Abstract
Background
Prolonged severe hypoglycemia (SH) in hospitalized patients is associated with increased morbidity and mortality. This study was undertaken to identify risk factors for SH, to apply that knowledge to the development of a prediction algorithm, and to institute a prevention program at a tertiary medical center.
Methods
We analyzed SH events for 172 patients and developed computer algorithms to predict SH that were tested on a population of 3028 inpatients who were found to have blood glucose (BG) <90 mg/dl during their hospital stay. Variables with significant bivariate associations were entered into partition analyses to identify interactions. Logistic regression was performed by calculating parameters related to the odds of hypoglycemia below each cut point. Sensitivity and specificity were determined at various cut points. The cut points resulting in 50% sensitivity for each hypoglycemia level were determined. These algorithms were tested against the initial 172 adjudicated patients.
Results
Variables related to the BG <40 mg/dl cut off point were basal and adjustment scale insulin doses, weight, and creatinine clearance, while variables related to the 60 mg/dl and 70 mg/dl cut points were basal, prandial, and adjustment scale insulin doses, weight, creatinine clearance, and sulfonylurea use. The 50% sensitivity cut point developed using the <70 mg/dl algorithm correctly identified 71% of the adjudicated cases, while the <60 mg/dl and <40 mg/dl algorithms identified 70% and 55% respectively.
Conclusions
A validated prediction algorithm for SH can aid in the identification of patients at risk for SH and may be useful in the development of prevention strategies.
Keywords: diabetes, inpatient hypoglycemia, patient safety, prediction, prevention
Introduction
Treatment-related hypoglycemia is a potentially dangerous and common complication of insulin therapy in the hospital. Hypoglycemia has been associated with mortality in multiple intensive care unit studies, but this association is not as clear on the medical wards.1–4 Hypoglycemia in the outpatient setting is a recognized risk factor for mortality and morbidity including cardiovascular, cerebro-vascular, and patient fall events.5,6 The prevalence of hypoglycemia (<70 mg/dl) was reported to be 5.7% of all point-of-care blood glucose (BG) tests in a 2009 survey of 575 hospitals.7 The commonly used definition for severe hypoglycemia (SH) (a low BG level that requires the assistance of another person for recovery) does not applyin the hospital setting, so a defined BG level, <40 mg/dl, has been adopted as the level likely to cause harm in the hospital setting.6 It has been recognized that early therapeutic changes after treatment for mild hypoglycemia can prevent more SH episodes8 and that clinicians do not consistently adjust their patients’ antidiabetic regimens appropriately following treatment of hypoglycemia, placing the patient at additional risk.3 Treatment-related hypoglycemia is an iatrogenic event that should be prevented; however, the tools available are inadequate.
Inpatient hypoglycemia may be due to excessive insulin dose, inappropriate timing of insulin or antidiabetes therapy, unaddressed antecedent hypoglycemia or changes in the nutritional regimen, creatinine clearance, or steroid dose.9 Failure of effective communication between physicians and nurses is an underlying problem.9 The diverse nature of potential errors in the treatment of inpatients with hyperglycemia supports the need for a decision-making model that can be used to predict and prevent hypoglycemia. Development of practices to prevent hypoglycemia is the challenge addressed by this study.
We carefully analyzed the clinical and dosing factors associated with SH (BG <40 mg/dl) and less severe hypoglycemia (LSH BG <60 mg/dl and <70 mg/dl) at Barnes-Jewish Hospital during a 6-month interval. The data was then used to develop a predictive model that could interface with the Internet protocol electronic health record (EHR) to provide alerts.
Methods
Study 1 was conducted at Barnes-Jewish Hospital, a 1259-bed tertiary care center in St. Louis, MO, from June 1 to November 30, 2009, to assess risk factors predisposing to inpatient hypoglycemia and to identify proximate causes of hypoglycemia. The EHR was programmed to identify patients with BG <40 mg/dl within 12–36 h of the event. A clinical pharmacist completed a “patient hypoglycemic event review form” for all events associated with the administration of an antihyperglycemic oral agent or insulin. Data recorded on the form included: patient demographics, serum creatinine, hemoglobin A1c (A1C) (if available), BG levels, dietary intake, use of corticosteroid medications, and dose and administration times of all antihyperglycemic medications. The proximate cause of the hypoglycemia was identified using multiple factors that were not mutually exclusive. The category of excess insulin was used if the dose was the singular proximate cause of the hypoglycemic event as verified by the absence of subsequent hypoglycemia after the insulin dose was modified. The category of inadequate monitoring was used to describe patients who demonstrated hypoglycemia (BG <70 mg/dl) without subsequent diabetic medication dose reduction and subsequently progressed to experience SH. The other categories included administration errors, hypoglycemia occurring in the setting of the treatment of hyperkalemia, and computer order entry errors. Hypoglycemic event data were entered into the hospital’s computerized safety event system. A study physician evaluated the completed forms and provided patient management recommendations and comments. The study physician then emailed the document to the patient’s hospital physician(s) for educational purposes. Patients’ physicians were asked to complete an evaluation form to assess the value of this educational tool in their care of diabetes patients.
