Abstract
Psoriasis vulgaris is a chronic, debilitating skin disease that affects millions of people worldwide. There is no mouse model that accurately reproduces all facets of the disease, but the accessibility of skin tissue from patients has facilitated the elucidation of many pathways involved in the pathogenesis of psoriasis and highlighted the importance of the immune system in the disease. The pathophysiological relevance of these findings has been supported by genetic studies that identified polymorphisms in genes associated with NFκB activation, IL-23 signaling and T helper 17 (Th17)-cell adaptive immune responses, and in genes associated with the epidermal barrier. Recently developed biologic agents that selectively target specific components of the immune system are highly effective for treating psoriasis. In particular, emerging therapeutics are focused on targeting the IL-23–Th17-cell axis, and several agents that block IL-17 signaling have shown promising results in early-phase clinical trials. This review discusses lessons learned about the pathogenesis of psoriasis from mouse-and patient-based studies, emphasizing how the outcomes of clinical trials with T-cell-targeted and cytokine-blocking therapies have clarified our understanding of the disease.
Introduction
Psoriasis is a debilitating skin disease affecting approximately 125 million persons in Europe, the USA and Japan (Langley et al., 2005). It is a chronic disease, generally characterized by periods of exacerbation and remission. Clinically, psoriasis is characterized by red plaques (due to dilation of blood vessels) with silver or white scales (due to rapid keratinocyte proliferation) that are clearly demarcated from adjacent, normal appearing, non-lesional skin (Fig. 1A). Thus, individuals with psoriasis have areas of involved skin (lesional skin) as well as areas of normal-appearing uninvolved skin (non-lesional skin). Lesions often occur at sites of epidermal trauma, such as the elbows and knees, but can appear anywhere on the body. In addition, it is becoming increasingly clear that psoriasis is not just skin deep. For example, the frequency of seronegative arthritis in individuals with psoriasis has been estimated to be approximately 7–8%, but can be up to 30% in some study populations (Christophers, 2001; Zachariae, 2003). Other co-morbidities observed in individuals with psoriasis can include cardiovascular disease, diabetes mellitus (mainly type 2), metabolic syndrome, obesity, impaired quality of life and depression (Christophers, 2001; Gelfand et al., 2006; Azfar and Gelfand, 2008; Davidovici et al., 2010; Mehta et al., 2010; Nijsten and Stern, 2012). For example, a recent meta-analysis of 22 studies that included over 3 million patients suggested that those with psoriasis had a 1.42-fold increased risk of diabetes (Cheng et al., 2012).
Fig. 1.
Clinical and histological features of psoriasis before and after effective treatment. (A) Clinical presentation of psoriasis showing clearly demarcated red plaques with silver scales. After 12 weeks of treatment with the TNFα inhibitor etanercept, there was marked lesion resolution. (B) Comparative hematoxylin and eosin staining of psoriatic lesional skin showed marked epidermal thickening and cellular infiltration compared with non-lesional skin. These features were reversed 12 weeks post-treatment with etanercept. (C) Increased infiltration of CD3+ T cells in lesional skin compared with non-lesional skin; this infiltration decreased with treatment (week 12). (D) Increased CD11c+ DCs in lesional skin were reduced with treatment (week 12). [Images are unpublished, from a study reported in Zaba et al. (Zaba et al., 2007a).]
Almost 90% of individuals with psoriasis have the most common form of the disease, known as psoriasis vulgaris or plaque psoriasis (Nestle et al., 2009). Many affected individuals have a mild form and can be treated with topical agents, but up to one third of patients have moderate-to-severe psoriasis (affecting >10% body surface area) and require additional therapies (Griffiths and Barker, 2007), including ultraviolet light therapy or systemic medications. Individuals with moderate-to-severe psoriasis often receive ‘rotational’ therapy, whereby drugs are changed after a certain time period to minimize the toxicity of a particular systemic treatment. Although available treatments are successful in many individuals, they do not ‘cure’ the disease, and the associated toxicities mean that improved therapies that target the underlying pathological mechanisms more specifically are urgently needed.
The pathophysiology of psoriasis is complex and dynamic, involving skin cells and immune cells. Cellular studies of mice and patient samples have been complemented by genetic studies (Box 1), which have helped to clarify and confirm many aspects of disease pathophysiology. Histologically, the disease is characterized by acanthosis (thickening of the epidermis) and parakeratosis (retention of nuclei in the stratum corneum, the outermost layer of the epidermis), and thus was once thought to be solely a hyperproliferative disease of keratinocytes (Fig. 1B). However, over the past decade, a large amount of evidence has defined a role for the immune system and its interactive network of leukocytes and cytokines in disease pathogenesis. Psoriatic lesions are highly infiltrated with immune cells, most notably CD3+ T cells and CD11c+ dendritic cells (DCs) (Chamian et al., 2005; Lowes et al., 2005b) (Fig. 1C,D). Pro-inflammatory cytokines produced by these cells – including tumor necrosis factor-α (TNFα), interferon-γ (IFNγ), interleukin-17 (IL-17), IL-22, IL-23, IL-12 and IL-1β – have been linked to the pathogenesis of psoriasis, through causing activation of keratinocytes and other resident cutaneous cells. As discussed in detail below, drugs that inhibit some of these cytokines have shown promise in the clinic. For example, Fig. 1A illustrates an example of successful therapy with the TNFα inhibitor etanercept (Enbrel) (Zaba et al., 2007a). Marked decreases in the number of T cells and DCs, as well as in the cytokines they produce, were observed after effective anti-psoriatic therapy (Zaba et al., 2007a; Johnson-Huang et al., 2010).
Box 1. The genetics of psoriasis.
Many observational studies have supported a role for genetic factors in the development of psoriasis, starting with a positive family history in approximately a third of patients, to the increased incidence in twins (approximately 70% in monozygotic and up to 20% in dizygotic twins, depending on the population studied) (Ruth and Neaton, 1991; Bataille et al., 2012; Kwon et al., 2012). However, finding the genes responsible for the psoriatic phenotype has proven challenging. Whereas Mendelian diseases are caused directly by rare mutations, in common diseases such as psoriasis, the genetic contribution is usually a complex sum of common genetic variants, with many individual alleles conferring modest risk. Early approaches for identifying these genes were linkage studies, later followed by genome-wide association studies (GWAS), which identify single nucleotide polymorphisms (SNPs) – subtle coding variations between individuals – that have a statistical association with a given disease.
The first and most influential psoriasis susceptibility locus, PSORS1 (psoriasis susceptibility 1), was found on chromosome 6p21.3 (Trembath et al., 1997; Nair et al., 2000). This locus contains several genes of potential interest, including HLA-C (human leukocyte antigen C), CCHCR1 (coiled-coil α-helical rod protein 1) and CDSN (corneodesmosin). The HLA-Cw6 allele has been associated with psoriasis in many different populations, indicating that it might be the causal disease susceptibility allele at the PSORS1 locus (Capon et al., 2002; Nair et al., 2006). However, the penetrance of HLA-Cw6 is approximately 10%, so it is not sufficient to explain all of psoriasis heritability (Roberson and Bowcock, 2010). Now, many other PSORS loci have been discovered that have potential roles in epidermal pathology as well as immune dysregulation [comprehensively reviewed by Capon et al. (Capon et al., 2012)]. For example, polymorphisms have been found in genes involved in the epidermal barrier (LCE3B, LCE3C, LCE3D), NFκB activation (REL, TNIP1, TRAF3IP2, TNFAIP3, NFKBIA, FBXL19), and IL-23 signaling and Th17 cell adaptive immune responses (IL23R, IL12B, IL23A) (Capon et al., 2012). Although a relationship between a polymorphism and in vitro responses was recently shown for an IL23R variant (Di Meglio et al., 2011), for most gene associations, functional effects of SNPs remain to be determined.
In 2011, there were several reports that a loss-of-function mutation in the gene encoding an IL-36 receptor antagonist (IL36RN) causes familial pustular psoriasis (Marrakchi et al., 2011; Onoufriadis et al., 2011). This gene encodes an anti-inflammatory innate immune cytokine in the IL-1 family, and implicates the innate immune system in this systemic presentation of psoriasis. This finding might also offer a novel therapeutic target for individuals with this particular form of psoriasis.
Advances in understanding the immune-mediated pathological mechanisms of psoriasis based on both animal and human studies have opened up new therapeutic avenues. In turn, recent data from clinical trials using immune-modifying drugs (Tables 1 and 2) have further informed the research community about the involvement of specific immune-cell types and cytokines in disease pathology. In this Commentary, we review recent lessons learned through animal and human research of immune-mediated pathology in psoriasis, and discuss the outlook for future therapeutic development.
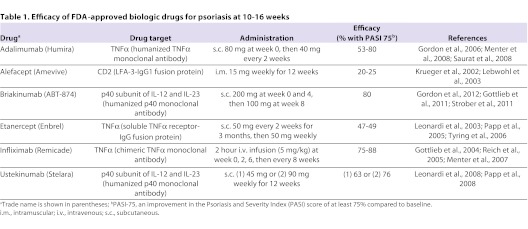
Table 1.
Efficacy of FDA-approved biologic drugs for psoriasis at 10–16 weeks
Table 2.
Psoriasis drugs currently under development
Approaches for studying the pathogenesis of psoriasis
The pathogenic mechanisms of many diseases have been elucidated using animal models. However, many of the key studies that led to a better understanding of the pathogenesis of psoriasis, and to the development of novel therapeutics, have been carried out directly in patients. This is due in part to the fact that there is no currently available mouse model that recapitulates all facets of human psoriasis (Swindell et al., 2011). Nevertheless, several mouse models have been used to provide mechanistic insight into the pathogenesis of psoriasis, as outlined below.
