Abstract
This article discusses human factors (HF) processes and how they are applied during the development of a medical device to minimize the risk that the user interface design could lead to patient errors, adverse events, and product recalls. This process is best defined as “prevention through design.” The HF design process is exemplified by three distinct phases: (1) preliminary analysis, (2) formative design evaluation and modification, and (3) design validation. Additional benefits of employing HF principles during medical device development are briefly reviewed, including reduced patient risk by eliminating design flaws, increased patient adherence through the reduction in the complexity of therapeutic regimes, and reduced likelihood for product recalls.
Keywords: engineering psychology, human factors, human factors engineering, human factors research, usability
Introduction
There are approximately 450,000 people in the United States who use insulin pumps to manage their diabetes. All these people interact with their pumps several times every day to administer insulin into their bodies to manage their blood glucose levels. If you have ever interacted with a piece of technology regularly—for example, the automated ticket machine for the train, a smart phone, or the check-in kiosk at the airport—and you have thought to yourself, if only the designers and engineers had done something a little differently, it would be so much easier to use, then you would have been thinking in terms of how people interact with technological systems and of how important it is (especially with medical devices) that those interactions are consistently safe, error free, and efficient.
Human Factors Defined
Human factors (HF) is a unique field because it draws from a variety of disciplines, including psychology, engineering, design, statistics, ethnography, and anthro-pometry. Specifically, a human factor is any physical, perceptual, cognitive, or behavioral aspect of a human being that impacts a technological system or environment. The most important aspect of HF is that it is truly an empirical science, which means it is based almost entirely on observation. The power of the HF discipline is that its process can be wielded to collect information on how humans interact with technology, and that knowledge can be applied to the design and development of technologies that better fit human behaviors, needs, and limitations. The focus of HF is expressed by the seminal work of Sanders and McCormick:
Human factors focuses on human beings and their interaction with products, equipment, facilities, procedures, and environments, used in work and everyday living. The emphasis is on human beings (as opposed to engineering, where the emphasis is more strictly technical engineering considerations) and how the design of things influences people. Human factors, then, seeks to change the things people use and the environments in which they use these things to better match the capabilities, limitations and needs of people.1
A common misperception is that HF research is similar or related to market research. Market research typically utilizes methodologies such as focus groups, customer preference surveys, or executive reviews to gather data that center on opinions, attitudes, and speculation about a product. Focus groups and other kinds of market research methodologies are not specific tests of medical device use or risks in medical device interaction. They provide a different kind of information, which can be useful during the very early stages of product development when developing user profiles, creating device-use scenarios, and better understanding use environments.
The Human Factors Process and Medical Device Development
The health care field has come under major scrutiny because of poorly designed medical systems and devices with inherent design flaws that induce user errors. There have been numerous examples of infusion pump recalls due to poor designs. For example, in August 2004, the N’Vision® Clinical Programmer was recalled by the Food and Drug Administration (FDA) because users were able to enter infusion times with incorrect units. Minutes were entered into the hours field, which caused patient overdose.2 The Colleague® IV infusion pump could shut down while delivering critical medication to patients. The reason for this was that the “on/off” key was so close to the “start” key that nurses would often inadvertently turn the pump off when they intended to start delivery. Over 206,000 Colleague infusion pumps, used mostly in hospitals, were recalled by the FDA.3,4
The HF process in the medical device field takes a very risk-centric approach, generally referred to as a “prevention through design” strategy. The primary objective is to design out characteristics of a system or device that could lead to human error. The HF process generally includes three primary phases: (1) preliminary analysis, (2) formative evaluation and design modification, and (3) validation testing. The t:slim™ insulin delivery system (t:slim pump) user interface was developed with this process. This process resulted in a pump that utilizes a touch screen to facilitate data entry, along with a home screen to display critical information users need to manage their diabetes. The home screen displays critical information such as the amount of insulin in the cartridge, battery life, time and date, and insulin on board, as well as provides direct access to bolus functions and pump system functions (“options”).