Severe hypoglycemia event tracking at our institution utilizes a “hypoglycemic harm score,” which is generated and reviewed on a monthly basis. The numerator for the harm score is the total number of BG values <40 mg/dl occurring in the setting of active orders for diabetes therapy. Hypoglycemia events are tracked if they involve a BG level between 15 and 39 mg/dl in a patient with an order for a hypoglycemic agent that is active within 24 h of the event. Erroneous levels are identified and removed from the audit if a subsequent BG >39 mg/dl is generated within 10 min of the suspect level. The use of this rule eliminates about 10% of the total events, a rate that has been stable over time. The denominator for the harm score is defined as “at risk patient days” and involves patients with an order for a hypoglycemic agent whose hospital stays minimally include a midnight within the stay.
Study 2 used a population of inpatients found to have any BG <90 mg/dl (n = 9995 patients) during their hospital stay, corresponding to the same time period as study 1. After limiting study patients to those that had received insulin while in the hospital, 3028 patients were assessed at three levels of hypoglycemia: <70 mg/dl (n = 1525), <60 mg/dl (n = 955), and <40 mg/dl (n = 232). The risk factors identified in the study 1 analysis were used. Chi-square analyses and t-tests were run to identify bivariate associations. Significant variables were entered into a partition analysis to identify interactions. Logistic regression was performed to verify the significance of associations and interactions and to calculate parameters related to the odds of hypoglycemia below each cut point. This resulted in three separate models. Receiver operating characteristic curve analysis was used to determine sensitivity and specificity at various cut points. The cut points that resulted in 50% sensitivity for each hypo-glycemia level were determined. PASW® Statistics 18, release version 18.0.0 (IBM, Armonk, NY) and the R-part package in the R software program10,11 were used to run these analyses.
Results
Study 1 showed 244 hypoglycemic events (BG <40 mg/dl)reported during the 6-month period. Forty-five events involved erroneous BG readings, and 27 reports involved patients without orders for insulin or oral antihyper-glycemic therapy. The remaining 172 events (70% of reports) were investigated and are included in this study.Twenty patients had a second hypoglycemic event, 6 patients had a third event, and 3 patients had more than 3 events. Hypoglycemia occurred between 12:00 a.m. and 6:59 a.m. in 48% of patients, between 7:00 a.m. and 3:59 p.m.in 30% of patients, and between 4:00 p.m. and 11:59 p.m. in 22% of patients. In 20% of the events (34 patients), the patient’s physician was contacted by the study pharmacist, and a drug therapy modification was implemented during that hospital stay. Otherwise, contact occurred via email after discharge. Physicians found this educational feedback process useful to their medical practice: 33% very useful; 60% useful; 0% marginally useful; 6% not useful. Physicians rated the future impact of the intervention on their care of diabetes patients: 36% significant impact; 50% important impact; 7% slight impact; 7% no impact. Patient variables and proximate causes associated with the hypoglycemic events are presented in Figures 1 and 2. As a consequence of these efforts, the hypoglycemia harm score was 5.01 events in 2008, compared with 3.90 in 2010.
Figure 1.
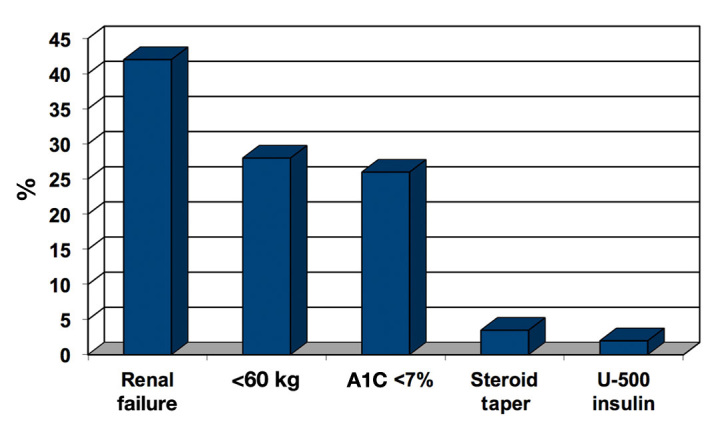
Percentage of patient variables associated with hypoglycemic events.