Mouse models of psoriasis
Humanized mouse models in which psoriatic skin is xenografted onto immunodeficient mice have been used as a means to study the immune pathways leading to the development and resolution of psoriatic lesions. Initial studies transplanted psoriatic skin onto athymic nude mice (which lack T cells) or severe combined immunodeficient (SCID) mice (which lack both T and B cells). Lesion development can be induced in non-lesional (normal appearing) skin grafts from psoriasis patients after injection of superantigen-activated leukocytes, providing strong evidence that T cells play a key role in the pathology of the disease (Boehncke et al., 1996; Wrone-Smith and Nickoloff, 1996). More recently, a new model of xenotransplantation has been developed whereby non-lesional skin is engrafted onto AGR129 mice (which lack T and B cells, and also have severely impaired natural killer cell responses). Non-lesional skin grafts transplanted onto AGR129 mice spontaneously convert to lesional skin, suggesting that all of the elements required for the development of psoriasis lesions are present in non-lesional skin (Boyman et al., 2004). The development of lesions in this model is associated with enhanced proliferation of T cells that are resident in non-lesional skin and increased TNFα production. Blocking either of these components prevented development of lesions, supporting the idea that T cells participate in the development of the psoriatic inflammatory cascade (Boyman et al., 2004). Additionally, neutralizing other factors in this model – such as α1β1 integrin (required for the accumulation of T cells in the epidermis), IFNα/β receptor, BDCA-2 [which blocks IFN production by plasmacytoid DCs (pDCs)] or the p19 subunit of IL-23 (which is involved in Th17 cell activation; see Box 2) – has also established the potential contributions of these elements in the generation of psoriasis lesions (Nestle et al., 2005; Conrad et al., 2007; Tonel et al., 2010). Xenotransplantation models have also been used to examine the effects of novel biologic agents in preclinical studies (Villadsen et al., 2003; Schafer et al., 2010).
Box 2. The IL-23–Th17-cell axis.
It was previously thought that helper T cells differentiated into either IFNγ-producing Th1 cells or IL-4-producing Th2 cells, but it is now clear that there are additional distinct Th cell subsets whose differentiation and function do not rely on the transcription factors or cytokines that regulate Th1 or Th2 cells. Among these additional Th cell subsets are Th17 cells, characterized mainly by the production of IL-17, as well as IL-22 and TNFα. IL-17 is a highly pro-inflammatory cytokine that induces production of IL-6, granulocyte macrophage colony stimulating factor (GM-CSF) and various chemokines that are important for the mobilization of other inflammatory cells, including neutrophils (Kolls and Linden, 2004). Th17 cells are involved in the immune response to fungi and extracellular bacteria, and also play a major role in autoimmunity and inflammation.
The factors involved in the differentiation of Th17 cells have been a source of much debate because there seem to be different species-specific requirements. In mice, TGFβ and IL-6 induce Th17 cell differentiation from naive T cell precursors, whereas IL-23 is thought to be involved in the maintenance of IL-17 production (Aggarwal et al., 2003; Bettelli et al., 2006; Mangan et al., 2006). By contrast, in humans, IL-23 (in conjunction with IL-1, IL-6 and in some cases TGFβ) plays a more direct role in the polarization of Th17 cells (Acosta-Rodriguez et al., 2007; Chen et al., 2007; Wilson et al., 2007; Manel et al., 2008; Volpe et al., 2008; Yang et al., 2008; Santarlasci et al., 2009). Elegant mouse studies demonstrated that transfer of Th17 cells generated in the presence of IL-23, but not TGFβ, caused autoimmunity in mice (McGeachy et al., 2007; Ghoreschi et al., 2010). These studies, coupled with the association between polymorphisms in IL-23 genes (IL23A, IL12B and IL23R) and psoriasis susceptibility, highlight the importance of the IL-23–Th17-cell axis in autoimmunity (Capon et al., 2007; Cargill et al., 2007; Nair et al., 2008; Nair et al., 2009).
Injection of certain inflammatory cytokines (e.g. IL-23) or transgenic overexpression of growth factors or signaling molecules [such as endothelial-specific receptor tyrosine kinase, constitutively active STAT3, amphiregulin or a latent form of transforming growth factor-β (TGFβ1)] in mouse keratinocytes induces epidermal hyperplasia that mirrors some features of psoriatic lesional skin (Cook et al., 1997; Kopp et al., 2003; Li et al., 2004; Sano et al., 2005; Zheng et al., 2007; Wolfram et al., 2009; Rizzo et al., 2011). However, although these models might help in understanding the functions of particular genes that are elevated in psoriasis, they are inherently limited because the phenotype resulting from fixed transgenes cannot be reversed in the same way that the inflammatory pathways in human psoriasis are ‘shut-off’ with effective treatment. Additionally, although these models might mimic the epidermal hyperplasia seen in human psoriasis, they do not accurately reflect all of the genomic changes that occur in psoriatic lesional skin, in which hundreds of inflammation-associated genes are altered compared with uninvolved, non-lesional skin (Suarez-Farinas et al., 2010a; Swindell et al., 2011). An imiquimod-induced dermatitis mouse model has also been proposed to reproduce psoriasis-like skin changes (van der Fits et al., 2009). This has been a useful model to study the T cell phenotype in skin inflammation (Cai et al., 2011) and the role of IL-22 in psoriasiform hyperplasia (Van Belle et al., 2012). Notably, a recent study used a microarray-based analytical approach to evaluate five different mouse models and found that each model had transcriptomic convergences and divergences from human psoriasis (Swindell et al., 2011). Knowledge of which mouse model best represents the pathways of interest for a given study could facilitate more tailored research into specific areas of psoriasis pathogenesis. It is important to note that the structure of mouse and human skin differs substantially, not only in epidermal thickness and the density of hair follicles, but also in the makeup of the inflammatory milieu of mouse and human cells (Lowes et al., 2007).
Patient-based research
The shortcomings of mouse models of psoriasis support a need for patient-based research. The accessibility of human skin has greatly facilitated psoriasis research and there are now many tools available to researchers. For example, serial biopsies of an index plaque can be obtained at various time points during clinical trials to investigate the cellular and molecular changes that occur with treatment. Biopsies of non-lesional and lesional skin are routinely used for comparative histological staining to assess various cell populations, as well as for genomic analysis to identify disease-related genes. Microarray techniques have provided an unbiased tool to generate hypotheses of potential disease-related genes and pathways, and have identified many dramatic genomic modifications in psoriasis compared with non-lesional and normal skin (Zhou et al., 2003; Gudjonsson et al., 2009; Suarez-Farinas et al., 2010a). In addition, immune cells can be isolated from shave biopsies of lesional skin to perform functional ex vivo studies. Using these techniques for determining the mechanisms of action of several different drugs during clinical trials has enabled the elucidation of the interactive immunological networks contributing to psoriasis.
Lessons learned from T-cell-targeted therapies
Early descriptive studies using immunohistochemistry of lesional skin biopsies showed that T cells are prevalent in psoriatic skin (Bos et al., 1983). Phenotyping of these T cells revealed that they were mainly activated memory T cells expressing CD2, CD3, CD5, CLA, CD45RO and activation markers including CD25, HLA-DR and CD27 (Bos and De Rie, 1999; Ferenczi et al., 2000). Furthermore, a skewed Th1 cell polarization profile with increased production of IFNγ and TNFα was observed (Austin et al., 1999). More recently, it has been appreciated that lesional T cells that secrete IL-17 (i.e. Th17 cells; see Box 2) are important contributors to psoriasis pathogenesis (Blauvelt, 2008; Kryczek et al., 2008; Lowes et al., 2008) and that many of these dermal T cells might contain a non-variant γδ T cell receptor (TCR) (Cai et al., 2011; Gray et al., 2011; Sumaria et al., 2011).
Perhaps the most compelling early evidence that psoriasis is a T-cell-mediated disease came from a series of clinical trials. A trial in which individuals with rheumatoid arthritis (who also had psoriasis and psoriatic arthritis) were treated with cyclosporine, a calcineurin antagonist that inhibits T cell activation, led to the finding that this drug also suppressed the clinical appearance of psoriasis (Ellis et al., 1986). Treatment with tacrolimus (FK506), another calcineurin inhibitor, showed similar results (Jegasothy et al., 1992). However, because these agents also inhibited keratinocyte proliferation, the success of treatment could not be attributed solely to effects on T cells. The first clear evidence that T cells contributed to the epidermal abnormalities seen in psoriasis was demonstrated by treatment with the fusion toxin DAB389IL-2, a protein that induces apoptosis of cells expressing functional IL-2 receptors and thus is a highly selective antagonist of activated T cells. DAB389IL-2 treatment successfully depleted lesional T cells and reversed keratinocyte proliferation and regenerative epidermal growth (Gottlieb et al., 1995), highlighting the fact that activated T cells are central to psoriasis.
