Abstract
In 2007, five blood glucose meters (BGMs) were introduced with integrated speech output necessary for use by persons with vision loss. One of those five meters had fully integrated speech output, allowing a person with vision loss independence in accessing all features and functions of the meter. In comparison, 13 BGMs with integrated speech output were available in 2011. Accessibility attributes of these 11 meters were tabulated and product design features examined.
All 13 meters were found to be usable by persons with vision loss to obtain a blood glucose measurement. However, only 4 of them featured the fully integrated speech output necessary for a person with vision loss to access all features and functions independently.
Keywords: accessibility, blindness, blood glucose meters, diabetes, visual impairment
Introduction
Because of the close relationship between diabetes and visual impairment, it is important that blood glucose meters (BGMs) are designed to be accessible to people who are blind or visually impaired. In 2010, 3.9 million adults with diabetes in the United States reported having a visual impairment, with 3.4 million projected to have vision-threatening diabetic retinopathy.1 Diabetes is also the leading cause of new cases of blindness among adults between 20 and 74 years old.2
A BGM is accessible to a person with vision loss if it is designed so that the person can independently operate it. Speech output of display data is the most important factor that determines a BGM’s accessibility. In their 2008 analysis of BGM accessibility, Uslan and colleagues3 established the following six criteria by which to measure the accessibility of a BGM:
Spoken display data
A high-contrast, large-font display
Control buttons that are both tactilely identifiable and distinctive in color contrast
Test strip calibration that is accessible (e.g., automatic)
An accessible operating manual [i.e., in an electronic format that can be read by personal computer (PC) screen-reading/magnifying software
Accessible PC software (i.e., the format of the downloaded data can be read by PC screen-reading/magnifying software)
We did not test accuracy of any of the meters; instead we relied on Food and Drug Administration approval as a qualification of being included in this article. We also did not concern ourselves with the print information on test strip or control solution packaging.
Because of significant usability problems related to the physical design of some talking BGMs available at the time, Uslan and colleagues3 also established criteria related to convenience and ease of use. However, those criteria, which dealt with portability, blood sample size, and speed of processing results, were not considered in this paper. Those criteria are no longer relevant because all the current talking BGMs are highly portable and have a blood sample size and processing speed that is comparable to nonaccessible meters on the market.
Blood Glucose Meters with Accessibility Features in 2007
In their analysis of BGMs on the market in 2007, Uslan and colleagues3 identified five BGMs available with integrated speech output: Prodigy, Prodigy Autocode, Prodigy Voice, Advocate, and Advocate Redi-Code (see Figure 1).
Figure 1.
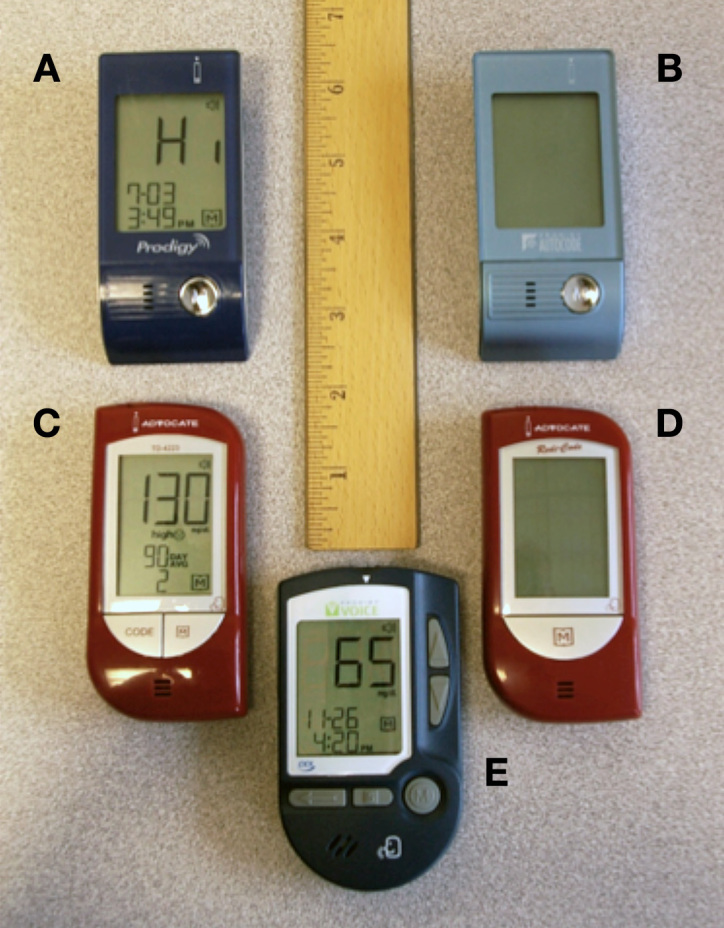
The BGMs with integrated speech available in 2007: (A) Prodigy, (B) Prodigy Autocode; (C) Advocate; (D) Advocate Redi-Code; (E) Prodigy Voice.