Figure 2.
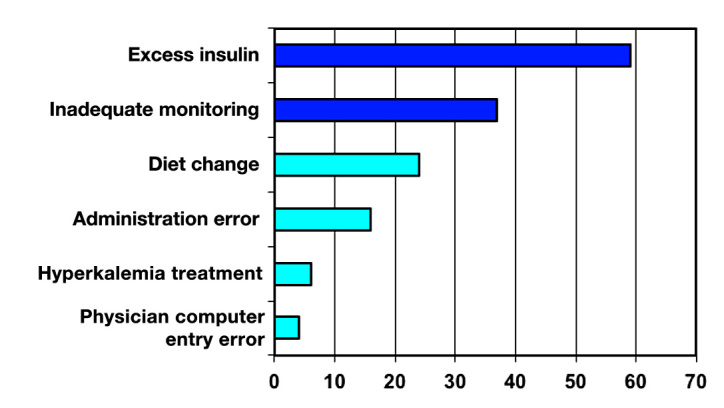
Incidence percentage of proximate causes of hypoglycemia.
Study 2 developed models that predicted levels of hypo-glycemia and used the adjudicated population to define sensitivity and specificity for prediction. The results of the bivariate tests are shown in Table 1. Blood glucose levels were related to receiving both prandial and adjust-ment scale insulin (chi-square p < .05, linear association p < .01), basal dose (chi-square p < .001, linear association p < .001), weight (chi-square p < .001, linear association p < .001), and creatinine clearance (chi-square p < .001, linear association p < .001). Significant linear associations were found for the combination of sulfonylurea and adjustment scale insulin (p < .05) and an interaction of sulfonylurea use by dose of basal insulin (p < .05).
Table 1.
Bivariate Associations with Glucose Level (mg/dl)
| Variable and level | BG ≥60 and <90 | BG ≥40 and <60 | BG <40 | Total BG <90 | Chi-square p-value | Linear association p-value | |
|---|---|---|---|---|---|---|---|
| Prandial insulin | Yes | 238 (11.5%) | 96 (13.3%) | 33 (14.2%) | 367 (12.1%) | 0.263 | 0.107 |
| No | 1835 (88.5%) | 627 (86.7%) | 199 (85.8%) | 2661 (87.9%) | |||
| Adjustment scale insulin | Yes | 1614 (77.9%) | 553 (76.5%) | 194 (83.6%) | 2361 (78.0%) | 0.072 | 0.278 |
| No | 459 (22.1%) | 170 (23.5%) | 38 (16.4%) | 667 (22.0%) | |||
| Sulfonylurea | Yes | 162 (7.8%) | 70 (9.7%) | 13 (5.6%) | 245 (8.1%) | 0.100 | 0.996 |
| No | 1911 (92.2%) | 653 (90.3%) | 219 (94.4%) | 2783 (91.9%) | |||
| Prandial and adjustment scale | Yes | 43 (49.4%) | 34 (61.8%) | 19 (82.6%) | 96 (58.2%) | 0.013 | 0.004 |
| No | 44 (50.6%) | 21 (38.2%) | 4 (17.4%) | 69 (41.8%) | |||
| Sulfonylurea and adjustment scale | Yes | 24 (75.0%) | 17 (94.4%) | 6 (100.0%) | 47 (83.9%) | 0.105 | 0.043 |
| No | 8 (25.0%) | 1 (5.6%) | 0 (0.0%) | 9 (16.1%) | |||
| Sulfonylurea and basal dose | Yes | 32 (19.8%) | 18 (25.7%) | 6 (46.2%) | 56 (22.9%) | 0.074 | 0.037 |
| No | 130 (80.2%) | 52 (74.3%) | 7 (53.8%) | 189 (77.1%) | |||
| Three level basal dose | ≥.25 | 256 (12.3%) | 175 (24.2%) | 63 (27.2%) | 494 (16.3%) | <.001 | <.001 |
| >0 and <.25 | 282 (13.6%) | 166 (23.0%) | 61 (26.3%) | 509 (16.8%) | |||
| No basal dose | 1535 (74.0%) | 382 (52.8%) | 108 (46.6%) | 2025 (66.9%) | |||
| Four level weight | ≥80 | 1142 (57.2%) | 345 (50.1%) | 90 (40.5%) | 1577 (54.2%) | <.001 | <.001 |
| ≥70 and <80 | 337 (16.9%) | 111 (16.1%) | 45 (20.3%) | 493 (17.0%) | |||
| ≥60 and <70 | 263 (13.2%) | 105 (15.3%) | 44 (19.8%) | 412 (14.2%) | |||