Subsequently developed therapies aimed to target T cell activation more specifically. T cell activation is negatively regulated by cytotoxic T lymphocyte antigen 4 (CTLA4), the expression of which is upregulated on the cell surface after T cell activation (June et al., 1990). When a soluble CTLA4 immunoglobulin (CTLA4Ig) that targets this inhibitory pathway was tested on individuals with psoriasis in a clinical trial, a 50% clinical improvement was achieved in almost half of the patients, with associated reductions in epidermal hyperplasia and lesional T cell numbers (Abrams et al., 1999; Abrams et al., 2000). Subsequently, alefacept (Amevive), which more selectively blocks the activation of effector memory T cells, but not naive T cells, was developed specifically for the treatment of psoriasis (Ellis and Krueger, 2001; Chamian et al., 2007). In clinical trials with this drug, PASI-75 [i.e. an improvement in the Psoriasis and Severity Index (PASI) score of at least 75% compared with baseline] was achieved in 28% of treated individuals, compared with only 8% of those in the placebo group (Krueger et al., 2002). The greatest clinical responses were correlated with the largest reduction in circulating memory T cell numbers, decreased numbers of lesional T cells, and decreased tissue expression of IFNγ, induced nitric oxide synthase (iNOS), IL-8 and IL-23 (Ortonne et al., 2003; Chamian et al., 2005).
Efalizumab (Raptiva) is a humanized monoclonal antibody that blocks interactions between T-cell-associated CD11a/LFA-1 and ICAM-1 on antigen presenting cells, as well as T cell activation and trafficking to skin. In a clinical trial with this drug, PASI-75, as well as a decreased number of lesional T cells and reduced epidermal thickness, were seen in 27% of patients compared with only 4% of patients that received placebo (Gordon et al., 2003). However, after discontinuation of efalizumab, up to 14% of patients had a dramatic worsening of psoriasis (Carey et al., 2006). Furthermore, a few cases of progressive multifocal leukoencephalopathy (PML), an often-fatal brain infection, were reported in efalizumab-treated patients (Sterry et al., 2009), and this drug was withdrawn from the market.
In general, T-cell-targeted therapies for psoriasis are only effective in a small percentage of patients and carry the risk of severe immunosuppression due to the fact that they globally suppress T cell activation and cytokine production. Thus, there is still an urgent need for more targeted therapies.
Lessons learned from anti-cytokine therapies
TNFα blockade
TNFα is prevalent in many inflammatory diseases, including psoriasis. In psoriasis, TNFα is produced by keratinocytes, DCs [particularly TNFα- and iNOS-producing DCs (TIP-DCs)] and by Th1, Th17 and Th22 cells (Lowes et al., 2005b; Lowes et al., 2008; Eyerich et al., 2009). TNFα is not only highly pro-inflammatory on its own, but is also able to synergistically enhance the effects of other pathogenic cytokines in psoriasis (Shen et al., 2006; Eyerich et al., 2009; Chiricozzi et al., 2011).
Similar to T-cell-targeted agents, the therapeutic benefit of TNFα neutralization in psoriasis was found serendipitously when an individual with refractory inflammatory bowel disease and concomitant psoriasis was treated with infliximab (a monoclonal antibody against TNFα; trade name Remicade; Table 1) (Oh et al., 2000). Since then, two additional TNFα inhibitors have been approved for use in psoriasis: adalimumab (Humira) and etanercept. Each agent targets TNFα in a slightly different manner. Infliximab is a chimeric monoclonal antibody that neutralizes soluble and membrane bound forms of TNFα (Reich et al., 2005). Adalimumab is a fully humanized IgG1 monoclonal antibody (Menter et al., 2008). Etanercept is a recombinant soluble receptor consisting of the extracellular ligand-binding domain of the TNFαreceptor fused to the Fc domain of human IgG1; it can neutralize soluble TNFα as well as lymphotoxin-α (also known as TNFβ) (Papp et al., 2005).
TNFα inhibitors were approved for the treatment of rheumatoid arthritis years before being approved for psoriasis. In rheumatoid arthritis, these agents act therapeutically by downregulating the expression of innate pro-inflammatory cytokines, including IL-1β, IL-6 and IL-8. However, in psoriasis, the mechanism of action of etanercept is different. Genomic comparisons between psoriasis patients that respond to etanercept treatment and those that do not revealed that, although both groups downregulate these innate cytokines, successful treatment outcomes were correlated with decreased expression of genes associated with the differentiation and function of Th17 cells (thought to be downstream of TNFα production) (Zaba et al., 2007a; Zaba et al., 2009a). Non-responding patients maintained the expression of these genes. Furthermore, rapid suppression of IL-23 and the Th17 cell axis preceded downregulation of IFNγ-associated genes, which correlated with final disease resolution (Zaba et al., 2007a; Zaba et al., 2009a). Recently, TNFα and IL-17 have been shown to synergistically induce many keratinocyte pro-inflammatory products that are involved in psoriasis (Chiricozzi et al., 2011). Thus, blocking either of these pathways (TNFα or IL-23–Th17) might have dramatic effects on keratinocyte production of pathogenic downstream molecules.
TNFαinhibitors are highly effective for the treatment of psoriasis (Table 1). However, the degree of immunosuppression induced by blocking TNFα can lead to adverse events. Patients treated with these agents have increased risk of developing serious infections, including sepsis and opportunistic infections, and of reactivating latent tuberculosis (Galloway et al., 2011). Furthermore, some studies have linked anti-TNFα therapy, particularly when used in conjunction with other drugs, to an increased incidence of lymphoma and other malignancies (Lakatos and Miheller, 2010; Mariette et al., 2010; Herrinton et al., 2011). Recently, it was reported that the long-term (>4 years) adherence rate of individuals with psoriasis to anti-TNFα drug therapy was 40% for etanercept and adalimumab and 70% for infliximab; reasons for discontinuation of treatment included loss of efficacy and the occurrence of adverse events (Gniadecki et al., 2011).
Targeting the p40 subunit common to IL-12 and IL-23
IL-23 is a heterodimeric cytokine composed of a unique p19 subunit (encoded by IL23A) and the p40 subunit (encoded by IL12B); together with p35, p40 also forms part of IL-12. IL-12 and IL-23 are primarily produced by myeloid DCs and macrophages, and are involved in the activation of Th1 and Th17 cells, respectively (Lee et al., 2004; Zhou et al., 2007). The expression of both subunits of IL-23 was found to be significantly upregulated in lesional psoriatic skin, whereas the unique IL-12 subunit, p35, was not (Lee et al., 2004; Tonel et al., 2010), implying that the effects of IL-23 might be dominant in psoriasis. This hypothesis was further supported by genetic studies (Box 1). For example, GWAS identified the IL-23 signaling pathway (IL23A, IL12 and IL23R) as a risk factor for the development of psoriasis (Capon et al., 2007; Cargill et al., 2007; Nair et al., 2008; Nair et al., 2009). Furthermore, in a more recent study, the IL23R R381Q gene variant was actually found to be protective against psoriasis by impairing the IL-23-induced Th17 cell effector response (Di Meglio et al., 2011). Selective neutralization of IL-23p19 in a humanized mouse model (see above) prevented the development of psoriasis (Tonel et al., 2010), further implicating IL-23 in the development of psoriasis lesions.
Ustekinumab (Stelara), a fully humanized monoclonal antibody that neutralizes the shared p40 subunit of IL-12 and IL-23, is now FDA-approved. In clinical trials, PASI-75 was achieved in greater than 60% of psoriasis patients treated with ustekinumab (Table 1), compared with only 3% in the placebo-treated group, after 12 weeks (Leonardi et al., 2008; Papp et al., 2008). Clinical trials with another p40 inhibitor, briakinumab (ABT874; Table 1), were similarly successful, with just over 80% of patients achieving a PASI-75 at 12 weeks (Gottlieb et al., 2011; Strober et al., 2011; Gordon et al., 2012).
Clearly, TNFα and IL-23 are both involved in the pathology of psoriasis, but it is still not clear which of these might represent the best drug target. One randomized trial compared treatment with ustekinumab (p40 neutralization) to treatment with etanercept (TNFα inhibition), and found that, although both drugs resulted in PASI-75 at week 12 in most patients (68% with 45 mg and 74% with 90 mg ustekinumab; 57% with etanercept) and downregulation of the same inflammatory genes, ustekinumab was clinically superior to etanercept as evaluated by the physicians’ global assessment (Griffiths et al., 2010; Krueger et al., 2010). Additionally, approximately half of the patients that did not respond to etanercept achieved PASI-75 after switching to ustekinumab (Griffiths et al., 2010). This study supports the idea that IL-23 is dominant over TNFα in the pathogenesis of psoriasis. This might be attributed to the fact that IL-23 has direct effects on T cell activation and cytokine production, whereas TNFα might have a more indirect role in T cell activation through inducing IL-23. Thus, in patients in which TNFα inhibition is ineffective, IL-23 levels remain high, resulting in sustained Th17 cell activation and cytokine production (Zaba et al., 2009a). Notably, antagonism of IL-23 dampens downstream T cell responses while preserving innate immune responses elicited by TNFα, and thus should be safer and induce fewer adverse events.
New emerging targets
As discussed, the cytokine antagonists that have been FDA-approved have been shown to be efficacious for the treatment of psoriasis (Table 1). However, as the key players in the pathogenic cascade of psoriasis were more clearly defined, it became clear that more specific molecules could be developed for treatment. More selective targeting should enable suppression of pathogenic immune responses while minimizing the risks of global immunosuppression seen with other agents (e.g. TNFα inhibitors). Several drugs in the pipeline (Table 2) are directed against components of the IL-23–Th17 cell axis, including the cytokines IL-23 (p19 subunit), IL-17 and its receptor, and IL-22. Many of the clinical studies for these agents are still underway.