Preliminary analysis started prior to the design of the t:slim and sought to better understand who the users are, what their needs are, what kind of environment they use the device in, what their limitations are, what specific tasks they need to accomplish, and a risk analysis of those tasks as they related to device use. This phase also included analysis of the user interface designs of products already in use. The methodologies, study focus, and outcomes that occurred during the preliminary analysis phase are summarized in Table 1. From the data collected in the preliminary analysis, an early interface design was developed, as shown in Figure 1.
Table 1.
Preliminary Analysis Phase
| Study methodology | Sample description | Study focus | Outcomes |
|---|---|---|---|
| Web-based survey | 350 patients, 125 clinicians | Focus on touch screen concept | Participants preferred touch screen interface |
| Web-based survey | 45 patient insulin pumpers | Prioritization of information to be included on the graphical user interface home screen | Initiated design based on user feedback of home screen function priorities |
| Web-based survey | 116 patients, 31 diabetes educators | Prioritization of all user tasks, including critical tasks, beyond the graphical user interface home screen | Used to select the architecture of the user task workflows |
| Focus group | 40 patients | Focus on various options for terms and icons in the user interface workflow | Used to select specific terms and icons for user workflows that minimize confusion |
| Web-based survey | 61 patients | Study color schemes used within the graphical user interface that maximize user recognition and minimize confusion | Selection of color scheme used throughout the graphical user interface |
Figure 1.
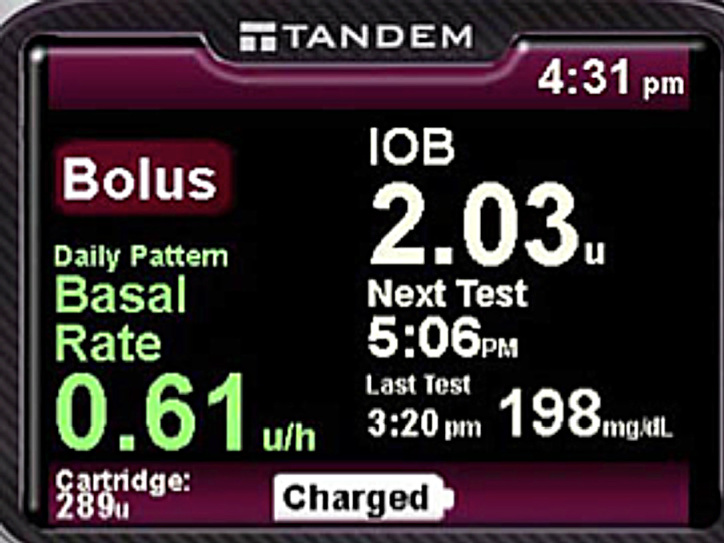
Early home screen design.
The next phase of t:slim pump development involved HF activities that included formative evaluation and design modification. This phase of the process was accomplished via risk-based iterative usability testing. The primary focus of this testing was on safety and usability of the product’s design. A typical usability study is relatively small (5–8 participants), with actual users being observed by a researcher in a controlled environment. The participants go through a set of task-based scenarios that are representative of real device use. The researcher observes each participant’s behavior as they are going through the tasks and records data on their interactions.5,6 Data collected are used to inform the next iteration of the design. Table 2 shows a summary of the formative studies that were conducted during the development of the t:slim pump.
Table 2.
Formative Evaluation Phase
| Study methodology | Sample description | Study focus | Outcomes |
|---|---|---|---|
| Usability study | 21 representative users | Workflow testing for completing critical tasks and for ease of use | Identified improvements required on critical tasks |
| Usability study | 30 representative users | Focus on detailed critical task list and user errors and other HF | Usability errors identified and mitigated through changes to the graphical user interface |
| Usability study | 33 representative users | Focus on test to ensure design changes made to the graphical-user-interface-mitigated errors | Design changes to increase task success rate |
| Usability study | 15 representative users | Collect end user feedback on design changes made to user interface based on previous outcomes | Usability issues identified and design improvements implemented |
| Usability study | 9 representative users | Graphical user interface navigation and presentation, software control usage and hardware control usage | Usability issues identified and design improvements implemented |
| Usability study | 5 representative users | Graphical user interface navigation and presentation, software control usage and hardware control usage | Usability issues identified and design improvements implemented |
| Usability study | 7 representative users | Graphical user interface navigation and presentation, software control usage and hardware control usage | Usability issues identified and design improvements implemented |
During several of the early usability tests, it was revealed that participants struggled with data entry, navigation, and information retrieval tasks. One example of design improvements to the home screen that occurred as a result of this iterative usability testing is exemplified in Figure 2.