| <60 | 255 (12.8%) | 127 (18.5%) | 43 (19.4%) | 425 (14.6%) | |||
| Three level creatinine clearance | ≥48 | 1264 (65.7%) | 343 (50.7%) | 101 (45.9%) | 1708 (60.5%) | <.001 | <.001 |
| ≥38 and <48 | 187 (9.7%) | 82 (12.1%) | 17 (7.7%) | 286 (10.1%) | |||
| <38 | 474 (24.6%) | 252 (37.2%) | 102 (46.4%) | 828 (29.3%) | |||
The logistic regressions indicated that variables related to BG <40 mg/dl were basal and adjustment scale insulin dose, weight, and creatinine clearance, while variables related to 60 mg/dl and 70 mg/dl cut points were basal, prandial, and adjustment scale insulin dose, weight, creatinine clearance, and sulfonylurea use (Table 2). The area under the curve (AUC) value for the <60 mg/dlequation was 0.692 and the Nagelkerke R2 was 0.134 (Table 3). The 50% sensitivity cut point correctly identified 70% of the adjudicated patients. The same analysis for <40 mg/dl gave an AUC value of 0.697, and the Nagelkerke R2 was 0.095. The 50% sensitivity cut point correctly identified 55% of the adjudicated cases. For <70 mg/dl, an AUC value of 0.679 and a Nagelkerke R2 of 0.129 were found. The 50% sensitivity cut point correctly identified 71% of the adjudicated cases. Eliminating events where a value below 60 mg/dl occurred as an initial value, we were able to predict 60.3% of events <60 mg/dl with our equation. This reflected 324 patients, of whom 78 (24.1%) had BG <40 mg/dl and met the criteria for SH. A <60 mg/dl alert was triggered in 28.1% of patients, where a value <60 mg/dl did not occur while in the hospital. The equation used for the alert was:
Table 2.
Model Parameters
| Risk score | Constant | Basal <.25 units/kg | Basal ≥.25 units/kg | Weight 60–69 kga | Weight 70–79 kg | Weight ≥80 kg | Creatinine clearance 38–47b | Creatinine clearance ≥48 | Adjustment dose yes | Meal dose yes | Adjustment and meal | Sulfonylurea yes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucose <40 | –2.490 | 1.090 | 1.693 | -0.375 | 0.065 | -0.375 | -0.907 | -0.792 | 0.402 | — | — | — |
| With weight 60–69 | — | 0.791 | 0.042 | — | — | — | — | — | — | — | — | — |
| With weight 70–79 | — | –0.139 | –1.166 | — | — | — | — | — | — | — | — | — |
| With weight ≥80 | — | –0.551 | –1.020 | — | — | — | — | — | — | — | — | — |
| Glucose <60c | -0.055 | 1.062 | 1.234 | –0.294 | –0.540 | –0.786 | –0.389 | –0.680 | –0.239 | –0.556 | 0.951 | 0.336 |
| Glucose <70 | 0.577 | 0.908 | 1.254 | –0.153 | –0.360 | –0.690 | –0.402 | –0.626 | –0.101 | –0.062 | 0.702 | 0.296 |
Referent category weight <60 kg
Referent category creatinine clearance <38
Used for alert score
Table 3.