Thus far, although drugs that specifically target the p19 subunit of IL-23 have not yet been FDA-approved for psoriasis treatment, encouraging results from a Phase 1 study of a single dose of anti-IL-23p19 (CNTO 1959; Table 2) in individuals with moderate-to-severe psoriasis were recently reported at the 6th International Congress of Psoriasis: from Gene to Clinic meeting in November 2011 (Sofen et al., 2011). PASI-75 was observed in all patients in a 300 mg treatment cohort, indicating that targeting the p19 subunit of IL-23 might be even more beneficial than blockade of the p40 subunit shared by IL-12 and IL-23. In addition, one anti-IL-17 agent (AIN457) has shown tremendous success for treatment of psoriasis, as well as for rheumatoid arthritis and uveitis (Hueber et al., 2010). Data on the effectiveness of three subcutaneous doses of another anti-IL-17 agent (LY2439821) in chronic psoriasis were recently published (Krueger et al., 2012). In this case, all eight patients receiving the highest dose of AIN457 at weeks 0, 2 and 4 achieved a PASI-75 by week 6. Additionally, JAK3 inhibitors, which prevent signaling of common γ-chain cytokines (IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21) that are involved in T cell proliferation, activation and survival, have also shown potential success in Phase 1 trials (Boy et al., 2009).
Putting together the puzzle: current model of psoriasis pathogenesis
Coupling basic science research with data obtained from clinical trials has established a paradigm of psoriasis disease pathogenesis that involves genetic risk factors and a complex network of inflammatory cells and cytokines. Psoriasis pathogenesis can be divided into two phases: initiation and maintenance. The abovementioned studies have mostly been conducted after psoriasis has been established and have given many insights into the maintenance phase of psoriasis. A current model of this maintenance phase of psoriasis, which integrates the contributions of CD11c+ myeloid DCs and T cells and the cytokines they produce, is presented in Fig. 2. In addition to resident myeloid DCs (marked by CD11c and CD1c) found in steady-state normal skin, psoriatic lesions contain an additional population of inflammatory DCs that express CD11c, but lack CD1c (Zaba et al., 2007b; Zaba et al., 2009b). Effective treatment of psoriasis with efalizumab, alefacept, cyclosporine, etanercept or narrow-band ultraviolet B radiation was correlated with decreased numbers of these inflammatory myeloid DCs in psoriasis lesions (Chamian et al., 2005; Lowes et al., 2005a; Zaba et al., 2007a; Haider et al., 2008; Johnson-Huang et al., 2010), strengthening the argument that CD11c+CD1c− inflammatory DCs are involved in disease pathogenesis. These DCs express inflammatory molecules (such as TLR1, TLR2, S100A7 and TRAIL) and secrete mediators (including TNFα, iNOS, IL-23 and IL-20) that are involved in downstream activation of T cells and keratinocytes (Lowes et al., 2005b; Zaba et al., 2007a; Guttman-Yassky et al., 2008; Zaba et al., 2010). DCs isolated from psoriasis lesions induced the production of IFNγ and IL-17 by Th1 and Th17 cells, respectively, as well as by a population of Th cells that produced both cytokines (Zaba et al., 2009b). All of these Th cell populations were abundant in psoriasis lesions (Lowes et al., 2008). Th17 cells have also been shown to be the main producers of IL-22, although recently, cells that exclusively produce IL-22 (termed Th22 cells) have been identified as a distinct Th cell subset that is present in lesional psoriatic skin (Eyerich et al., 2009; Nograles et al., 2009; Trifari et al., 2009). Whereas IFNγ and IL-17 induce chemokine expression by keratinocytes and the subsequent recruitment of immune cells to the skin, IL-22 leads to aberrant keratinocyte proliferation and epidermal hyperplasia (Boniface et al., 2005; Sa et al., 2007; Nograles et al., 2008). Moreover, IL-17 and IL-22 cooperatively induce the expression of antimicrobial peptides by keratinocytes (Liang et al., 2006).
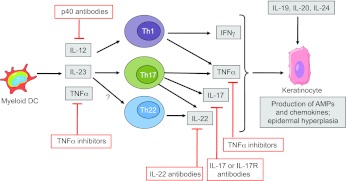
Fig. 2.
Current model of the maintenance phase of psoriasis, showing the targets of approved or emerging psoriasis drugs. Myeloid DCs produce cytokines that induce IFNγ production by Th1 cells and IL-17 production by Th17 cells. IL-23 also induces production of IL-22 by Th17 and possibly Th22 cells. Th cell cytokines IFNγ, IL-17 and TNFα cooperate to induce the production of anti-microbial peptides (AMPs) and chemokines by keratinocytes, thereby enhancing immune-cell recruitment and inflammation in lesions. IL-22 is also involved in promoting epidermal hyperplasia. The IL-20 subfamily cytokines (IL-19, IL-20 and IL-24), which are mainly produced by monocytes, also contribute to epidermal hyperplasia. Drugs that are currently FDA-approved target upstream molecules in this pathway (anti-p40 antibodies and TNF inhibitors), whereas drugs in the pipeline (antibodies targeting IL-17, IL-17R or IL-22) are directed against downstream molecules.
Much is known about the immunological circuits that maintain established psoriasis lesions, but how these lesions initially develop is still unclear. pDCs have been implicated in the development of psoriasis lesions. IFNα-producing pDCs have been shown to be increased in psoriasis lesions compared with normal skin (Nestle et al., 2005), and an activated type I IFN (IFNα/β) genomic signature in psoriasis has been reported, suggesting that this cytokine family contributes to the disease (Yao et al., 2008). As discussed above, blocking pDC production of IFNα was found to prevent spontaneous development of psoriasis in xenograft transplant mouse models (Nestle et al., 2005). Furthermore, recent evidence has pointed to a role for the antimicrobial peptide LL-37 (cathelicidin) in the induction of IFNα production by pDCs. LL-37 is induced by skin infections or injury (Marrakchi et al., 2011) and is upregulated in lesional psoriatic skin. LL-37 forms complexes with self-DNA (released from dying cells) and, through TLR9 engagement, induces IFNα production by pDCs and subsequent activation of myeloid DCs (Lande et al., 2007). Additionally, LL-37 complexes with self-RNA to directly mature myeloid DCs and induce production of TNFα, IL-6 and potentially IL-23 in a TLR7-and TLR8-dependent manner (Ganguly et al., 2009). Interestingly, self-RNA–LL-37 complexes colocalize with DC-LAMP+ mature myeloid DCs in psoriatic lesions (Ganguly et al., 2009). Hence, pDCs might play a role in lesion initiation, leading to the recruitment and activation of myeloid DCs and T cells that are responsible for lesion maintenance.
Remaining questions
Although there have been many advances in our knowledge of psoriasis pathogenesis in recent years, questions remain. First, it will be important to determine whether knowledge of a given individual’s genomic signature can predict who will develop psoriasis, determine which drug will be most effective or predict responsiveness to a particular drug. This concept of personalized medicine was recently examined in a study involving psoriasis patients treated with alefacept: analysis of microarray data showed that changes in a specific group of genes could predict whether a patient would be a responder or non-responder to the drug (Suarez-Farinas et al., 2010b). Furthermore, in an elegant, novel application of recently implicated psoriasis genetic loci, Chen et al. proposed the use of a ‘weighted genetic risk score’ (wGRS), which combines ten psoriasis risk loci, as a tool to predict risk of developing psoriasis (Chen et al., 2011). The ten single nucleotide polymorphisms (SNPs), which are common risk variants chosen from the results of GWAS on the basis of significance in at least one independent study, are IL23R, LCE3C/3D, IL13, TNIP1/ANXA6, IL12B, CDKAL1, HLA-C, TNFAIP3, IL23A/STAT2 and ZNF313. This combination of SNPs collectively captures more risk than any SNP considered alone. A higher wGRS was found to be associated with early disease onset and a positive family history in a large group of European patients. However, only 11.6% of psoriasis heritability was captured by this combination of common risk variants, and the most dominant risk locus was HLA-C, which has a predictive capability as strong as that of the other nine non-MHC loci combined. Nevertheless, this study suggests how emerging data on common genetic variants might be used in the future to predict risk of developing psoriasis.
Second, although more sophisticated genomic analyses continue to uncover previously unknown genetic mutations implicated in psoriasis pathogenesis, it is still unclear whether certain mutations (e.g. in innate versus adaptive immune genes) are associated with different clinical forms of the disease. For example, as discussed in Box 1, two recent studies linked the innate immune gene IL-36 receptor antagonist (IL36RN) to generalized pustular psoriasis (Marrakchi et al., 2011; Onoufriadis et al., 2011). Further studies are needed to prospectively study the relationships between different clinical phenotypes of psoriasis and specific genetic variants.
Third, because it is now appreciated that psoriasis is not merely ‘skin deep’ but also linked to many co-morbidities (Davidovici et al., 2010), it will be interesting to determine whether reversal of the skin manifestations also minimizes systemic effects. Two retrospective studies showed that successful treatment of psoriasis (with TNFα inhibitors or anti-p40 agents) did not reduce the risk of adverse cardiovascular events (Abuabara et al., 2011; Ryan et al., 2011). However, there were limitations to these studies and additional careful prospective studies are required to address this important issue. Positron emission tomography-computed tomography (PET-CT) scans could be used to non-invasively measure systemic and cutaneous inflammation (Mehta et al., 2011) in studies that address how co-morbidities are affected by psoriasis treatments. Continuing to expand our knowledge of disease pathogenesis will help to inform the development of newer and more targeted therapeutics.