Figure 2.
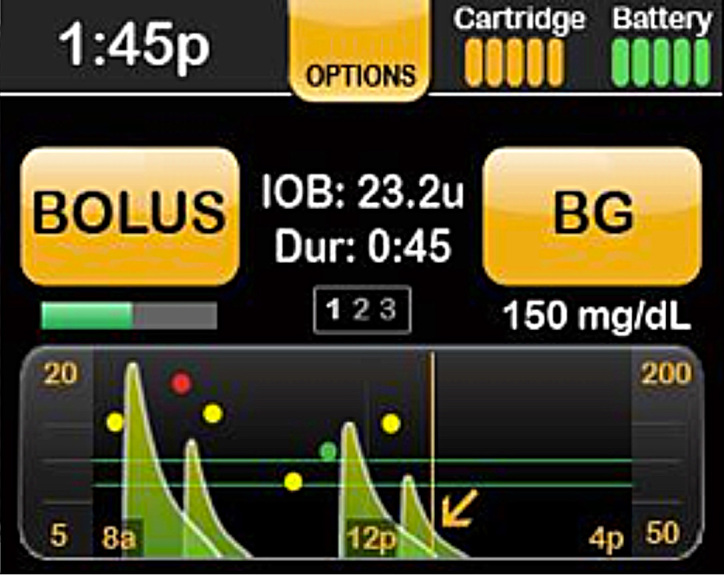
Design iteration of the home screen.
Modifications were continually made to the user interface based on observations made during the formative design evaluation phase. At the end of the development process and formative tests, a HF validation test was conducted to prove that the t:slim pump was safe and effective for human use.
The purpose of the validation study was to confirm that the t:slim pump could be used safely and effectively by representative users in the intended use environment. Study participants included both people with diabetes who were insulin pump users and people with diabetes being treated with multiple daily injections. Participants performed a comprehensive, realistic set of basic and advanced task-based scenarios with the t:slim pump. Data were collected via a software data log, participant journals, follow-up interviews, and video/audio tape recordings. The results indicated that, during certain tasks, participants made programming errors when inputting information into the pump. To mitigate potential programming errors, a software solution was developed that forced users to actively confirm their inputs before continuing through the workflow. In order to prevent automaticity7,8 or response chaining, in which users could act habitually in an automatic fashion and tap through confirmation screens that were presented too frequently, the confirmation screens appear only when users make changes to settings that impact insulin delivery, such as delivering a bolus or setting a basal rate. A revalidation study was conducted to evaluate the effectiveness of the active confirmation screens. The goal was to demonstrate and validate the effectiveness of the active confirmation screens on the t:slim pump with a sample of representative users.
There were several events that occurred during the revalidation study that revealed that the active confirmation screens did prevent incorrect entries. The reason the active confirmation screens may have impacted participant behavior is likely due to several related factors, including the unique screen layout, a summary of all critical information in one location, and the active confirmation required from the participant. The home screen of the validated design is shown in Figure 3.
Figure 3.