Testing
| Testing Against Each Cut Point | ||||||||
|---|---|---|---|---|---|---|---|---|
| Glucose cut | Nagelkerke R2 | AUC rounded | Sensitivity at cut point | Specificity at cut point | Predictive value of a positive test | Predictive value of a negative test | False positive number (% of those at glucose level or above) | False negative number (% of those below glucose level) |
| 40 | 0.10 | 0.70 | 0.50 | 0.79 | 0.17 | 0.95 | 541 (21%) | 107 (50%) |
| 60 | 0.13 | 0.69 | 0.54 | 0.74 | 0.49 | 0.78 | 492 (26%) | 400 (46%) |
| 70 | 0.13 | 0.68 | 0.51 | 0.76 | 0.69 | 0.60 | 325 (24%) | 692 (49%) |
| Testing Against Severe Hypoglycemia (BG <40 mg/dl) | ||||||||
|---|---|---|---|---|---|---|---|---|
| BG cut point mg/dl | Sensitivity at cut point | Specificity at cut point | Predictive value of a positive test | Predictive value of a negative test | False positives | False negatives | Number (%) of those in 6-month study identified | Number (%) of those in 6-month study not identified |
| 40 | 0.50 | 0.79 | 0.17 | 0.95 | 541 (21%) | 107 (50%) | 63 (54.8) | 52 (45.2) |
| 60 | 0.58 | 0.67 | 0.13 | 0.95 | 837 (33%) | 90 (42%) | 81 (70.4) | 34 (29.6) |
| 70 | 0.61 | 0.65 | 0.13 | 0.95 | 903 (36%) | 84 (39%) | 82 (71.3) | 33 (28.7) |
(value <60) = –0.055 + 1.062 × (basal <0.25 U/kg) + 1.234 × (basal ≥0.25 U/kg) – 0.294 × (weight 60–69 kg) – 0.540 × (weight 70–79 kg) – 0.786 × (weight ≥80 kg) –0.389 × (creatinine clearance 38–47) – 0.680 × (creatinine clearance ≥48) – 0.239 × (sliding yes) – 0.556 × (meal yes) + 0.951 × (sliding and meal) + 0.336 × (sulfonylurea yes)
Risk Score = 100 × [exp (value <60)/(1 + exp (value <60)].
Discussion
Treatment of hyperglycemia in hospitalized patients carries a significant risk for iatrogenic hypoglycemia. The risk for harm is less clear because the events are often transient and rapidly treated in the inpatient setting.1,3,12–14 Outpatient studies have more complete data on the prevalence of severe events requiring intervention. The Diabetes Control and Complications Trial reported 61 episodes per 100 patient years of SH in the intensive treatment arm,15 and the 2007 U.K.2 Hypoglycemia Study Group in patients with either type 1 or type 2 diabetes reported 110 episodes per 100 patient years.16 Overall, the risk of hypoglycemia is less prevalent in type 2 diabetes patients, but with advanced disease, the rate approaches that seen in type 1 diabetes. The U.K.2 reported 1020 episodes of mild hypoglycemia and 70 episodes of SH per 100 patient years in type 2 diabetes patients treated with insulin for >5 years. Based on this data, patients admitted to the hospital on antihyperglycemic therapy are clearly at risk for further events.
Development of hypoglycemia has been linked to mortality and morbidity, although direct causality has been questioned through two arguments. The first relates to the timing of the events in relationship to the morbid outcome, and the second is the suggestion that SH simply identifies persons with greater underlying illness severity. The timing argument is clearly flawed because an event can create a cascade that predisposes to a fatal outcome such as an arrhythmia.12,17–19 Additionally, sampling of the blood glucose may miss the hypoglycemia event entirely. Continuous glucose monitoring system data clearly show that hypoglycemic events are being under-reported by self-monitoring of blood glucose and may be clinically silent.1,20,21 Data linking SH to brain death and arrhythmia has been reported in humans and in animal models. The level of hypoglycemia needed to cause cell death is not entirely clear, but once the process has been programmed, treatment with glucose may have detrimental effects on survival.22,23 These findings support the notion that prediction and prevention of hypoglycemia should be the top priority and that an intervention before cell death is needed to avoid morbidity and mortality.
Hospitalized patients with diabetes have an increased incidence of medical errors, incurring >0.53 errors per patient days and >1.72 errors per patient period.9 The office of the inspector general has listed SH as a “diabetes never event.”24 Given the prevalence of diabetes in the general population and in the hospital setting, it is not surprising that “diabetes never events” are the most common among serious medical errors. Interestingly, setting higher glucose targets does not necessarily prevent hypoglycemia.20 Clearly there is a need for clinicians to improve their understanding of risk factors for inpatient SH and for health system initiatives to facilitate an improved flow of actionable information to the patient care providers.