Footnotes
COMPETING INTERESTS
J.G.K. has served as a consultant for many pharmaceutical companies, including those mentioned in this paper.
FUNDING
This work was supported by National Institutes of Health (NIH) grant UL1 RR024143 from the National Center for Research Resources (NCRR). L.M.J.-H. is supported by the Linda and Leonard Berkowitz Postdoctoral Fellowship. M.A.L. is supported by NIH 1R01AR060222.
REFERENCES
- Abrams J. R., Lebwohl M. G., Guzzo C. A., Jegasothy B. V., Goldfarb M. T., Goffe B. S., Menter A., Lowe N. J., Krueger G., Brown M. J., et al. (1999). CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J. Clin. Invest. 103, 1243–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams J. R., Kelley S. L., Hayes E., Kikuchi T., Brown M. J., Kang S., Lebwohl M. G., Guzzo C. A., Jegasothy B. V., Linsley P. S., et al. (2000). Blockade of T lymphocyte costimulation with cytotoxic T lymphocyte-associated antigen 4-immunoglobulin (CTLA4Ig) reverses the cellular pathology of psoriatic plaques, including the activation of keratinocytes, dendritic cells, and endothelial cells. J. Exp. Med. 192, 681–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuabara K., Lee H., Kimball A. B. (2011). The effect of systemic psoriasis therapies on the incidence of myocardial infarction: a cohort study. Br. J. Dermatol. 165, 1066–1073 [DOI] [PubMed] [Google Scholar]
- Acosta-Rodriguez E. V., Napolitani G., Lanzavecchia A., Sallusto F. (2007). Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 8, 942–949 [DOI] [PubMed] [Google Scholar]
- Aggarwal S., Ghilardi N., Xie M. H., de Sauvage F. J., Gurney A. L. (2003). Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278, 1910–1914 [DOI] [PubMed] [Google Scholar]
- Austin L. M., Ozawa M., Kikuchi T., Walters I. B., Krueger J. G. (1999). The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J. Invest. Dermatol. 113, 752–759 [DOI] [PubMed] [Google Scholar]
- Azfar R. S., Gelfand J. M. (2008). Psoriasis and metabolic disease: epidemiology and pathophysiology. Curr. Opin. Rheumatol. 20, 416–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataille V., Lens M., Spector T. D. (2012). The use of the twin model to investigate the genetics and epigenetics of skin diseases with genomic, transcriptomic and methylation data. J. Eur. Acad. Dermatol. Venereol. [Epub ahead of print] doi: 10.1111/j.1468-3083.2011.04444.x [DOI] [PubMed] [Google Scholar]
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T. B., Oukka M., Weiner H. L., Kuchroo V. K. (2006). Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 [DOI] [PubMed] [Google Scholar]
- Blauvelt A. (2008). T-helper 17 cells in psoriatic plaques and additional genetic links between IL-23 and psoriasis. J. Invest. Dermatol. 128, 1064–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehncke W. H., Dressel D., Zollner T. M., Kaufmann R. (1996). Pulling the trigger on psoriasis. Nature 379, 777. [DOI] [PubMed] [Google Scholar]
- Boniface K., Bernard F. X., Garcia M., Gurney A. L., Lecron J. C., Morel F. (2005). IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J. Immunol. 174, 3695–3702 [DOI] [PubMed] [Google Scholar]
- Bos J. D., De Rie M. A. (1999). The pathogenesis of psoriasis: immunological facts and speculations. Immunol. Today 20, 40–46 [DOI] [PubMed] [Google Scholar]
- Bos J. D., Hulsebosch H. J., Krieg S. R., Bakker P. M., Cormane R. H. (1983). Immunocompetent cells in psoriasis. In situ immunophenotyping by monoclonal antibodies. Arch. Dermatol. Res. 275, 181–189 [DOI] [PubMed] [Google Scholar]
- Boy M. G., Wang C., Wilkinson B. E., Chow V. F., Clucas A. T., Krueger J. G., Gaweco A. S., Zwillich S. H., Changelian P. S., Chan G. (2009). Double-blind, placebo-controlled, dose-escalation study to evaluate the pharmacologic effect of CP-690,550 in patients with psoriasis. J. Invest. Dermatol. 129, 2299–2302 [DOI] [PubMed] [Google Scholar]
- Boyman O., Hefti H. P., Conrad C., Nickoloff B. J., Suter M., Nestle F. O. (2004). Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-alpha. J. Exp. Med. 199, 731–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Shen X., Ding C., Qi C., Li K., Li X., Jala V. R., Zhang H. G., Wang T., Zheng J., et al. (2011). Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity 35, 596–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capon F., Munro M., Barker J., Trembath R. (2002). Searching for the major histocompatibility complex psoriasis susceptibility gene. J. Invest. Dermatol. 118, 745–751 [DOI] [PubMed] [Google Scholar]
- Capon F., Di Meglio P., Szaub J., Prescott N. J., Dunster C., Baumber L., Timms K., Gutin A., Abkevic V., Burden A. D., et al. (2007). Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum. Genet. 122, 201–206 [DOI] [PubMed] [Google Scholar]
- Capon F., Burden A. D., Trembath R. C., Barker J. N. (2012). Psoriasis and other complex trait dermatoses: from Loci to functional pathways. J. Invest. Dermatol. 132, 915–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey W., Glazer S., Gottlieb A. B., Lebwohl M., Leonardi C., Menter A., Papp K., Rundle A. C., Toth D. (2006). Relapse, rebound, and psoriasis adverse events: an advisory group report. J. Am. Acad. Dermatol. 54, S171–S181 [DOI] [PubMed] [Google Scholar]
- Cargill M., Schrodi S. J., Chang M., Garcia V. E., Brandon R., Callis K. P., Matsunami N., Ardlie K. G., Civello D., Catanese J. J., et al. (2007). A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am. J. Hum. Genet. 80, 273–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamian F., Lowes M. A., Lin S. L., Lee E., Kikuchi T., Gilleaudeau P., Sullivan-Whalen M., Cardinale I., Khatcherian A., Novitskaya I., et al. (2005). Alefacept reduces infiltrating T cells, activated dendritic cells, and inflammatory genes in psoriasis vulgaris. Proc. Natl. Acad. Sci. USA 102, 2075–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamian F., Lin S. L., Lee E., Kikuchi T., Gilleaudeau P., Sullivan-Whalen M., Cardinale I., Khatcherian A., Novitskaya I., Wittkowski K. M., et al. (2007). Alefacept (anti-CD2) causes a selective reduction in circulating effector memory T cells (Tem) and relative preservation of central memory T cells (Tcm) in psoriasis. J. Transl. Med. 5, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Poon A., Yeung C., Helms C., Pons J., Bowcock A. M., Kwok P. Y., Liao W. (2011). A genetic risk score combining ten psoriasis risk loci improves disease prediction. PLoS ONE 6, e19454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Tato C. M., Muul L., Laurence A., O’Shea J. J. (2007). Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum. 56, 2936–2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Kuai D., Zhang L., Yang X., Qiu B. (2012). Psoriasis increased the risk of diabetes: a meta-analysis. Arch. Dermatol. Res. 304, 119–125 [DOI] [PubMed] [Google Scholar]
- Chiricozzi A., Guttman-Yassky E., Suarez-Farinas M., Nograles K. E., Tian S., Cardinale I., Chimenti S., Krueger J. G. (2011). Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J. Invest. Dermatol. 131, 677–687 [DOI] [PubMed] [Google Scholar]
- Christophers E. (2001). Psoriasis-epidemiology and clinical spectrum. Clin. Exp. Dermatol. 26, 314–320 [DOI] [PubMed] [Google Scholar]
- Conrad C., Boyman O., Tonel G., Tun-Kyi A., Laggner U., de Fougerolles A., Kotelianski V., Gardner H., Nestle F. O. (2007). Alpha1beta1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat. Med. 13, 836–842 [DOI] [PubMed] [Google Scholar]
- Cook P. W., Piepkorn M., Clegg C. H., Plowman G. D., DeMay J. M., Brown J. R., Pittelkow M. R. (1997). Transgenic expression of the human amphiregulin gene induces a psoriasis-like phenotype. J. Clin. Invest. 100, 2286–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovici B. B., Sattar N., Prinz J. C., Puig L., Emery P., Barker J. N., van de Kerkhof P., Stahle M., Nestle F. O., Girolomoni G., et al. (2010). Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J. Invest. Dermatol. 130, 1785–1796 [DOI] [PubMed] [Google Scholar]
- Di Meglio P., Di Cesare A., Laggner U., Chu C. C., Napolitano L., Villanova F., Tosi I., Capon F., Trembath R. C., Peris K., et al. (2011). The IL23R R381Q gene variant protects against immune-mediated diseases by impairing IL-23-induced Th17 effector response in humans. PLoS ONE 6, e17160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis C. N., Krueger G. G. (2001). Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. N. Engl. J. Med. 345, 248–255 [DOI] [PubMed] [Google Scholar]
- Ellis C. N., Gorsulowsky D. C., Hamilton T. A., Billings J. K., Brown M. D., Headington J. T., Cooper K. D., Baadsgaard O., Duell E. A., Annesley T. M., et al. (1986). Cyclosporine improves psoriasis in a double-blind study. JAMA 256, 3110–3116 [PubMed] [Google Scholar]