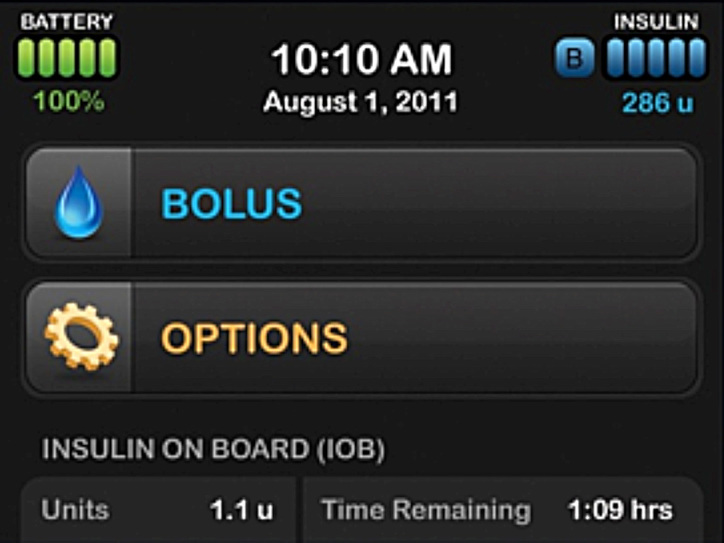
Validated home screen design.
The improvement in the usability of the interface was further substantiated through a system usability scale (SUS) questionnaire that participants completed after their sessions. The SUS yields a single number which represents the overall usability of the system.9 Figure 4 shows higher SUS scores for the new interface versus the old.
Figure 4.
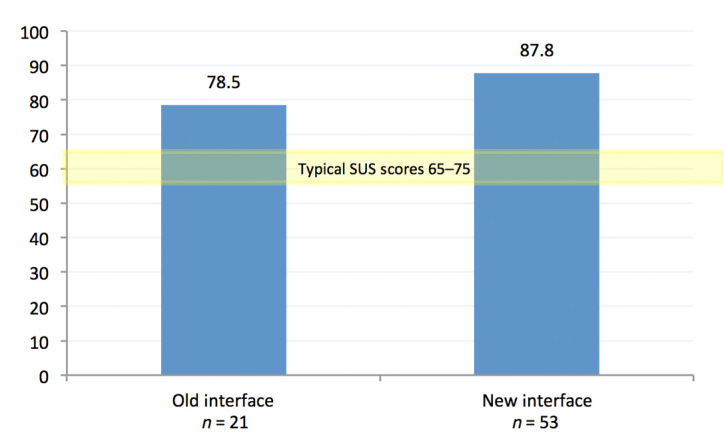
Mean SUS score across all participants.
The results of these validation studies were the culmination of successful HF/usability design efforts, which included preliminary analysis, iterative formative evaluations, and design modifications. The current regulatory environment has required an increased focus on the application of the HF process to the development of medical devices in order to eliminate design flaws that could lead to unsafe behavior. These studies exemplified how applying that process helped create a user-centric system that reduced risk, increased effectiveness, and improved ease of use.
Human Factors Standards
There are several HF standards that practitioners and companies can use to guide them through the practical application of HF processes. The Association for the Advancement of Medical Instrumentation is an organization that brings together experts in order to create standards that are widely recognized by other organizations, including the FDA. Many of these documents contain comprehensive information that covers HF design processes as well as HF design principles.10–12
Conclusion
There are several primary benefits to employing a HF process during the design of medical devices. The most important benefit is increased patient safety. This safety comes in the form of reduced patient risk through a design that has been heavily tested with representative users in its intended use environment in order to eliminate design flaws. Another benefit may be increased patient adherence. There is evidence indicating that reducing the complexity of therapeutic regimes may increase patient adherence.13–16 Reduced therapeutic complexity, in part, can be derived from improved device usability. Secondary benefits of a systematic HF process may include reducing or eliminating the need for costly modification(s) after product launch, reducing the likelihood of product recalls due to design flaws, improving overall ease of use (improved ease of use is a byproduct of good HF practices, not the sole outcome), and enhancing look and feel. There is also evidence to suggest that patients may pay a higher premium for more usable products.17 All of the benefits resulting from the systematic implementation of a sound HF process will converge to generate a medical product that creates a patient experience that is safer, more efficient, and more satisfactory.
Acknowledgments
I thank all the HF practitioners and user-experience designers who contributed their time, energy, and expertise to the development of the t:slim pump, including Kathryn Rieger-King, Ph.D., Brian Bureson, and Jason Farnan.