Implementation of the initial hypoglycemic surveillance and education program at Barnes-Jewish Hospital has produced increased awareness of the risks of hypo-glycemia among the hospital’s administrative, nursing, pharmacy, and medical staffs. The data outlined in the original 172-patient data set confirms the findings of other investigators in demonstrating that nocturnal hypo-glycemia is a common problem. We have demonstrated that risk factors for hypoglycemia include acute kidney injury or end-stage renal disease, low body weight (weight <60 kg), A1C <7%, and corticosteroid tapering therapy. The proximate causes of hypoglycemia in our study population included excessive insulin doses, inadequate monitoring of blood glucose trends, changes in nutritional status without a change in the antidiabetic therapy, nursing administration errors, and insufficient glucose with insulin for acute treatment of hyperkalemia. Consequently, interventions were developed that included: a recommended change in the treatment for acute hyperkalemia,25 creation of a nursing educational tool that focuses on using critical thinking skills in the assessment of patients receiving insulin, implementation of a case-based insulin dosing curriculum for all medical staff, and a redesign of the hospital’s supplementary insulin dosing protocols for bedtime administration. These changes have decreased the rate of SH <40 mg/dl from a rate of 5.01 events per 1000 at-risk patient days in 2008 to 3.90 per 1000 at-risk patient days in 2010 at Barnes-Jewish Hospital. This improvement confirms that changes in systems and policies can have an impact. However, further improvement will require a more creative approach.
The presence of antecedent mild hypoglycemia is commonly reported.8,26 We saw antecedent hypoglycemia in >60% of the 172 severe events, suggesting that identification of at-risk individuals and prevention is possible. The difference in the reported rates for antecedent hypoglycemia in the literature is related to the target that is used in the analysis and the threshold for the mild event as well as the definition of the hypoglycemic target. The target chosen at our institution for the analysis was a BG ≤90 mg/dl, based on the 2006 American College ofEndocrinology Guidelines, which contrasts with a target BG <100 mg/dl in the American Diabetes Association consensus guideline and the Society of Hospital Medicine guidelines.27,28 Despite this change, the use of the 90 mg/dl cut point benefits our analysis because it allows the partition analysis to be more specific to the development of true or risk-related hypoglycemia and should limit the false positive rates in the predictive equation.
We analyzed our data at three cut points to create the predictive equations for <70 mg/dl, <60 mg/dl, and <40 mg/dl. These levels were somewhat arbitrary but have scientific backing. The accepted level for the definition of hypoglycemia in outpatients is 70 mg/dl, which corresponds to the level at which counterregulatory hormones are released.29,30 The basis for 60 mg/dl as a population grouping was it is clearly in the low range but is still above the 50 mg/dl level where cognitive impairment occurs. A widely accepted definition of SH in the hospital is any glucose <40 mg/dl. This definition is somewhat arbitrary, since the effects of “mild” hypo-glycemia can be severe in individuals who have underlying cardiac or cerebrovascular disease. Variables that defined the populations <70 mg/dl, <60 mg/dl, and <40 mg/dl were similar, but the cut points varied, with many of them showing a trend for linearity. Rubin published an analysis31 of the total daily dose (TDD) of insulin as it relates to the odds ratio of hypoglycemia at <70 mg/dl, showing an increasing odds ratio from 1.08 at a TDD of 0.2–0.4 units/kg to 2.95 at a TDD >0.8 units/kg. Our bivariate and the logistic regression analyses informed the variables that were used to develop the model. Variables related to BG <60 mg/dl were basal, prandial, and adjustment scale insulin dose, weight, creatinine clearance, and sulfonylurea use. The <40 mg/dl group had fewer predictive variables but included an interaction of basal dose and weight that was not seen in the other models.
Predictive areas were similar to each other, with the equations designed at 60 mg/dl and 70 mg/dl having the best R2 values. The 60 mg/dl group was found to be superior compared to the 70 mg/dl group in regard to the predictive value of a negative test without a substantial change in the false positive or false negative rates (26 and 46% vs 24 and 49%). The 60 mg/dl proved to be superior to the 70 mg/dl equation in its ability to predict and identify the SH group with fewer falsepositives and a smaller number of total alerts (927 vs 987) based on the 6-month analysis. This analysis suggests that the 60 mg/dl equation would represent a reasonable compromise. By eliminating events where an antecedent glucose <60 mg/dl occurred, we were able to predict 60.3% of events <60 mg/dl with our equation. The threshold occurred in 40% before an alert could be activated, suggesting the need to look at prehospital variables in a more standardized way.