Of these five meters, only the Prodigy Voice had speech output that supported all the functions of the meter, including current measurement, settings, and memory functions. The other four spoke only the current measure-ment. All five featured tactilely identifiable control buttons and had buttons whose colors contrasted well with the background panel. Although all five used large fonts on their displays, no meter had high-contrast displays, accessible manuals, nor PC software that was compatible with screen-reading technology used by persons with vision loss. All but the Prodigy and the Advocate featured automatic test strip calibration.
Blood Glucose Meters with Accessibility Features in 2011
In November 2011, 13 BGMs with integrated speech output were available on the U.S. market: Clever Check, Easy Max V, Fora V10, Fora V20, Fora V22, Fora V30a, Advocate Redi-Code, Omnis Health Embrace, Prodigy Autocode, Prodigy Voice, VeroStar TK, Vocal Point, and SOLO V2. Testing determined that only 4 of these meters have speech output that fully supports all functions, namely: Prodigy Voice from Prodigy Diabetes Care LLC, Solo V2 from Biosense Medical Devices, and both Fora V20 and Fora V22 from Fora Care Inc. (see Figure 2).
Figure 2.
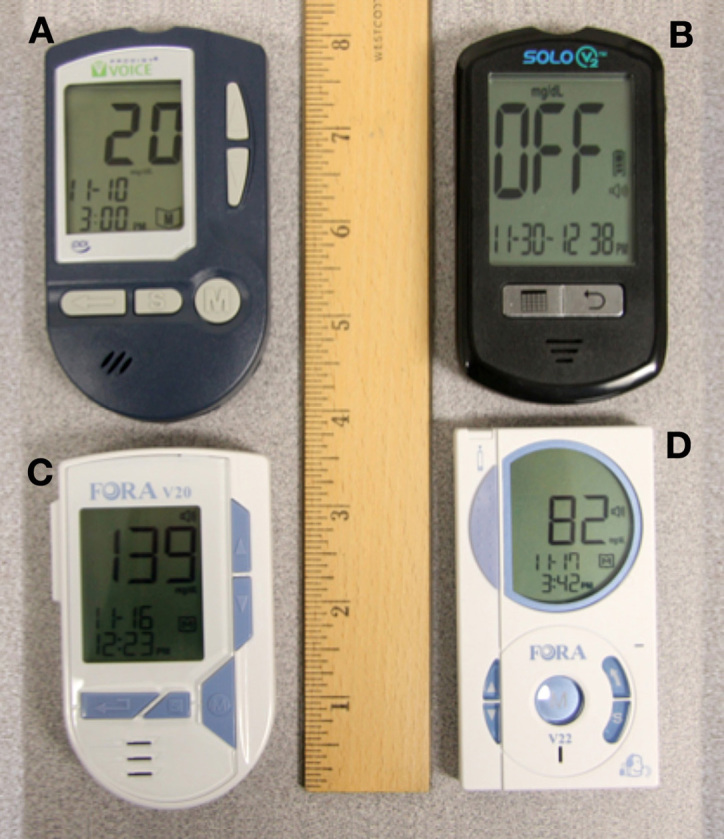
The BGMs with fully integrated speech available in 2011: (A) Prodigy Voice; (B) Solo V2; (C) Fora V20; (D) Fora V22.
Table 1 represents the results of further testing to determine the extent to which those four meters meet the accessibility criteria established by Uslan and colleagues.3
Table 1.
Accessibility Features of Blood Glucose Meters with Speech Supporting All Functions
| Prodigy Voice | Solo V2 | Fora V20 | Fora V22 | |
|---|---|---|---|---|
| Spoken glucose level | 5 | 5 | 5 | 5 |
| Spoken settings | 5 | 5 | 5 | 5 |
| Spoken memory (Glucose level, Date, Time) | 5 | 3 (Time is not spoken) | 5 | 5 |
| Ease of identifying buttons (1-5 Scale)a | 4 | 3 | 4 | 2 |
| Spoken error 0tifications | 5 | 5 | 5 | 5 |
| Spoken “Not enough blood” warning | 5 | 5 | 0 | 0 |
| Headphone jack | 5 | 0 | 5 | 5 |
| Manual compatible with screen-reading software | 5 | 5 | 3 (Partially compatible) | 5 |
| Software compatible with screen-reading software | 3 (Partially compatible) | 3 (Partially compatible) | 0 (Not compatible) | 0 (Not compatible) |
| Large-font/high-contrast screen | (3) | (3) | (3) | (3) |
| Total Score (out of 50) | 45 | 38 | 35 | 35 |
Rating scale for ease of identifying buttons: (1) buttons cannot be identified or used tactilely; (2) buttons are very difficult to identify and use tactilely; (3) buttons can be identified and used, but there is a definite need for improvement. (4) buttons are easy to identify and use, but minor improvements would help; and (5) buttons are very easy to identify and use tactilely.
