 145: 1526–1536, 1994 [PMC free article] [PubMed] [Google Scholar]
- 52.Nolan CM, Creek KE, Grubb JH, Sly WS: Antibody to the phosphomannosyl receptor inhibits recycling of receptor in fibroblasts. J Cell Biochem 35: 137–151, 1987 [DOI] [PubMed] [Google Scholar]
- 53.Geering K, Theulaz I, Verrey F, Häuptle MT, Rossier BC: A role for the beta-subunit in the expression of functional Na+-K+-ATPase in Xenopus oocytes. Am J Physiol 257: C851–C858, 1989 [DOI] [PubMed] [Google Scholar]
- 54.Ramsey IS, DeFelice LJ: Serotonin transporter function and pharmacology are sensitive to expression level: Evidence for an endogenous regulatory factor. J Biol Chem 277: 14475–14482, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Groves JD, Falson P, le Maire M, Tanner MJ: Functional cell surface expression of the anion transport domain of human red cell band 3 (AE1) in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci USA 93: 12245–12250, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Girotti M, Banting G: TGN38-green fluorescent protein hybrid proteins expressed in stably transfected eukaryotic cells provide a tool for the real-time, in vivo study of membrane traffic pathways and suggest a possible role for ratTGN38. J Cell Sci 109: 2915–2926, 1996 [DOI] [PubMed] [Google Scholar]
- 57.MacVinish LJ, Cope G, Ropenga A, Cuthbert AW: Chloride transporting capability of Calu-3 epithelia following persistent knockdown of the cystic fibrosis transmembrane conductance regulator, CFTR. Br J Pharmacol 150: 1055–1065, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ihrke G, Martin GV, Shanks MR, Schrader M, Schroer TA, Hubbard AL: Apical plasma membrane proteins and endolyn-78 travel through a subapical compartment in polarized WIF-B hepatocytes. J Cell Biol 141: 115–133, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.