The alert development process has had an impact on the incidence of SH at our institution and is a model that can be implemented at other institutions. The failure to predict 40% of the events in the original analysis suggests that a predictive equation is only one tool among many that will need to be developed to protect all inpatients from harm. The work presented here shows that decision analysis can be driven by risk data and that interventions can be designed to reduce the risk of hypoglycemia in hospitalized patients.
Glossary
Abbreviations
- (A1C)
hemoglobin A1c
- (AUC)
area under the curve
- (BG)
blood glucose
- (EHR)
electronic health record
- (LSH)
less severe hypoglycemia
- (SH)
severe hypoglycemia
- (TDD)
total daily dose
Funding
This work was funded by the Barnes-Jewish Hospital Foundation and the Diabetes Research and Training Center Grant #P60 DK20579.
Acknowledgements
This work was made possible by the contributions of the entire Barnes-Jewish Hospital Diabetes Management Committee as well as the enthusiastic support of the Barnes-Jewish nursing leadership shown by Elizabeth Pratt, DNP. The creation of the alert program code was accomplished and supported by Kevin Heard, Richard Reichley RPh, and Nicholas Hampton PharmD at Barnes-Jewish Hospital.
Disclosures
Dr. Garry S. Tobin is a member of the following speakers’ bureaus: Eli Lilly, Amylin, Boehringer Ingelheim, Takeda and Santarus pharmaceuticals. Dr. Janet B. McGill has received research grants from Novartis, GSK, Mannkind, Takeda, Andromeda, AstraZeneca, and Bristol Meyer Squibb Pharmaceuticals.
References
- 1.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 2.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. Epub 2009 Mar 24. [DOI] [PubMed] [Google Scholar]
- 3.Boucai L, Southern WN, Zonszein J. Hypoglycemia-associated mortality is not drug-associated but linked to comorbidities. Am J Med. 2011;124(11):1028–1035. doi: 10.1016/j.amjmed.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kansagara D, Fu R, Freeman M, Wolf F, Helfand M. Intensive insulin therapy in hospitalized patients: a systematic review. Ann Intern Med. 2011;154(4):268–282. doi: 10.7326/0003-4819-154-4-201102150-00008. [DOI] [PubMed] [Google Scholar]
- 5.Desouza C, Salazar H, Cheong B, Murgo J, Fonseca V. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care. 2003;26(5):1485–1489. doi: 10.2337/diacare.26.5.1485. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz AV, Vittinghoff E, Sellmeyer DE, Feingold KR, de Rekeneire N, Strotmeyer ES, Shorr RI, Vinik AI, Odden MC, Park SW, Faulkner KA, Harris TB. Health, Aging, and Body Composition Study. Diabetes-related complications, glycemic control, and falls in older adults. Diabetes Care. 2008;31(3):391–396. doi: 10.2337/dc07-1152. Epub 2007 Dec 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swanson CM, Potter DJ, Kongable GL, Cook CB. Update on inpatient glycemic control in hospitals in the United States. Endocr Pract. 2011;17(6):853–861. doi: 10.4158/EP11042.OR. [DOI] [PubMed] [Google Scholar]
- 8.DiNardo M, Noschese M, Korytkowski M, Freeman S. The medical emergency team and rapid response system: finding, treating, and preventing hypoglycemia. Jt Comm J Qual Patient Saf. 2006;32(10):591–595. doi: 10.1016/s1553-7250(06)32077-6. [DOI] [PubMed] [Google Scholar]
- 9.Deal EN, Liu A, Wise LL, Honick KA, Tobin GS. Inpatient insulin orders: Are patients getting what is prescribed? Journal of Hospital Medicine. 2011;6(9):526–529. doi: 10.1002/jhm.938. Epub 2011 Oct 31. [DOI] [PubMed] [Google Scholar]
- 10.R Development Core Team (2010) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org/
- 11.Therneau TM, Atkinson B. R port by Ripley B. rpart: Recursive Partitioning. The R Project for Statistical Computing. http://CRAN.R-project.org/package=rpart.