* Ratings Scored on a scale of 0–5, where 5 is fully accessible.
The four devices are rated based on the following criteria on a scale of 0 to 5 for each category, for a total of 50 possible points. In categories such as spoken glucose level, where the device either speaks or does not, speaking results in 5 points and not speaking results in 0 points.
Although all four BGMs do support all their functions with high-quality speech output, the Prodigy Voice scores the best based on the listed criteria. The Prodigy Voice and the two Fora meters speak both the time and date of memory readings, but the Solo V2 does not. The Prodigy Voice and the Solo V2 speak a “not enough blood” warning, but the Fora meters do not. That spoken warning can help users with vision loss to avoid false low test results. The lack of this spoken warning on the Fora meters may be a problem that is serious enough for people who are blind to avoid using them.
Although it can be difficult to quantify how tactilely identifiable a control button is, the buttons on the Prodigy Voice and the Fora V20 are definitely the easiest to distinguish from one another nonvisually. The Solo V2’s “memory” and “back” buttons are right next to each other and could be difficult to distinguish from one another. Although the Solo V2’s “settings” buttons are easy to feel, it does take considerable force to activate them. Most of the Fora V22’s buttons are nearly flush with the panel and are very difficult to feel and activate nonvisually. However, even though the buttons on the Solo V2 and Fora V22 can be difficult to use, proper practice and training from a health care professional should make them usable.
All four BGMs feature automatic test-strip calibration and large fonts on the display, but none feature a high-contrast display. All but the Solo V2 have a headphone jack, useful for private use or to attach an assisted listening device for a person who is hard of hearing.
The Prodigy Voice, the Solo V2, and the Fora V22 all have manuals available in an electronic format that is compatible with screen-reading software used by people with vision loss. However, the electronic manual for the Fora V20 is only partially compatible, as several graphics and important instructions cannot be accessed.
The PC software for the two Fora meters was not compatible with screen-reading technology at all, but parts of the Prodigy Voice and Solo V2 software were compatible. The Prodigy Voice had more compatible components than the Solo V2. Neither would allow a screen-reader user to access reports of test results, but both could export the reports to accessible spreadsheets.
Conclusion
Since 2007, the number of BGMs available with integrated speech that will voice the current measurement value has increased from 5 to 13. Of those 13, 4 meters feature fully integrated speech output that supports all their features and functions, which is an increase over the 1 available in 2007. Additionally, the Prodigy Voice and the Solo V2 now have manuals available in an accessible electronic format, further facilitating their independent use by people with vision loss.
While these facts show a positive trend in the develop-ment of accessible BGMs, the quality of the meters’ visual displays has not improved. The meters all still feature low-contrast displays that are difficult to see for people with low vision. This is a significant concern for people with age-related vision loss who prefer to use their remaining vision to manage their diabetes. Additionally, none of the talking meters are among the most popular in use today or among the top-selling meters as listed by Amazon.com.4 This is an ongoing problem for people who have diabetes and vision loss, because the most popular meters, such as the Accu-Chek Compact and the OneTouch Ultra2, are far more likely to be prescribed by physicians and are also more likely to be covered by insurance carriers. When the leading BGM manufacturers build accessible meters, more people with vision loss will have greater access to the proper tools to independently manage their diabetes.
Glossary
Abbreviations
- (BGM)
blood glucose meter
- (PC)
personal computer
Funding
Support was provided by the Teubert Foundation.
Disclosures
The American Foundation for the Blind, which employs the authors of this article, gave Prodigy Diabetes Care their 2009 Access Award for developing the Prodigy Voice blood glucose meter, which is reviewed in this article.
References
- 1.Centers for Disease Control and Prevention. Self-reported visual impairment among persons with diagnosed diabetes—United States, 1997–2010. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6045a2.htm?s_cid=mm6045a2_w. Accessed November 30, 2011.
- 2.Centers for Disease Control and Prevention. National diabetes fact sheet, 2011. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf Accessed February 9, 2012. [Google Scholar]
- 3.Uslan MM, Burton DM, Clements CW. Blood glucose meters that are accessible to blind and visually impaired persons. J Diabetes Sci Technol. 2008;2(2):284–287. doi: 10.1177/193229680800200219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Amazon.com. Diabetes monitors and kits. http://www.amazon.com/gp/bestsellers/hpc/3761851/ref=pd_zg_hrsr_hpc_1_4_last#1. Accessed November 30, 2011. [Google Scholar]