- Eyerich S., Eyerich K., Pennino D., Carbone T., Nasorri F., Pallotta S., Cianfarani F., Odorisio T., Traidl-Hoffmann C., Behrendt H., et al. (2009). Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J. Clin. Invest. 119, 3573–3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi K., Burack L., Pope M., Krueger J. G., Austin L. M. (2000). CD69, HLA-DR and the IL-2R identify persistently activated T cells in psoriasis vulgaris lesional skin: blood and skin comparisons by flow cytometry. J. Autoimmun. 14, 63–78 [DOI] [PubMed] [Google Scholar]
- Galloway J. B., Hyrich K. L., Mercer L. K., Dixon W. G., Fu B., Ustianowski A. P., Watson K. D., Lunt M., Symmons D. P. (2011). Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology (Oxford) 50, 124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly D., Chamilos G., Lande R., Gregorio J., Meller S., Facchinetti V., Homey B., Barrat F. J., Zal T., Gilliet M. (2009). Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 206, 1983–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand J. M., Neimann A. L., Shin D. B., Wang X., Margolis D. J., Troxel A. B. (2006). Risk of myocardial infarction in patients with psoriasis. JAMA 296, 1735–1741 [DOI] [PubMed] [Google Scholar]
- Ghoreschi K., Laurence A., Yang X. P., Tato C. M., McGeachy M. J., Konkel J. E., Ramos H. L., Wei L., Davidson T. S., Bouladoux N., et al. (2010). Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature 467, 967–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gniadecki R., Kragballe K., Dam T. N., Skov L. (2011). Comparison of drug survival rates for adalimumab, etanercept and infliximab in patients with psoriasis vulgaris. Br. J. Dermatol. 164, 1091–1096 [DOI] [PubMed] [Google Scholar]
- Gordon K. B., Papp K. A., Hamilton T. K., Walicke P. A., Dummer W., Li N., Bresnahan B. W., Menter A. (2003). Efalizumab for patients with moderate to severe plaque psoriasis: a randomized controlled trial. JAMA 290, 3073–3080 [DOI] [PubMed] [Google Scholar]
- Gordon K. B., Langley R. G., Leonardi C., Toth D., Menter M. A., Kang S., Heffernan M., Miller B., Hamlin R., Lim L., et al. (2006). Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension study. J. Am. Acad. Dermatol. 55, 598–606 [DOI] [PubMed] [Google Scholar]
- Gordon K. B., Langley R. G., Gottlieb A. B., Papp K. A., Krueger G. G., Strober B. E., Williams D. A., Gu Y., Valdes J. M. (2012). A phase III, randomized, controlled trial of the fully human IL-12/23 mAb briakinumab in moderate-to-severe psoriasis. J. Invest. Dermatol. 132, 304–314 [DOI] [PubMed] [Google Scholar]
- Gottlieb A. B., Evans R., Li S., Dooley L. T., Guzzo C. A., Baker D., Bala M., Marano C. W., Menter A. (2004). Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J. Am. Acad. Dermatol. 51, 534–542 [DOI] [PubMed] [Google Scholar]
- Gottlieb A. B., Leonardi C., Kerdel F., Mehlis S., Olds M., Williams D. A. (2011). Efficacy and safety of briakinumab vs. etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br. J. Dermatol. 165, 652–660 [DOI] [PubMed] [Google Scholar]
- Gottlieb S. L., Gilleaudeau P., Johnson R., Estes L., Woodworth T. G., Gottlieb A. B., Krueger J. G. (1995). Response of psoriasis to a lymphocyte-selective toxin (DAB389IL-2) suggests a primary immune, but not keratinocyte, pathogenic basis. Nat. Med. 1, 442–447 [DOI] [PubMed] [Google Scholar]
- Gray E. E., Suzuki K., Cyster J. G. (2011). Cutting edge: Identification of a motile IL-17-producing gammadelta T cell population in the dermis. J. Immunol. 186, 6091–6095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths C. E., Barker J. N. (2007). Pathogenesis and clinical features of psoriasis. Lancet 370, 263–271 [DOI] [PubMed] [Google Scholar]
- Griffiths C. E., Strober B. E., van de Kerkhof P., Ho V., Fidelus-Gort R., Yeilding N., Guzzo C., Xia Y., Zhou B., Li S., et al. (2010). Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N. Engl. J. Med. 362, 118–128 [DOI] [PubMed] [Google Scholar]
- Gudjonsson J. E., Ding J., Li X., Nair R. P., Tejasvi T., Qin Z. S., Ghosh D., Aphale A., Gumucio D. L., Voorhees J. J., et al. (2009). Global gene expression analysis reveals evidence for decreased lipid biosynthesis and increased innate immunity in uninvolved psoriatic skin. J. Invest. Dermatol. 129, 2795–2804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman-Yassky E., Lowes M. A., Fuentes-Duculan J., Zaba L. C., Cardinale I., Nograles K. E., Khatcherian A., Novitskaya I., Carucci J. A., Bergman R., et al. (2008). Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J. Immunol. 181, 7420–7427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider A., Lowes M., Suárez-Fariñas M., Zaba L., Cardinale I., Khatcherian A., Novitskaya I., Wittkowski K. M., Krueger J. (2008). Identification of cellular pathways of “type 1,” Th17 T cells, and TNF- and inducible nitric oxide synthase-producing dendritic cells in autoimmune inflammation through pharmacogenomic study of cyclosporine A in psoriasis. J. Immunol. 180, 1913–1920 [DOI] [PubMed] [Google Scholar]
- Herrinton L. J., Liu L., Weng X., Lewis J. D., Hutfless S., Allison J. E. (2011). Role of thiopurine and anti-TNF therapy in lymphoma in inflammatory bowel disease. Am. J. Gastroenterol. 106, 2146–2153 [DOI] [PubMed] [Google Scholar]
- Hueber W., Patel D. D., Dryja T., Wright A. M., Koroleva I., Bruin G., Antoni C., Draelos Z., Gold M. H., Durez P., et al. (2010). Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci. Transl. Med. 2, 52ra72. [DOI] [PubMed] [Google Scholar]
- Jegasothy B. V., Ackerman C. D., Todo S., Fung J. J., Abu-Elmagd K., Starzl T. E. (1992). Tacrolimus (FK 506) – a new therapeutic agent for severe recalcitrant psoriasis. Arch. Dermatol. 128, 781–785 [PMC free article] [PubMed] [Google Scholar]
- Johnson-Huang L. M., Suarez-Farinas M., Sullivan-Whalen M., Gilleaudeau P., Krueger J. G., Lowes M. A. (2010). Effective narrow-band UVB radiation therapy suppresses the IL-23/IL-17 axis in normalized psoriasis plaques. J. Invest. Dermatol. 130, 2654–2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Linsley P. S., Thompson C. B. (1990). Role of the CD28 receptor in T-cell activation. Immunol. Today 11, 211–216 [DOI] [PubMed] [Google Scholar]
- Kolls J. K., Linden A. (2004). Interleukin-17 family members and inflammation. Immunity 21, 467–476 [DOI] [PubMed] [Google Scholar]
- Kopp T., Lenz P., Bello-Fernandez C., Kastelein R. A., Kupper T. S., Stingl G. (2003). IL-23 production by cosecretion of endogenous p19 and transgenic p40 in keratin 14/p40 transgenic mice: evidence for enhanced cutaneous immunity. J. Immunol. 170, 5438–5444 [DOI] [PubMed] [Google Scholar]
- Krueger G. G., Papp K. A., Stough D. B., Loven K. H., Gulliver W. P., Ellis C. N. (2002). A randomized, double-blind, placebo-controlled phase III study evaluating efficacy and tolerability of 2 courses of alefacept in patients with chronic plaque psoriasis. J. Am. Acad. Dermatol. 47, 821–833 [DOI] [PubMed] [Google Scholar]
- Krueger J., Brodmerkel C., Li K., Suarez-Farinas M. (2010). The molecular profile of psoriatic skin in responders to ustekinumab or etanercept after 12 weeks of treatment: Results from the ACCEPT trial. J. Am. Acad. Dermatol. 62, AB13 [Google Scholar]
- Krueger J. G., Fretzin S., Suarez-Farinas M., Haslett P. A., Phipps K. M., Cameron G. S., McColm J., Katcherian A., Cueto I., White T., et al. (2012). IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J. Allergy Clin. Immunol. [Epub ahead of print] doi:1016/j.jaci.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I., Bruce A. T., Gudjonsson J. E., Johnston A., Aphale A., Vatan L., Szeliga W., Wang Y., Liu Y., Welling T. H., et al. (2008). Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J. Immunol. 181, 4733–4741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H. H., Na S. J., Jo S. J., Youn J. I. (2012). Epidemiology and clinical features of pediatric psoriasis in tertiary referral psoriasis clinic. J. Dermatol. 39, 260–264 [DOI] [PubMed] [Google Scholar]
- Lakatos P. L., Miheller P. (2010). Is there an increased risk of lymphoma and malignancies under anti-TNF therapy in IBD? Curr. Drug Targets 11, 179–186 [DOI] [PubMed] [Google Scholar]
- Lande R., Gregorio J., Facchinetti V., Chatterjee B., Wang Y., Homey B., Cao W., Wang Y., Su B., Nestle F. O., et al. (2007). Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449, 564–569 [DOI] [PubMed] [Google Scholar]
- Langley R. G., Krueger G. G., Griffiths C. E. (2005). Psoriasis: epidemiology, clinical features, and quality of life. Ann. Rheum. Dis. 64 Suppl. 2, ii18–23; discussion ii24–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebwohl M., Christophers E., Langley R., Ortonne J. P., Roberts J., Griffiths C. E. (2003). An international, randomized, double-blind, placebo-controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasis. Arch. Dermatol. 139, 719–727 [DOI] [PubMed] [Google Scholar]