N’Vision® is a registered trademark of Medtronic Inc. Colleague® is a registered trademark of Baxter Healthcare Corp.
Glossary
Abbreviations
- (FDA)
Food and Drug Administration
- (HF)
human factors
- (SUS)
system usability scale
Funding
This work was funded by Tandem Diabetes Care Inc., San Diego, CA.
Disclosure
Noel E. Schaeffer, Ph.D., is a full-time employee at Tandem Diabetes Care.
References
- 1.Sanders MS, McCormick EJ. 7th ed. New York: McGraw-Hill; 1993. Human factors in engineering and design. [Google Scholar]
- 2.Food and Drug Administration. Medtronic 8870 Software Application Card Version AAA 02: class I recall. http://www.fda.gov/MedicalDevices/Safety/RecallsCorrectionsRemovals/ListofRecalls/ucm064764.htm. Accessed October 31, 2011.
- 3.Food and Drug Administration. Baxter Healthcare Corp. COLLEAGUE® Volumetric Infusion Pumps: class 1 recall. http://www.fda.gov/MedicalDevices/Safety/RecallsCorrectionsRemovals/ListofRecalls/ucm063713.htm. Accessed October 31, 2011.
- 4. Reuters. F.D.A. orders Baxter recall of IV pumps. New York Times; July 14, 2010.
- 5.Tullis T, Albert B. Amsterdam: Elsevier; 2008. Measuring the user experience: collecting, analyzing, and presenting usability metrics. [Google Scholar]
- 6.Kuniavsky M. San Francisco: Morgan Kaufmann; 2003. Observing the user experience: a practitioner’s guide to user research. [Google Scholar]
- 7.Schneider W, Shiffrin RM. Controlled and automatic human information processing. I. Detection, search, and attention. Psychol Rev. 1977;84(1):1–66. [Google Scholar]
- 8.Shiffrin RM, Schneider W. Controlled and automatic human information processing. II. Perceptual learning, automatic attending and a general theory. Psychol Rev. 1977;84(2):127–190. [Google Scholar]
- 9.Brooke J. SUS: a “quick and dirty” usability scale. In: Jordan PW, Thomas B, Weerdmeester BA, McClelland AL, editors. Usability evaluation in industry. London: Taylor and Francis; 1996. [Google Scholar]
- 10.Association for the Advancement of Medical Instrumentation; American National Standards Institute. Human factors engineering: design of medical devices. ANSI/AAMI HE75:2009. www.aami.org/publications/standards/HE75_Ch16_Access_Board.pdf. Accessed January 31, 2012. [Google Scholar]
- 11.Association for the Advancement of Medical Instrumentation; American National Standards Institute. Medical devices: application of usability engineering to medical devices. ANSI/AAMI/IEC 62366. 2007.
- 12.Food and Drug Administration. Applying human factors and usability engineering to optimize medical device design. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm259748.htm. Accessed January 31, 2012. [Google Scholar]
- 13.Paes AH, Bakker A, Soe-Agnie CJ. Impact of dosage frequency on patient compliance. Diabetes Care. 1997;20(10):1512–1517. doi: 10.2337/diacare.20.10.1512. [DOI] [PubMed] [Google Scholar]
- 14.Viller F, Guillemin F, Briançon S, Moum T, Suurmeijer T, van den Heuvel W. Compliance to drug treatment of patients with rheumatoid arthritis: a 3 year longitudinal study. J Rheumatol. 1999;26(10):2114–2122. [PubMed] [Google Scholar]
- 15.Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18(8):1023–1031. doi: 10.1007/s00198-006-0322-8. [DOI] [PubMed] [Google Scholar]
- 16.Zahn JD. Analysis: desirable attributes of insulin injection pens that drive patient preference and compliance. J Diabetes Sci Technol. 2011;5(5):1210–1211. doi: 10.1177/193229681100500528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cambridge Consultants. Patients prescribe ease-of-use for the medical device industry. http://www.cambridgeconsultants.com/news_pr296.html. Accessed October 31, 2011. [Google Scholar]