- 12.Cryer PE. Death during intensive glycemic therapy of diabetes: mechanisms and implications. Am J Med. 2011;124(11):993–996. doi: 10.1016/j.amjmed.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kagansky N, Levy S, Rimon E, Cojocaru L, Fridman A, Ozer Z, Knobler H. Hypoglycemia as a predictor of mortality in hospitalized elderly patients. Arch Intern Med. 2003;163(15):1825–1829. doi: 10.1001/archinte.163.15.1825. [DOI] [PubMed] [Google Scholar]
- 14.Kosiborod M, Inzucchi SE, Krumholz HM, Xiao L, Jones PG, Fiske S, Masoudi FA, Marso SP, Spertus JA. Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes-based measure of risk. Circulation. 2008;117(8):1018–1027. doi: 10.1161/CIRCULATIONAHA.107.740498. Epub 2008 Feb 11. [DOI] [PubMed] [Google Scholar]
- 15.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 16.UK Hypoglycaemia Study Group Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50(6):1140–1147. doi: 10.1007/s00125-007-0599-y. Epub 2007 Apr 6. [DOI] [PubMed] [Google Scholar]
- 17.Bergner DW, Goldberger JJ. Diabetes mellitus and sudden cardiac death: what are the data? Cardiol J. 2010;17(2):117–129. [PubMed] [Google Scholar]
- 18.Robinson RT, Harris ND, Ireland RH, Lee S, Newman C, Heller SR. Mechanisms of abnormal cardiac repolarization during insulin-induced hypoglycemia. Diabetes. 2003;52(6):1469–1474. doi: 10.2337/diabetes.52.6.1469. [DOI] [PubMed] [Google Scholar]
- 19.Gill GV, Woodward A, Casson IF, Weston PJ. Cardiac arrhythmia and nocturnal hypoglycaemia in type 1 diabetes--the ‘dead in bed’ syndrome revisited. Diabetologia. 2009;52(1):42–45. doi: 10.1007/s00125-008-1177-7. Epub 2008 Oct 30. [DOI] [PubMed] [Google Scholar]
- 20.Munshi MN, Segal AR, Suhl E, Staum E, Desrochers L, Sternthal A, Giusti J, McCartney R, Lee Y, Bonsignore P, Weinger K. Frequent hypoglycemia among elderly patients with poor glycemic control. Arch Intern Med. 2011;171(4):362–364. doi: 10.1001/archinternmed.2010.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L, Woodward M, Ninomiya T, Neal B, MacMahon S, Grobbee DE, Kengne AP, Marre M, Heller S, ADVANCE Collaborative Group Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363(15):1410–1418. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 22.Cryer PE. Hypoglycemia, functional brain failure, and brain death. J Clin Invest. 2007;117(4):868–870. doi: 10.1172/JCI31669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest. 2007;117(4):910–918. doi: 10.1172/JCI30077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Quality Forum Serious Reportable Events (SREs) Transparency, accountability critical to reducing medical errors and harm. http://www.qualityforum.org/projects/completed/sre/fact-sheet.asp.
- 25.Schafers S, Naunheim R, Vijayan A, Tobin G. Incidence of hypo-glycemia following insulin-based acute stabilization of hyperkalemia treatment. Journal of Hospital Medicine. doi: 10.1002/jhm.977. Epub 2011 Nov 15. [DOI] [PubMed] [Google Scholar]
- 26.Galati S-J, Hendrickson KC, Lipska KJ, Bozzo JE, Lin Z, Inzucchi SE. Blood glucose trends and prediction of severe hypo-glycemia in hospitalized patients. San Diego: American Diabetes Association; 2011. 887–P. [Google Scholar]
- 27.ACE/ADA Task Force on Inpatient Diabetes American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control. Diabetes Care. 2006;29(8):1955–1962. doi: 10.2337/dc06-9913. [DOI] [PubMed] [Google Scholar]
- 28.Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez GE, American Association of Clinical Endocrinologists; American Diabetes Association American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119–1131. doi: 10.2337/dc09-9029. Epub 2009 May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitrakou A, Ryan C, Veneman T, Mokan M, Jenssen T, Kiss I, Durrant J, Cryer P, Gerich J. Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol. 1991;260(1 Pt 1):E67–74. doi: 10.1152/ajpendo.1991.260.1.E67. [DOI] [PubMed] [Google Scholar]
- 30.Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26(6):1902–1912. doi: 10.2337/diacare.26.6.1902. [DOI] [PubMed] [Google Scholar]
- 31.Rubin DJ, Rybin D, Doros G, McDonnell ME. Weight-based, insulin dose-related hypoglycemia in hospitalized patients with diabetes. Diabetes Care. 2011;34(8):1723–1728. doi: 10.2337/dc10-2434. Epub 2011 Jun 23. [DOI] [PMC free article] [PubMed] [Google Scholar]