- Lee E., Trepicchio W. L., Oestreicher J. L., Pittman D., Wang F., Chamian F., Dhodapkar M., Krueger J. G. (2004). Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J. Exp. Med. 199, 125–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi C. L., Powers J. L., Matheson R. T., Goffe B. S., Zitnik R., Wang A., Gottlieb A. B. (2003). Etanercept as monotherapy in patients with psoriasis. N. Engl. J. Med. 349, 2014–2022 [DOI] [PubMed] [Google Scholar]
- Leonardi C. L., Kimball A. B., Papp K. A., Yeilding N., Guzzo C., Wang Y., Li S., Dooley L. T., Gordon K. B. (2008). Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 371, 1665–1674 [DOI] [PubMed] [Google Scholar]
- Li A. G., Wang D., Feng X. H., Wang X. J. (2004). Latent TGFbeta1 overexpression in keratinocytes results in a severe psoriasis-like skin disorder. EMBO J. 23, 1770–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S. C., Tan X. Y., Luxenberg D. P., Karim R., Dunussi-Joannopoulos K., Collins M., Fouser L. A. (2006). Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203, 2271–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes M., Bowcock A. M., Krueger J. (2007). Pathogenesis and therapy of psoriasis. Nature 445, 866–873 [DOI] [PubMed] [Google Scholar]
- Lowes M. A., Turton J. A., Krueger J. G., Barnetson R. S. (2005a). Psoriasis vulgaris flare during efalizumab therapy does not preclude future use: a case series. BMC Dermatol. 5, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes M. A., Chamian F., Abello M. V., Fuentes-Duculan J., Lin S. L., Nussbaum R., Novitskaya I., Carbonaro H., Cardinale I., Kikuchi T., et al. (2005b). Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a). Proc. Natl. Acad. Sci. USA 102, 19057–19062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes M. A., Kikuchi T., Fuentes-Duculan J., Cardinale I., Zaba L. C., Haider A. S., Bowman E. P., Krueger J. G. (2008). Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J. Invest. Dermatol. 128, 1207–1211 [DOI] [PubMed] [Google Scholar]
- Manel N., Unutmaz D., Littman D. R. (2008). The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat. Immunol. 9, 641–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan P. R., Harrington L. E., O’Quinn D. B., Helms W. S., Bullard D. C., Elson C. O., Hatton R. D., Wahl S. M., Schoeb T. R., Weaver C. T. (2006). Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441, 231–234 [DOI] [PubMed] [Google Scholar]
- Mariette X., Tubach F., Bagheri H., Bardet M., Berthelot J. M., Gaudin P., Heresbach D., Martin A., Schaeverbeke T., Salmon D., et al. (2010). Lymphoma in patients treated with anti-TNF: results of the 3-year prospective French RATIO registry. Ann. Rheum. Dis. 69, 400–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrakchi S., Guigue P., Renshaw B. R., Puel A., Pei X. Y., Fraitag S., Zribi J., Bal E., Cluzeau C., Chrabieh M., et al. (2011). Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N. Engl. J. Med. 365, 620–628 [DOI] [PubMed] [Google Scholar]
- McGeachy M. J., Bak-Jensen K. S., Chen Y., Tato C. M., Blumenschein W., McClanahan T., Cua D. J. (2007). TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 8, 1390–1397 [DOI] [PubMed] [Google Scholar]
- Mehta N. N., Azfar R. S., Shin D. B., Neimann A. L., Troxel A. B., Gelfand J. M. (2010). Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur. Heart J. 31, 1000–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta N. N., Yu Y., Saboury B., Foroughi N., Krishnamoorthy P., Raper A., Baer A., Antigua J., Van Voorhees A. S., Torigian D. A., et al. (2011). Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch. Dermatol. 147, 1031–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menter A., Feldman S. R., Weinstein G. D., Papp K., Evans R., Guzzo C., Li S., Dooley L. T., Arnold C., Gottlieb A. B. (2007). A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J. Am. Acad. Dermatol. 56, e31–e15 [DOI] [PubMed] [Google Scholar]
- Menter A., Tyring S. K., Gordon K., Kimball A. B., Leonardi C. L., Langley R. G., Strober B. E., Kaul M., Gu Y., Okun M., et al. (2008). Adalimumab therapy for moderate to severe psoriasis: A randomized, controlled phase III trial. J. Am. Acad. Dermatol. 58, 106–115 [DOI] [PubMed] [Google Scholar]
- Nair R. P., Stuart P., Henseler T., Jenisch S., Chia N. V., Westphal E., Schork N. J., Kim J., Lim H. W., Christophers E., et al. (2000). Localization of psoriasis-susceptibility locus PSORS1 to a 60-kb interval telomeric to HLA-C. Am. J. Hum. Genet. 66, 1833–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair R. P., Stuart P. E., Nistor I., Hiremagalore R., Chia N. V., Jenisch S., Weichenthal M., Abecasis G. R., Lim H. W., Christophers E., et al. (2006). Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am. J. Hum. Genet. 78, 827–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair R. P., Ruether A., Stuart P. E., Jenisch S., Tejasvi T., Hiremagalore R., Schreiber S., Kabelitz D., Lim H. W., Voorhees J. J., et al. (2008). Polymorphisms of the IL12B and IL23R genes are associated with psoriasis. J. Invest. Dermatol. 128, 1653–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair R. P., Duffin K. C., Helms C., Ding J., Stuart P. E., Goldgar D., Gudjonsson J. E., Li Y., Tejasvi T., Feng B. J., et al. (2009). Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat. Genet. 41, 199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle F. O., Conrad C., Tun-Kyi A., Homey B., Gombert M., Boyman O., Burg G., Liu Y. J., Gilliet M. (2005). Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J. Exp. Med. 202, 135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle F. O., Kaplan D. H., Barker J. (2009). Psoriasis. N. Engl. J. Med. 361, 496–509 [DOI] [PubMed] [Google Scholar]
- Nijsten T., Stern R. S. (2012). How epidemiology has contributed to a better understanding of skin disease. J. Invest. Dermatol. 132, 994–1002 [DOI] [PubMed] [Google Scholar]
- Nograles K. E., Zaba L. C., Guttman-Yassky E., Fuentes-Duculan J., Suarez-Farinas M., Cardinale I., Khatcherian A., Gonzalez J., Pierson K. C., White T. R., et al. (2008). Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br. J. Dermatol. 159, 1092–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nograles K. E., Zaba L. C., Shemer A., Fuentes-Duculan J., Cardinale I., Kikuchi T., Ramon M., Bergman R., Krueger J. G., Guttman-Yassky E. (2009). IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J. Allergy Clin. Immunol. 123, 1244–1252.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh C. J., Das K. M., Gottlieb A. B. (2000). Treatment with anti-tumor necrosis factor alpha (TNF-alpha) monoclonal antibody dramatically decreases the clinical activity of psoriasis lesions. J. Am. Acad. Dermatol. 42, 829–830 [DOI] [PubMed] [Google Scholar]
- Onoufriadis A., Simpson M. A., Pink A. E., Di Meglio P., Smith C. H., Pullabhatla V., Knight J., Spain S. L., Nestle F. O., Burden A. D., et al. (2011). Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am. J. Hum. Genet. 89, 432–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortonne J. P., Lebwohl M., Griffiths C. E. M. (2003). Alefacept-induced decreases in circulating blood lymphocyte counts correlate with clinical response in patients with chronic plaque psoriasis. Eur. J. Dermatol. 13, 117–123 [PubMed] [Google Scholar]
- Papp K. A., Tyring S., Lahfa M., Prinz J., Griffiths C. E., Nakanishi A. M., Zitnik R., van de Kerkhof P. C., Melvin L. (2005). A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br. J. Dermatol. 152, 1304–1312 [DOI] [PubMed] [Google Scholar]
- Papp K. A., Langley R. G., Lebwohl M., Krueger G. G., Szapary P., Yeilding N., Guzzo C., Hsu M. C., Wang Y., Li S., et al. (2008). Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 371, 1675–1684 [DOI] [PubMed] [Google Scholar]
- Reich K., Nestle F. O., Papp K., Ortonne J. P., Evans R., Guzzo C., Li S., Dooley L. T., Griffiths C. E. (2005). Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet 366, 1367–1374 [DOI] [PubMed] [Google Scholar]
- Rizzo H. L., Kagami S., Phillips K. G., Kurtz S. E., Jacques S. L., Blauvelt A. (2011). IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A. J. Immunol. 186, 1495–1502 [DOI] [PubMed] [Google Scholar]
- Roberson E. D., Bowcock A. M. (2010). Psoriasis genetics: breaking the barrier. Trends Genet. 26, 415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruth K. J., Neaton J. D. (1991). Evaluation of two biological markers of tobacco exposure. MRFIT Research Group. Prev. Med. 20, 574–589 [DOI] [PubMed] [Google Scholar]
- Ryan C., Leonardi C. L., Krueger J. G., Kimball A. B., Strober B. E., Gordon K. B., Langley R. G., de Lemos J. A., Daoud Y., Blankenship D., et al. (2011). Association between biologic therapies for chronic plaque psoriasis and cardiovascular events: a meta-analysis of randomized controlled trials. JAMA 306, 864–871 [DOI] [PubMed] [Google Scholar]
- Sa S. M., Valdez P. A., Wu J., Jung K., Zhong F., Hall L., Kasman I., Winer J., Modrusan Z., Danilenko D. M., et al. (2007). The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J. Immunol. 178, 2229–2240 [DOI] [PubMed] [Google Scholar]
- Sano S., Chan K. S., Carbajal S., Clifford J., Peavey M., Kiguchi K., Itami S., Nickoloff B. J., DiGiovanni J. (2005). Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat. Med. 11, 43–49 [DOI] [PubMed] [Google Scholar]
- Santarlasci V., Maggi L., Capone M., Frosali F., Querci V., De Palma R., Liotta F., Cosmi L., Maggi E., Romagnani S., et al. (2009). TGF-beta indirectly favors the development of human Th17 cells by inhibiting Th1 cells. Eur. J. Immunol. 39, 207–215 [DOI] [PubMed] [Google Scholar]
- Saurat J. H., Stingl G., Dubertret L., Papp K., Langley R. G., Ortonne J. P., Unnebrink K., Kaul M., Camez A. (2008). Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br. J. Dermatol. 158, 558–566 [DOI] [PubMed] [Google Scholar]
- Schafer P. H., Parton A., Gandhi A. K., Capone L., Adams M., Wu L., Bartlett J. B., Loveland M. A., Gilhar A., Cheung Y. F., et al. (2010). Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br. J. Pharmacol. 159, 842–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F., Hu Z., Goswami J., Gaffen S. L. (2006). Identification of common transcriptional regulatory elements in interleukin-17 target genes. J. Biol. Chem. 281, 24138–24148 [DOI] [PubMed] [Google Scholar]
- Sofen H., Smith S., Matheson R., Leonardi C., Calderon C., Bouman-Thio E., Brodmerkel C., Li K., Marciniak S., Petty K., et al. (2011). Results of a single ascending dose study to assess the safety and tolerability of CNTO 1959 following intravenous or subcutaneous administration in healthy subjects and in subjects with moderate to severe psoriasis. FC-21. Psoriasis: from Gene to Clinic 6th International Congress, 1–3 December 2011. Br. J. Dermatol. 165, e1–e46 [Google Scholar]
- Sterry W., Bagot M., Ferrandiz C., Kragballe K., Papp K., Stingl G. (2009). Immunosuppressive therapy in dermatology and PML. J. Dtsch. Dermatol. Ges. 7, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober B. E., Crowley J. J., Yamauchi P. S., Olds M., Williams D. A. (2011). Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br. J. Dermatol. 165, 661–668 [DOI] [PubMed] [Google Scholar]
- Suarez-Farinas M., Lowes M. A., Zaba L. C., Krueger J. G. (2010a). Evaluation of the psoriasis transcriptome across different studies by gene set enrichment analysis (GSEA). PLoS ONE 5, e10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Farinas M., Shah K. R., Haider A. S., Krueger J. G., Lowes M. A. (2010b). Personalized medicine in psoriasis: developing a genomic classifier to predict histological response to Alefacept. BMC Dermatol. 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumaria N., Roediger B., Ng L. G., Qin J., Pinto R., Cavanagh L. L., Shklovskaya E., Fazekas de St Groth B., Triccas J. A., Weninger W. (2011). Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. J. Exp. Med. 208, 505–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell W. R., Johnston A., Carbajal S., Han G., Wohn C., Lu J., Xing X., Nair R. P., Voorhees J. J., Elder J. T., et al. (2011). Genome-wide expression profiling of five mouse models identifies similarities and differences with human psoriasis. PLoS ONE 6, e18266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonel G., Conrad C., Laggner U., Di Meglio P., Grys K., McClanahan T. K., Blumenschein W. M., Qin J. Z., Xin H., Oldham E., et al. (2010). Cutting edge: a critical functional role for IL-23 in psoriasis. J. Immunol. 185, 5688–5691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trembath R. C., Clough R. L., Rosbotham J. L., Jones A. B., Camp R. D., Frodsham A., Browne J., Barber R., Terwilliger J., Lathrop G. M., et al. (1997). Identification of a major susceptibility locus on chromosome 6p and evidence for further disease loci revealed by a two stage genome-wide search in psoriasis. Hum. Mol. Genet. 6, 813–820 [DOI] [PubMed] [Google Scholar]
- Trifari S., Kaplan C. D., Tran E. H., Crellin N. K., Spits H. (2009). Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat. Immunol. 10, 864–871 [DOI] [PubMed] [Google Scholar]
- Tyring S., Gottlieb A., Papp K., Gordon K., Leonardi C., Wang A., Lalla D., Woolley M., Jahreis A., Zitnik R., et al. (2006). Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet 367, 29–35 [DOI] [PubMed] [Google Scholar]
- Van Belle A. B., de Heusch M., Lemaire M. M., Hendrickx E., Warnier G., Dunussi-Joannopoulos K., Fouser L. A., Renauld J. C., Dumoutier L. (2012). IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J. Immunol. 188, 462–469 [DOI] [PubMed] [Google Scholar]
- van der Fits L., Mourits S., Voerman J. S., Kant M., Boon L., Laman J. D., Cornelissen F., Mus A. M., Florencia E., Prens E. P., et al. (2009). Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 182, 5836–5845 [DOI] [PubMed] [Google Scholar]
- Villadsen L. S., Schuurman J., Beurskens F., Dam T. N., Dagnaes-Hansen F., Skov L., Rygaard J., Voorhorst-Ogink M. M., Gerritsen A. F., van Dijk M. A., et al. (2003). Resolution of psoriasis upon blockade of IL-15 biological activity in a xenograft mouse model. J. Clin. Invest. 112, 1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe E., Servant N., Zollinger R., Bogiatzi S. I., Hupe P., Barillot E., Soumelis V. (2008). A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat. Immunol. 9, 650–657 [DOI] [PubMed] [Google Scholar]
- Wilson N. J., Boniface K., Chan J. R., McKenzie B. S., Blumenschein W. M., Mattson J. D., Basham B., Smith K., Chen T., Morel F., et al. (2007). Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 8, 950–957 [DOI] [PubMed] [Google Scholar]
- Wolfram J. A., Diaconu D., Hatala D. A., Rastegar J., Knutsen D. A., Lowther A., Askew D., Gilliam A. C., McCormick T. S., Ward N. L. (2009). Keratinocyte but not endothelial cell-specific overexpression of Tie2 leads to the development of psoriasis. Am. J. Pathol. 174, 1443–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrone-Smith T., Nickoloff B. J. (1996). Dermal injection of immunocytes induces psoriasis. J. Clin. Invest. 98, 1878–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Anderson D. E., Baecher-Allan C., Hastings W. D., Bettelli E., Oukka M., Kuchroo V. K., Hafler D. A. (2008). IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature 454, 350–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Richman L., Morehouse C., de los Reyes M., Higgs B. W., Boutrin A., White B., Coyle A., Krueger J., Kiener P. A., et al. (2008). Type I interferon: potential therapeutic target for psoriasis? PLoS ONE 3, e2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba L. C., Cardinale I., Gilleaudeau P., Sullivan-Whalen M., Suárez-Fariñas M., Fuentes-Duculan J., Novitskaya I., Khatcherian A., Bluth M. J., Lowes M., et al. (2007a). Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J. Exp. Med. 204, 3183–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba L. C., Fuentes-Duculan J., Steinman R. M., Krueger J. G., Lowes M. A. (2007b). Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J. Clin. Invest. 117, 2517–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba L. C., Suarez-Farinas M., Fuentes-Duculan J., Nograles K. E., Guttman-Yassky E., Cardinale I., Lowes M. A., Krueger J. G. (2009a). Effective treatment of psoriasis with etanercept is linked to suppression of IL-17 signaling, not immediate response TNF genes. J. Allergy Clin. Immunol. 124, 1022–1010 e395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba L. C., Fuentes-Duculan J., Eungdamrong N. J., Abello M. V., Novitskaya I., Pierson K. C., Gonzalez J., Krueger J. G., Lowes M. A. (2009b). Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J. Invest. Dermatol. 129, 79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba L. C., Fuentes-Duculan J., Eungdamrong N. J., Johnson-Huang L. M., Nograles K. E., White T. R., Pierson K. C., Lentini T., Suarez-Farinas M., Lowes M. A., et al. (2010). Identification of TNF-related apoptosis-inducing ligand and other molecules that distinguish inflammatory from resident dendritic cells in patients with psoriasis. J. Allergy Clin. Immunol. 125, 1261–1268.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariae H. (2003). Prevalence of joint disease in patients with psoriasis: implications for therapy. Am. J. Clin. Dermatol. 4, 441–447 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Danilenko D. M., Valdez P., Kasman I., Eastham-Anderson J., Wu J., Ouyang W. (2007). Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445, 648–651 [DOI] [PubMed] [Google Scholar]
- Zhou L., Ivanov I. I., Spolski R., Min R., Shenderov K., Egawa T., Levy D. E., Leonard W. J., Littman D. R. (2007). IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 8, 967–974 [DOI] [PubMed] [Google Scholar]
- Zhou X., Krueger J. G., Kao M. C., Lee E., Du F., Menter A., Wong W. H., Bowcock A. M. (2003). Novel mechanisms of T-cell and dendritic cell activation revealed by profiling of psoriasis on the 63,100-element oligonucleotide array. Physiol. Genomics 13, 69–78 [DOI] [PubMed] [Google Scholar]